3D Printable Composite Biomaterials Based on GelMA and Hydroxyapatite Powders Doped with Cerium Ions for Bone Tissue Regeneration
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Ceramic Powders, GelMA and Composite Materials
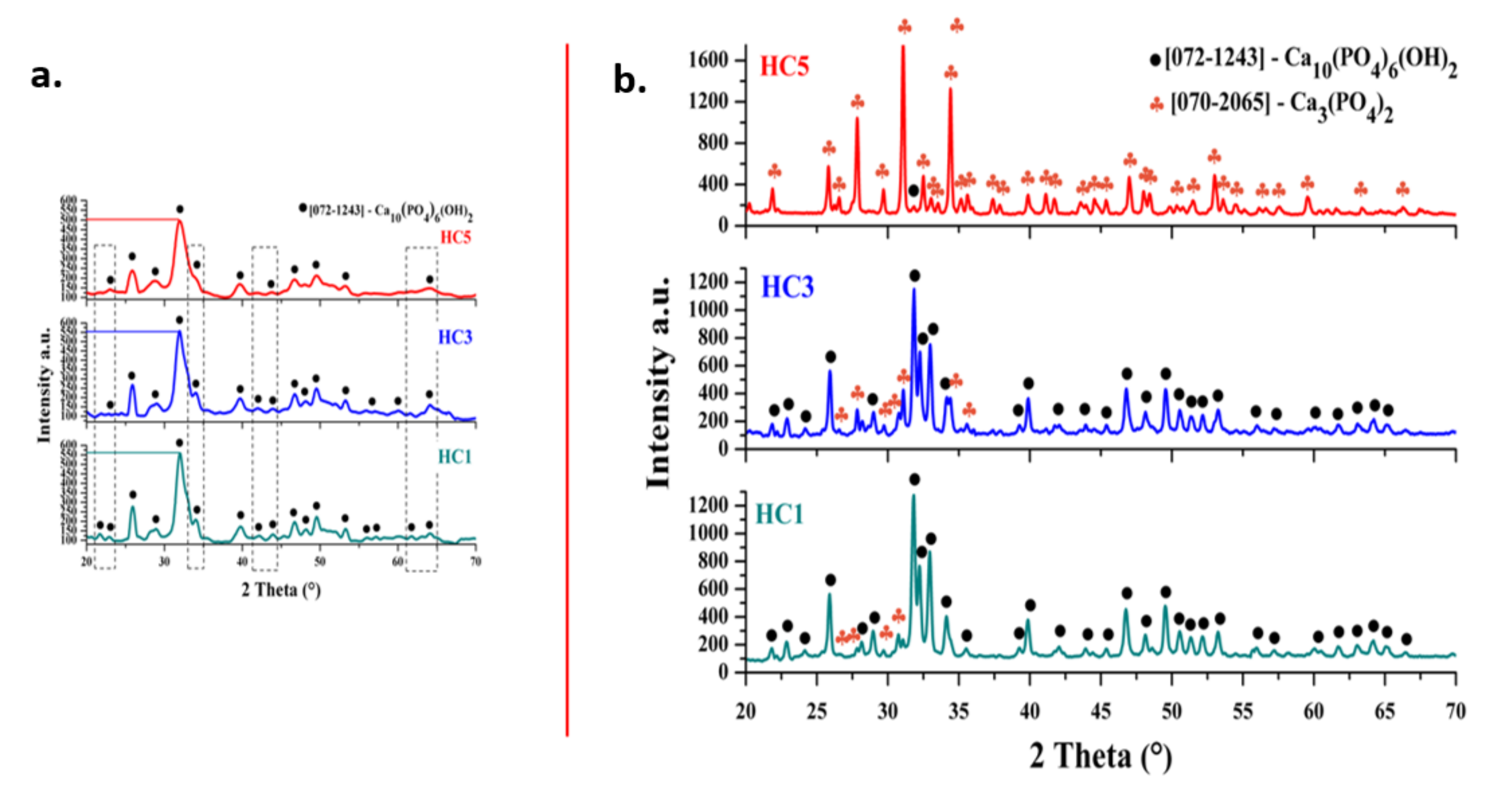
2.1.1. X-ray Diffraction (XRD)
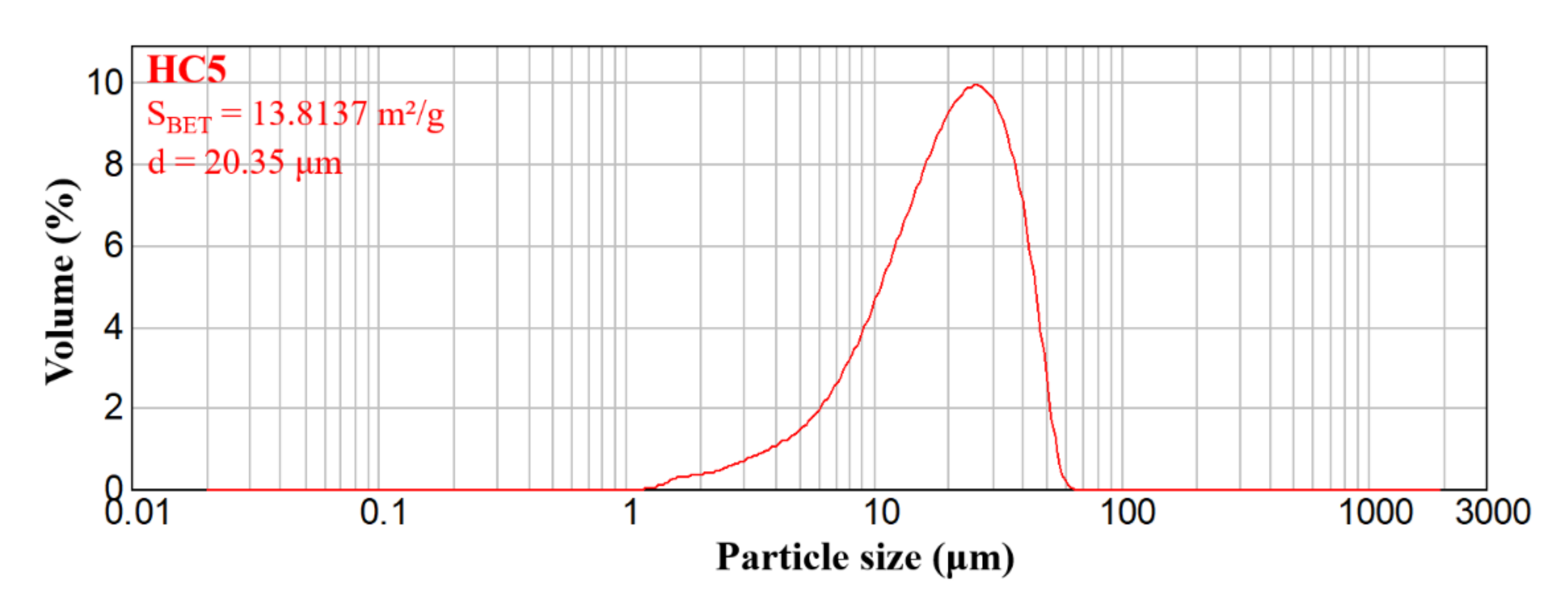
2.1.2. Laser Granulometry
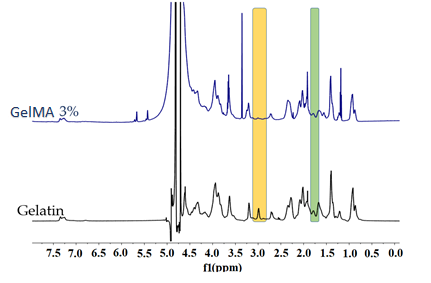
2.1.3. 1H-NMR Spectrometry
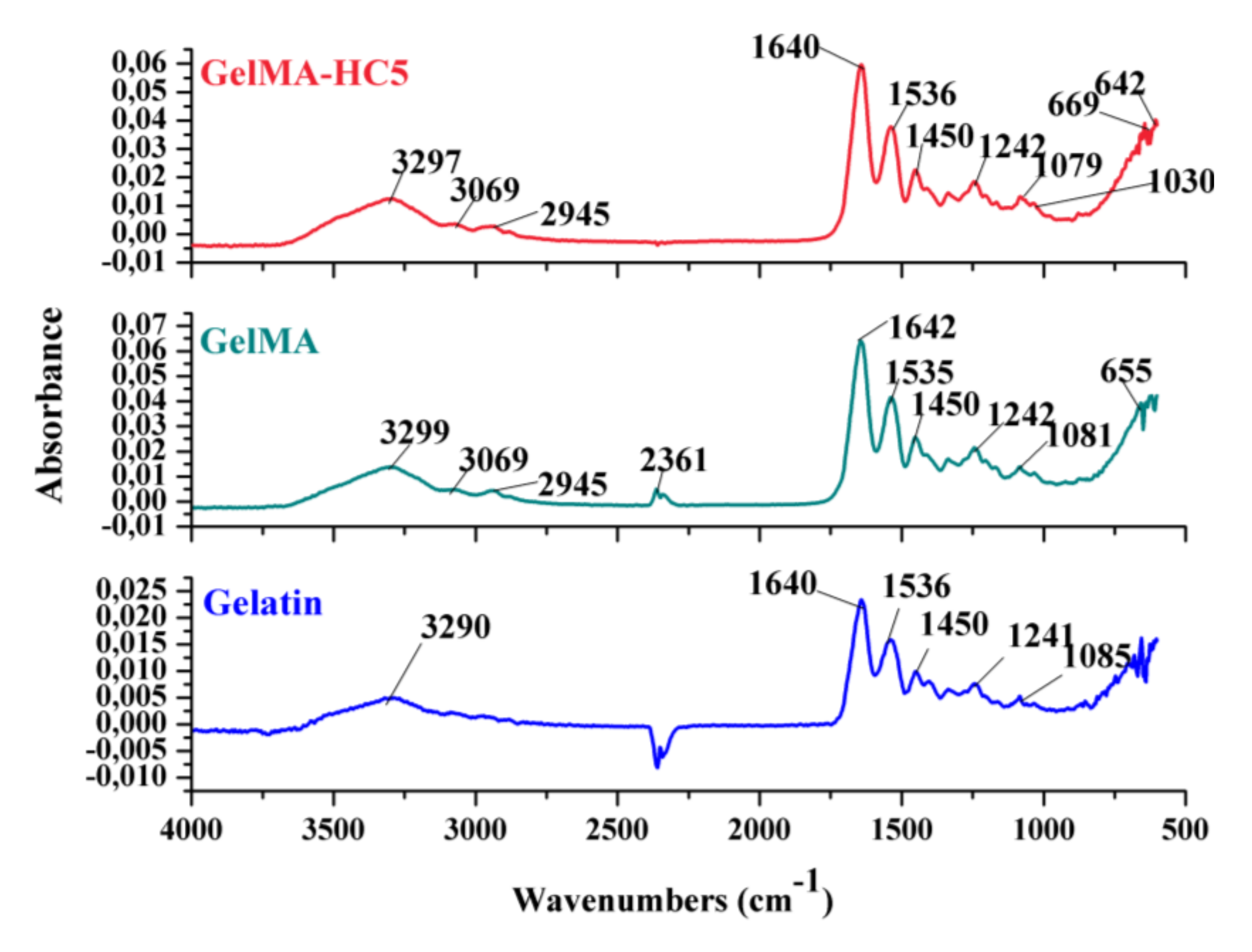
2.1.4. Fourier Transform Infrared Spectrometry—FTIR
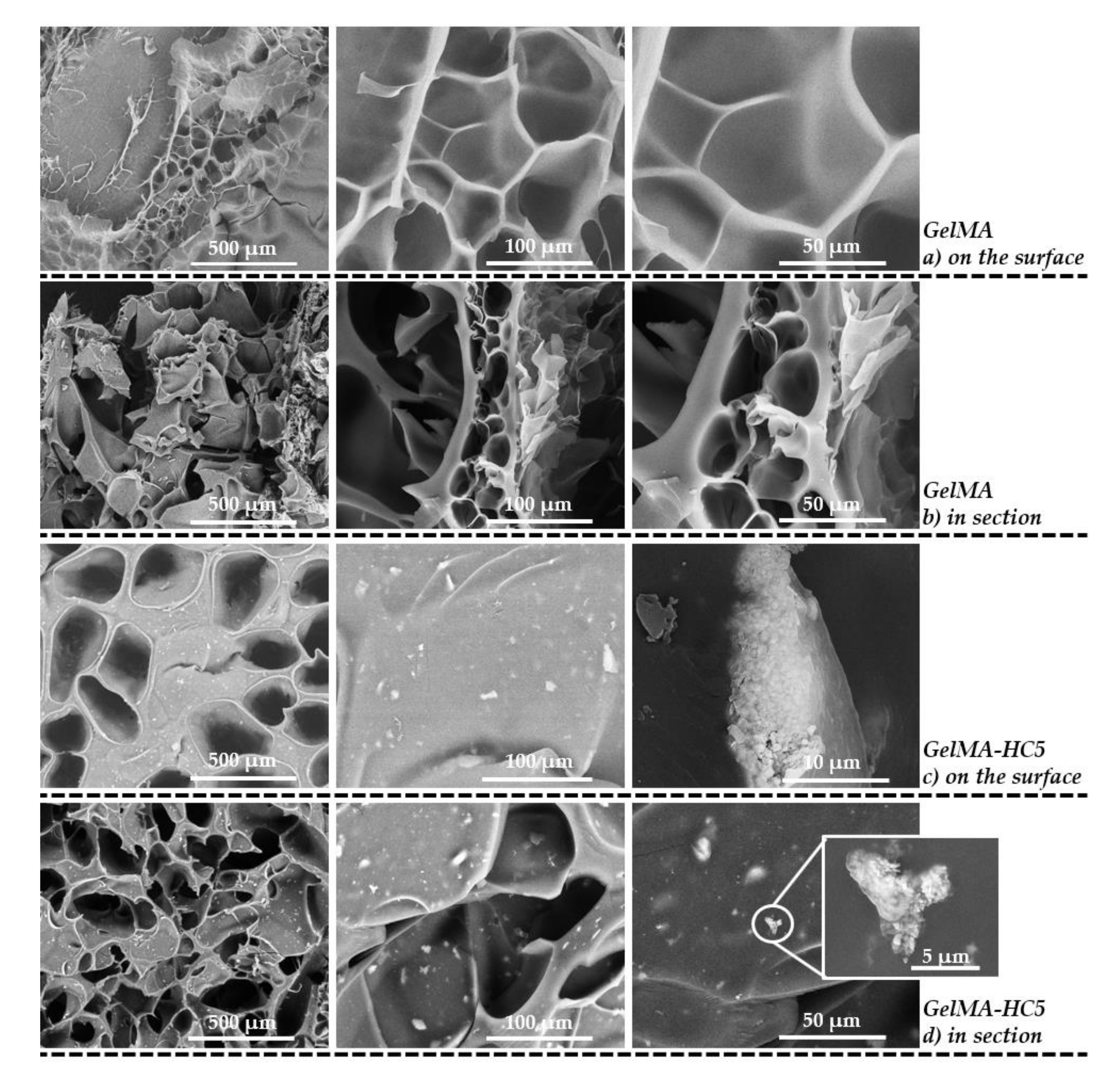
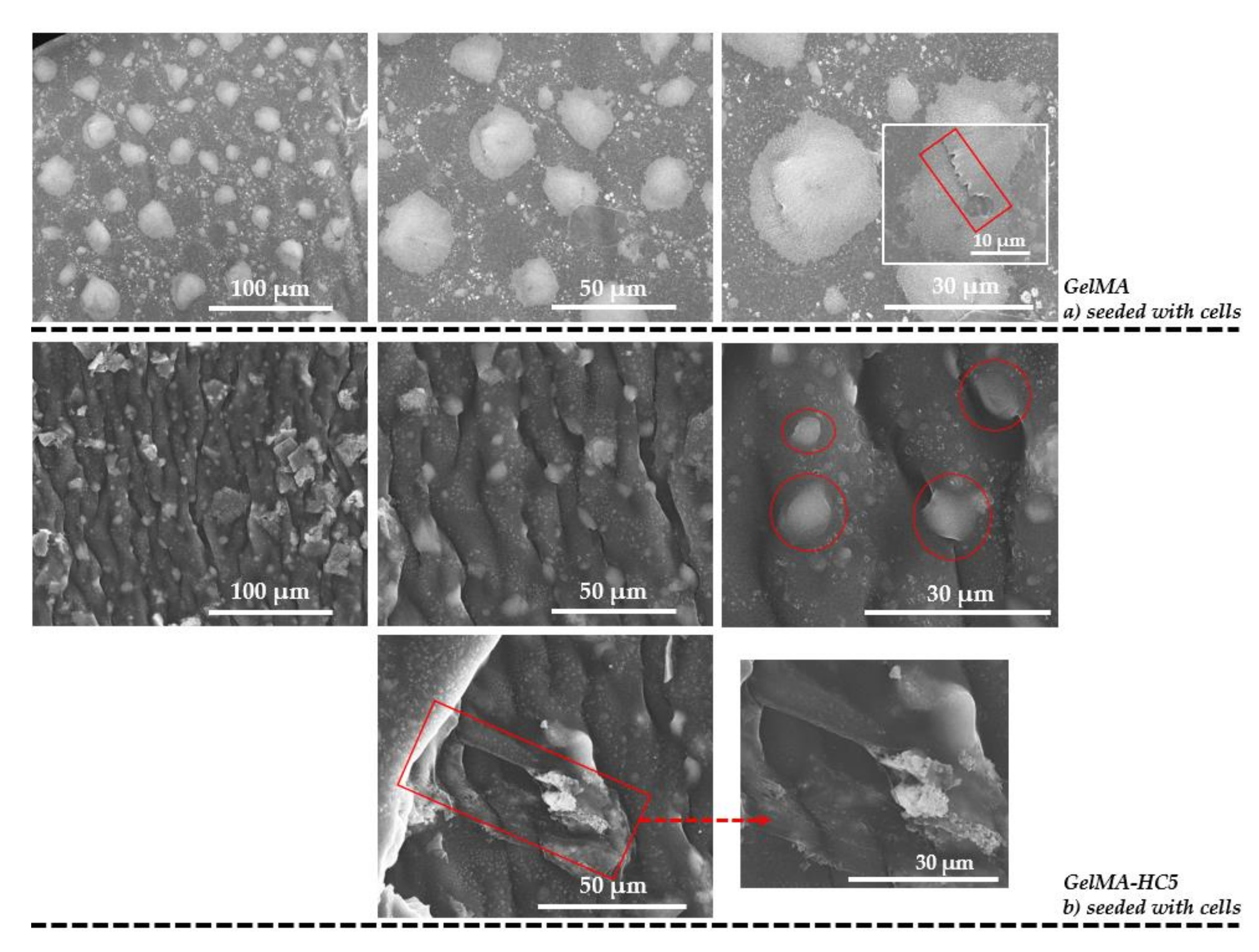
2.1.5. Scanning Electron Microscopy
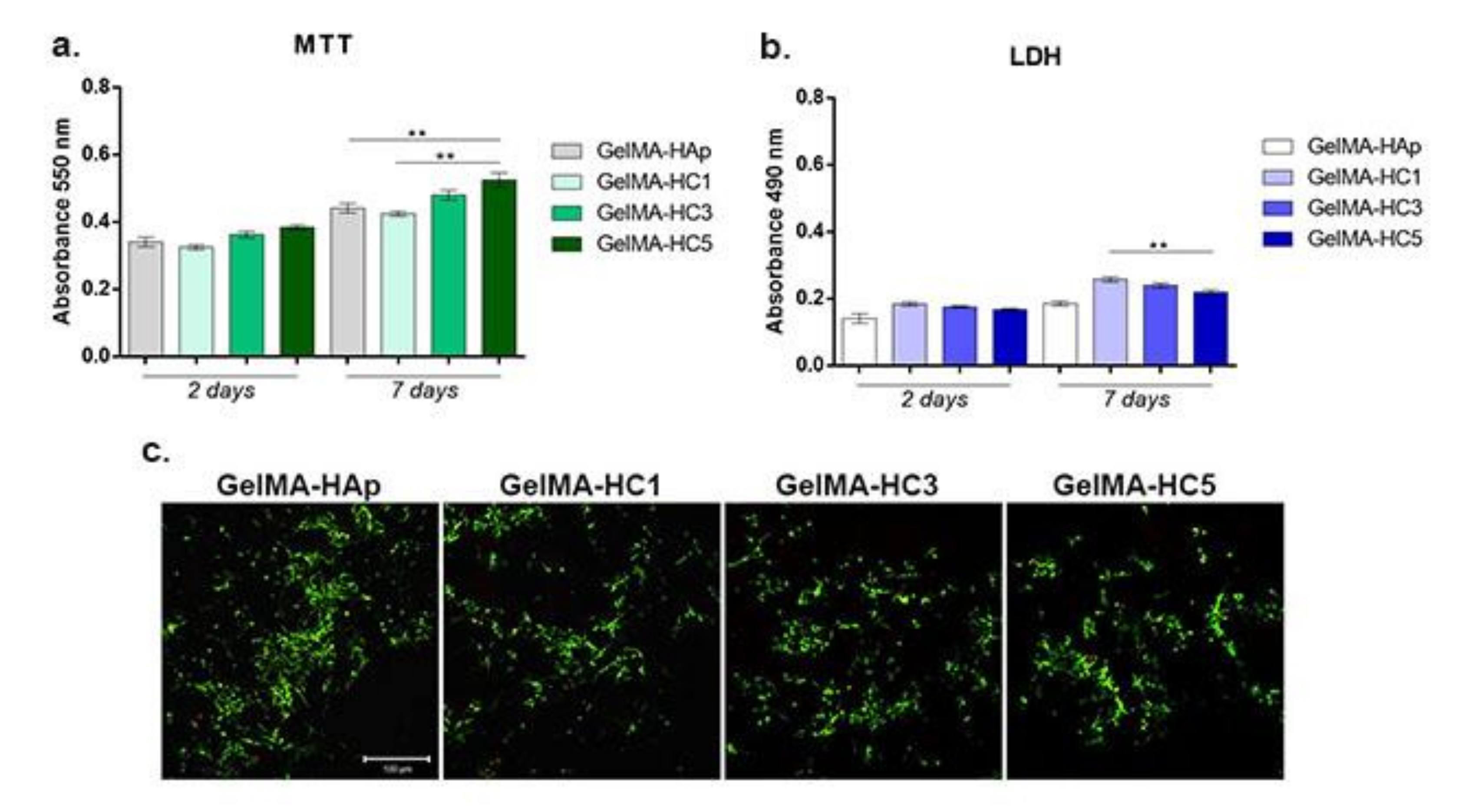
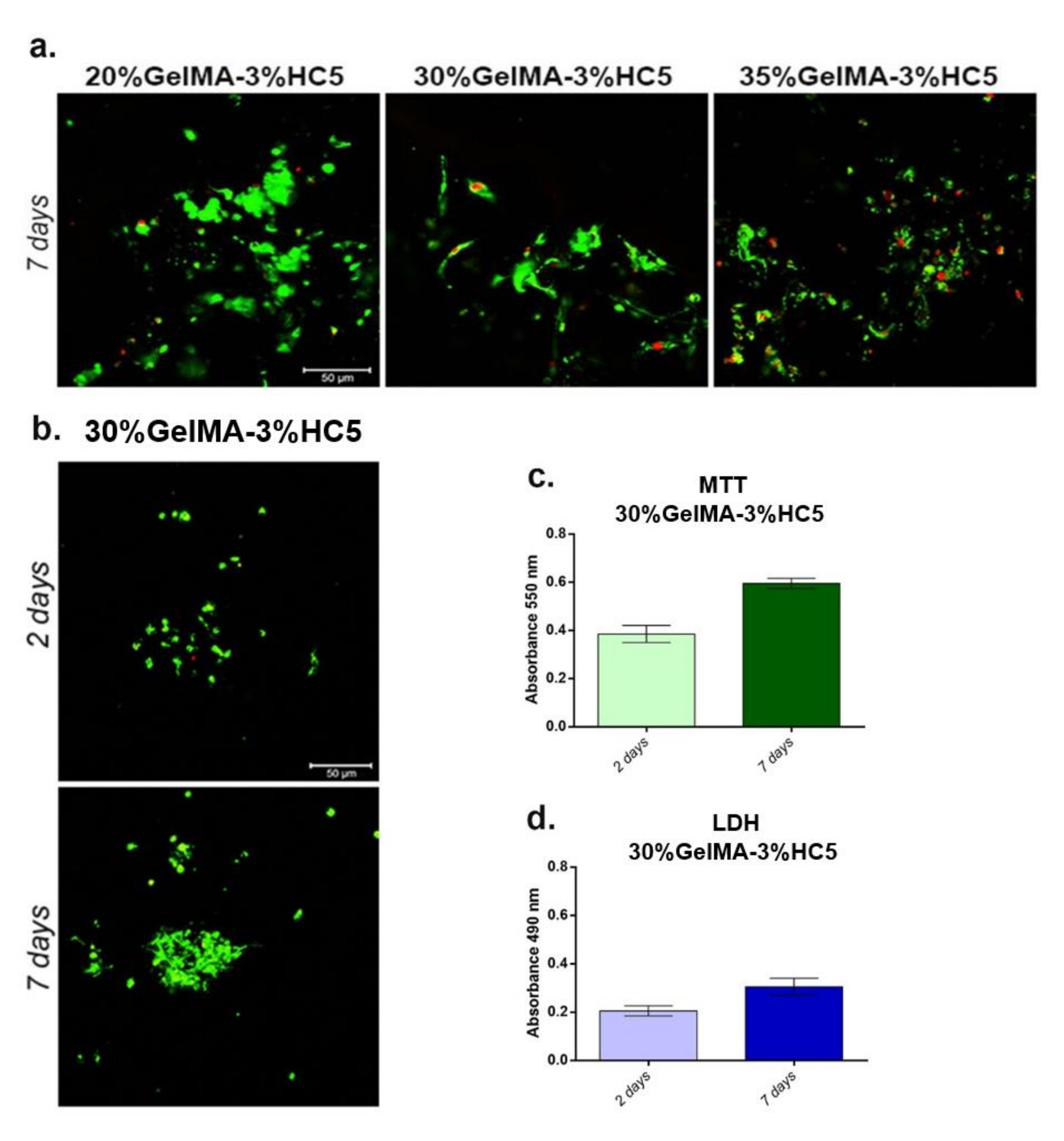
2.1.6. Biological Analyses
- Cell viability
- Materials cytotoxicity
- Live/Dead assay
2.2. Characterization of Scaffolds Obtained from Nanocomposite Hydrogel Printing Inks—20%GelMA-3%HC5, 30%GelMA-3%HC5, 35%GelMA-3%HC5
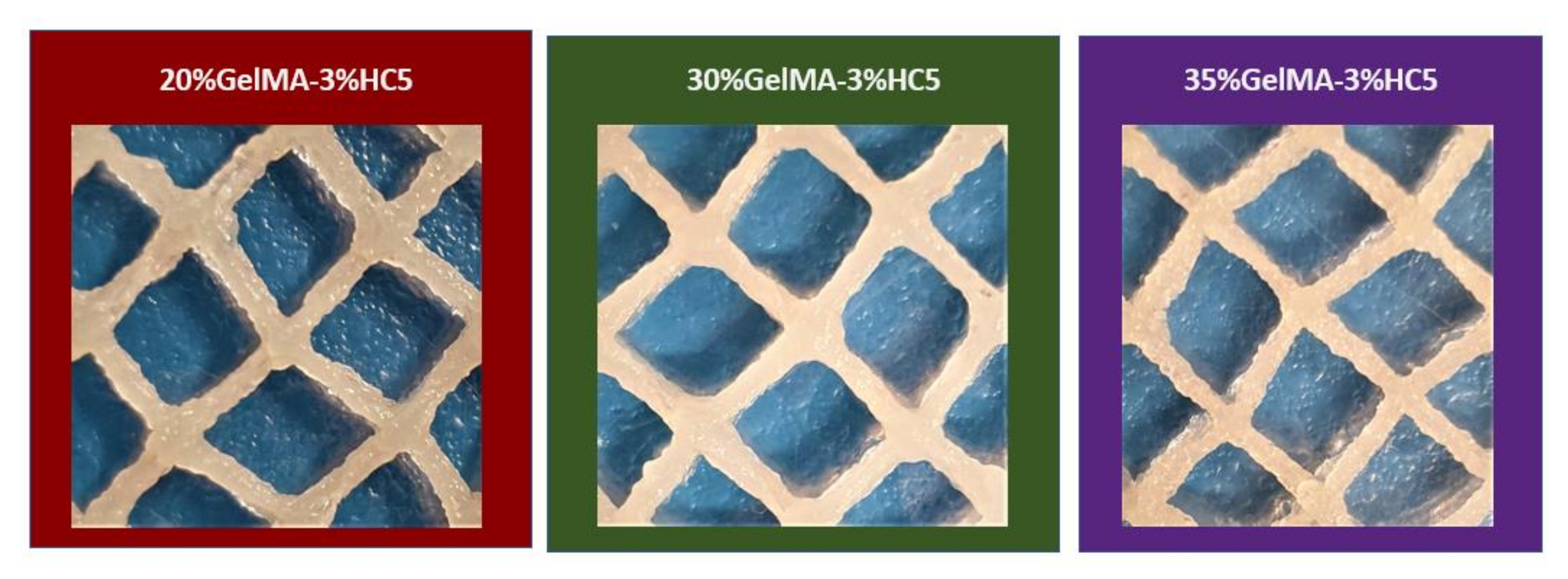
2.2.1. Printability
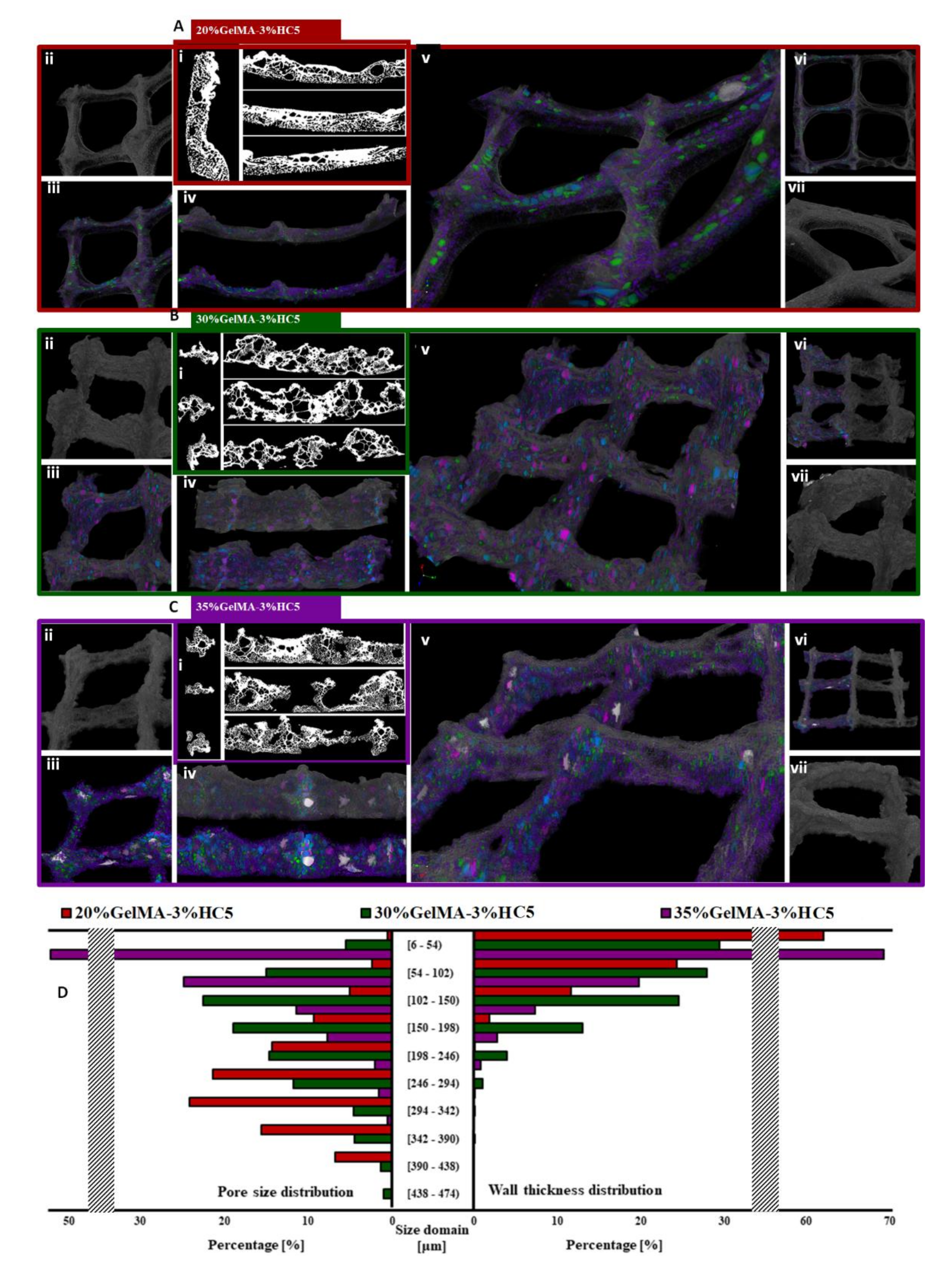
2.2.2. Micro-CT
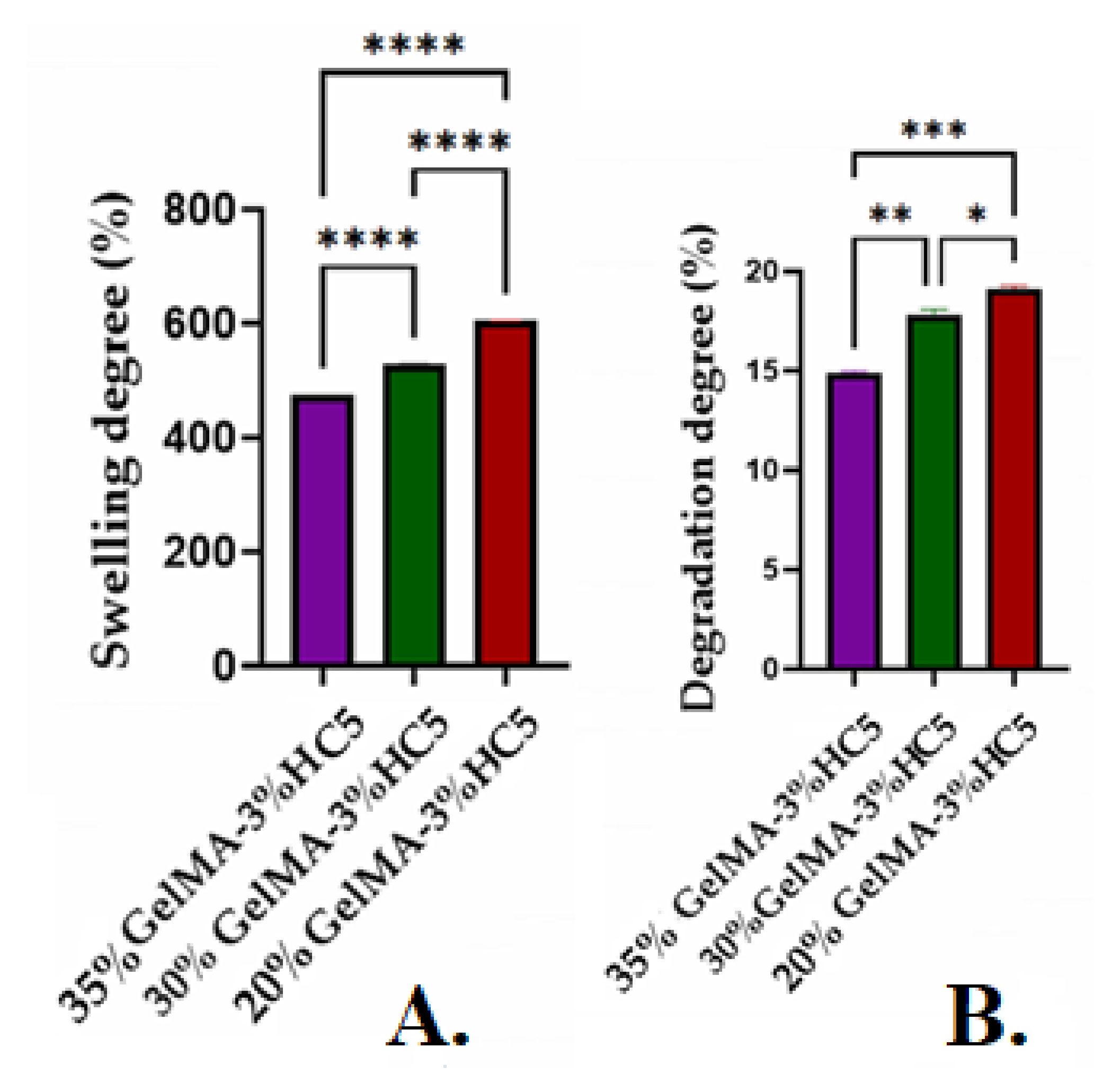
2.2.3. Swelling Degree, Degradability of the 3D Printed Hydrogel Based on GelMA
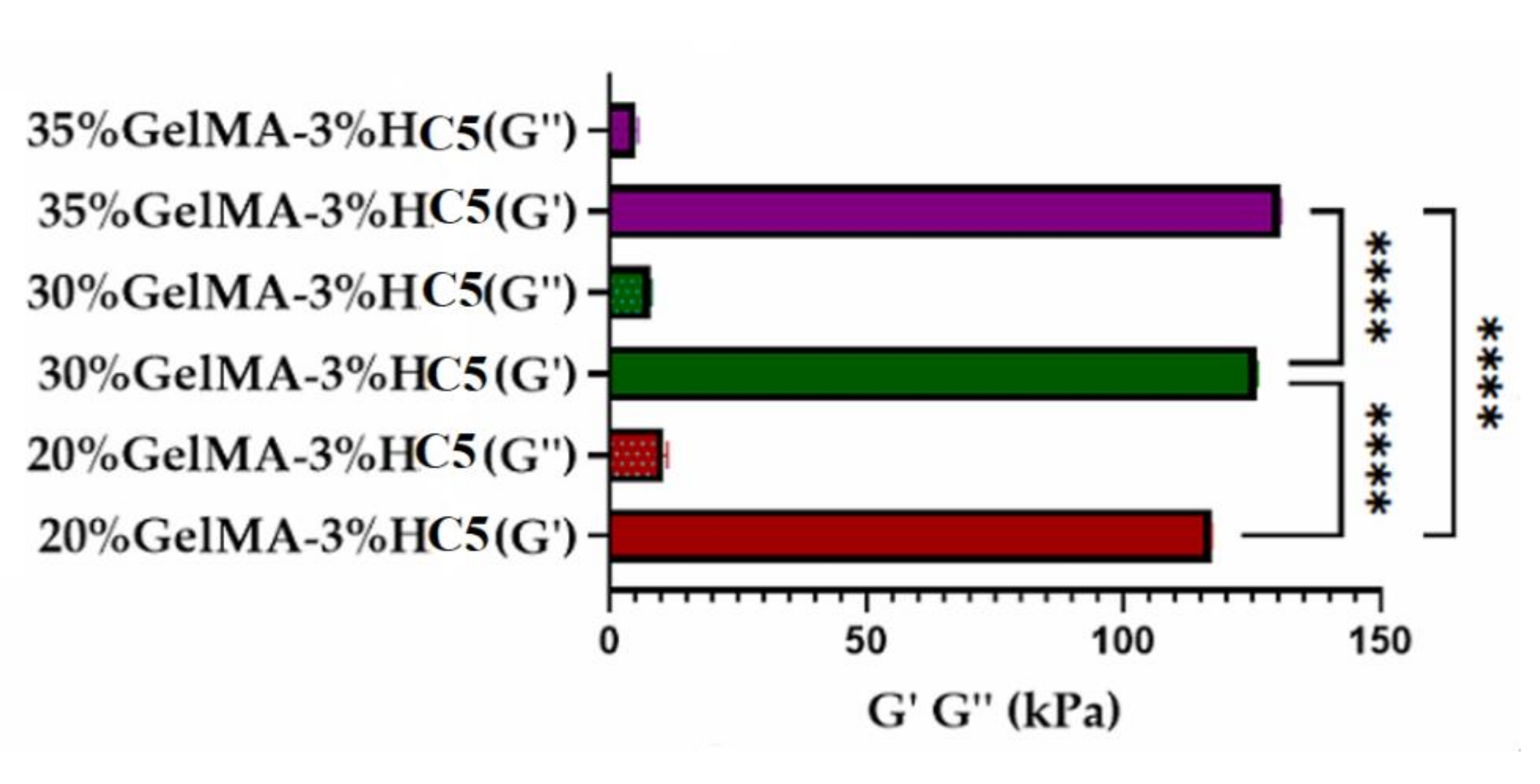
2.2.4. Nanoindentation
2.2.5. Evaluation of Cellular Response toward 3D Printed Scaffolds
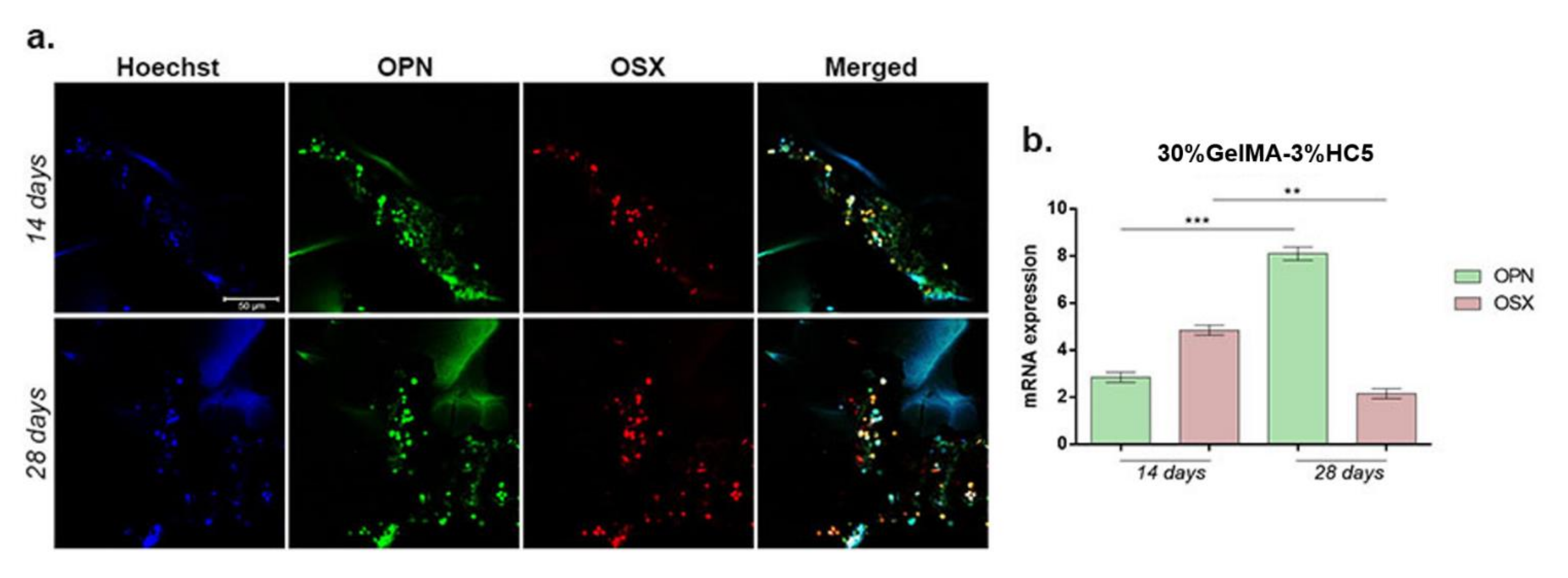
2.2.6. Evaluation of 30%GelMA-3%HC5 Printed Scaffold Effect on Osteogenic Differentiation
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Ceramic Powders
3.3. Synthesis of GelMA
3.4. Synthesis of Composite Materials
3.5. Characterization Techniques of Ceramic Powders, GelMA and Composite Materials
3.5.1. X-ray Diffraction (XRD)
3.5.2. Brunauer–Emmett–Teller (BET)
3.5.3. Laser Diffraction Granulometer
3.5.4. Scanning Electron Microscopy SEM
3.5.5. 1H-NMR Spectrometry
3.5.6. FTIR Spectrometry
3.5.7. Biological Analyses
- Cell culture model. Murine preosteoblasts from MC3T3-E1 cell line (ATCC CRL-2593) were used for the biocompatibility assessment. MC3T3-E1 cells were cultured in DMEM media supplemented with 10% FBS and 1% antibiotic antimycotic solution and maintained at 37 °C, 5% CO2, in a humidified atmosphere.
- Biocompatibility assays. To evaluate the biocompatibility of the GelMA-Hap enriched 3D scaffolds, cells’ viability, proliferation profile and materials’ cytotoxicity were evaluated at two and seven days after cells seeding. Cell viability and proliferation profile were quantitatively determined by MTT assay and qualitatively by Live/Dead fluorescence staining, while the materials’ cytotoxicity was quantitatively assessed by LDH assay.
- MTT assay. Cells’ viability and proliferation profile were qualitatively determined using spectrophotometric MTT assay, at two and seven days after seeding. The 3D systems were incubated with 1 mg/mL MTT solution prepared in simple culture media, for 4 h at 37 °C, allowing the formation of formazan crystals by living cells. The formazan crystals were solubilized with isopropanol and the optic density was measured by spectrophotometry at 550 nm on Flex Station 3 (Molecular Devices, LLC. 3860 N First Street San Jose, CA 95134, USA)). The values obtained were directly proportional with the amount of live cells.
- LDH assay. The cytotoxic potential of GelMA-HAp enriched 3D scaffolds was determined based on LDH quantification, released from damaged cells, which lost their membrane integrity. The culture medium was collected from the 3D systems and mixed with LDH Assay Kit reagents, according to the manufacturer’s instructions. The mix was stored in the dark for 10–15 min and the optic density was measured by spectrophotometry at 490 nm on Flex Station 3. The quantity of LDH was directly proportional with the number of dead cells.
- Live/Dead fluorescence microscopy. The ratio between live cells and dead cells was qualitatively evaluated via Live/Dead fluorescence staining. The assay provided two fluorescent dyes, calcein acetomethoxy (AM), which marks live cells in green fluorescence, and ethidium bromide homodimer (EtBr), which marks dead cells in red fluorescence. The staining solution was prepared according to the manufacturer’s indications and incubated with the 3D systems for 1 h at room temperature, in the dark. The images were captured using confocal microscope Zeiss 710 and processed using Zeiss Zen software.
- Statistical analysis. All experiments were performed in triplicate (n = 3). Statistical analysis of the data was performed using GraphPad Prism software, one-way ANOVA method and Bonferroni algorithm to compare between groups. Values were considered significant for p < 0.05.
3.6. Characterization of 3D Scaffolds Obtained from Nanocomposite Hydrogel Printing Inks—20% GelMA-3% HC, 30% GelMA-3% HC, 35% GelMA-3% HC
3.6.1. Printability
3.6.2. Swelling Degree, Degradability of the 3D Printed Scaffolds
3.6.3. Microcomputer Tomography (µCT)
3.6.4. Nanoindentation
3.6.5. Evaluation of Osteogenic Markers Gene and Protein Expression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Archunan, M.W.; Petronis, S. Bone Grafts in Trauma and Orthopaedics. Cureus 2021, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone grafts and substitutes in dentistry: A review of current trends and developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.M.; Mazza, E.; Colombo, M.; Ripamonti, C. The use of bone-graft substitutes in large bone defects: Any specific needs? Injury 2011, 42 (Suppl. S2), S56–S63. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, C.; Knuth, C.; Farrell, E. Endochondral Ossification: Recapitulating Bone Development for Bone Defect Repair; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Hossan, M.J.; Gafur, M.A.; Kadir, M.; Karim, M.M. Preparation and Characterization of Gelatin- Hydroxyapatite Composite for Bone Tissue Engineering. Int. J. Eng. Technol. 2014, 14, 113–122. [Google Scholar]
- Sreekumaran, S.; Radhakrishnan, A.; Rauf, A.A.; Kurup, G.M. Nanohydroxyapatite incorporated photocrosslinked gelatin methacryloyl/poly (ethylene glycol) diacrylate hydrogel for bone tissue engineering. Prog. Biomater. 2021, 10, 43–51. [Google Scholar] [CrossRef]
- Stahl, A.; Yang, Y.P. Regenerative Approaches for the Treatment of Large Bone Defects. Tissue Eng. Part B Rev. 2021, 27, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.; Antunović, M.; Rogina, A.; Ivanković, M.; Ivanković, H. Bone-mimetic porous hydroxyapatite/whitlockite scaffolds: Preparation, characterization and interactions with human mesenchymal stem cells. J. Mater. Sci. 2020, 56, 3947–3969. [Google Scholar] [CrossRef]
- Azami, M.; Samadikuchaksaraei, A.; Poursamar, S.A. Synthesis and characterization of a laminated hydroxyapatite/gelatin nanocomposite scaffold with controlled pore structure for bone tissue engineering. Int. J. Artif. Organs 2010, 33, 86–95. [Google Scholar] [CrossRef]
- Vinicius Beserra dos Santos, M.; Bastos Nogueira Rocha, L.; Gomes Vieira, E.; Leite Oliveira, A.; Oliveira Lobo, A.; de Carvalho, M.A.M.; Anteveli Osajima, J.; Cavalcanti Silva-Filho, E. Development of Composite Scaffolds Based on Cerium Doped-Hydroxyapatite and Natural Gums—Biological and Mechanical Properties. Materials 2019, 12, 2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, E.; Silva, M.; Maia-Filho, A.; Ferreira, D.; Figuerêdo-Silva, J.; Rovaris, K.; Fialho, A.C.; Leite-Oliveira, A.; Menezes de Oliveira, A.L.; da Fonseca, M.G.; et al. Effect of cerium-containing hydroxyapatite in bone repair in female rats with osteoporosis induced by ovariectomy. Minerals 2021, 11, 377. [Google Scholar] [CrossRef]
- Na, K.; Shin, S.; Lee, H.; Shin, D.; Baek, J.; Kwak, H.; Park, M.; Shin, J.; Hyun, J. Effect of solution viscosity on retardation of cell sedimentation in DLP 3D printing of gelatin methacrylate/silk fibroin bioink. J. Ind. Eng. Chem. 2018, 61, 340–347. [Google Scholar] [CrossRef]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Liu, T. 3D printing of polycaprolactone-based composites with diversely tunable mechanical gradients via multi-material fused deposition modeling. Compos. Commun. 2021, 23, 100600. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Q.; Xu, S.; Zheng, Q.; Cao, X. Preparation and properties of 3D printed alginate-chitosan polyion complex hydrogels for tissue engineering. Polymers 2018, 10, 664. [Google Scholar] [CrossRef] [Green Version]
- Alexa, R.L.; Iovu, H.; Trica, B.; Zaharia, C.; Serafim, A.; Alexandrescu, E.; Radu, I.C.; Vlasceanu, G.; Preda, S.; Ninciuleanu, C.M.; et al. Assessment of naturally sourced mineral clays for the 3D printing of biopolymer-based nanocomposite inks. Nanomaterials 2021, 11, 703. [Google Scholar] [CrossRef] [PubMed]
- Alexa, R.L.; Iovu, H.; Ghitman, J.; Serafim, A.; Stavarache, C.; Marin, M.M.; Ianchis, R. 3D-printed gelatin methacryloyl-based scaffolds with potential application in tissue engineering. Polymers 2021, 13, 727. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Liu, X.; Zhang, H.J.; Zhu, X. Novel Gelatin-based Eco-friendly Adhesive with a Hyperbranched Cross-linked Structure. Ind. Eng. Chem. Res. 2020, 59, 5500–5511. [Google Scholar] [CrossRef]
- Ismail, H.M.; Zamani, S.; Elrayess, M.A.; Kafienah, W.; Younes, H.M. New three-dimensional poly(decanediol-co-tricarballylate) elastomeric fibrous mesh fabricated by photoreactive electrospinning for cardiac tissue engineering applications. Polymers 2018, 10, 455. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and properties of gelatin methacryloyl (GelMA) hydrogels and their recent applications in load-bearing tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef] [Green Version]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Park, C.H.; Kim, C.S. Microcylinder-laden gelatin-based bioink engineered for 3D bioprinting. Mater. Lett. 2018, 233, 24–27. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Shi, X.; Wang, Y. Injectable alendronate-functionalized GelMA hydrogels for mineralization and osteogenesis. RSC Adv. 2018, 8, 22764–22776. [Google Scholar] [CrossRef] [Green Version]
- Bektas, C.K.; Hasirci, V. Cell loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering. Biomater. Sci. 2020, 8, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Kim, D.; Kang, D.; Yang, G.H.; Jung, B.; Yeo, M.; Yeo, M.-J.; An, S.; Lee, K.; Kim, J.S.; et al. 3D-printed gelatin methacrylate (GelMA)/ silanated silica scaffold assisted by two-stage cooling system for hard tissue regeneration. Regen. Biomater. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Gao, Q.; Niu, X.; Shao, L.; Zhou, L.; Lin, Z.; Sun, A.; Fu, Z.; Chen, Z.; Hu, J.; Liu, Y.; et al. 3D Printing of Complex GelMA-based Scaffolds with Nanoclay. Biofabrication 2019, 11, 035006. [Google Scholar] [CrossRef]
- Buyuksungur, S.; Hasirci, V.; Hasirci, N. 3D printed hybrid bone constructs of PCL and dental pulp stem cells loaded GelMA. J. Biomed. Mater. Res. 2021, 109, 2425–2437. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Suo, H.; Yan, M.; Yin, J.; Fu, J. 3D printing of biomimetic multi-layered GelMA/nHA scaffold for osteochondral defect repair. Mater. Des. 2019, 171, 107708. [Google Scholar] [CrossRef]
- Phatai, P.; Futalan, C.M.; Utara, S.; Khemthong, P.; Kamonwannasit, S. Structural characterization of cerium-doped hydroxyapatite nanoparticles synthesized by an ultrasonic-assisted sol-gel technique. Results Phys. 2018, 10, 956–963. [Google Scholar] [CrossRef]
- Ciobanu, G.; Maria Bargan, A.; Luca, C. New cerium(IV)-substituted hydroxyapatite nanoparticles: Preparation and characterization. Ceram. Int. 2015, 41, 12192–12201. [Google Scholar] [CrossRef]
- Modaresifar, K.; Hadjizadeh, A.; Niknejad, H. Design and fabrication of GelMA/chitosan nanoparticles composite hydrogel for angiogenic growth factor delivery. Artif. Cells. Nanomed. Biotechnol. 2018, 46, 1799–1808. [Google Scholar] [CrossRef] [Green Version]
- Rahimi Mamaghani, K.; Morteza Naghib, S.; Zahedi, A.; Mozafari, M. Synthesis and microstructural characterization of GelMa/PEGDA hybrid hydrogel containing graphene oxide for biomedical purposes. Mater. Today Proc. 2018, 5, 15635–15644. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, X.; Wei, D.; Sun, J.; Xiao, W.; Zhao, H.; Guo, L.; Wei, Q.; Fan, H.; Zhang, X. Photo-cross-linkable methacrylated gelatin and hydroxyapatite hybrid hydrogel for modularly engineering biomimetic osteon. ACS Appl. Mater. Interfaces 2015, 7, 10386–10394. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, K.H.; Kim, D.H.; Chae, H.J.; Sung, G.H.; Kim, Y.O. In vitro assessments of bone microcomputed tomography in an aged male rat model supplemented with Panax ginseng. Saudi J. Biol. Sci. 2018, 25, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Vlasceanu, G.M.; Șelaru, A.; Dinescu, S.; Balta, C.; Herman, H.; Gharbia, S.; Hermenean, A.; Ioniță, M.; Costache, M. Comprehensive appraisal of graphene–oxide ratio in porous biopolymer hybrids targeting bone-tissue regeneration. Nanomaterials 2020, 10, 1444. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Lee, J.J.; Ho, C.C.; Yen, S.Y.; Ng, H.Y.; Chen, Y.W. Effects of Gelatin Methacrylate Bio-ink Concentration on Mechano-Physical Properties and Human Dermal Fibroblast Behavior. Polymers 2020, 12, 1930. [Google Scholar] [CrossRef]
- Yoon, H.J.; Shin, S.R.; Cha, J.M.; Lee, S.H.; Kim, J.H.; Do, J.T.; Song, H.; Bae, H. Cold water fish gelatin methacryloyl hydrogel for tissue engineering application. PLoS ONE 2016, 11, e0163902. [Google Scholar] [CrossRef] [Green Version]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the Debye-Scherrer equation. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Ghitulica, C.D.; Damian-Buda, A.I.; Cucuruz, A.; Voicu, G. Synthesis and Characterization of ZnO(MgO)-CaO-SiO2-P2O5 Bioglass Obtained by Sol-Gel Method in Presence of Surfactant Agent. Gels 2021, 7, 187. [Google Scholar] [CrossRef]
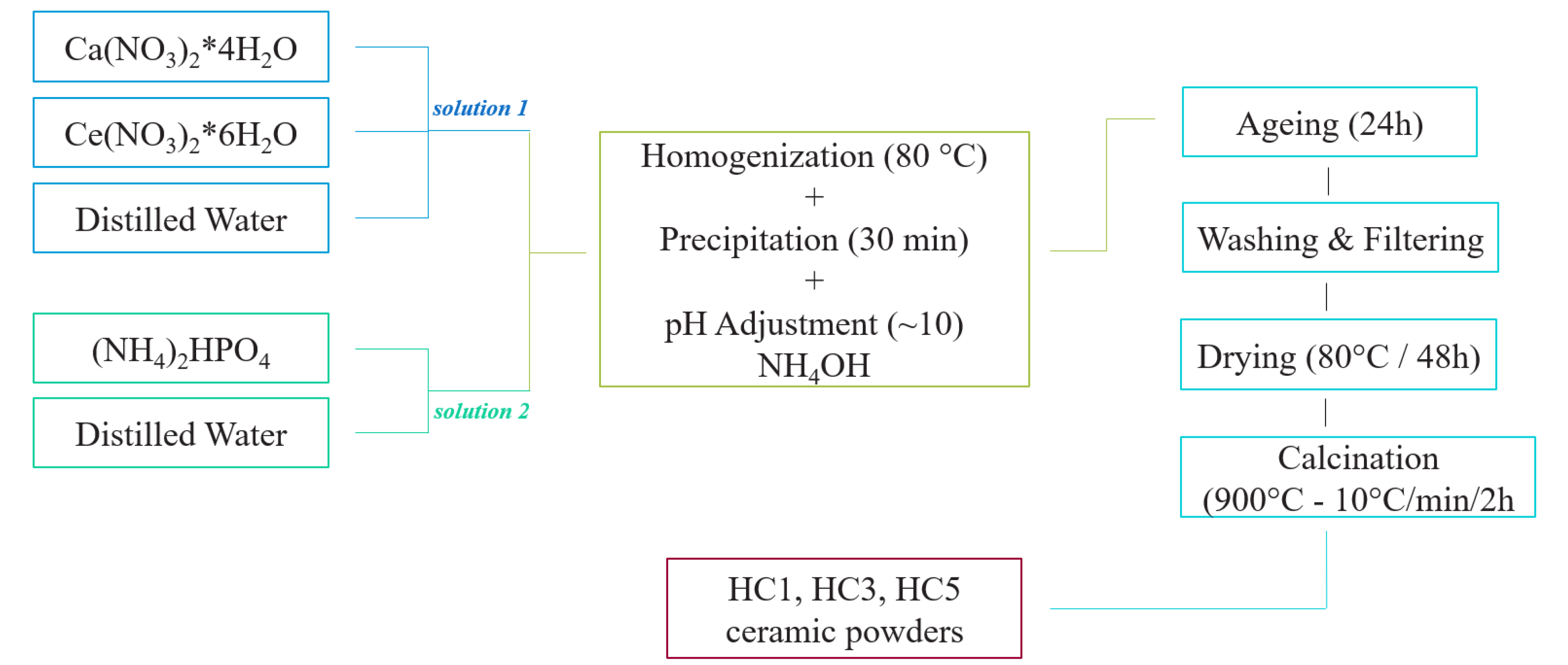
| Sample | Cerium Ion Concentration (Molar %) |
|---|---|
| HC1 | 0.1 |
| HC3 | 0.3 |
| HC5 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leu Alexa, R.; Cucuruz, A.; Ghițulică, C.-D.; Voicu, G.; Stamat, L.-R.; Dinescu, S.; Vlasceanu, G.M.; Stavarache, C.; Ianchis, R.; Iovu, H.; et al. 3D Printable Composite Biomaterials Based on GelMA and Hydroxyapatite Powders Doped with Cerium Ions for Bone Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 1841. https://doi.org/10.3390/ijms23031841
Leu Alexa R, Cucuruz A, Ghițulică C-D, Voicu G, Stamat L-R, Dinescu S, Vlasceanu GM, Stavarache C, Ianchis R, Iovu H, et al. 3D Printable Composite Biomaterials Based on GelMA and Hydroxyapatite Powders Doped with Cerium Ions for Bone Tissue Regeneration. International Journal of Molecular Sciences. 2022; 23(3):1841. https://doi.org/10.3390/ijms23031841
Chicago/Turabian StyleLeu Alexa, Rebeca, Andreia Cucuruz, Cristina-Daniela Ghițulică, Georgeta Voicu, Liliana-Roxana Stamat (Balahura), Sorina Dinescu, George Mihail Vlasceanu, Cristina Stavarache, Raluca Ianchis, Horia Iovu, and et al. 2022. "3D Printable Composite Biomaterials Based on GelMA and Hydroxyapatite Powders Doped with Cerium Ions for Bone Tissue Regeneration" International Journal of Molecular Sciences 23, no. 3: 1841. https://doi.org/10.3390/ijms23031841
APA StyleLeu Alexa, R., Cucuruz, A., Ghițulică, C.-D., Voicu, G., Stamat, L.-R., Dinescu, S., Vlasceanu, G. M., Stavarache, C., Ianchis, R., Iovu, H., & Costache, M. (2022). 3D Printable Composite Biomaterials Based on GelMA and Hydroxyapatite Powders Doped with Cerium Ions for Bone Tissue Regeneration. International Journal of Molecular Sciences, 23(3), 1841. https://doi.org/10.3390/ijms23031841











