Calcium-Dependent Cytosolic Phospholipase A2α as Key Factor in Calcification of Subdermally Implanted Aortic Valve Leaflets
Abstract
1. Introduction
2. Results
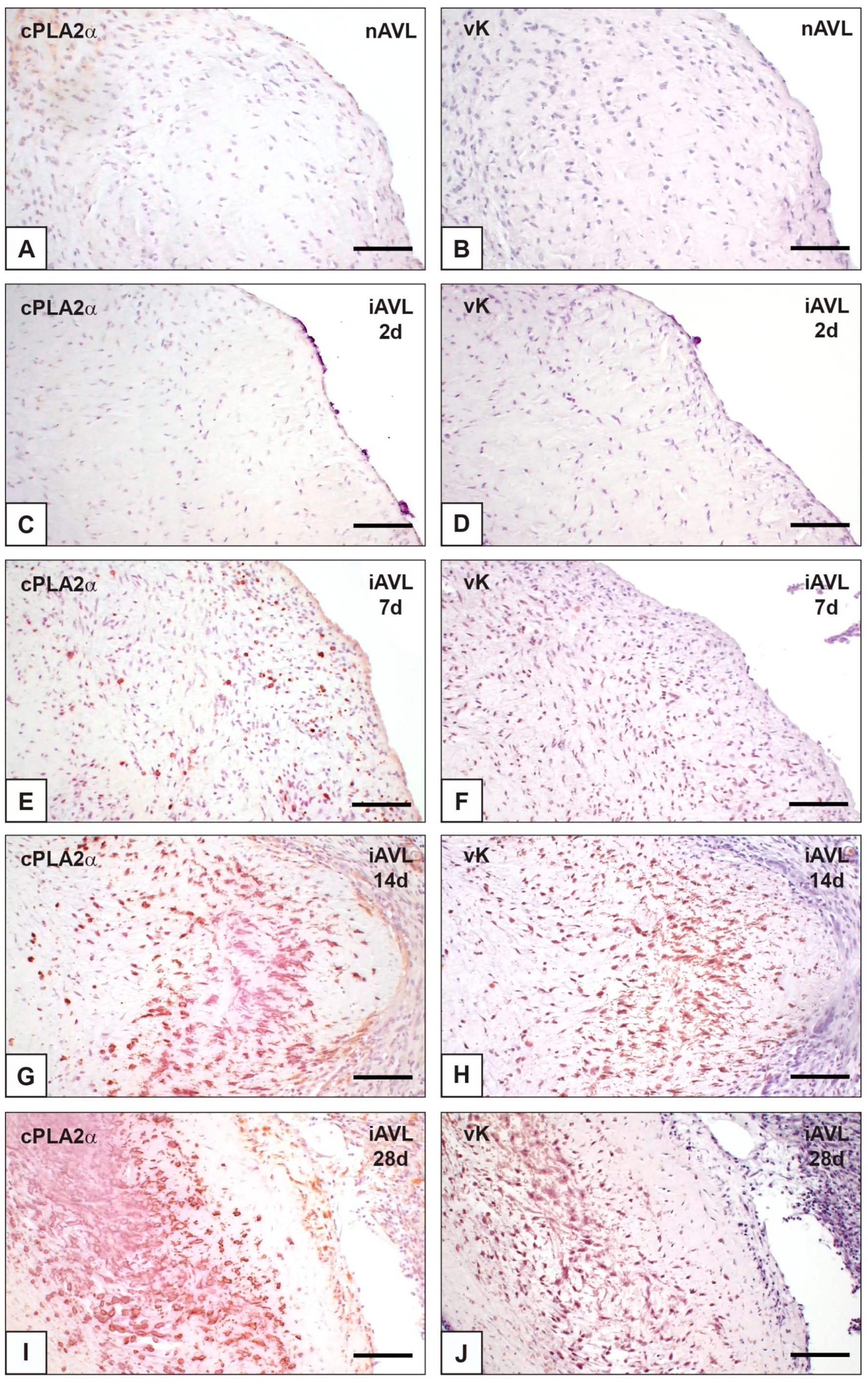
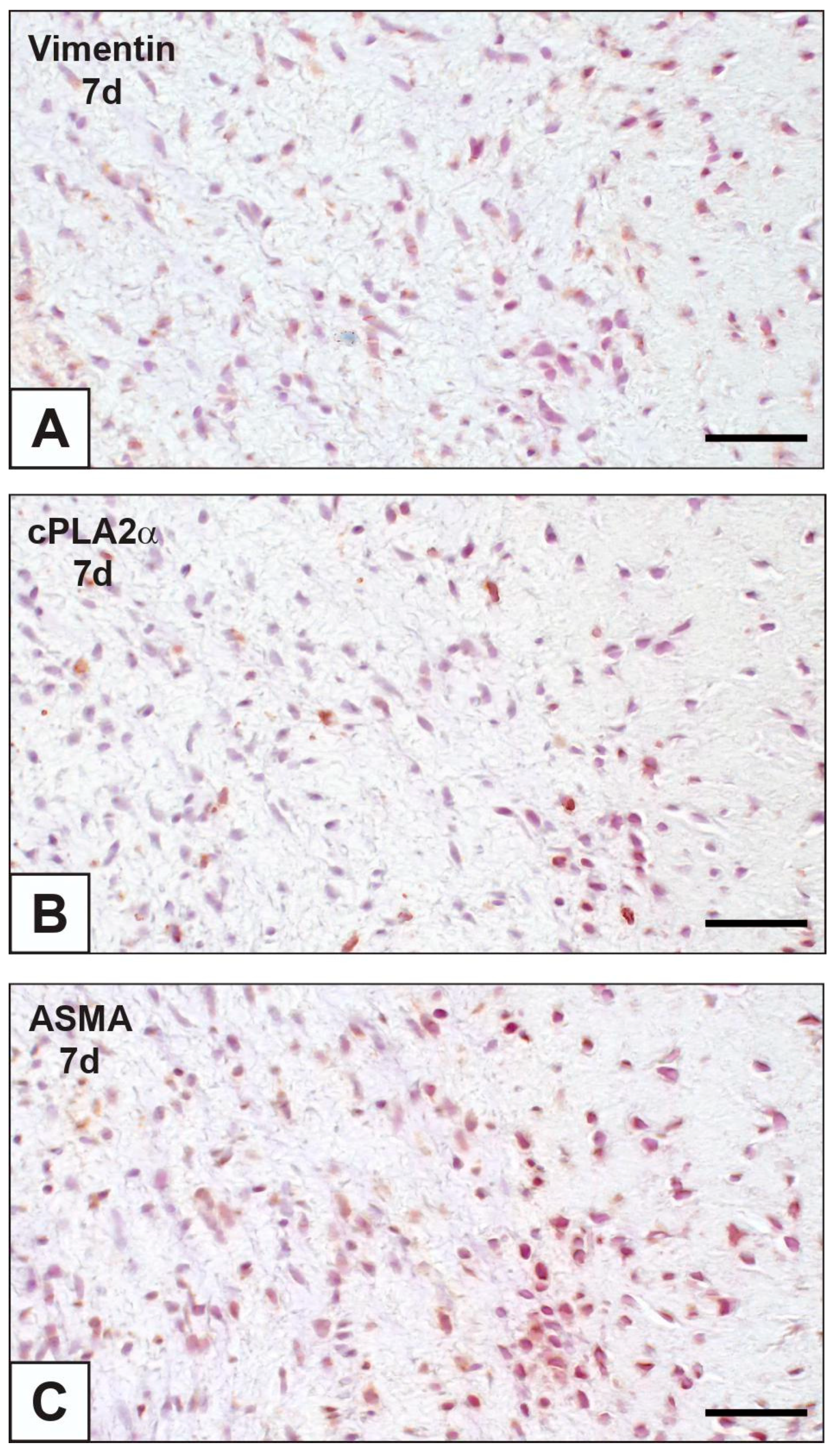
2.1. cPLA2α Expression and iAVL Calcification
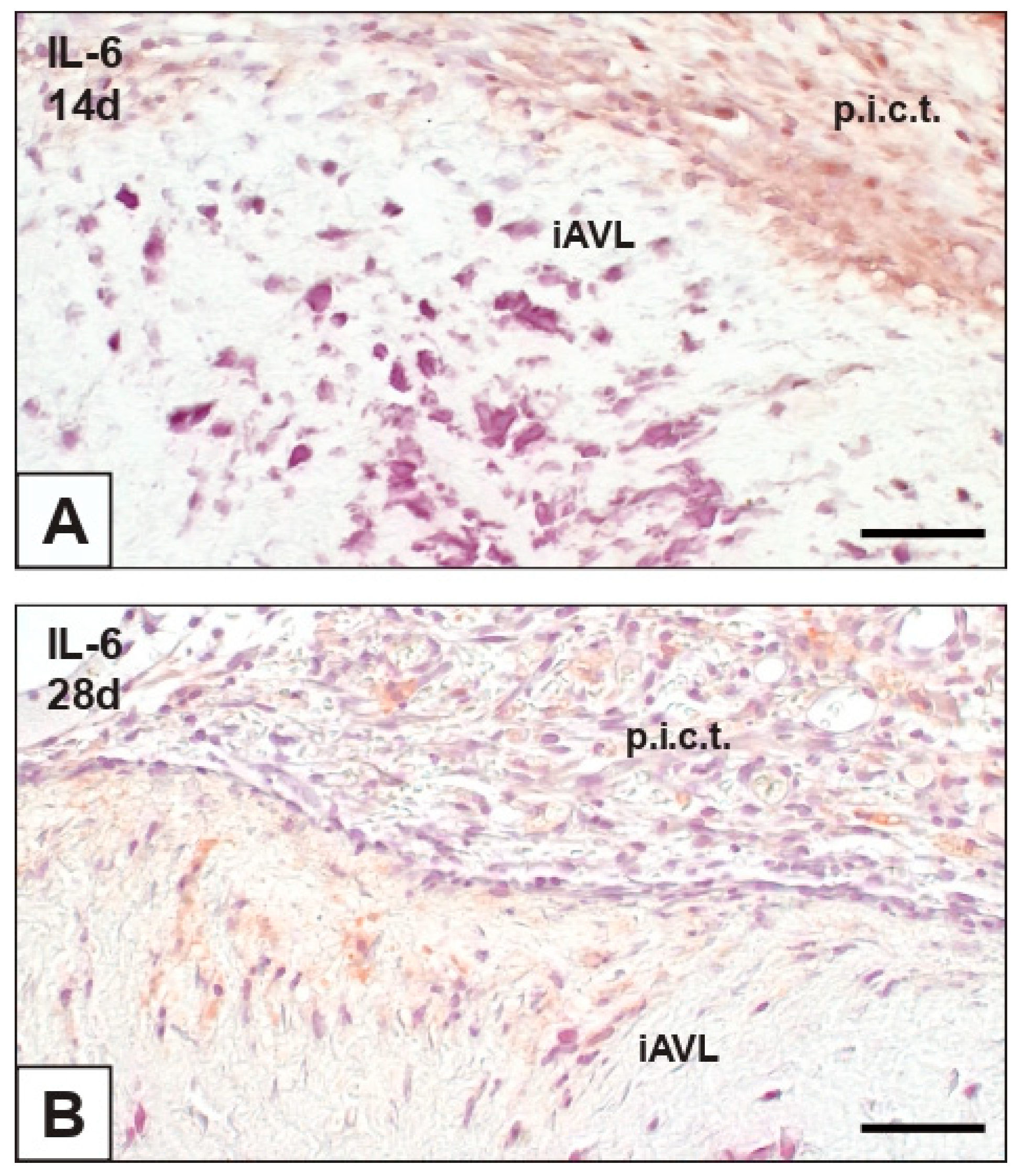
2.2. Interleukin-6 (IL-6) Expression
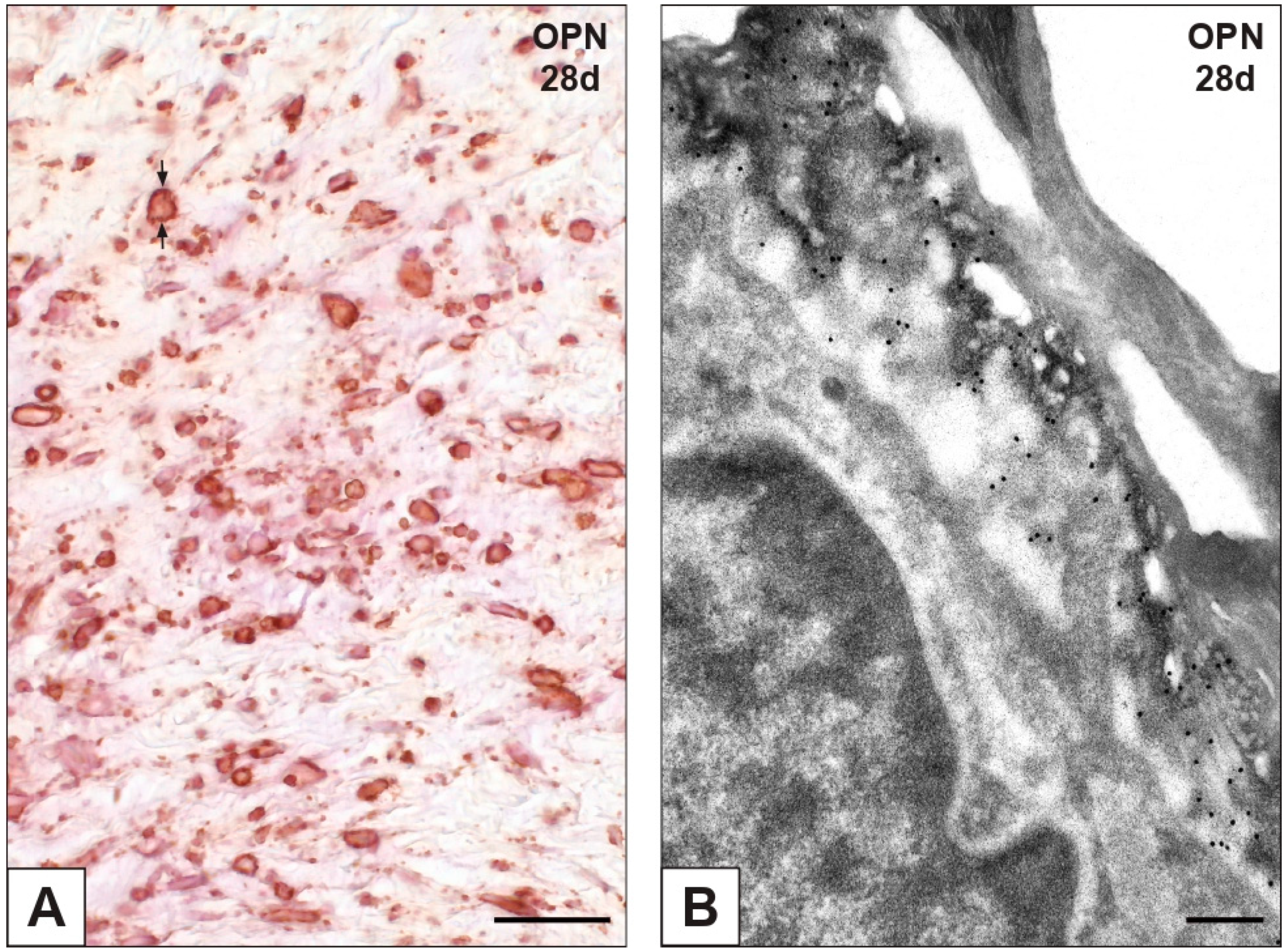
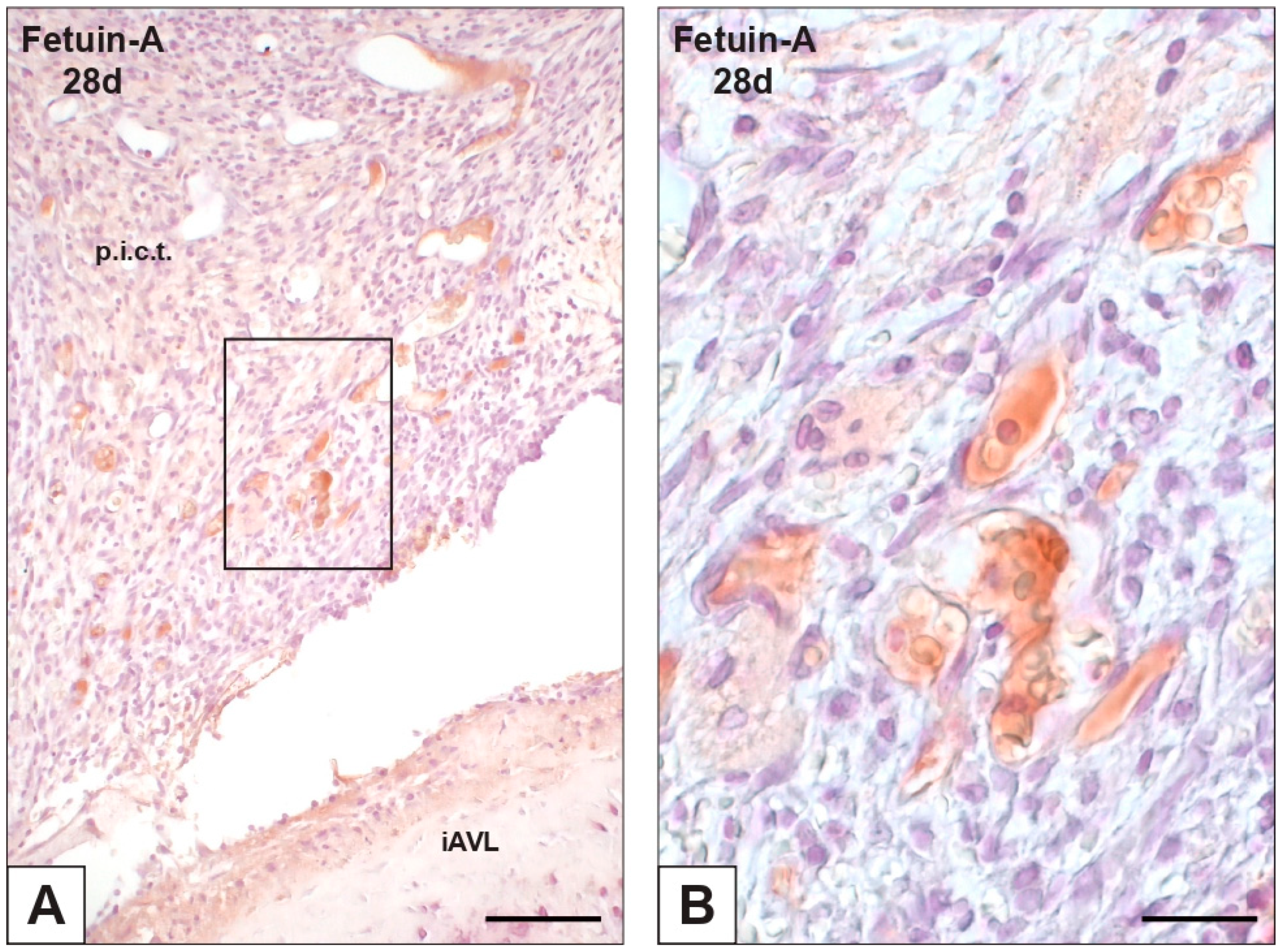
2.3. Expression of OPN and Fetuin-A
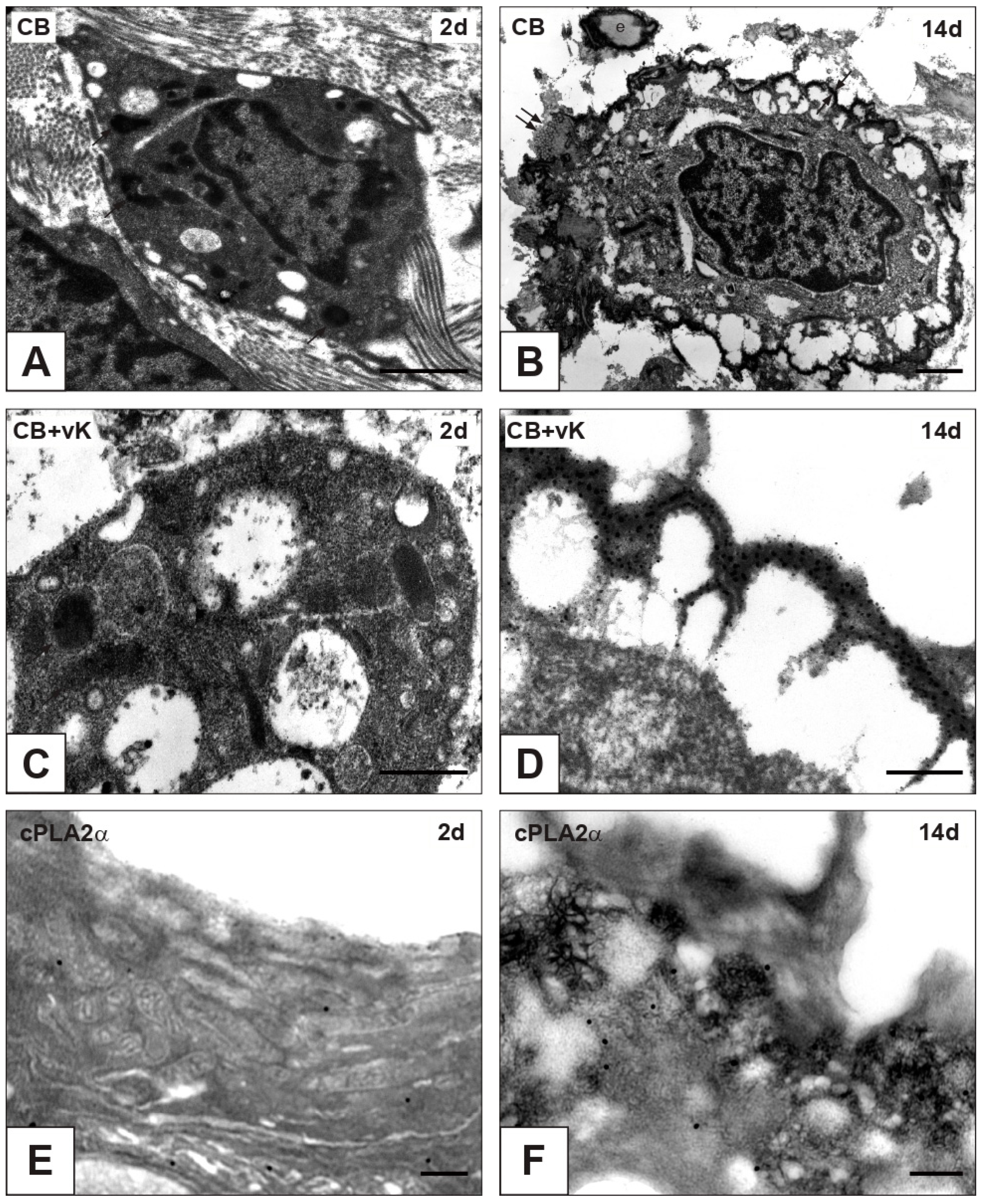
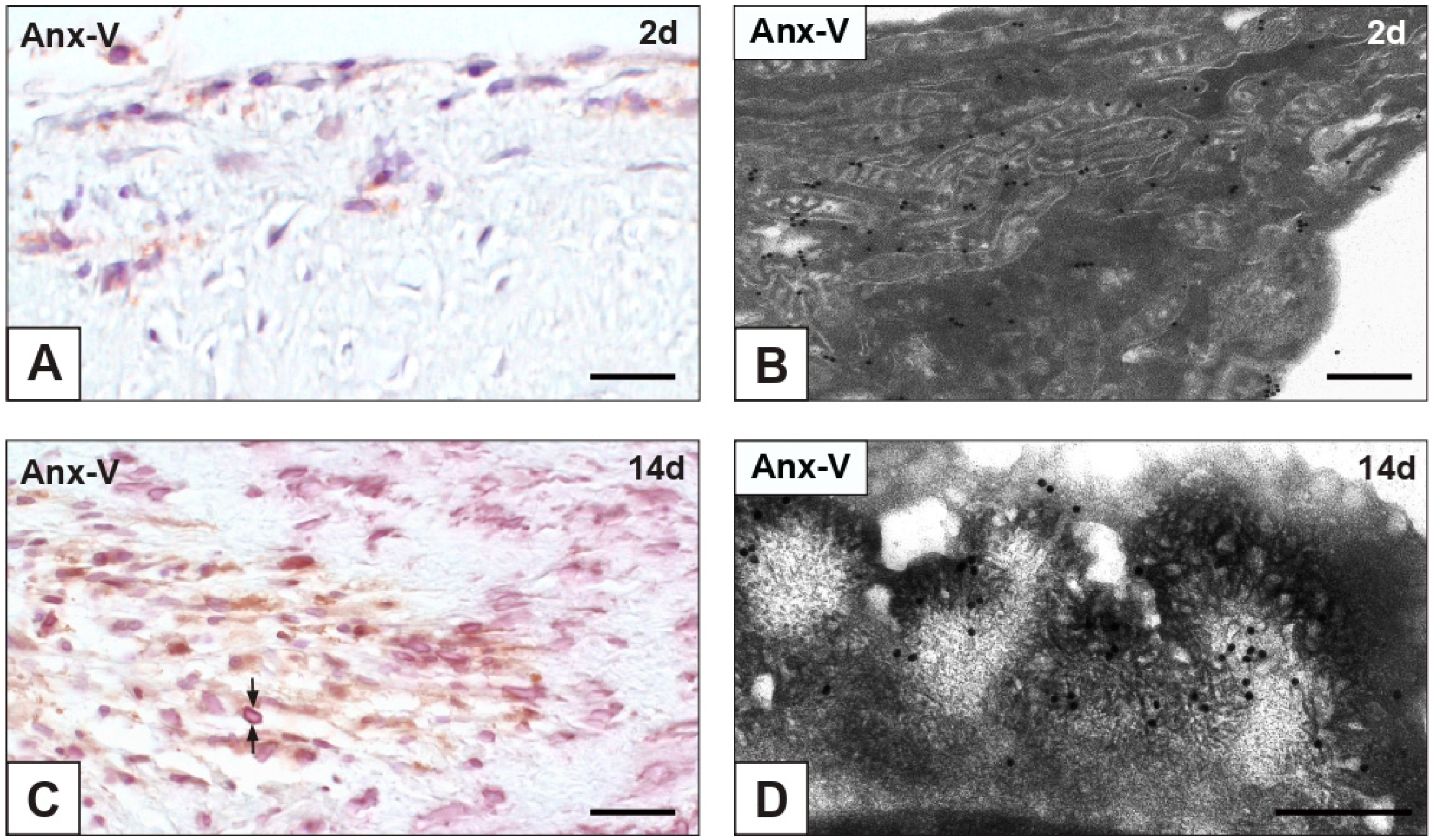
2.4. Expression of Cell Death Markers
3. Discussion
3.1. cPLA2α as Key Pro-Calcific Trigger
3.2. Non-Canonical Cell Death in iAVL Calcification
4. Materials and Methods
4.1. Sampling
4.2. Immunohistochemical Assays
4.3. Von Kossa Silver Staining for Calcium Binding Site Detection
4.4. Transmission Electron Microscopy
4.5. Post-Embedding von Kossa Reaction
4.6. Immunogold Labelling
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capoulade, R.; Clavel, M.A.; Mathieu, P.; Côté, N.; Dumesnil, J.G.; Arsenault, M.; Bédard, E.; Pibarot, P. Impact of hypertension and renin-angiotensin system inhibitors in aortic stenosis. Eur. J. Clin. Investig. 2013, 43, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Fondard, O.; Detaint, D.; Iung, B.; Choqueux, C.; Adle-Biassette, H.; Jarraya, M.; Hvass, U.; Couetil, J.P.; Henin, D.; Michel, J.B.; et al. Extracellular matrix remodelling in human aortic valve disease: The role of matrix metalloproteinases and their tissue inhibitors. Eur. Heart J. 2005, 26, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.H.; Tsimikas, S.; Pawade, T.; Kroon, J.; Jenkins, W.S.A.; Doris, M.K.; White, A.C.; Timmers, N.K.L.M.; Hjortnaes, J.; Rogers, M.A.; et al. Lipoprotein (a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J. Am. Coll. Cardiol. 2019, 73, 2150–2162. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Chu, Y.; Brooks, R.M.; Richenbacher, W.E.; Peña-Silva, R.; Heistad, D.D. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J. Am. Coll. Cardiol. 2008, 52, 843–850. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, C.; Parra-Izquierdo, I.; Castaños-Mollor, I.; López, J.; San Román, J.A.; Sánchez Crespo, M. Toll-like receptors, inflammation, and calcific aortic valve disease. Front. Physiol. 2018, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, A.; Zanobini, M.; Myasoedova, V.A.; Valerio, V.; Songia, P.; Saccocci, M.; Di Minno, M.N.D.; Tremoli, E.; Poggio, P. Could circulating fetuin A be a biomarker of aortic valve stenosis? Int. J. Cardiol. 2017, 249, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Akat, K.; Kaden, J.J.; Schmitz, F.; Ewering, S.; Anton, A.; Klomfass, S.; Hoffmann, R.; Ortlepp, J.R. Calcium metabolism in adults with severe aortic valve stenosis and preserved renal function. Am. J. Cardiol. 2010, 105, 862–864. [Google Scholar] [CrossRef]
- Soini, Y.; Salo, T.; Satta, J. Angiogenesis is involved in the pathogenesis of nonrheumatic aortic valve stenosis. Hum. Pathol. 2003, 34, 756–763. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Z. Osteogenesis in calcified aortic valve disease: From histopathological observation towards molecular understanding. Prog. Biophys. Mol. Biol. 2016, 122, 156–161. [Google Scholar] [CrossRef]
- Kim, K.M. Apoptosis and calcification. Scanning Microsc. 1995, 9, 1137–1178. [Google Scholar]
- Somers, P.; Knaapen, M.; Kockx, M.; van Cauwelaert, P.; Bortier, H.; Mistiaen, W. Histological evaluation of autophagic cell death in calcified aortic valve stenosis. J. Heart Valve Dis. 2006, 15, 43–48. [Google Scholar] [PubMed]
- Galeone, A.; Brunetti, G.; Oranger, A.; Greco, G.; Di Benedetto, A.; Mori, G.; Colucci, S.; Zallone, A.; Paparella, D.; Grano, M. Aortic valvular interstitial cells apoptosis and calcification are mediated by TNF-related apoptosis-inducing ligand. Int. J. Cardiol. 2013, 169, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Matsukuma, S.; Takeo, H.; Kono, T.; Sato, K. Fat cells and membranous fat necrosis of aortic valves: A clinicopathological study. Pathol. Int. 2013, 63, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Srivatsa, S.S.; Harrity, P.J.; Maercklein, P.B.; Kleppe, L.; Veinot, J.; Edwards, W.D.; Johnson, C.M.; Fitzpatrick, L.A. Increased cellular expression of matrix proteins that regulate mineralization is associated with calcification of native human and porcine xenograft bioprosthetic heart valves. J. Clin. Investig. 1997, 99, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Pepin, A.; Charest, A.; Perron, J.; Doyle, D.; Voisine, P.; Dagenais, F.; Pibarot, P.; Mathieu, P. Expression of bone-regulatory proteins in human valve allografts. Heart 2006, 92, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M. Calcification of matrix vesicles in human aortic valve and aortic media. Fed. Proc. 1976, 35, 156–162. [Google Scholar]
- Mathieu, P.; Voisine, P.; Pépin, A.; Shetty, R.; Savard, N.; Dagenais, F. Calcification of human valve interstitial cells is dependent on alkaline phosphatase activity. J. Heart Valve Dis. 2005, 14, 353–357. [Google Scholar]
- Hung, M.Y.; Witztum, J.L.; Tsimikas, S. New therapeutic targets for calcific aortic valve stenosis: The lipoprotein(a)-lipoprotein-associated phospholipase A2-oxidized phospholipid axis. J. Am. Coll. Cardiol. 2014, 63, 478–480. [Google Scholar] [CrossRef]
- Mahmut, A.; Boulanger, M.C.; El Husseini, D.; Fournier, D.; Bouchareb, R.; Després, J.P.; Pibarot, P.; Bossé, Y.; Mathieu, P. Elevated expression of lipoprotein-associated phospholipase A2 in calcific aortic valve disease: Implications for valve mineralization. J. Am. Coll. Cardiol. 2014, 63, 460–469. [Google Scholar] [CrossRef]
- Wuthier, R.E. The role of phospholipids in biological calcification: Distribution of phospholipase activity in calcifying epiphyseal cartilage. Clin. Orthop. 1973, 90, 191–200. [Google Scholar]
- Higashi, S.; Ohishi, H.; Kudo, I. Augmented prostaglandin E2 generation resulting from increased activities of cytosolic and secretory phospholipase A2 and induction of cyclooxygenase-2 in interleukin-1 beta-stimulated rat calvarial cells during the mineralizing phase. Inflamm. Res. 2000, 49, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M. The quest for a medical treatment of aortic stenosis: Putative therapeutic targets. Eur. Med. J. Cardiol. 2014, 2, 78–86. [Google Scholar]
- Bäck, M.; Larsson, S.C. Bioactive lipids in aortic valve stenosis-a possible link to atherosclerosis? Cardiovasc. Res. 2017, 113, 1276–1278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ortolani, F.; Petrelli, L.; Tubaro, F.; Spina, M.; Marchini, M. Novel ultrastructural features as revealed by phthalocyanine reactions indicate cell priming for calcification in subdermally implanted aortic valves. Connect. Tissue Res. 2002, 43, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, F.; Tubaro, F.; Petrelli, L.; Gandaglia, A.; Spina, M.; Marchini, M. Copper retention, calcium release and ultrastructural evidence indicate specific Cuprolinic Blue uptake and peculiar modifications in mineralizing aortic valves. Histochem. J. 2002, 34, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, F.; Petrelli, L.; Nori, S.L.; Gerosa, G.; Spina, M.; Marchini, M. Malachite green and phthalocyanine-silver reactions reveal acidic phospholipid involvement in calcification of porcine aortic valves in rat subdermal model. Histol. Histopathol. 2003, 18, 1131–1140. [Google Scholar] [PubMed]
- Ortolani, F.; Bonetti, A.; Tubaro, F.; Petrelli, L.; Contin, M.; Nori, S.L.; Spina, M.; Marchini, M. Ultrastructural characterization of calcification onset and progression in subdermally implanted aortic valves. Histochemical and spectrometric data. Histol. Histopathol. 2007, 22, 261–272. [Google Scholar] [PubMed]
- Ortolani, F.; Rigonat, L.; Bonetti, A.; Contin, M.; Tubaro, F.; Rattazzi, M.; Marchini, M. Pro-calcific responses by aortic valve interstitial cells in a novel in vitro model simulating dystrophic calcification. Ital. J. Anat. Embryol. 2010, 115, 135–139. [Google Scholar]
- Bonetti, A.; Marchini, M.; Ortolani, F. Ectopic mineralization in heart valves: New insights from in vivo and in vitro procalcific models and promising perspectives on noncalcifiable bioengineered valves. J. Thorac. Dis. 2019, 11, 2126–2143. [Google Scholar] [CrossRef]
- Bonetti, A.; Della Mora, A.; Contin, M.; Tubaro, F.; Marchini, M.; Ortolani, F. Ultrastructural and spectrophotometric study on the effects of putative triggers on aortic valve interstitial cells in in vitro models simulating metastatic calcification. Anat. Rec. 2012, 295, 1117–1127. [Google Scholar] [CrossRef]
- Bonetti, A.; Della Mora, A.; Contin, M.; Gregoraci, G.; Tubaro, F.; Marchini, M.; Ortolani, F. Survival-related autophagic activity versus procalcific death in cultured aortic valve interstitial cells treated with critical normophosphatemic-like phosphate concentrations. J. Histochem. Cytochem. 2017, 65, 125–138. [Google Scholar] [CrossRef]
- Bonetti, A.; Allegri, L.; Baldan, F.; Contin, M.; Battistella, C.; Damante, G.; Marchini, M.; Ortolani, F. Critical involvement of calcium-dependent cytosolic phospholipase A2a in aortic valve interstitial cell calcification. Int. J. Mol. Sci. 2020, 21, 6398. [Google Scholar] [CrossRef]
- Bonetti, A.; Bonifacio, A.; Della Mora, A.; Livi, U.; Marchini, M.; Ortolani, F. Carotenoids co-localize with hydroxyapatite, cholesterol, and other lipids in calcified stenotic aortic valves. Ex vivo Raman maps compared to histological patterns. Eur. J. Histochem. 2015, 59, 2505. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.J.; Schoen, F.J.; Levy, J.T.; Nelson, A.C.; Howard, S.L.; Oshry, L.J. Biologic determinants of dystrophic calcification and osteocalcin deposition in glutaraldehyde-preserved porcine aortic valve leaflets implanted subcutaneously in rats. Am. J. Pathol. 1983, 113, 143–155. [Google Scholar] [PubMed]
- Lin, L.L.; Lin, A.Y.; DeWitt, D.L. Interleukin-1 alpha induces the accumulation of cytosolic phospholipase A2 and the release of prostaglandin E2 in human fibroblasts. J. Biol. Chem. 1992, 267, 23451–23454. [Google Scholar] [CrossRef]
- Murakami, M.; Kuwata, H.; Amakasu, Y.; Shimbara, S.; Nakatani, Y.; Atsumi, G.; Kudo, I. Prostaglandin E2 amplifies cytosolic phospholipase A2- and cyclooxygenase-2-dependent delayed prostaglandin E2 generation in mouse osteoblastic cells. Enhancement by secretory phospholipase A2. J. Biol. Chem. 1997, 272, 19891–19897. [Google Scholar] [CrossRef]
- Lo, M.V.; Woodruff, T.M. Complement: Bridging the innate and adaptive immune systems in sterile inflammation. J. Leukoc. Biol. 2020, 108, 339–351. [Google Scholar] [CrossRef]
- Human, P.; Zilla, P. Characterization of the immune response to valve bioprostheses and its role in primary tissue failure. Ann. Thorac. Surg. 2001, 71, S385–S388. [Google Scholar] [CrossRef]
- Kim, M.S.; Jeong, S.; Lim, H.G.; Kim, Y.J. Differences in xenoreactive immune response and patterns of calcification of porcine and bovine tissues in α-Gal knock-out and wild-type mouse implantation models. Eur. J. Cardiothorac. Surg. 2015, 48, 392–399. [Google Scholar] [CrossRef]
- Manji, R.A.; Zhu, L.F.; Nijjar, N.K.; Rayner, D.C.; Korbutt, G.S.; Churchill, T.A.; Rajotte, R.V.; Koshal, A.; Ross, D.B. Glutaraldehyde-fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejection. Circulation 2006, 114, 318–327. [Google Scholar] [CrossRef]
- Hadad, N.; Tuval, L.; Elgazar-Carmom, V.; Levy, R.; Levy, R. Endothelial ICAM-1 protein induction is regulated by cytosolic phospholipase A2α via both NF-κB and CREB transcription factors. J. Immunol. 2011, 186, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Li, R.C.; Zhang, J.; Klaus, J.A.; Kibler, K.K.; Doré, S.; Koehler, R.C.; Sapirstein, A.J. Cytosolic phospholipase A2 alpha amplifies early cyclooxygenase-2 expression, oxidative stress and MAP kinase phosphorylation after cerebral ischemia in mice. Neuroinflammation 2010, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Maranto, A.R.; Schoen, F.J. Phosphatase enzyme activity is retained in glutaraldehyde treated bioprosthetic heart valves. ASAIO Trans. 1988, 34, 827–830. [Google Scholar] [PubMed]
- Baudry, A.; Bitard, J.; Mouillet-Richard, S.; Locker, M.; Poliard, A.; Launay, J.M.; Kellermann, O. Serotonergic 5-HT2B receptor controls tissue-nonspecific alkaline phosphatase activity in osteoblasts via eicosanoids and phosphatidylinositol-specific phospholipase C. J. Biol. Chem. 2010, 285, 26066–26073. [Google Scholar] [CrossRef]
- O’Brien, K.D.; Kuusisto, J.; Reichenbach, D.D.; Ferguson, M.; Giachelli, C.; Alpers, C.E.; Ottto, C.M. Osteopontin is expressed in human aortic valvular lesions. Circulation 1995, 92, 2163–2168. [Google Scholar] [CrossRef]
- Mohler, E.R., 3rd; Adam, L.P.; McClelland, P.; Graham, L.; Hathaway, D.R. Detection of osteopontin in calcified human aortic valves. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 547–552. [Google Scholar] [CrossRef]
- Shen, M.; Marie, P.; Farge, D.; Carpentier, S.; De Pollak, C.; Hott, M.; Chen, L.; Martinet, B.; Carpentier, A. Osteopontin is associated with bioprosthetic heart valve calcification in humans. C. R. Acad. Sci. III 1997, 320, 49–57. [Google Scholar] [CrossRef]
- Hunter, G.K. Role of osteopontin in modulation of hydroxyapatite formation. Calcif. Tissue Int. 2013, 93, 348–354. [Google Scholar] [CrossRef]
- Boskey, A.L.; Maresca, M.; Ullrich, W.; Doty, S.B.; Butler, W.T.; Prince, C.W. Osteopontin-hydroxyapatite interactions in vitro: Inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993, 22, 147–159. [Google Scholar] [CrossRef]
- Sainger, R.; Grau, J.B.; Poggio, P.; Branchetti, E.; Bavaria, J.E.; Gorman, J.H., 3rd; Gorman, R.C.; Ferrari, G. Dephosphorylation of circulating human osteopontin correlates with severe valvular calcification in patients with calcific aortic valve disease. Biomarkers 2012, 17, 111–118. [Google Scholar] [CrossRef]
- Rajamannan, N.M.; Subramaniam, M.; Rickard, D.; Stock, S.R.; Donovan, J.; Springett, M.; Orszulak, T.; Fullerton, D.A.; Tajik, A.J.; Bonow, R.O.; et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 2003, 107, 2181–2184. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Fullerton, D.A.; Ao, L.; Zheng, D.; Zhao, K.; Meng, X. BMP-2 and TGF-β1 mediate biglycan-induced pro-osteogenic reprogramming in aortic valve interstitial cells. J. Mol. Med. 2015, 93, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wu, H.; Bai, Y.; Gong, D.; Xia, C.; Li, Q.; Lu, F.; Xu, Z. Evidence of osteogenic regulation in calcific porcine aortic valves. Heart Surg. Forum. 2018, 21, E375–E381. [Google Scholar] [CrossRef] [PubMed]
- Mohler, E.R., 3rd; Gannon, F.; Reynolds, C.; Zimmerman, R.; Keane, M.G.; Kaplan, F.S. Bone formation and inflammation in cardiac valves. Circulation 2001, 103, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Steiner, I.; Kasparová, P.; Kohout, A.; Dominik, J. Bone formation in cardiac valves: A histopathological study of 128 cases. Virchows Arch. 2007, 450, 653–657. [Google Scholar] [CrossRef]
- Kaden, J.J.; Reinöhl, J.O.; Blesch, B.; Brueckmann, M.; Haghi, D.; Borggrefe, M.; Schmitz, F.; Klomfass, S.; Pillich, M.; Ortlepp, J.R. Systemic and local levels of fetuin-A in calcific aortic valve stenosis. Int. J. Mol. Med. 2007, 20, 193–197. [Google Scholar] [CrossRef]
- Kubota, N.; Testuz, A.; Boutten, A.; Robert, T.; Codogno, I.; Duval, X.; Tubiana, S.; Hekimian, G.; Arangalage, D.; Cimadevilla, C.; et al. Impact of Fetuin-A on progression of calcific aortic valve stenosis—The COFRASA-GENERAC study. Int. J. Cardiol. 2018, 265, 52–57. [Google Scholar] [CrossRef]
- Jirak, P.; Stechemesser, L.; Moré, E.; Franzen, M.; Topf, A.; Mirna, M.; Paar, V.; Pistulli, R.; Kretzschmar, D.; Wernly, B.; et al. Clinical implications of fetuin-A. Adv. Clin. Chem. 2019, 89, 79–130. [Google Scholar]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Adam-Klages, S.; Schwandner, R.; Lüschen, S.; Ussat, S.; Kreder, D.; Krönke, M. Caspase-mediated inhibition of human cytosolic phospholipase A2 during apoptosis. J. Immunol. 1998, 161, 5687–5694. [Google Scholar] [PubMed]
- Buckland, A.G.; Wilton, D.C. Inhibition of human cytosolic phospholipase A2 by human annexin V. Biochem. J. 1998, 329, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.P.; Dubois, T.; Oudinet, J.P.; Lukowski, S.; Russo-Marie, F.; Geny, B. Inhibition of cytosolic phospholipase A2 by annexin V in differentiated permeabilized HL-60 cells. Evidence of crucial importance of domain I type II Ca2+-binding site in the mechanism of inhibition. J. Biol. Chem. 1997, 272, 10474–10482. [Google Scholar] [CrossRef]
- Kirsch, T.; Harrison, G.; Golub, E.E.; Nah, H.D. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J. Biol. Chem. 2000, 275, 35577–35583. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kirsch, T. Collagen/annexin V interactions regulate chondrocyte mineralization. J. Biol. Chem. 2008, 283, 10310–10317. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Jones, J.W.; Hegdekar, N.; Thayer, J.A.; Kumar, A.; Faden, A.I.; Kane, M.A.; Lipinski, M.M. PLA2G4A/cPLA2-mediated lysosomal membrane damage leads to inhibition of autophagy and neurodegeneration after brain trauma. Autophagy 2020, 16, 466–485. [Google Scholar] [CrossRef] [PubMed]
- Schoen, F.J.; Levy, R.J.; Nelson, A.C.; Bernhard, W.F.; Nashef, A.; Hawley, M. Onset and progression of experimental bioprosthetic heart valve calcification. Lab. Investig. 1985, 52, 523–532. [Google Scholar]
| nAVL | 2d-iAVL | 7d-iAVL | 14d-iAVL | 28d-iAVL | |
|---|---|---|---|---|---|
| cPLA2α | ± | ± | ++ | +++ | ++++ |
| IL-6 | − | − | −/± | ± | + |
| OPN | ± | ± | ++ | +++ | ++++ |
| Fetuin-A | ± | − | − | − | − |
| Anx-V | ± | ± | + | + | + |
| cl-C3 | − | − | − | − | − |
| MAP1 | − | − | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonetti, A.; Contin, M.; Tonon, F.; Marchini, M.; Ortolani, F. Calcium-Dependent Cytosolic Phospholipase A2α as Key Factor in Calcification of Subdermally Implanted Aortic Valve Leaflets. Int. J. Mol. Sci. 2022, 23, 1988. https://doi.org/10.3390/ijms23041988
Bonetti A, Contin M, Tonon F, Marchini M, Ortolani F. Calcium-Dependent Cytosolic Phospholipase A2α as Key Factor in Calcification of Subdermally Implanted Aortic Valve Leaflets. International Journal of Molecular Sciences. 2022; 23(4):1988. https://doi.org/10.3390/ijms23041988
Chicago/Turabian StyleBonetti, Antonella, Magali Contin, Federica Tonon, Maurizio Marchini, and Fulvia Ortolani. 2022. "Calcium-Dependent Cytosolic Phospholipase A2α as Key Factor in Calcification of Subdermally Implanted Aortic Valve Leaflets" International Journal of Molecular Sciences 23, no. 4: 1988. https://doi.org/10.3390/ijms23041988
APA StyleBonetti, A., Contin, M., Tonon, F., Marchini, M., & Ortolani, F. (2022). Calcium-Dependent Cytosolic Phospholipase A2α as Key Factor in Calcification of Subdermally Implanted Aortic Valve Leaflets. International Journal of Molecular Sciences, 23(4), 1988. https://doi.org/10.3390/ijms23041988







