Structural Analysis of Human Serum Albumin in Complex with the Fibrate Drug Gemfibrozil
Abstract
:1. Introduction
2. Results
2.1. Overview of the Crystal Structure of HSA in Complex with GEM Ligands
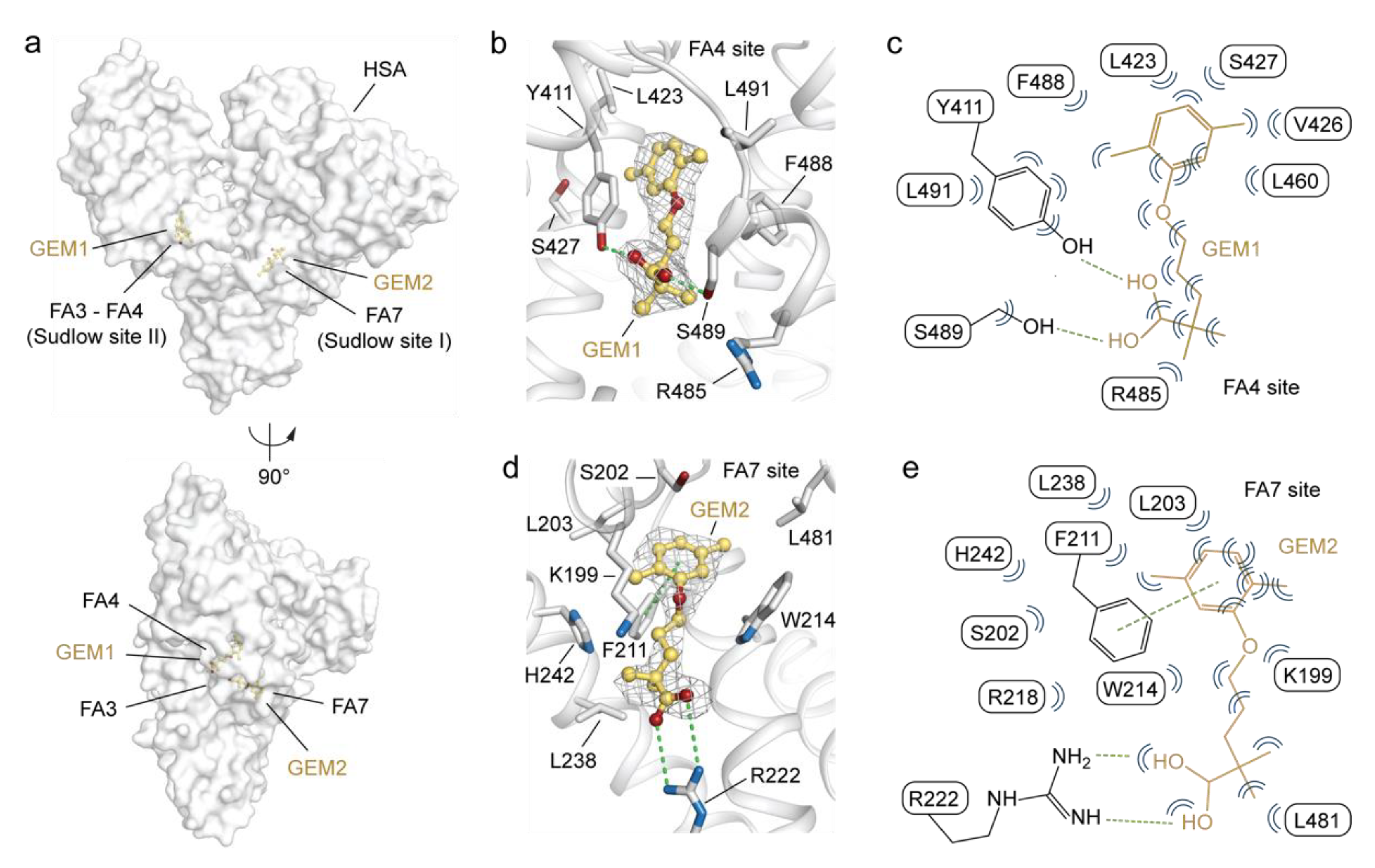
2.2. Molecular Binding Mode of GEM Ligands to HSA
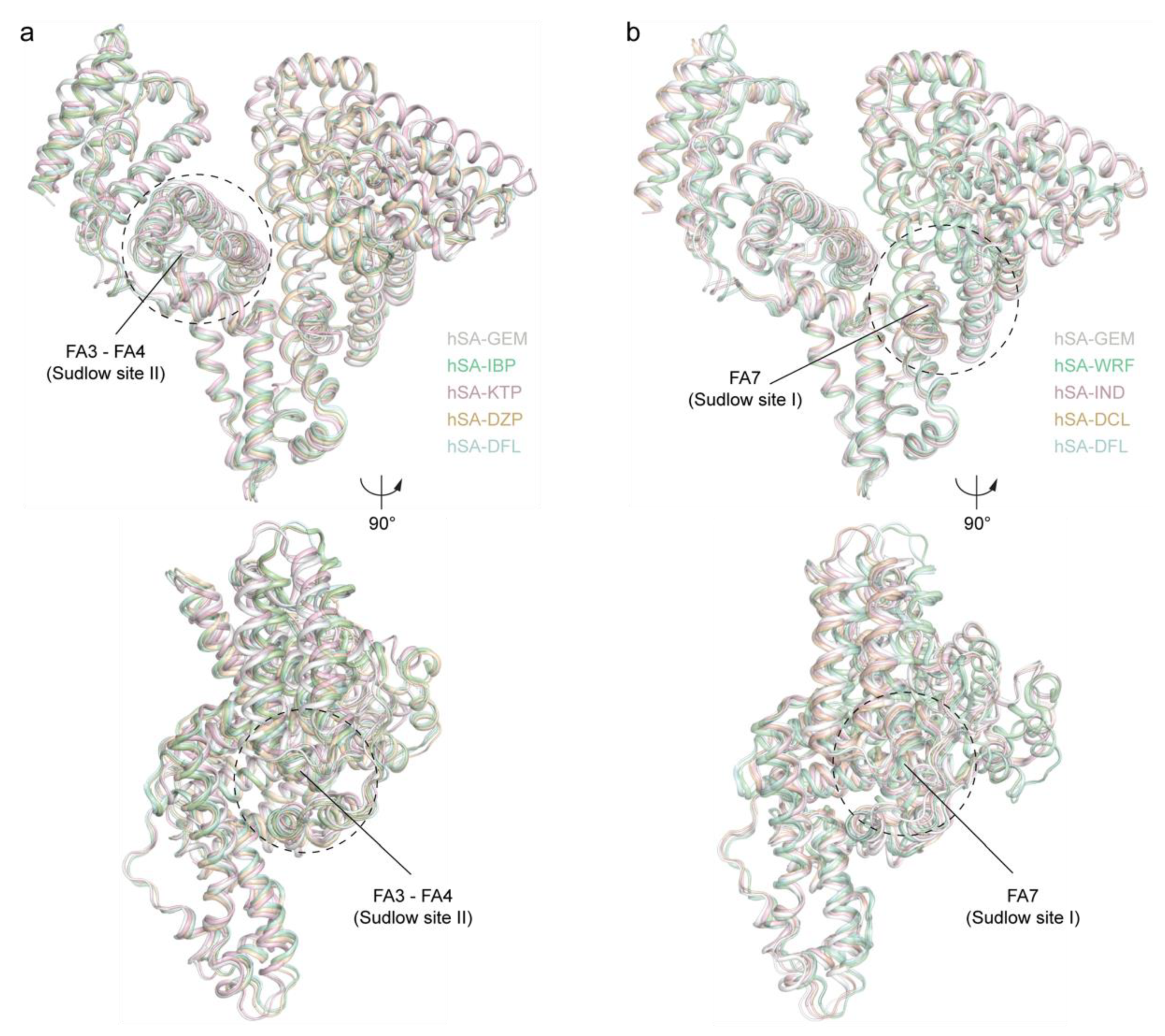
2.3. Structural Comparison of the Ligand Binding Modes of GEM and Other Known Drugs Binding HSA Sudlow’s Sites I and II
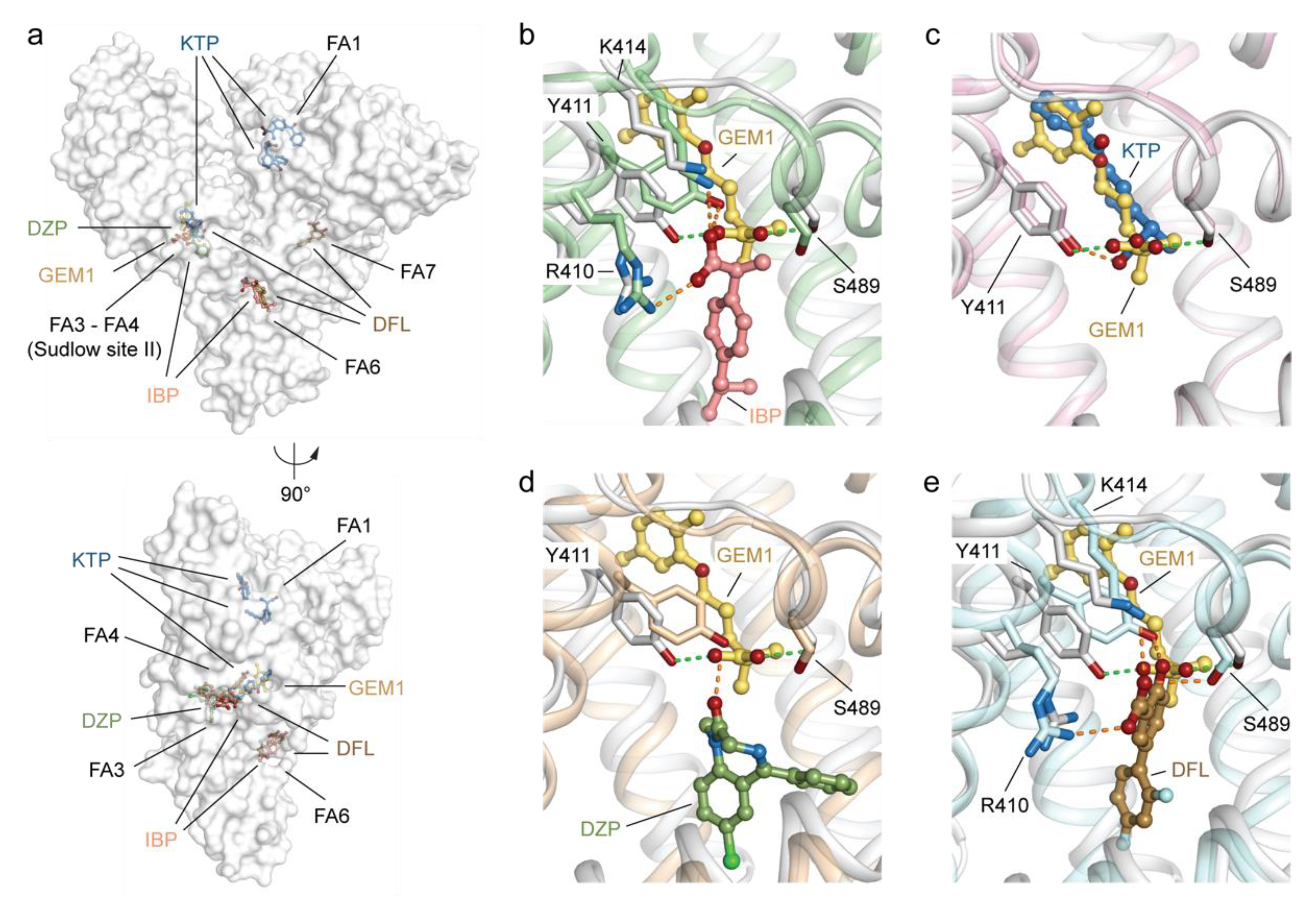
2.3.1. Differences between GEM1 and Other Known Drugs Binding HSA Sudlow’s Site II (FA3–FA4)
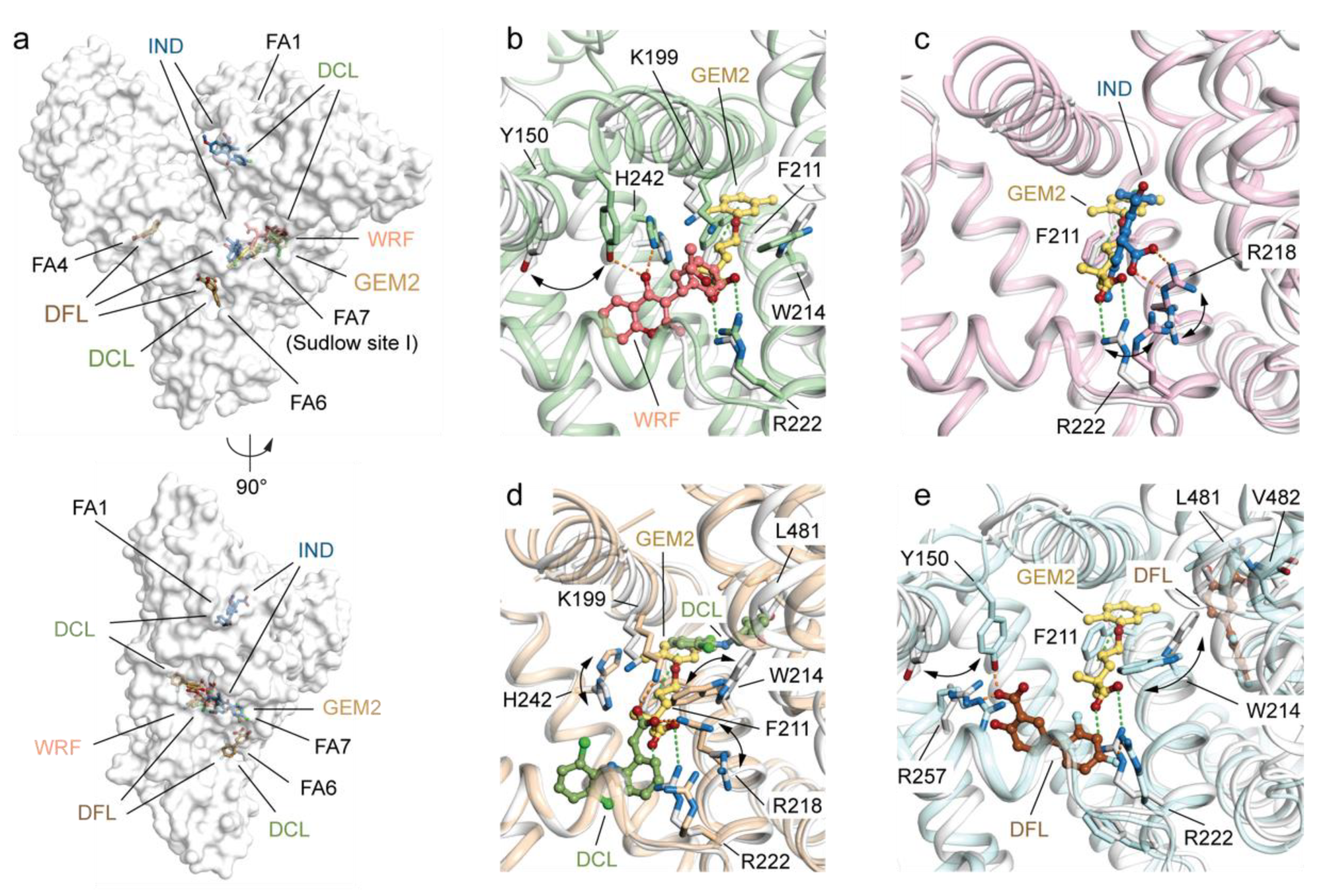
2.3.2. Differences between GEM2 and Other Known Drugs Binding HSA Sudlow’s Site I (FA7)
3. Discussion
4. Materials and Methods
4.1. Protein and Chemicals
4.2. Protein Preparation and Purification
4.3. Crystallisation
4.4. X-ray Diffraction Data Collection and Processing
4.5. Structure Determination and Model Refinement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Todd, P.A.; Ward, A. Gemfibrozil. Drugs 1988, 36, 314–339. [Google Scholar] [CrossRef] [PubMed]
- Hermens, W.A.J.J.; Lohman, J.J.H.M. Gemfibrozil (Lopid®). Pharm. Weekbl. 1990, 125, 984–986. [Google Scholar]
- Kundu, S.C.; Roxy, S.; Batabyal, S.K. Gemfibrozil in dyslipidaemia. J. Assoc. Physicians India 1990, 38, 156–159. [Google Scholar] [PubMed]
- Gaw, A.; Packard, C.J.; Shepherd, J. Fibrates-Principles and Treatment of Lipoprotein Disorders; Schettler, G., Habenicht, A.J.R., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 325–348. ISBN 978-3-642-78426-2. [Google Scholar]
- Duval, C.; Müller, M.; Kersten, S. PPARα and dyslipidemia. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2007, 1771, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Saku, K.; Gartside, P.S.; Hynd, B.A.; Kashyap, M.L. Mechanism of action of gemfibrozil on lipoprotein metabolism. J. Clin. Investig. 1985, 75, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. The Pathogenesis of Atherosclerosis—An Update. N. Engl. J. Med. 1986, 314, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Poplawski, J.; Lozowicka, B.; Dubis, A.T.; Lachowska, B.; Witkowski, S.; Siluk, D.; Petrusewicz, J.; Kaliszan, R.; Cybulski, J.; Strzalkowska, M.; et al. Synthesis and hypolipidemic and antiplatelet activities of α-asarone isomers in humans (in vitro), mice (in vivo), and rats (In Vivo). J. Med. Chem. 2000, 43, 3671–3676. [Google Scholar] [CrossRef]
- Roy, A.; Pahan, K. Gemfibrozil, stretching arms beyond lipid lowering. Immunopharmacol. Immunotoxicol. 2009, 31, 339–351. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Kleinman, H.K.; Lee, H.-J.; Pahan, K. Safety and potential efficacy of gemfibrozil as a supportive treatment for children with late infantile neuronal ceroid lipofuscinosis and other lipid storage disorders. Orphanet J. Rare Dis. 2017, 12, 113. [Google Scholar] [CrossRef]
- Ghosh, A.; Rangasamy, S.B.; Modi, K.K.; Pahan, K. Gemfibrozil, food and drug administration-approved lipid-lowering drug, increases longevity in mouse model of late infantile neuronal ceroid lipofuscinosis. J. Neurochem. 2017, 141, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Farnier, M. Combination Therapy with an HMG-CoA Reductase Inhibitor and a Fibric Acid Derivative. Am. J. Cardiovasc. Drugs 2003, 3, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Shek, A.; Ferrill, M.J. Statin—Fibrate Combination Therapy. Ann. Pharmacother. 2001, 35, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Backman, J.T.; Kyrklund, C.; Neuvonen, M.; Neuvonen, P.J. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin. Pharmacol. Ther. 2002, 72, 685–691. [Google Scholar] [CrossRef]
- Rindone, J.P.; Keng, H.-C. Gemfibrozil-Warfarin Drug Interaction Resulting in Profound Hypoprothrombinemia. Chest 1998, 114, 641–642. [Google Scholar] [CrossRef]
- Lilja, J.J.; Backman, J.T.; Neuvonen, P.J. Effect of gemfibrozil on the pharmacokinetics and pharmacodynamics of racemic warfarin in healthy subjects. Br. J. Clin. Pharmacol. 2005, 59, 433–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, D.L.; Williams, V.G. Interaction Between Gemfibrozil and Warfarin: Case Report and Review of the Literature. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2009, 29, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.A.; de Vries, M.; Krauwinkel, W.; Ohtsu, Y.; Noukens, J.; van der Walt, J.-S.; Mol, R.; Mordenti, J.; Ouatas, T. Pharmacokinetic Drug Interaction Studies with Enzalutamide. Clin. Pharmacokinet. 2015, 54, 1057–1069. [Google Scholar] [CrossRef] [Green Version]
- Menon, R.M.; Badri, P.S.; Wang, T.; Polepally, A.R.; Zha, J.; Khatri, A.; Wang, H.; Hu, B.; Coakley, E.P.; Podsadecki, T.J.; et al. Drug-drug interaction profile of the all-oral anti-hepatitis C virus regimen of paritaprevir/ritonavir, ombitasvir, and dasabuvir. J. Hepatol. 2015, 63, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Gan, J.; Chen, W.; Shen, H.; Gao, L.; Hong, Y.; Tian, Y.; Li, W.; Zhang, Y.; Tang, Y.; Zhang, H.; et al. Repaglinide-gemfibrozil drug interaction: Inhibition of repaglinide glucuronidation as a potential additional contributing mechanism. Br. J. Clin. Pharmacol. 2010, 70, 870–880. [Google Scholar] [CrossRef] [Green Version]
- Niemi, M.; Backman, J.T.; Neuvonen, M.; Neuvonen, P.J. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics and pharmacodynamics of repaglinide: Potentially hazardous interaction between gemfibrozil and repaglinide. Diabetologia 2003, 46, 347–351. [Google Scholar] [CrossRef]
- Bruderer, S.; Petersen-Sylla, M.; Boehler, M.; Remeňová, T.; Halabi, A.; Dingemanse, J. Effect of gemfibrozil and rifampicin on the pharmacokinetics of selexipag and its active metabolite in healthy subjects. Br. J. Clin. Pharmacol. 2017, 83, 2778–2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okerholm, R.A.; Keeley, F.J.; Peterson, F.E.; Glazko, A.J. The metabolism of gemfibrozil. Proc. R. Soc. Med. 1976, 69 (Suppl. S2), 11–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giampietro, L.; Ammazzalorso, A.; Amoroso, R.; De Filippis, B. Development of Fibrates as Important Scaffolds in Medicinal Chemistry. ChemMedChem 2019, 14, 1051–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, D.C. Crystallographic Survey of Albumin Drug Interaction and Preliminary Applications in Cancer Chemotherapy. Burger’s Med. Chem. Drug Discov. 2010, 437–468. [Google Scholar] [CrossRef]
- Zsila, F. Circular Dichroism Spectroscopic Detection of Ligand Binding Induced Subdomain IB Specific Structural Adjustment of Human Serum Albumin. J. Phys. Chem. B 2013, 117, 10798–10806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegedus, L.; Krempels, K.; Paal, K.; Petho, G. Pharmaceutical compositions containing plasma protein. U.S. patent 7,501,455 B2, March 2009. [Google Scholar]
- Sallustio, B.C.; Fairchild, B.A.; Pannall, P.R. Interaction of human serum albumin with the electrophilic metabolite 1-O-gemfibrozil-beta-D-glucuronide. Drug Metab. Dispos. 1997, 25, 55–60. [Google Scholar]
- Janson, G.; Zhang, C.; Prado, M.G.; Paiardini, A. PyMod 2.0: Improvements in protein sequence-structure analysis and homology modeling within PyMOL. Bioinformatics 2017, 33, 444–446. [Google Scholar] [CrossRef] [Green Version]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef]
- Davies, N.M. Clinical Pharmacokinetics of Ibuprofen. Clin. Pharmacokinet. 1998, 34, 101–154. [Google Scholar] [CrossRef]
- Varrassi, G.; Pergolizzi, J.V.; Dowling, P.; Paladini, A. Are all NSAIDs the Same? A Narrative Review. Adv. Ther. 2020, 37, 61–82. [Google Scholar] [CrossRef] [Green Version]
- Kokki, H. Ketoprofen Pharmacokinetics, Efficacy, and Tolerability in Pediatric Patients. Pediatr. Drugs 2010, 12, 313–329. [Google Scholar] [CrossRef]
- Curry, S.; Mandelkow, H.; Brick, P.; Franks, N. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat. Struct. Biol. 1998, 5, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Birkett, D.J.; Myers, S.P.; Sudlow, G. Effects of Fatty Acids on Two Specific Drug Binding Sites on Human Serum Albumin. Mol. Pharmacol. 1977, 13, 987–992. [Google Scholar] [PubMed]
- Afkham, S.; Hanaee, J.; Zakariazadeh, M.; Fathi, F.; Shafiee, S.; Soltani, S. Molecular mechanism and thermodynamic study of Rosuvastatin interaction with human serum albumin using a surface plasmon resonance method combined with a multi-spectroscopic, and molecular modeling approach. Eur. J. Pharm. Sci. 2022, 168, 106005. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-H.; Wang, Q.; Pan, D.-Q.; Liu, T.-T.; Jiang, M. Characterization of interactions of simvastatin, pravastatin, fluvastatin, and pitavastatin with bovine serum albumin: Multiple spectroscopic and molecular docking. J. Biomol. Struct. Dyn. 2017, 35, 1529–1546. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, C.; Jiang, M.; Zhu, Y.; Wang, J.; Chen, J.; Shi, J. Binding interaction of atorvastatin with bovine serum albumin: Spectroscopic methods and molecular docking. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 156, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Petitpas, I.; Bhattacharya, A.A.; Twine, S.; East, M.; Curry, S. Crystal structure analysis of warfarin binding to human serum albumin. Anatomy of drug site I. J. Biol. Chem. 2001, 276, 22804–22809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovbude, S.T.; Tao, P.; Li, Z.; Hage, D.S. High-Performance affinity chromatographic studies of repaglinide and nateglinide interactions with normal and glyoxal- or methylglyoxal-modified human albumin serum. J. Pharm. Biomed. Anal. 2021, 201, 114097. [Google Scholar] [CrossRef]
- Maso, L.; Trande, M.; Liberi, S.; Moro, G.; Daems, E.; Linciano, S.; Sobott, F.; Covaceuszach, S.; Cassetta, A.; Fasolato, S.; et al. Unveiling the binding mode of perfluorooctanoic acid to human serum albumin. Protein Sci. 2021, 30, 830–841. [Google Scholar] [CrossRef]
- Potterton, L.; Agirre, J.; Ballard, C.; Cowtan, K.; Dodson, E.; Evans, P.R.; Jenkins, H.T.; Keegan, R.; Krissinel, E.; Stevenson, K.; et al. CCP4i2: The new graphical user interface to the CCP4 program suite. Acta Crystallogr. Sect. D 2018, 74, 68–84. [Google Scholar] [CrossRef] [Green Version]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vagin, A.A.; Steiner, R.A.; Lebedev, A.A.; Potterton, L.; McNicholas, S.; Long, F.; Murshudov, G.N. REFMAC5 dictionary: Organization of prior chemical knowledge and guidelines for its use. Acta Crystallogr. Sect. D 2004, 60, 2184–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.-W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laskowski, R.A.; Watson, J.D.; Thornton, J.M. ProFunc: A server for predicting protein function from 3D structure. Nucleic Acids Res. 2005, 33, W89–W93. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liberi, S.; Linciano, S.; Moro, G.; De Toni, L.; Cendron, L.; Angelini, A. Structural Analysis of Human Serum Albumin in Complex with the Fibrate Drug Gemfibrozil. Int. J. Mol. Sci. 2022, 23, 1769. https://doi.org/10.3390/ijms23031769
Liberi S, Linciano S, Moro G, De Toni L, Cendron L, Angelini A. Structural Analysis of Human Serum Albumin in Complex with the Fibrate Drug Gemfibrozil. International Journal of Molecular Sciences. 2022; 23(3):1769. https://doi.org/10.3390/ijms23031769
Chicago/Turabian StyleLiberi, Stefano, Sara Linciano, Giulia Moro, Luca De Toni, Laura Cendron, and Alessandro Angelini. 2022. "Structural Analysis of Human Serum Albumin in Complex with the Fibrate Drug Gemfibrozil" International Journal of Molecular Sciences 23, no. 3: 1769. https://doi.org/10.3390/ijms23031769
APA StyleLiberi, S., Linciano, S., Moro, G., De Toni, L., Cendron, L., & Angelini, A. (2022). Structural Analysis of Human Serum Albumin in Complex with the Fibrate Drug Gemfibrozil. International Journal of Molecular Sciences, 23(3), 1769. https://doi.org/10.3390/ijms23031769





