Functional Characterization of Tomato Phytochrome A and B1B2 Mutants in Response to Heat Stress
Abstract
:1. Introduction
2. Results
2.1. The Phenotype of phy Mutants in Response to HS
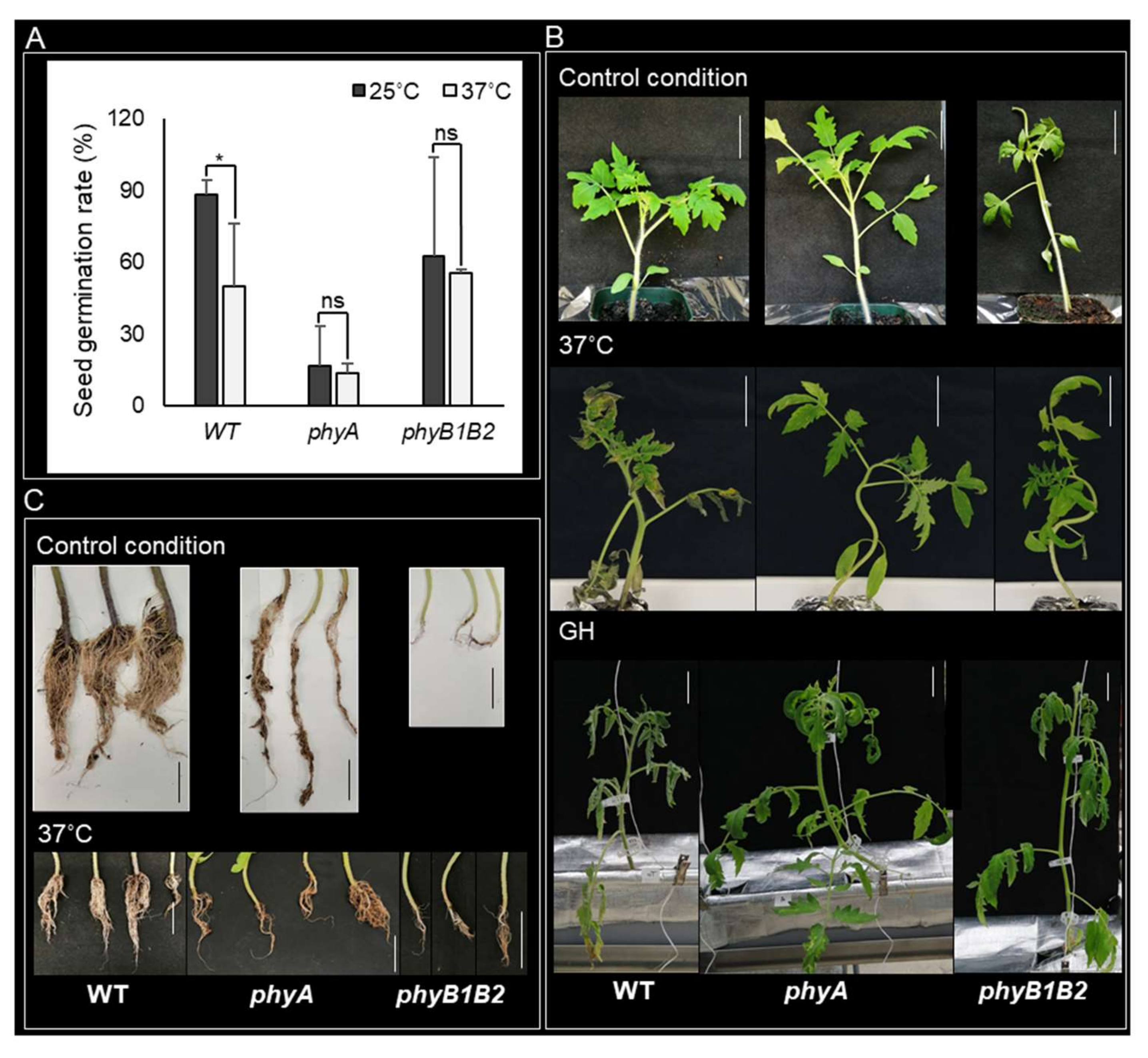
2.1.1. Conservation of the Seed Germination Rate in phyA and phyB1B2 under HS
2.1.2. Enhancement of Tomato Tolerance toward HS by phyA and phyB1B2 Mutation
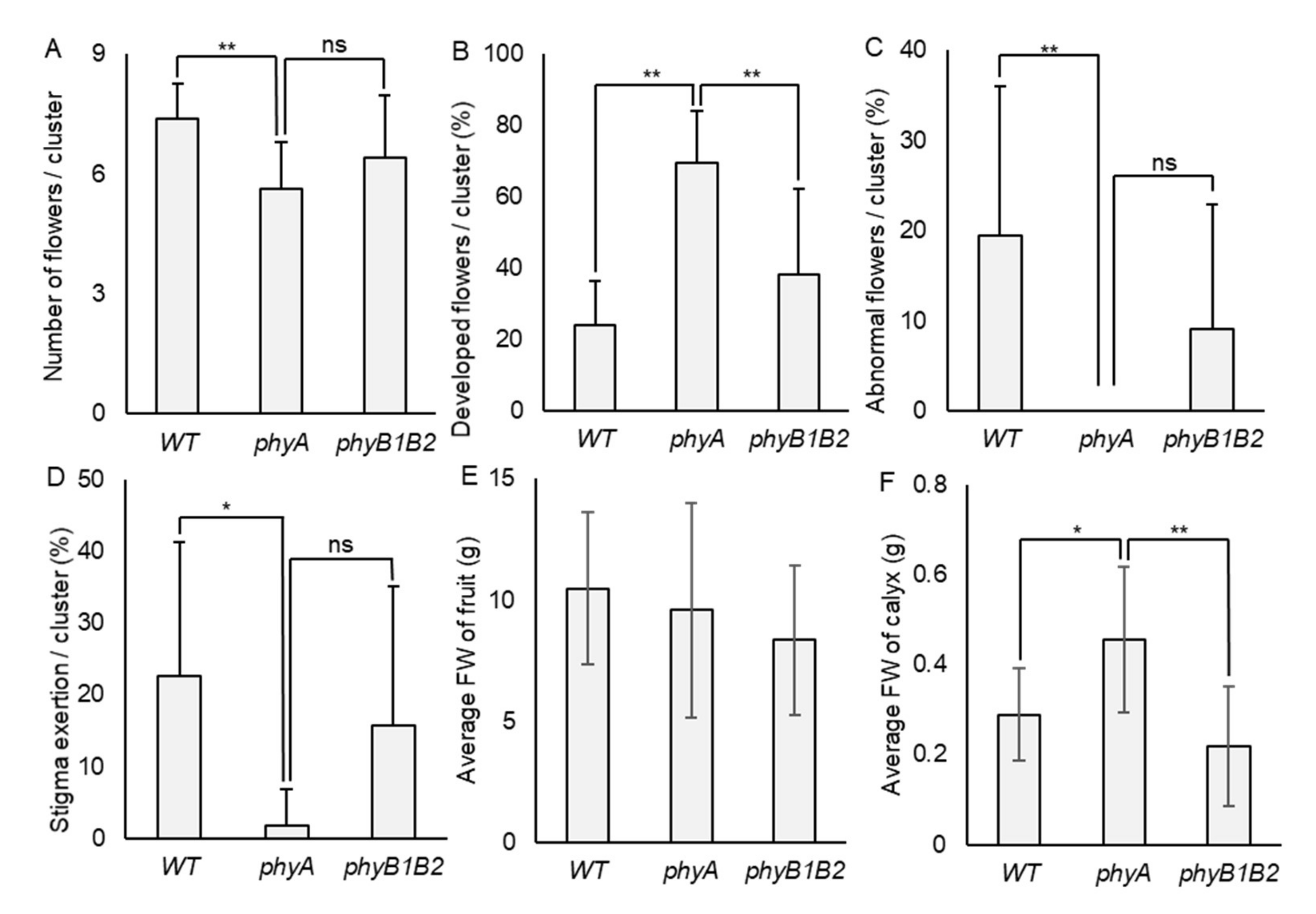
2.1.3. The phyA and phyB1B2 Flower and Fruit Phenotypic Response under HS
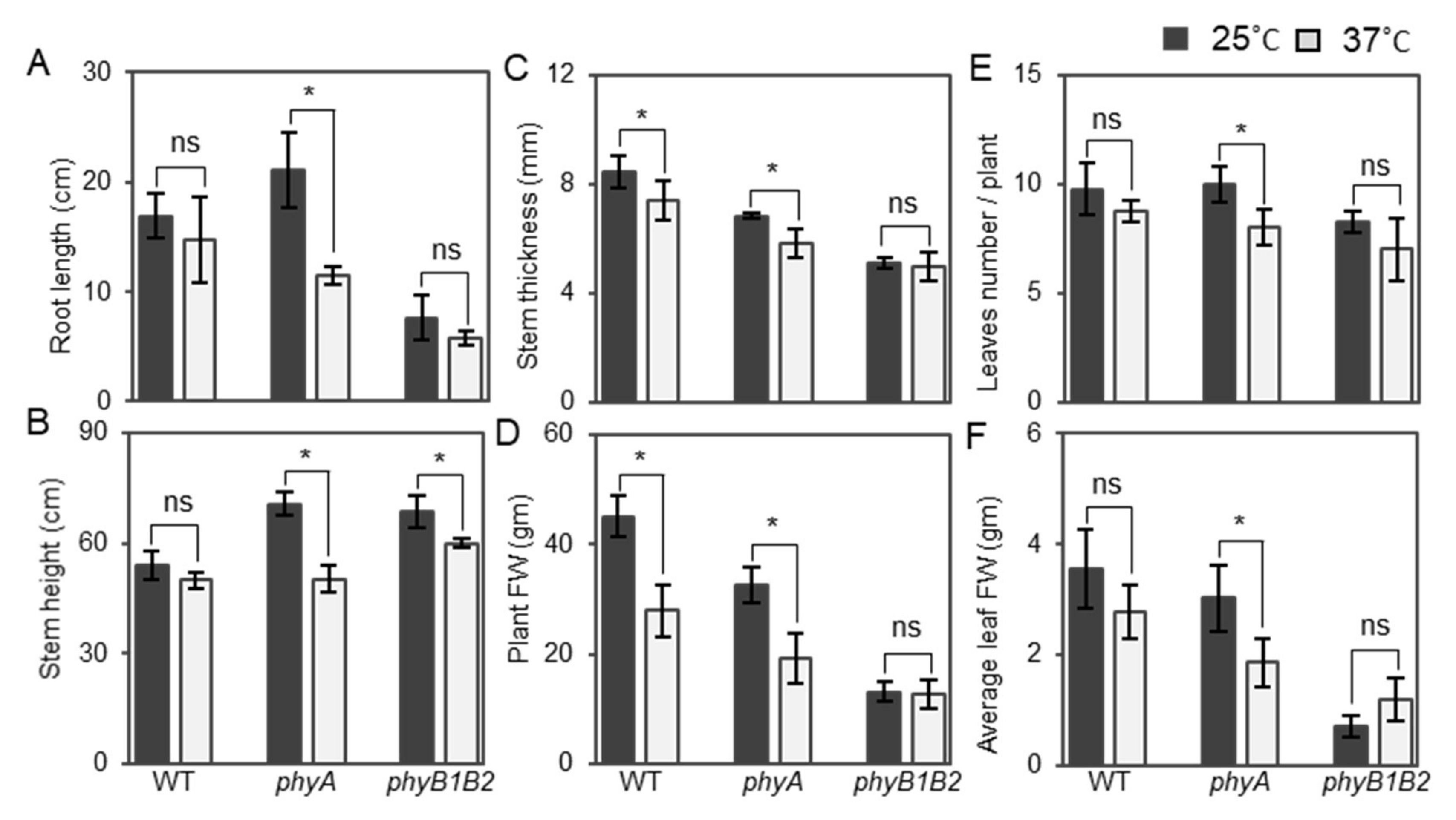
2.1.4. Changes in Plant Vegetative Characteristics of phy Mutants in Response to HS
2.1.5. Tomato phyA Mutant Inhibited Undesirable Flower Traits under HS
2.1.6. Negative Effects of HS on Fruit Characteristics
2.2. Tomato phyA and phyB1B2 Physiological Response under HS Conditions
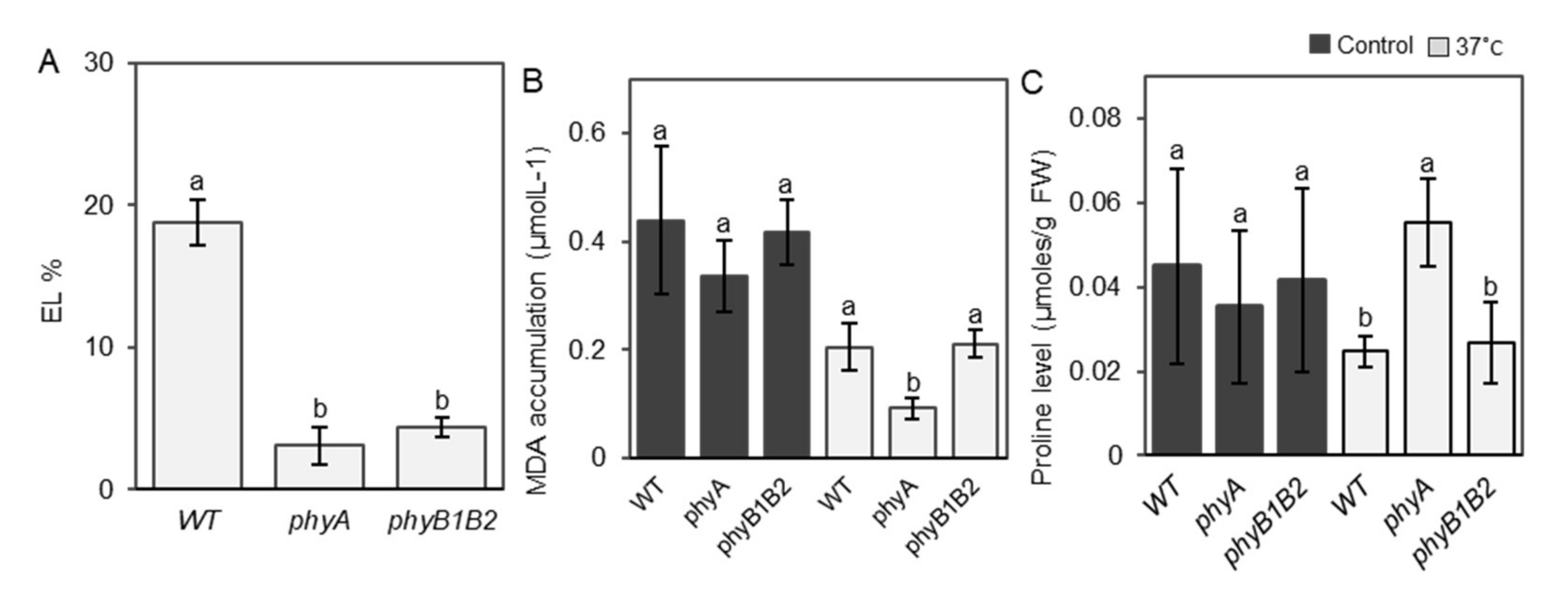
2.2.1. Inhibiting EL and MDA Accumulation and Enhancing Proline Accumulation under HS by Tomato phyA mutation
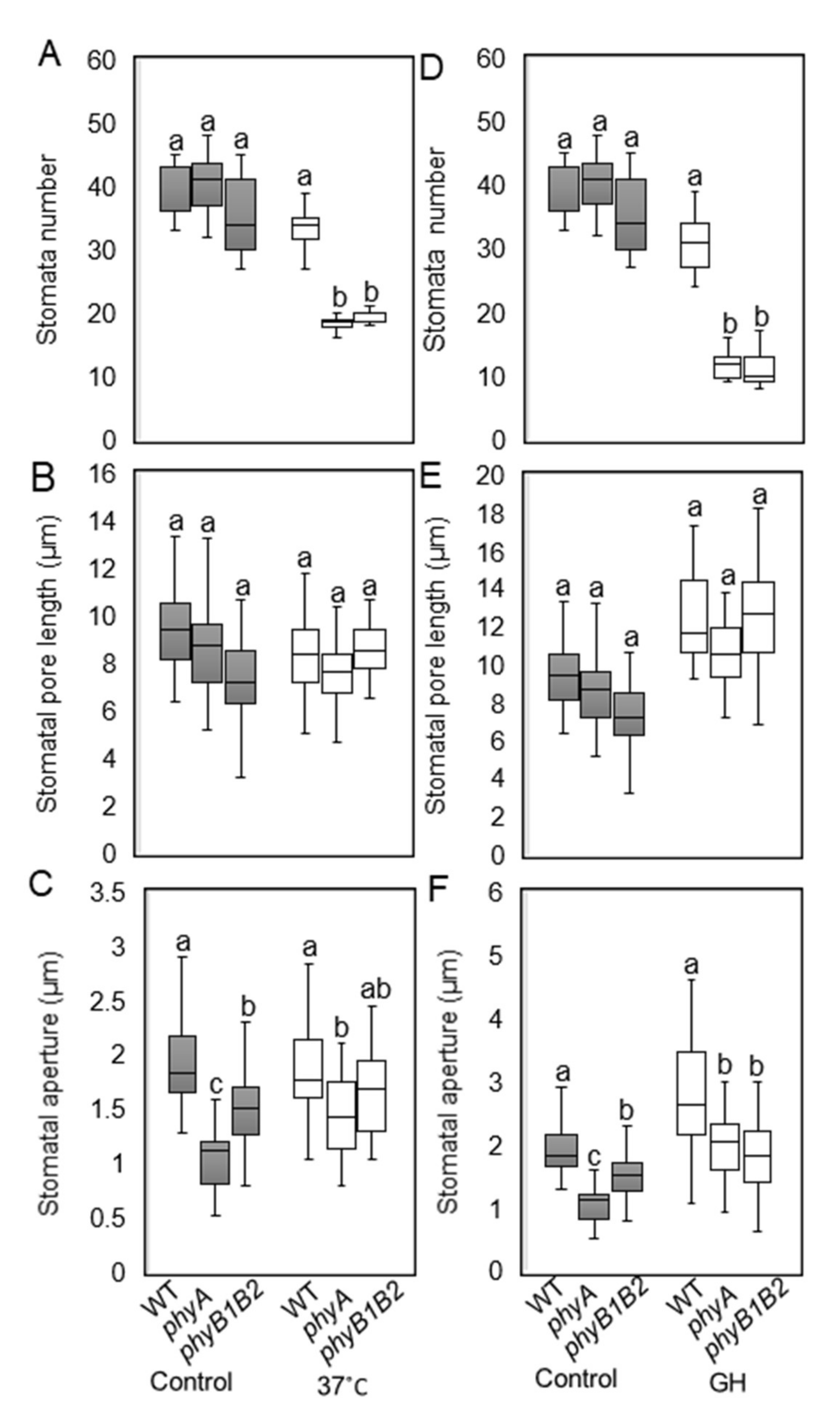
2.2.2. Tomato phyA and phyB1B2 Can Change Stomata Features under HS Conditions
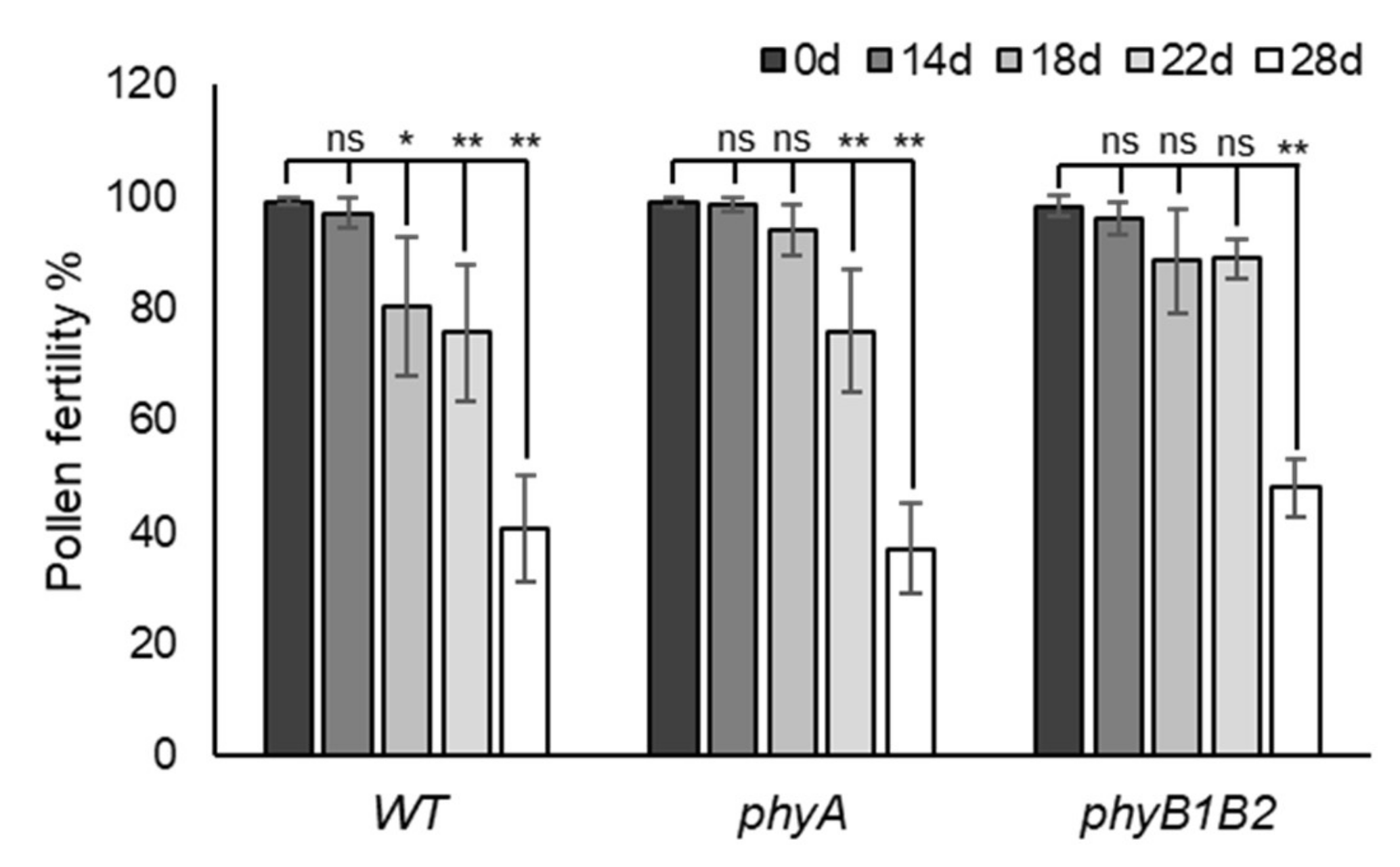
2.2.3. Enhancing Pollen Viability under HS in Tomato phyA and phyB1B2
2.2.4. Inhibiting of Pollen Tube Growth by HS
2.3. Tomato phyA and phyB1B2 Molecular Response under HS Conditions
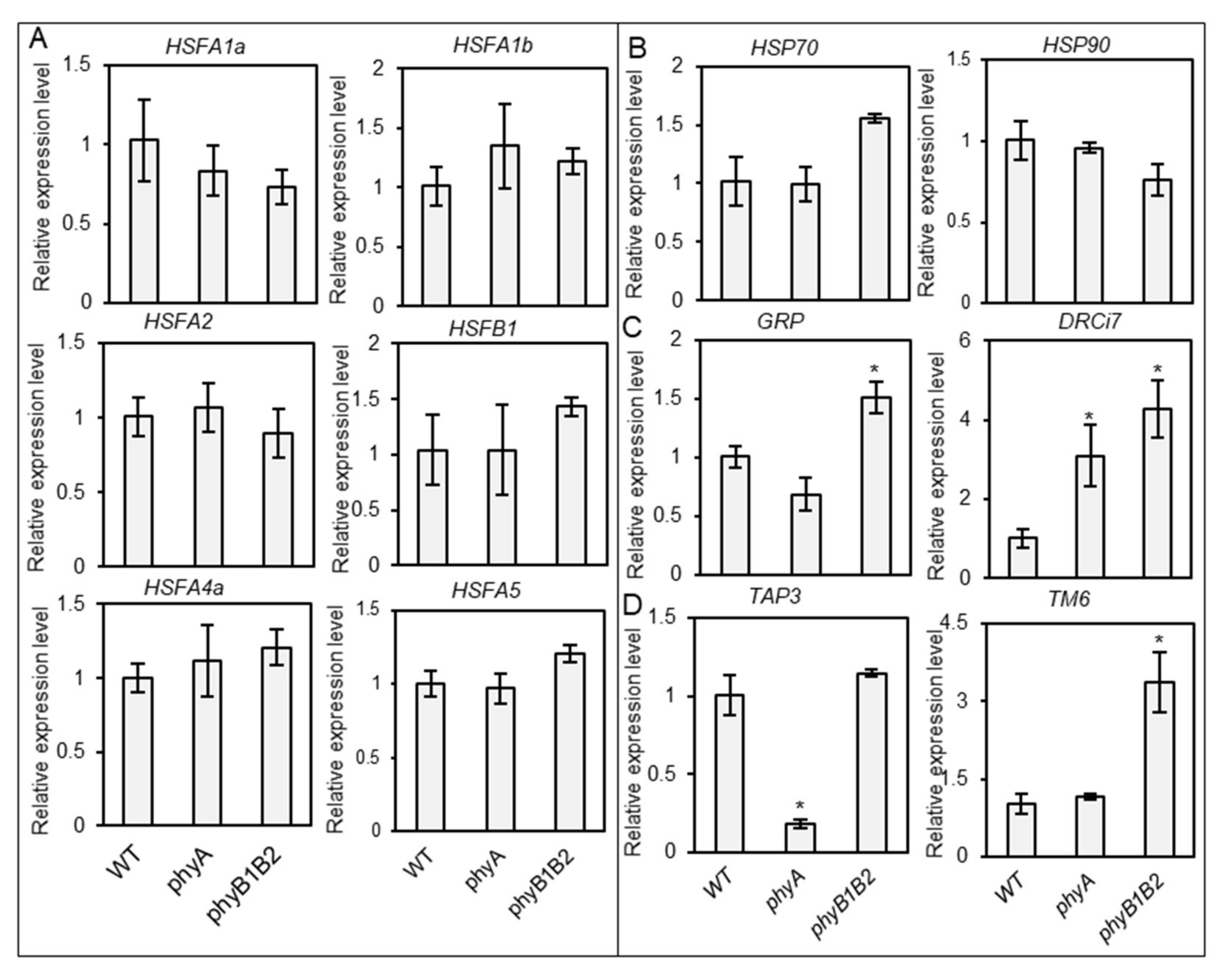
2.3.1. Tomato phyA and phyB1B2 Enhanced the Expression of Some Heat- and Stress-Responsive Genes under HS during the Vegetative Growth Stage
2.3.2. Relative Expression of Some Heat- and Stress-Responsive Genes as Well as Flower Controller Genes during the Flowering Stage
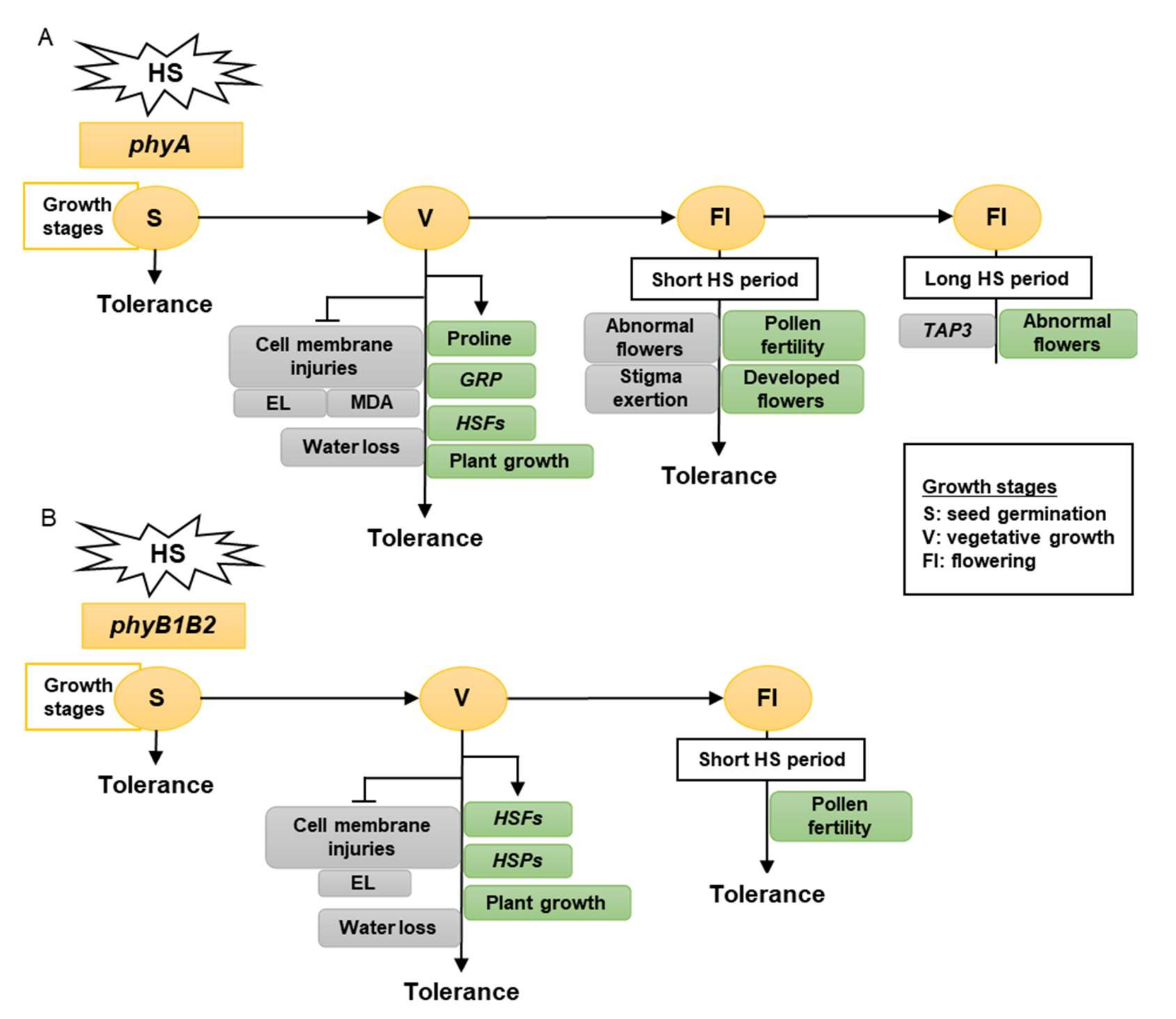
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Phy Responses to HS during the Seed Germination Stage
Seed Germination Rate
4.3. Phy Responses to HS during the Vegetative Growth Stage
4.3.1. Morphological Phenotype
4.3.2. EL
4.3.3. Measuring Proline and MDA Levels
4.3.4. Microscopic Analysis for Stomata
4.3.5. RNA Isolation and Quantitative RT-PCR
4.4. Phy Responses to HS during Flowering and Fruiting Growth Stage
4.4.1. Pollen Fertility and Pollen Tube Growth Test
4.4.2. Flower Characteristics
4.4.3. Fruiting Characteristics
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, R.; Yu, X.; Ottosen, C.-O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought Stress Had a Predominant Effect over Heat Stress on Three Tomato Cultivars Subjected to Combined Stress. BMC Plant Biol. 2017, 17, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Heckathorn, S.A.; Mainali, K.; Tripathee, R. Timing Effects of Heat-Stress on Plant Ecophysiological Characteristics and Growth. Front. Plant Sci. 2016, 7, 1629. [Google Scholar] [CrossRef] [Green Version]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Silva, R.S.; Kumar, L.; Shabani, F.; Picanço, M.C. Assessing the Impact of Global Warming on Worldwide Open Field Tomato Cultivation through CSIRO-Mk3·0 Global Climate Model. J. Agric. Sci. 2017, 155, 407–420. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Yu, X.; Zhao, T.; Ottosen, C.-O.; Rosenqvist, E.; Wu, Z. Physiological Analysis and Transcriptome Sequencing Reveal the Effects of Combined Cold and Drought on Tomato Leaf. BMC Plant Biol. 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.B., Jr. Tomato Plant Culture: In the Field, Greenhouse, and Home Garden, Second Edition, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-8493-7395-4. [Google Scholar]
- Carvalho, R.F.; Campos, M.L.; Azevedo, R.A. The Role of Phytochromes in Stress Tolerance. In Salt Stress in Plants: Signalling, Omics and Adaptations; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2013; pp. 283–299. ISBN 9781461461081. [Google Scholar]
- Carvalho, R.F.; Campos, M.L.; Azevedo, R.A. The Role of Phytochrome in Stress Tolerance. J. Integr. Plant Biol. 2011, 53, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Alba, R.; Kelmenson, P.M.; Cordonnier-Pratt, M.M.; Pratt, L.H. The Phytochrome Gene Family in Tomato and the Rapid Differential Evolution of This Family in Angiosperms. Mol. Biol. Evol. 2000, 17, 362–373. [Google Scholar] [CrossRef] [Green Version]
- Whitelam, G.C.; Devlin, P.F. Roles of Different Phytochromes in Arabidopsis Photomorphogenesis. Plant Cell Environ. 1997, 20, 752–758. [Google Scholar] [CrossRef]
- Sun, W.; Hui Xu, X.; Lu, X.; Xie, L.; Bai, B.; Zheng, C.; Sun, H.; He, Y.; Xie, X. The Rice Phytochrome Genes, PHYA and PHYB, Have Synergistic Effects on Anther Development and Pollen Viability. Sci. Rep. 2017, 7, 6439. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Liu, Q.; Hu, B.; Wu, W. Photoreceptor PhyB Involved in Arabidopsis Temperature Perception and Heat-Tolerance Formation. Int. J. Mol. Sci. 2017, 18, 1194. [Google Scholar] [CrossRef] [Green Version]
- Legris, M.; Klose, C.; Burgie, E.S.; Rojas, C.C.R.; Neme, M.; Hiltbrunner, A.; Wigge, P.A.; Schäfer, E.; Vierstra, R.D.; Casal, J.J. Phytochrome B Integrates Light and Temperature Signals in Arabidopsis. Science 2016, 354, 897–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arico, D.; Legris, M.; Castro, L.; Garcia, C.F.; Laino, A.; Casal, J.J.; Mazzella, M.A. Neighbour Signals Perceived by Phytochrome B Increase Thermotolerance in Arabidopsis. Plant Cell Environ. 2019, 42, 2554–2566. [Google Scholar] [CrossRef] [PubMed]
- Auge, G.A.; Rugnone, M.L.; Cortés, L.E.; González, C.V.; Zarlavsky, G.; Boccalandro, H.E.; Sánchez, R.A. Phytochrome A Increases Tolerance to High Evaporative Demand. Physiol. Plant 2012, 146, 228–235. [Google Scholar] [CrossRef]
- Van Tuinen, A.; Kerckhoffs, L.H.J.; Nagatani, A.; Kendrick, R.E.; Koornneef, M. Far-Red Light-Insensitive, Phytochrome A-Deficient Mutants of Tomato. Molec. Gen. Genet. 1995, 246, 133–141. [Google Scholar] [CrossRef] [PubMed]
- D’Amico Damião, V.; Cruz, F.; Gavassi, M.; Santos, D.; Melo, H.C.; Carvalho, R. Photomorphogenic Modulation of Water Stress in Tomato (Solanum Lycopersicum L.): The Role of Phytochromes A, B1, and B2. J. Hortic. Sci. Biotechnol. 2015, 90, 25–30. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Cui, L.; Xie, L.; Zheng, C.; Zhou, G.; Zhou, J.; Xie, X. Phytochrome B Negatively Affects Cold Tolerance by Regulating OsDREB1 Gene Expression through Phytochrome Interacting Factor-Like Protein OsPIL16 in Rice. Front. Plant Sci. 2016, 7, 1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Lv, R.; Li, J.; Lin, H.; Xi, D. Phytochrome A and B Negatively Regulate Salt Stress Tolerance of Nicotiana Tobacum via ABA–Jasmonic Acid Synergistic Cross-Talk. Plant Cell Physiol. 2018, 59, 2381–2393. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, T.; Kimura, S. Plant Temperature Sensors. Sensors 2018, 18, 4365. [Google Scholar] [CrossRef] [Green Version]
- Saelim, S.; Zwiazek, J.J. Preservation of Thermal Stability of Cell Membranes and Gas Exchange in High Temperature Acclimated Xylia Xylocarpa Seedlings. J. Plant Physiol. 2000, 156, 380–385. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of Glycine Betaine and Proline in Improving Plant Abiotic Stress Resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline Accumulation in Plants: A Review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Fraire-Velázquez, S.; Balderas-Hernández, V.E. Abiotic Stress in Plants and Metabolic Responses. In Abiotic Stress—Plant Responses and Applications in Agriculture; Vahdati, K., Leslie, C., Eds.; IntechOpen: London, UK, 2013; pp. 25–48. Available online: https://www.intechopen.com/chapters/43341 (accessed on 8 January 2021). [CrossRef] [Green Version]
- Kong, W.; Liu, F.; Zhang, C.; Zhang, J.; Feng, H. Non-Destructive Determination of Malondialdehyde (MDA) Distribution in Oilseed Rape Leaves by Laboratory Scale NIR Hyperspectral Imaging. Sci. Rep. 2016, 6, 35393. [Google Scholar] [CrossRef] [PubMed]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Scharf, K.-D.; Berberich, T.; Ebersberger, I.; Nover, L. The Plant Heat Stress Transcription Factor (Hsf) Family: Structure, Function and Evolution. Biochim. Et Biophys. Acta (BBA)—Gene Regul. Mech. 2012, 1819, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Heat Stress Response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, W.; Zhang, H.; Liu, N.; Tian, S. Heat Shock Factors in Tomatoes: Genome-Wide Identification, Phylogenetic Analysis and Expression Profiling under Development and Heat Stress. PeerJ 2016, 4, e1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maestri, E.; Klueva, N.; Perrotta, C.; Gulli, M.; Nguyen, H.T.; Marmiroli, N. Molecular Genetics of Heat Tolerance and Heat Shock Proteins in Cereals. Plant Mol. Biol. 2002, 48, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Driedonks, N.; Rieu, I.; Vriezen, W.H. Breeding for Plant Heat Tolerance at Vegetative and Reproductive Stages. Plant Reprod. 2016, 29, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Comastri, A.; Janni, M.; Simmonds, J.; Uauy, C.; Pignone, D.; Nguyen, H.T.; Marmiroli, N. Heat in Wheat: Exploit Reverse Genetic Techniques to Discover New Alleles Within the Triticum Durum SHsp26 Family. Front. Plant Sci. 2018, 9, 1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paupière, M.J.; van Haperen, P.; Rieu, I.; Visser, R.G.F.; Tikunov, Y.M.; Bovy, A.G. Screening for Pollen Tolerance to High Temperatures in Tomato. Euphytica 2017, 213, 130. [Google Scholar] [CrossRef]
- Sato, S.; Peet, M.M.; Thomas, J.F. Physiological Factors Limit Fruit Set of Tomato (Lycopersicon Esculentum Mill.) under Chronic, Mild Heat Stress. Plant Cell Environ. 2000, 23, 719–726. [Google Scholar] [CrossRef]
- Sato, S.; Peet, M.M.; Gardner, R.G. Formation of Parthenocarpic Fruit, Undeveloped Flowers and Aborted Flowers in Tomato under Moderately Elevated Temperatures. Sci. Hortic. 2001, 90, 243–254. [Google Scholar] [CrossRef]
- Gavassi, M.A.; Monteiro, C.C.; Campos, M.L.; Melo, H.C.; Carvalho, R.F. Phytochromes Are Key Regulators of Abiotic Stress Responses in Tomato. Sci. Hortic. 2017, 222, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Correll, M.J.; Kiss, J.Z. The Roles of Phytochromes in Elongation and Gravitropism of Roots. Plant Cell Physiol. 2005, 46, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Alsamir, M.; Ahmad, N.M.; Keitel, C.; Mahmood, T.; Trethowan, R. Identification of High-Temperature Tolerant and Agronomically Viable Tomato (S. Lycopersicum) Genotypes from a Diverse Germplasm Collection. Adv. Crop Sci. Tech. 2017, 5, 10–4172. [Google Scholar] [CrossRef]
- Adams, S.; Cockshull, K.; Cave, C. Effect of Temperature on the Growth and Development of Tomato Fruits. Ann. Bot. 2001, 88, 869–877. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant Tolerance to High Temperature in a Changing Environment: Scientific Fundamentals and Production of Heat Stress-Tolerant Crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsamir, M.; Mahmood, T.; Trethowan, R.; Ahmad, N. An Overview of Heat Stress in Tomato (Solanum Lycopersicum L.). Saudi J. Biol. Sci. 2021, 28, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Yang, D.; Zhao, X.; Jiao, C.; Yan, Y.; Lamin-Samu, A.T.; Wang, Q.; Xu, X.; Fei, Z.; Lu, G. Tomato Stigma Exsertion Induced by High Temperature Is Associated with the Jasmonate Signalling Pathway. Plant Cell Environ. 2019, 42, 1205–1221. [Google Scholar] [CrossRef]
- Müller, F.; Xu, J.; Kristensen, L.; Wolters-Arts, M.; de Groot, P.F.M.; Jansma, S.Y.; Mariani, C.; Park, S.; Rieu, I. High-Temperature-Induced Defects in Tomato (Solanum Lycopersicum) Anther and Pollen Development Are Associated with Reduced Expression of B-Class Floral Patterning Genes. PLoS ONE 2016, 11, e0167614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peet, M.M.; Sato, S.; Gardner, R.G. Comparing Heat Stress Effects on Male-Fertile and Male-Sterile Tomatoes. Plant Cell Environ. 1998, 21, 225–231. [Google Scholar] [CrossRef]
- Srinivasan, A.; Takeda, H.; Senboku, T. Heat Tolerance in Food Legumes as Evaluated by Cell Membrane Thermostability and Chlorophyll Fluorescence Techniques. Euphytica 1996, 88, 35–45. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar]
- Claussen, W. Proline as a Measure of Stress in Tomato Plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A Key Player in Plant Abiotic Stress Tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Samakovli, D.; Tichá, T.; Šamaj, J. HSP90 Chaperones Regulate Stomatal Differentiation under Normal and Heat Stress Conditions. Plant Signal. Behav. 2020, 15, 1789817. [Google Scholar] [CrossRef]
- Karapanos, I.C.; Akoumianakis, K.A.; Olympios, C.M.; Passam, H.C. The Effect of Substrate, ADP and Uncoupler on the Respiration of Tomato Pollen during Incubation In Vitro at Moderately High Temperature. Sex. Plant Reprod. 2009, 22, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Perumal, R.; Jagadish, S.V.K.; Ciampitti, I.A.; Welti, R.; Prasad, P.V.V. Sensitivity of Sorghum Pollen and Pistil to High Temperature Stress. Plant Cell Environ. 2018, 41, 1065–1082. [Google Scholar] [CrossRef] [PubMed]
- Muhlemann, J.K.; Younts, T.L.B.; Muday, G.K. Flavonols Control Pollen Tube Growth and Integrity by Regulating ROS Homeostasis during High-Temperature Stress. Proc. Natl. Acad. Sci. USA 2018, 115, E11188–E11197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, F.; Shinozaki, K. Long-Distance Signaling in Plant Stress Response. Curr. Opin. Plant Biol. 2019, 47, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, J.-H.; Ma, X.; Luo, D.-X.; Gong, Z.-H.; Lu, M.-H. The Plant Heat Stress Transcription Factors (HSFs): Structure, Regulation, and Function in Response to Abiotic Stresses. Front Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.-X.; Zhang, H.-X.; Wei, A.-M.; Gong, Z.-H. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef] [Green Version]
- Czolpinska, M.; Rurek, M. Plant Glycine-Rich Proteins in Stress Response: An Emerging, Still Prospective Story. Front. Plant Sci. 2018, 9, 302. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T. The Role of Dehydrins in Plant Response to Cold. Biol. Plant 2007, 51, 601–617. [Google Scholar] [CrossRef]
- Fiorucci, A.-S.; Galvão, V.C.; Ince, Y.Ç.; Boccaccini, A.; Goyal, A.; Allenbach Petrolati, L.; Trevisan, M.; Fankhauser, C. PHYTOCHROME INTERACTING FACTOR 7 Is Important for Early Responses to Elevated Temperature in Arabidopsis Seedlings. New Phytol. 2020, 226, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wu, Z.; Hou, S. SPEECHLESS Speaks Loudly in Stomatal Development. Front. Plant Sci. 2020, 11, 114. [Google Scholar] [CrossRef]
- De Marcos, A.; Houbaert, A.; Triviño, M.; Delgado, D.; Martín-Trillo, M.; Russinova, E.; Fenoll, C.; Mena, M. A Mutation in the BHLH Domain of the SPCH Transcription Factor Uncovers a BR-Dependent Mechanism for Stomatal Development1. Plant Physiol. 2017, 174, 823–842. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Li, M.; Su, L.; Ge, K.; Li, L.; Li, X.; Liu, X.; Li, L. Negative Feedback Regulation of ABA Biosynthesis in Peanut (Arachis Hypogaea): A Transcription Factor Complex Inhibits AhNCED1 Expression during Water Stress. Sci. Rep. 2016, 6, 37943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.-F.F.; et al. Abscisic Acid Inhibits Type 2C Protein Phosphatases via the PYR/PYL Family of START Proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Shuqiang, W.; Lei, H.; Yongqiang, Q.; Huali, Z.; Haibo, X.; Zhenyuan, S. Gene Cloning and Expression of the Pyrroline-5-Carboxylate Reductase Gene of Perennial Ryegrass (Lolium Perenne). Hortic. Plant J. 2015, 1, 113–120. [Google Scholar] [CrossRef]
- Li, Q.; Huo, Q.; Wang, J.; Zhao, J.; Sun, K.; He, C. Expression of B-Class MADS-Box Genes in Response to Variations in Photoperiod Is Associated with Chasmogamous and Cleistogamous Flower Development in Viola Philippica. BMC Plant Biol. 2016, 16, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Martino, G.; Pan, I.; Emmanuel, E.; Levy, A.; Irish, V.F. Functional Analyses of Two Tomato APETALA3 Genes Demonstrate Diversification in Their Roles in Regulating Floral Development. Plant Cell 2006, 18, 1833–1845. [Google Scholar] [CrossRef] [Green Version]
- Dhanda, S.S.; Munjal, R. Cell Membrane Stability: Combining Ability and Gene Effects under Heat Stress Conditions. Cereal Res. Commun. 2009, 37, 409–417. [Google Scholar] [CrossRef]
- Alsadon, A.; Wahb-Allah, M.; Khalil, S. In Vitro Evaluation of Heat Stress Tolerance in Some Tomato Cultivars. J. King Saud Univ. 2006, 19, 13–24. [Google Scholar]
- Hurkman, W.J.; Vensel, W.H.; Tanaka, C.K.; Whitehand, L.; Altenbach, S.B. Effect of High Temperature on Albumin and Globulin Accumulation in the Endosperm Proteome of the Developing Wheat Grain. J. Cereal Sci. 2009, 49, 12–23. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Tarpley, L. Effects of High Night Temperature and Spikelet Position on Yield-Related Parameters of Rice (Oryza Sativa L.) Plants. Eur. J. Agron. 2010, 33, 117–123. [Google Scholar] [CrossRef]
- Asthir, B. Mechanisms of Heat Tolerance in Crop Plants. J. Plant Interact. 2015, 10, 1–21. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Staden, J.V. Dissecting the Roles of Osmolyte Accumulation during Stress. Plant Cell Environ. 1998, 21, 535–553. [Google Scholar] [CrossRef]
- Sairam, R.K.; Tyagi, A. Physiology and Molecular Biology of Salinity Stress Tolerance in Plants. Curr. Sci. 2004, 86, 407–421. [Google Scholar]
- Liu, J.; Sun, X.; Xu, F.; Zhang, Y.; Zhang, Q.; Miao, R.; Zhang, J.; Liang, J.; Xu, W. Suppression of OsMDHAR4 Enhances Heat Tolerance by Mediating H2O2-Induced Stomatal Closure in Rice Plants. Rice 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Balfagón, D.; Zandalinas, S.I.; Gómez-Cadenas, A. High Temperatures Change the Perspective: Integrating Hormonal Responses in Citrus Plants under Co-Occurring Abiotic Stress Conditions. Physiol. Plant 2018, 165, 183–197. [Google Scholar] [CrossRef]
- Hahn, A.; Bublak, D.; Schleiff, E.; Scharf, K.-D. Crosstalk between Hsp90 and Hsp70 Chaperones and Heat Stress Transcription Factors in Tomato. Plant Cell 2011, 23, 741–755. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.; et al. Heat Stress in Cultivated Plants: Nature, Impact, Mechanisms, and Mitigation Strategies—A Review. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2020, 155, 211–234. [Google Scholar] [CrossRef]
- Baniwal, S.K.; Bharti, K.; Chan, K.Y.; Fauth, M.; Ganguli, A.; Kotak, S.; Mishra, S.K.; Nover, L.; Port, M.; Scharf, K.-D.; et al. Heat Stress Response in Plants: A Complex Game with Chaperones and More than Twenty Heat Stress Transcription Factors. J. Biosci. 2004, 29, 471–487. [Google Scholar] [CrossRef]
- Pérez-Salamó, I.; Papdi, C.; Rigó, G.; Zsigmond, L.; Vilela, B.; Lumbreras, V.; Nagy, I.; Horváth, B.; Domoki, M.; Darula, Z.; et al. The Heat Shock Factor A4A Confers Salt Tolerance and Is Regulated by Oxidative Stress and the Mitogen-Activated Protein Kinases MPK3 and MPK61. Plant Physiol. 2014, 165, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Shim, D.; Hwang, J.-U.; Lee, J.; Lee, S.; Choi, Y.; An, G.; Martinoia, E.; Lee, Y. Orthologs of the Class A4 Heat Shock Transcription Factor HsfA4a Confer Cadmium Tolerance in Wheat and Rice. Plant Cell 2009, 21, 4031–4043. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Amaro, M.A.; Rodríguez-Hernández, A.A.; Rodríguez-Kessler, M.; Hernández-Lucero, E.; Rosales-Mendoza, S.; Ibáñez-Salazar, A.; Delgado-Sánchez, P.; Jiménez-Bremont, J.F. Overexpression of AtGRDP2, a Novel Glycine-Rich Domain Protein, Accelerates Plant Growth and Improves Stress Tolerance. Front. Plant Sci. 2015, 5, 782. [Google Scholar] [CrossRef] [Green Version]
- Pham, D.; Hoshikawa, K.; Fujita, S.; Fukumoto, S.; Hirai, T.; Shinozaki, Y.; Ezura, H. A Tomato Heat-Tolerant Mutant Shows Improved Pollen Fertility and Fruit-Setting under Long-Term Ambient High Temperature. Environ. Exp. Bot. 2020, 178, 104150. [Google Scholar] [CrossRef]
- Driedonks, N.J.W. From Flower to Fruit in the Heat—Reproductive Thermotolerance in Tomato and Its Wild Relatives. Ph.D.Thesis. Radboud University, Nijmegen, The Netherlands, 2018; p. 193. [Google Scholar]
- Yamamoto, T.; Kashojiya, S.; Kamimura, S.; Kameyama, T.; Ariizumi, T.; Ezura, H.; Miura, K. Application and Development of Genome Editing Technologies to the Solanaceae Plants. Plant Physiol. Biochem. 2018, 131, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Bhat, M.A.; Kumar, V.; Wani, I.A.; Bashir, H.; Shah, A.A.; Rahman, S.; Jan, A.T. The Era of Editing Plant Genomes Using CRISPR/Cas: A Critical Appraisal. J. Biotechnol. 2020, 324, 34–60. [Google Scholar] [CrossRef]
- Wenjing, W.; Chen, Q.; Singh, P.K.; Huang, Y.; Pei, D. CRISPR/Cas9 Edited HSFA6a and HSFA6b of Arabidopsis Thaliana Offers ABA and Osmotic Stress Insensitivity by Modulation of ROS Homeostasis. Plant Signal. Behav. 2020, 15, 1816321. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Wang, Y.; Zhou, G.; Wang, C.; Hussain, S.; Adnan; Lin, R.; Wang, T.; Wang, S. SlEAD1, an EAR Motif-Containing ABA down-Regulated Novel Transcription Repressor Regulates ABA Response in Tomato. GM Crops Food 2020, 11, 275–289. [Google Scholar] [CrossRef]
- Huang, Y.; Xuan, H.; Yang, C.; Guo, N.; Wang, H.; Zhao, J.; Xing, H. GmHsp90A2 Is Involved in Soybean Heat Stress as a Positive Regulator. Plant Sci. 2019, 285, 26–33. [Google Scholar] [CrossRef]
- Weller, J.L.; Schreuder, M.E.L.; Smith, H.; Koornneef, M.; Kendrick, R.E. Physiological Interactions of Phytochromes A, B1 and B2 in the Control of Development in Tomato. Plant J. 2000, 24, 345–356. [Google Scholar] [CrossRef]
- Miura, K.; Shiba, H.; Ohta, M.; Kang, S.W.; Sato, A.; Yuasa, T.; Iwaya-Inoue, M.; Kamada, H.; Ezura, H. SlICE1 Encoding a MYC-Type Transcription Factor Controls Cold Tolerance in Tomato, Solanum Lycopersicum. Plant Biotechnol. 2012, 29, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.H.; Khan, A.; Litaf, U.; Shah, A.S.; Khan, M.A.; Bilal, M.; Usman Ali, M. Effect of Drought Stress on Tomato Cv. Bombino. Food Process Technol. 2015, 6, 2. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, F.; Wen, J.; Wu, Z. Overexpression of Solanum Habrochaites MicroRNA319d (Sha-MiR319d) Confers Chilling and Heat Stress Tolerance in Tomato (S. Lycopersicum). BMC Plant Biol. 2019, 19, 214. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Jin, J.B.; Lee, J.; Yoo, C.Y.; Stirm, V.; Miura, T.; Ashworth, E.N.; Bressan, R.A.; Yun, D.-J.; Hasegawa, P.M. SIZ1-Mediated Sumoylation of ICE1 Controls CBF3/DREB1A Expression and Freezing Tolerance in Arabidopsis. Plant Cell 2007, 19, 1403–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Hoshikawa, K.; Fujita, S.; Thi, D.P.; Mizoguchi, T.; Ezura, H.; Ito, E. Evaluation of Internal Control Genes for Quantitative Realtime PCR Analyses for Studying Fruit Development of Dwarf Tomato Cultivar ‘Micro-Tom. ’ Plant Biotechnol. 2018, 35, 225–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulusoglu, M.; Cavusoglu, A. In Vitro Pollen Viability and Pollen Germination in Cherry Laurel (Prunus Laurocerasus L.). Sci. World J. 2014, 2014, 657123. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdellatif, I.M.Y.; Yuan, S.; Na, R.; Yoshihara, S.; Hamada, H.; Suzaki, T.; Ezura, H.; Miura, K. Functional Characterization of Tomato Phytochrome A and B1B2 Mutants in Response to Heat Stress. Int. J. Mol. Sci. 2022, 23, 1681. https://doi.org/10.3390/ijms23031681
Abdellatif IMY, Yuan S, Na R, Yoshihara S, Hamada H, Suzaki T, Ezura H, Miura K. Functional Characterization of Tomato Phytochrome A and B1B2 Mutants in Response to Heat Stress. International Journal of Molecular Sciences. 2022; 23(3):1681. https://doi.org/10.3390/ijms23031681
Chicago/Turabian StyleAbdellatif, Islam M. Y., Shaoze Yuan, Renhu Na, Shizue Yoshihara, Haruyasu Hamada, Takuya Suzaki, Hiroshi Ezura, and Kenji Miura. 2022. "Functional Characterization of Tomato Phytochrome A and B1B2 Mutants in Response to Heat Stress" International Journal of Molecular Sciences 23, no. 3: 1681. https://doi.org/10.3390/ijms23031681
APA StyleAbdellatif, I. M. Y., Yuan, S., Na, R., Yoshihara, S., Hamada, H., Suzaki, T., Ezura, H., & Miura, K. (2022). Functional Characterization of Tomato Phytochrome A and B1B2 Mutants in Response to Heat Stress. International Journal of Molecular Sciences, 23(3), 1681. https://doi.org/10.3390/ijms23031681






