High Iron and Iron Household Protein Contents in Perineuronal Net-Ensheathed Neurons Ensure Energy Metabolism with Safe Iron Handling
Abstract
:1. Introduction
2. Results
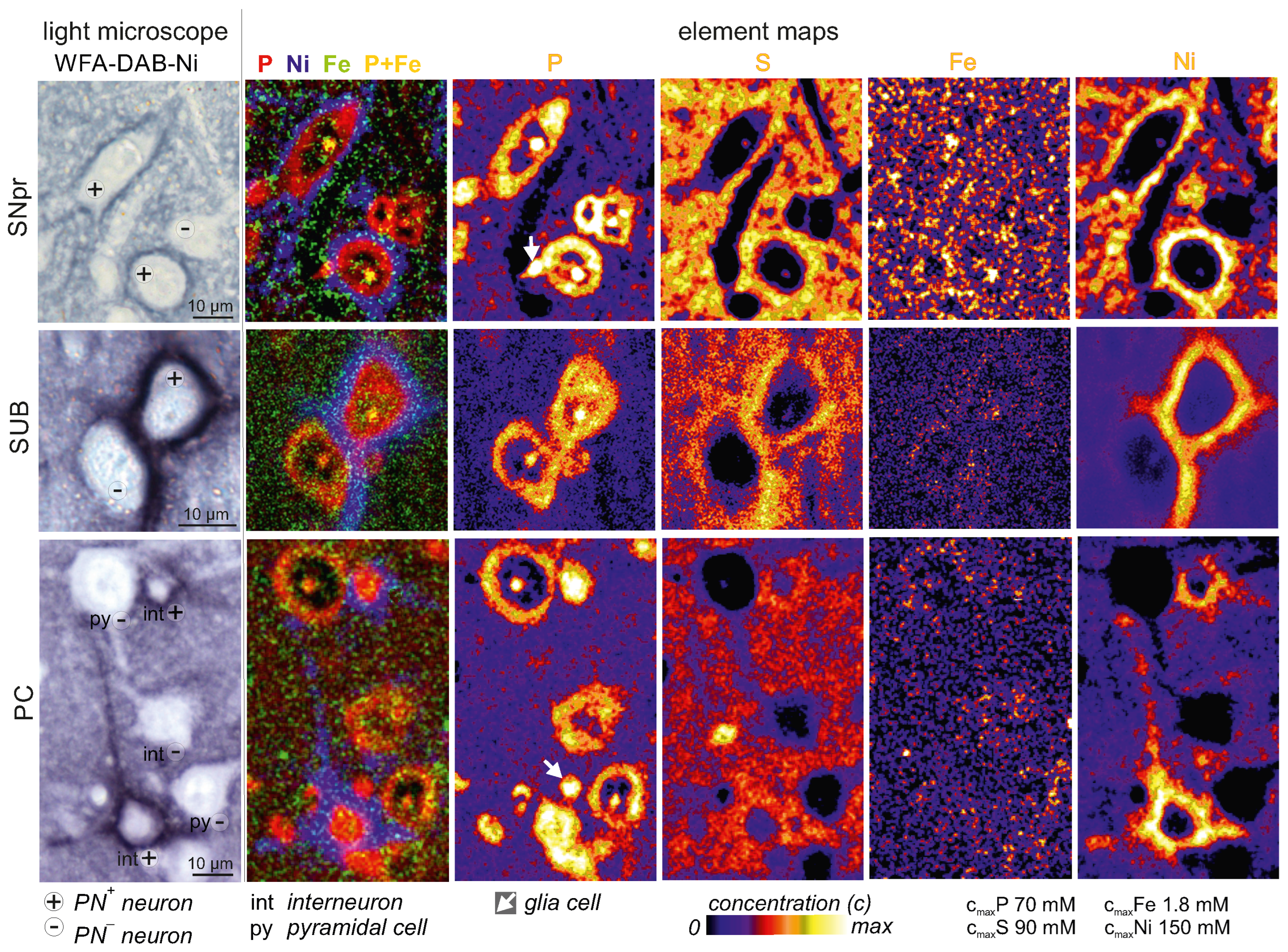
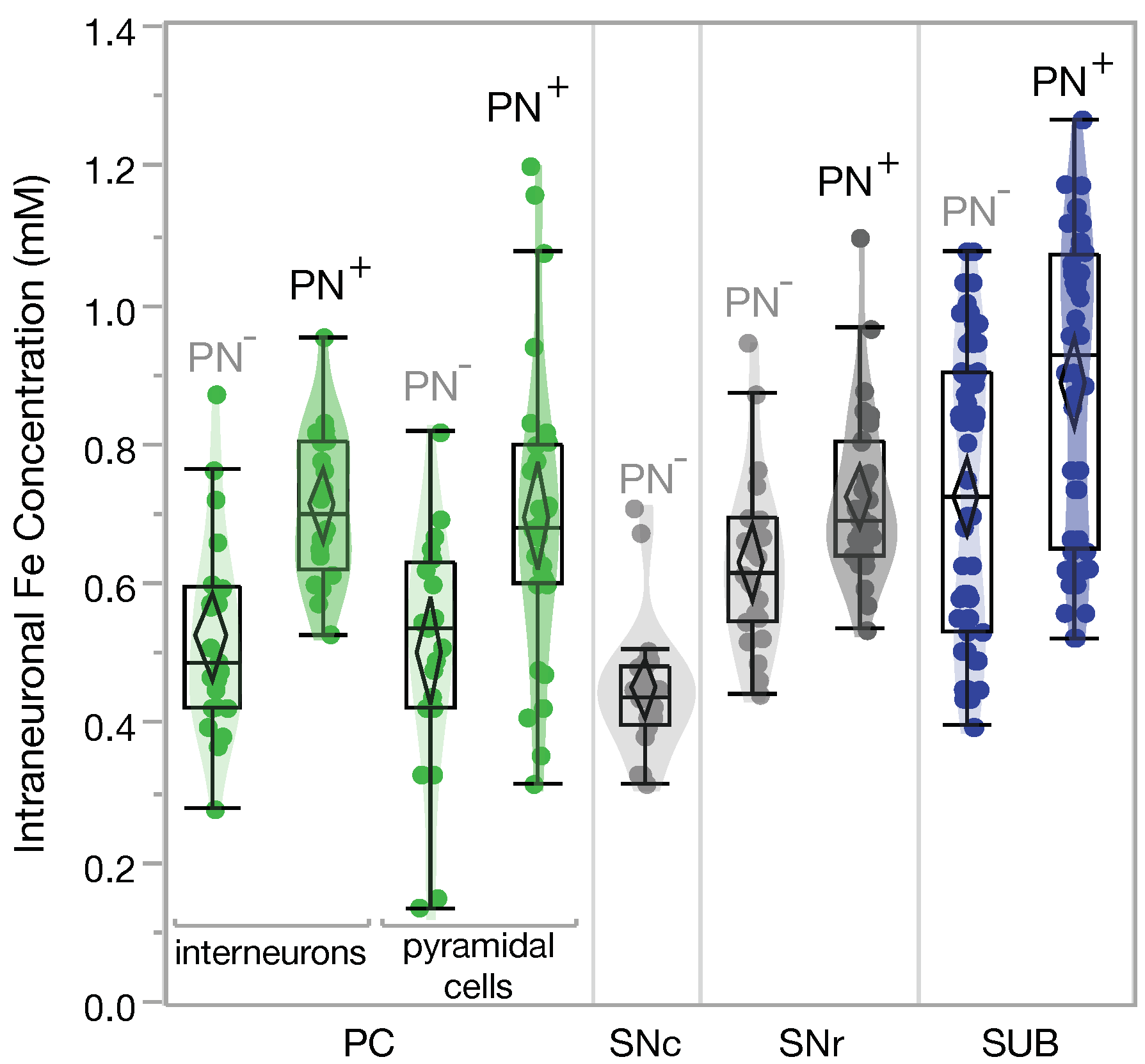
2.1. Higher Iron Concentrations in PN+ Neurons
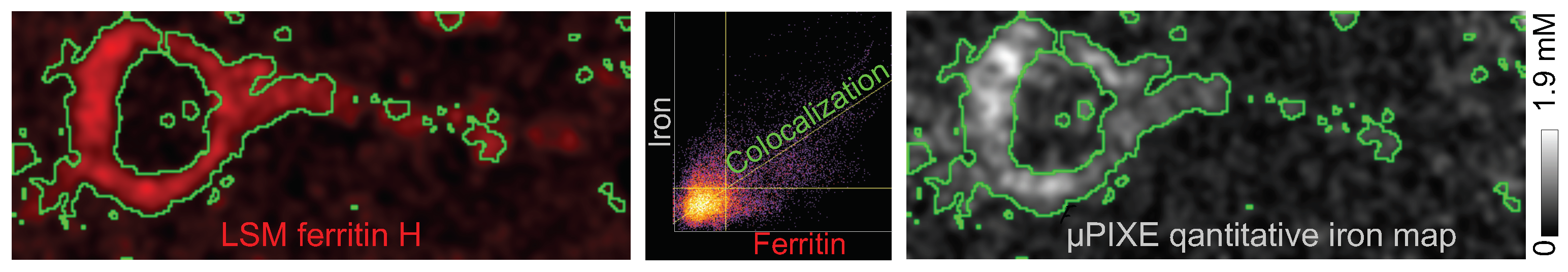
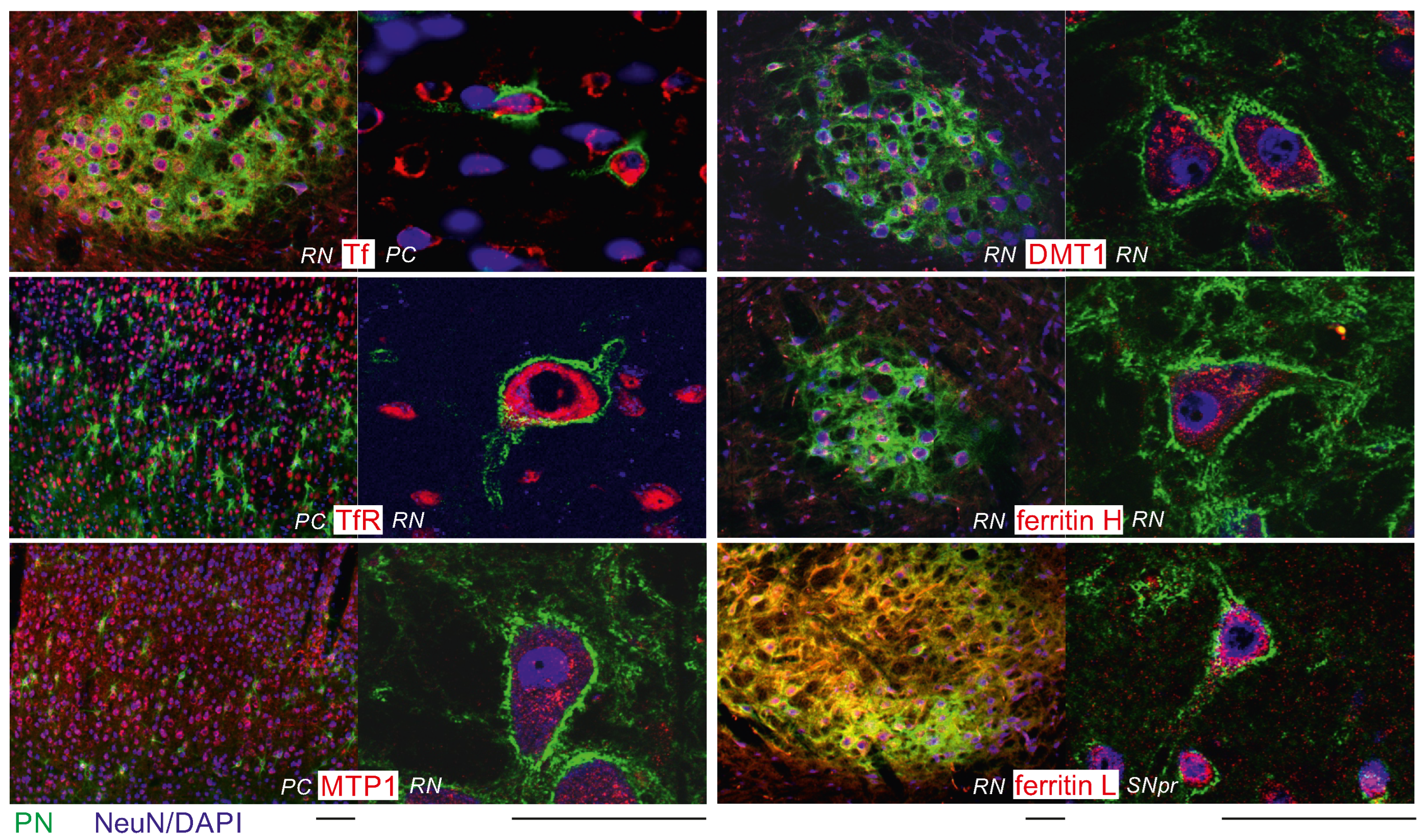
2.2. Intraneuronal Iron Protein Localization
2.3. Higher Iron Protein Levels in PN+ Neurons
2.4. Higher Iron Protein and mRNA Expression in PN-Rich Brain Tissue
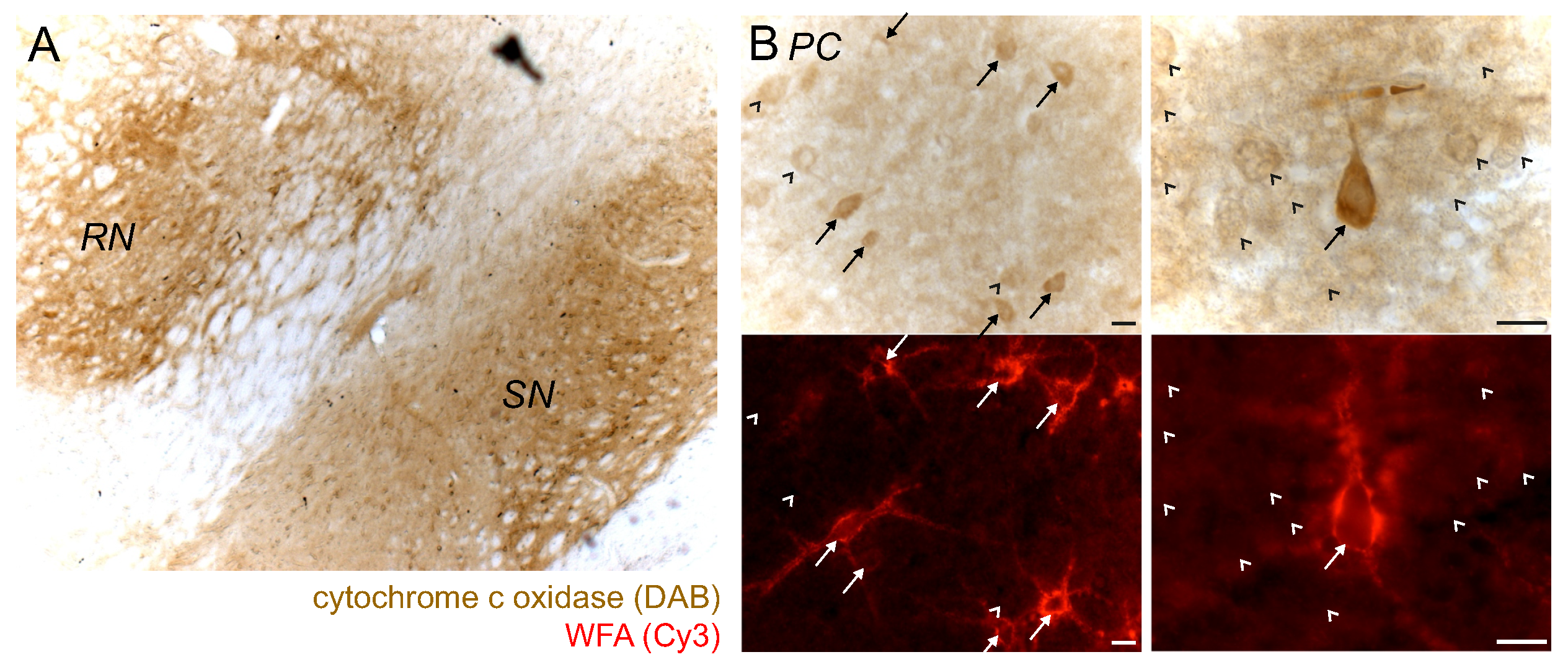
2.5. PN+ Neurons/Regions Show High Activity of Cytochrome C Oxidase
3. Discussion
3.1. Neuronal and Regional Iron Concentrations
3.1.1. PN and Iron Proteins Protect the Neuron by Handling Excessive Iron
3.1.2. PN+ Neurons May Have a Special Iron Household to Maintain a High Metabolic Rate
3.1.3. Limitations of the Study
4. Materials and Methods
4.1. Quantitative Element Mapping of Neurons in Brain Slices
4.1.1. Preparation of Brain Sections
4.1.2. Lectinhistochemistry and Embedding
4.1.3. µPIXE Analysis
4.2. Cellular Analysis of Iron Proteins
4.2.1. Preparation of Brain Sections
4.2.2. Multiple Lectin-/Immunohistochemistry
4.2.3. Slide-Based Cytometry
4.3. Cytochrome C Oxidase Enzyme Histochemistry
4.4. Regional Analysis of Iron Proteins
4.4.1. Quantitative Real-Time PCR
4.4.2. Protein Extraction and Western Blotting
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Fe | iron |
| Ni | nickel |
| PN(s) | perineuronal net(s) |
| PN+ | PN-ensheathed |
| PN− | PN-less |
| SBC | slide-based cytometry |
| PIXE | particle-induced X-ray emission |
| Tf | transferrin |
| TfR | transferrin receptor |
| DMT1 | divalent metal transporter 1 |
| MTP1 | metal transport protein 1 |
| DcytB | duodenal cytochrome B |
| WFA | Wisteria floribunda agglutinin |
| PC | parietal cortex |
| SN | substantia nigra |
| SUB | subiculum |
| RN | red nucleus |
| EC | entorhinal cortex |
| CytOx | cytochrome c oxidase |
| SEM | standard error |
Appendix A
| Target Antigen/Marker | Hostconj. | Dilution 1:x | RRID/Cat# | |
|---|---|---|---|---|
| WB | IH | |||
| PRIMARY Reaction | ||||
| iron proteins | ||||
| human transferrin (plasma protein) | chicken | 500 | 250 | 1 A80-156A |
| rat transferrin receptor, CD71 | mouse | 500 | 150 | 2‘RRID:AB-1120369 |
| human ferritin H (Y-16) | goat | 500 | 200 | 2 RRID:AB-2107172 |
| human ferritin L (Y-20) | goat | 500 | 200 | 2 RRID:AB-2107158 |
| rat DMT1+IRE | rabbit | 500 | 150 | 3 RRID:AB-1620356 |
| mouse MTP1 | rabbit | 500 | 150 | 3 RRID:AB-1619475 |
| mouse DcytB | rabbit | 500 | 150 | 3 RRID:AB-1614641 |
| PN marker | ||||
| GalNAc | WFAbiotin | 300 | 4 A1516 | |
| cell marker | ||||
| -actin | mouse | 20,000 | 4 RRID:AB-476697 | |
| NeuN | mouse | 150 | 5 RRID:AB-2298772 | |
| NeuroTrace fluor. Nissl stain green | 1000 | 6 | ||
| DAPI | 15,000 ★ | 6 | ||
| SECONDARY Reaction | ||||
| goat | donkeyCy5 | 200 | 7 | |
| rabbit | donkeyCy5 | 200 | 7 | |
| rabbit | donkeyCy3 | 300 | 7 | |
| rabbit | donkeyCy2 | 250 | 7 | |
| chicken | goatCy3 | 350 | 8 | |
| chicken | donkeyCy2 | 400 | 7 | |
| mouse | donkeyCy5 | 200 | 7 | |
| mouse | donkey IgMCy5 | 200 | 7 | |
| mouse | donkeyCy3 | 300 | 7 | |
| mouse | donkeyCy2 | 250 | 7 | |
| mouse | donkey IgMCy2 | 300 | 7 | |
| biotin | StreptavidinCy3 | 250 | 7 | |
| rabbit | donkeybiotin-SP | 1000 | 7 | |
| biotin | ExtrAvidinHRP | 1000 | 4 | |
| chicken | rabbitHRP | 10,000 | 4 | |
| goat | rabbitHRP | 10,000 | 9 | |
| mouse | goatHRP | 10,000 | 10 | |
| rabbit | goatHRP | 10,000 | 10 |
References
- Thompson, K.J.; Shoham, S.; Connor, J.R. Iron and neurodegenerative disorders. Brain Res. Bull. 2001, 55, 155–164. [Google Scholar] [CrossRef]
- Gerlach, M.; Ben-Shachar, D.; Riederer, P.; Youdim, M.B. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J. Neurochem. 1994, 63, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Hare, D.; Ayton, S.; Bush, A.; Lei, P. A delicate balance: Iron metabolism and diseases of the brain. Front. Aging Neurosci. 2013, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dusek, P.; Jankovic, J.; Le, W. Iron dysregulation in movement disorders. Neurobiol. Dis. 2012, 46, 1–18. [Google Scholar] [CrossRef]
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 2010, 345, 91–104. [Google Scholar] [CrossRef]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.H.; Singh, N.; Tay, H.; Walczyk, T. Imbalance of iron influx and efflux causes brain iron accumulation over time in the healthy adult rat. Metallomics 2014, 6, 1417–1426. [Google Scholar] [CrossRef]
- Genoud, S.; Roberts, B.R.; Gunn, A.P.; Halliday, G.M.; Lewis, S.J.; Ball, H.J.; Hare, D.J.; Double, K.L. Subcellular compartmentalisation of copper, iron, manganese, and zinc in the Parkinson’s disease brain. Metallomics 2017, 9, 1447–1455. [Google Scholar] [CrossRef] [Green Version]
- Ayton, S.; Wang, Y.; Diouf, I.; Schneider, J.A.; Brockman, J.; Morris, M.C.; Bush, A.I. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol. Psychiatry 2020, 25, 2932–2941. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Doll, S.; Conrad, M. Iron and ferroptosis: A still ill-defined liaison. IUBMB Life 2017, 69, 423–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinert, T.; Morawski, M.; Arendt, T.; Butz, T. Quantitative microanalysis of perineuronal nets in brain tissue. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 210, 395–400. [Google Scholar] [CrossRef]
- Morawski, M.; Brückner, M.K.; Riederer, P.; Brückner, G.; Arendt, T. Perineuronal nets potentially protect against oxidative stress. Exp. Neurol. 2004, 188, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, A.; Reinert, T.; Morawski, M.; Brückner, G.; Arendt, T.; Butz, T. Intracellular iron concentration of neurons with and without perineuronal nets. Nucl. Instrum. Methods Phys. Res. Sect. B 2007, 260, 153–158. [Google Scholar] [CrossRef]
- Suttkus, A.; Rohn, S.; Jäger, C.; Arendt, T.; Morawski, M. Neuroprotection against iron-induced cell death by perineuronal nets—An in vivo analysis of oxidative stress. Am. J. Neurodegener. Dis. 2012, 1, 122–129. [Google Scholar]
- Suttkus, A.; Rohn, S.; Weigel, S.; Glöckner, P.; Arendt, T.; Morawski, M. Aggrecan, link protein and tenascin-R are essential components of the perineuronal net to protect neurons against iron-induced oxidative stress. Cell Death Dis. 2014, 5, e1119. [Google Scholar] [CrossRef] [Green Version]
- Cabungcal, J.H.; Steullet, P.; Morishita, H.; Kraftsik, R.; Cuenod, M.; Hensch, T.K.; Do, K.Q. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc. Natl. Acad. Sci. USA 2013, 110, 9130–9135. [Google Scholar] [CrossRef] [Green Version]
- Celio, M.R.; Blumcke, I. Perineuronal nets—A specialized form of extracellular matrix in the adult nervous system. Brain Res. Rev. 1994, 19, 128–145. [Google Scholar] [CrossRef]
- Brückner, G.; Härtig, W.; Kacza, J.; Seeger, J.; Welt, K.; Brauer, K. Extracellular matrix organization in various regions of rat brain grey matter. J. Neurocytol. 1996, 25, 333–346. [Google Scholar] [CrossRef]
- Brückner, G.; Pavlica, S.; Morawski, M.; Palacios, A.G.; Reichenbach, A. Organization of brain extracellular matrix in the Chilean fat-tailed mouse opossum Thylamys elegans (Waterhouse, 1839). J. Chem. Neuroanat. 2006, 32, 143–158. [Google Scholar] [CrossRef]
- Morawski, M.; Reinert, T.; Meyer-Klaucke, W.; Wagner, F.; Tröger, W.; Reinert, A.; Jäger, C.; Brückner, G.; Arendt, T. Ion exchanger in the brain: Quantitative analysis of perineuronally fixed anionic binding sites suggests diffusion barriers with ion sorting properties. Sci. Rep. 2015, 5, 16471. [Google Scholar] [CrossRef] [PubMed]
- Schüppel, K.; Brauer, K.; Härtig, W.; Grosche, J.; Earley, B.; Leonard, B.E.; Brückner, G. Perineuronal nets of extracellular matrix around hippocampal interneurons resist destruction by activated microglia in trimethyltin-treated rats. Brain Res. 2002, 958, 448–453. [Google Scholar] [CrossRef]
- Brückner, G.; Hausen, D.; Härtig, W.; Drlicek, M.; Arendt, T.; Brauer, K. Cortical areas abundant in extracellular matrix chondroitin sulphate proteoglycans are less affected by cytoskeletal changes in Alzheimer’s disease. Neuroscience 1999, 92, 791–805. [Google Scholar] [CrossRef]
- Morawski, M.; Pavlica, S.; Seeger, G.; Grosche, J.; Kouznetsova, E.; Schliebs, R.; Brückner, G.; Arendt, T. Perineuronal nets are largely unaffected in Alzheimer model Tg2576 mice. Neurobiol. Aging 2010, 31, 1254–1256. [Google Scholar] [CrossRef] [PubMed]
- Härtig, W.; Klein, C.; Brauer, K.; Schüppel, K.F.; Arendt, T.; Bigl, V.; Brückner, G. Hyperphosphorylated protein tau is restricted to neurons devoid of perineuronal nets in the cortex of aged bison. Neurobiol. Aging 2001, 22, 25–33. [Google Scholar] [CrossRef]
- Miyata, S.; Nishimura, Y.; Nakashima, T. Perineuronal nets protect against amyloid β-protein neurotoxicity in cultured cortical neurons. Brain Res. 2007, 1150, 200–206. [Google Scholar] [CrossRef]
- Yasuhara, O.; Akiyama, H.; McGeer, E.G.; McGeer, P.L. Immunohistochemical localization of hyaluronic acid in rat and human brain. Brain Res. 1994, 635, 269–282. [Google Scholar] [CrossRef]
- Seeger, G.; Brauer, K.; Härtig, W.; Brückner, G. Mapping of perineuronal nets in the rat brain stained by colloidal iron hydroxide histochemistry and lectin cytochemistry. Neuroscience 1994, 58, 371–388. [Google Scholar] [CrossRef]
- Brückner, G.; Morawski, M.; Arendt, T. Aggrecan-based extracellular matrix is an integral part of the human basal ganglia circuit. Neuroscience 2008, 151, 489–504. [Google Scholar] [CrossRef]
- Asher, R.A.; Scheibe, R.J.; Keiser, H.D.; Bignami, A. On the existence of a cartilage-like proteoglycan and link proteins in the central nervous system. Glia 1995, 13, 294–308. [Google Scholar] [CrossRef]
- Lander, C.; Zhang, H.; Hockfield, S. Neurons produce a neuronal cell surface-associated chondroitin sulfate proteoglycan. J. Neurosci. 1998, 18, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Brückner, G.; Grosche, J.; Schmidt, S.; Härtig, W.; Margolis, R.U.; Delpech, B.; Seidenbecher, C.I.; Czaniera, R.; Schachner, M. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J. Comp. Neurol. 2000, 428, 616–629. [Google Scholar] [CrossRef]
- Yamaguchi, Y. Lecticans: Organizers of the brain extracellular matrix. Cell. Mol. Life Sci. 2000, 57, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Cabulli, D.; Rhodes, K.E.; Fawcett, J.W. Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J. Comp. Neurol. 2007, 501, 83–94. [Google Scholar] [CrossRef]
- Galtrey, C.M.; Kwok, J.C.; Carulli, D.; Rhodes, K.E.; Fawcett, J.W. Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur. J. Neurosci. 2008, 27, 1373–1390. [Google Scholar] [CrossRef]
- Galtrey, C.M.; Fawcett, J.W. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res. Rev. 2007, 54, 1–18. [Google Scholar] [CrossRef]
- Arendt, T. Neurodegeneration and plasticity. Int. J. Dev. Neurosci. 2004, 22, 507–514. [Google Scholar] [CrossRef]
- Tsien, R.Y. Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc. Natl. Acad. Sci. USA 2013, 110, 12456–12461. [Google Scholar] [CrossRef] [Green Version]
- Brückner, G.; Brauer, K.; Härtig, W.; Wolff, J.R.; Rickmann, M.J.; Derouiche, A.; Delpech, B.; Girard, N.; Oertel, W.H.; Reichenbach, A. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia 1993, 8, 183–200. [Google Scholar] [CrossRef]
- Brückner, G.; Schütz, A.; Härtig, W.; Brauer, K.; Paulke, B.R.; Bigl, V. Projection of non-cholinergic basal forebrain neurons ensheathed with perineuronal nets to rat mesocortex. J. Chem. Neuroanat. 1994, 8, 11–18. [Google Scholar] [CrossRef]
- Härtig, W.; Derouiche, A.; Welt, K.; Brauer, K.; Grosche, J.; Mäder, M.; Reichenbach, A.; Brückner, G. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999, 842, 15–29. [Google Scholar] [CrossRef]
- Morris, N.P.; Henderson, Z. Perineuronal nets ensheath fast spiking, parvalbumin-immunoreactive neurons in the medial septum/diagonal band complex. Eur. J. Neurosci. 2000, 12, 828–838. [Google Scholar] [CrossRef]
- Wen, T.H.; Binder, D.K.; Ethell, I.M.; Razak, K.A. The Perineuronal ‘Safety’ Net? Perineuronal Net Abnormalities in Neurological Disorders. Front. Mol. Neurosci. 2018, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Albertini, R.; Passi, A.; Abuja, P.M.; De Luca, G. The effect of glycosaminoglycans and proteoglycans on lipid peroxidation. Int. J. Mol. Med. 2000, 6, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Ferlazzo, A.M.; Calatroni, A. Reduction of DNA fragmentation and hydroxyl radical production by hyaluronic acid and chondroitin-4-sulphate in iron plus ascorbate-induced oxidative stress in fibroblast cultures. Free Radic. Res. 2004, 38, 601–611. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Lee, D.Y.; Yee, A.; Cao, L.; Zhang, Y.; Kiani, C.; Yang, B.B. Versican protects cells from oxidative stress-induced apoptosis. Matrix Biol. 2005, 24, 3–13. [Google Scholar] [CrossRef]
- Dusek, P.; Schneider, S.A.; Aaseth, J. Iron chelation in the treatment of neurodegenerative diseases. J. Trace Elem. Med. Biol. 2016, 38, 81–92. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Ul-haq, M.I.; Creagh, A.L.; Haynes, C.A.; Kizhakkedathu, J.N. Iron Binding and Iron Removal Efficiency of Desferrioxamine Based Polymeric Iron Chelators: Influence of Molecular Size and Chelator Density. Macromol. Biosci. 2017, 17, 1600244. [Google Scholar] [CrossRef]
- Masaldan, S.; Bush, A.I.; Devos, D.; Rolland, A.S.; Moreau, C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radic. Biol. Med. 2019, 133, 221–233. [Google Scholar] [CrossRef]
- Das, N.; Raymick, J.; Sarkar, S. Role of metals in Alzheimer’s disease. Metab. Brain Dis. 2021, 36, 1627–1639. [Google Scholar] [CrossRef]
- Peng, Y.; Chang, X.; Lang, M. Iron Homeostasis Disorder and Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 12442. [Google Scholar] [CrossRef] [PubMed]
- MacGillivray, R.T.; Mason, A.B. Transferrins. In Molecular and Cellular Iron Transport, 1st ed.; Templeton, D.M., Ed.; Chapter Molecular Aspects of Iron Transport; CRC Press: Boca Raton, FL, USA, 2002; pp. 49–84. [Google Scholar] [CrossRef]
- Aisen, P.; Enns, C.; Wessling-Resnick, M. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 2001, 33, 940–959. [Google Scholar] [CrossRef]
- Garrick, M.D.; Dolan, K.G.; Horbinski, C.; Ghio, A.J.; Higgins, D.; Porubcin, M.; Moore, E.G.; Hainsworth, L.N.; Umbreit, J.N.; Conrad, M.E.; et al. DMT1: A mammalian transporter for multiple metals. BioMetals 2003, 16, 41–54. [Google Scholar] [CrossRef] [PubMed]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A.; et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Abboud, S.; Haile, D.J. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 2000, 275, 19906–19912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdo, J.R.; Menzies, S.L.; Simpson, I.A.; Garrick, L.M.; Garrick, M.D.; Dolan, K.G.; Haile, D.J.; Beard, J.L.; Connor, J.R. Distribution of Divalent Metal Transporter 1 and Metal Transport Protein 1 in the normal and Belgrade rat. J. Neurosci. Res. 2001, 66, 1198–1207. [Google Scholar] [CrossRef]
- Wu, L.J.C.; Leenders, A.G.; Cooperman, S.; Meyron-Holtz, E.; Smith, S.; Land, W.; Tsai, R.Y.; Berger, U.V.; Sheng, Z.H.; Rouault, T.A. Expression of the iron transporter ferroportin in synaptic vesicles and the blood-brain barrier. Brain Res. 2004, 1001, 108–117. [Google Scholar] [CrossRef]
- Jian, N.; Dowle, M.; Horniblow, R.D.; Tselepis, C.; Palmer, R.E. Morphology of the ferritin iron core by aberration corrected scanning transmission electron microscopy. Nanotechnology 2016, 27, 46LT02. [Google Scholar] [CrossRef]
- Dedman, D.J.; Treffry, A.; Candy, J.M.; Taylor, G.A.; Morris, C.M.; Bloxham, C.A.; Perry, R.H.; Edwardson, J.A.; Harrison, P.M. Iron and aluminium in relation to brain ferritin in normal individuals and Alzheimer’s-disease and chronic renal-dialysis patients. Biochem. J. 1992, 287, 509–514. [Google Scholar] [CrossRef]
- Reinert, A.; Morawski, M.; Seeger, J.; Arendt, T.; Reinert, T. Iron concentrations in neurons and glial cells with estimates on ferritin concentrations. BMC Neurosci. 2019, 20, 25. [Google Scholar] [CrossRef]
- Eisenstein, R.S. Iron regulatory proteins and the molecular control of mammalian iron metabolism. Annu. Rev. Nutr. 2000, 20, 627–662. [Google Scholar] [CrossRef]
- Dringen, R.; Pawlowski, P.G.; Hirrlinger, J. Peroxide detoxification by brain cells. J. Neurosci. Res. 2005, 79, 157–165. [Google Scholar] [CrossRef]
- Wegner, F.; Härtig, W.; Bringmann, A.; Grosche, J.; Wohlfarth, K.; Zuschratter, W.; Brückner, G. Diffuse perineuronal nets and modified pyramidal cells immunoreactive for glutamate and the GABAA receptor α1 subunit form a unique entity in rat cerebral cortex. Exp. Neurol. 2003, 184, 705–714. [Google Scholar] [CrossRef]
- Snyder, A.M.; Connor, J.R. Iron, the substantia nigra and related neurological disorders. Biochim. Et Biophys. Acta Gen. Subj. 2009, 1790, 606–614. [Google Scholar] [CrossRef]
- Morawski, M.; Meinecke, C.; Reinert, T.; Dörffel, A.C.; Riederer, P.; Arendt, T.; Butz, T. Determination of trace elements in the human substantia nigra. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 231, 224–228. [Google Scholar] [CrossRef]
- Carmona, A.; Roudeau, S.; Perrin, L.; Carcenac, C.; Vantelon, D.; Savasta, M.; Ortega, R. Mapping chemical elements and iron oxidation states in the substantia nigra of 6-hydroxydopamine lesioned rats using correlative immunohistochemistry with proton and synchrotron micro-analysis. Front. Neurosci. 2019, 13, 1014. [Google Scholar] [CrossRef] [Green Version]
- Brammerloh, M.; Morawski, M.; Friedrich, I.; Reinert, T.; Lange, C.; Pelicon, P.; Vavpetič, P.; Jankuhn, S.; Jäger, C.; Alkemade, A.; et al. Measuring the iron content of dopaminergic neurons in substantia nigra with MRI relaxometry. NeuroImage 2021, 239, 118255. [Google Scholar] [CrossRef]
- Friedrich, I.; Reimann, K.; Jankuhn, S.; Kirilina, E.; Stieler, J.; Sonntag, M.; Meijer, J.; Weiskopf, N.; Reinert, T.; Arendt, T.; et al. Cell specific quantitative iron mapping on brain slices by immuno-µPIXE in healthy elderly and Parkinson’s disease. Acta Neuropathol. Commun. 2021, 9, 47. [Google Scholar] [CrossRef]
- Celada, P.; Paladini, C.A.; Tepper, J.M. GABAergic control of rat substantia nigra dopaminergic neurons: Role of globus pallidus and substantia nigra pars reticulata. Neuroscience 1999, 89, 813–825. [Google Scholar] [CrossRef]
- Werkman, T.R.; Kruse, C.G.; Nievelstein, H.; Long, S.K.; Wadman, W.J. In vitro modulation of the firing rate of dopamine neurons in the rat substantia nigra pars compacta and the ventral tegmental area by antipsychotic drugs. Neuropharmacology 2001, 40, 927–936. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Poor and protracted myelination as a contributory factor to neurodegenerative disorders. Neurobiol. Aging 2004, 25, 19–23. [Google Scholar] [CrossRef]
- Griffin, J.W.; Thompson, W.J. Biology and pathology of nonmyelinating schwann cells. Glia 2008, 56, 1518–1531. [Google Scholar] [CrossRef]
- Double, K.L.; Maywald, M.; Schmittel, M.; Riederer, P.; Gerlach, M. In vitro studies of ferritin iron release and neurotoxicity. J. Neurochem. 1998, 70, 2492–2499. [Google Scholar] [CrossRef]
- Williams, R.J. The biological chemistry of the brain and its possible evolution. Inorganica Chim. Acta 2003, 356, 27–40. [Google Scholar] [CrossRef]
- Ke, Y.; Chang, Y.Z.; Duan, X.L.; Du, J.R.; Zhu, L.; Wang, K.; Yang, X.D.; Ho, K.P.; Qian, Z.M. Age-dependent and iron-independent expression of two mRNA isoforms of divalent metal transporter 1 in rat brain. Neurobiol. Aging 2005, 26, 739–748. [Google Scholar] [CrossRef]
- Balmer, T.S. Perineuronal nets enhance the excitability of fast-spiking neurons. eNeuro 2016, 3, 745–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morawski, M.; Sonntag, M. Perineuronal Nets in the Superior Olivary Complex. In The Oxford Handbook of the Auditory Brainstem; Oxford University Press: Oxford, UK, 2019; pp. 420–444. [Google Scholar] [CrossRef]
- Kann, O.; Papageorgiou, I.E.; Draguhn, A. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J. Cereb. Blood Flow Metab. 2014, 34, 1270–1282. [Google Scholar] [CrossRef] [Green Version]
- Yi, G.; Grill, W.M. Average firing rate rather than temporal pattern determines metabolic cost of activity in thalamocortical relay neurons. Sci. Rep. 2019, 9, 6940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasenstaub, A.; Otte, S.; Callaway, E.; Sejnowski, T.J. Metabolic cost as a unifying principle governing neuronal biophysics. Proc. Natl. Acad. Sci. USA 2010, 107, 12329–12334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morawski, M.; Reinert, T.; Meinecke, C.; Arendt, T.; Butz, T. Antibody meets the microbeam—Or how to find neurofibrillary tangles. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 231, 229–233. [Google Scholar] [CrossRef]
- Reinert, T.; Andrea, T.; Barapatre, N.; Hohlweg, M.; Koal, T.; Larisch, W.; Reinert, A.; Spemann, D.; Vogt, J.; Werner, R.; et al. Biomedical research at LIPSION—Present state and future developments. Nucl. Instrum. Methods Phys. Res. Sect. B 2011, 269, 2254–2259. [Google Scholar] [CrossRef]
- Ryan, C.G. PIXE and the nuclear microprobe: Tools for quantitative imaging of complex natural materials. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2011, 269, 2151–2162. [Google Scholar] [CrossRef]
- Ryan, C.G.; Van Achterbergh, E.; Jamieson, D.N.; Churms, C.L. Overlap corrected on-line PIXE imaging using the proton microprobe. Nuclear Instrum. Methods Phys. Res. Sect. Beam Interact. Mater. At. 1996, 109–110, 154–160. [Google Scholar] [CrossRef]
- Reinert, A.; Mittag, A.; Reinert, T.; Tárnok, A.; Arendt, T.; Morawski, M. On the quantification of intracellular proteins in multifluorescence-labeled rat brain slices using slide-based cytometry. Cytometry Part A 2011, 79 A, 485–491. [Google Scholar] [CrossRef]
- Divac, I.; Mojsilovic-Petrovic, J.; López-Figueroa, M.O.; Petrovic-Minic, B.; Møller, M. Improved contrast in histochemical detection of cytochrome oxidase: Metallic ions protocol. J. Neurosci. Methods 1995, 56, 105–113. [Google Scholar] [CrossRef]
- Rawer, D. Real-Time PCR: Optimierung und Evaluation, Etablierung von Housekeeping-Genen und die Expressionsanalyse bei Fallotscher Tetralogie. Ph.D. Thesis, Justus-Liebig-Universität, Giessen, Germany, 2005. [Google Scholar]
- Kubista, M.; Andrade, J.M.; Bengtsson, M.; Forootan, A.; Jonak, J.; Lind, K.; Sindelka, R.; Sjoback, R.; Sjogreen, B.; Strombom, L.; et al. The real-time polymerase chain reaction. Mol. Asp. Med. 2006, 27, 95–125. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
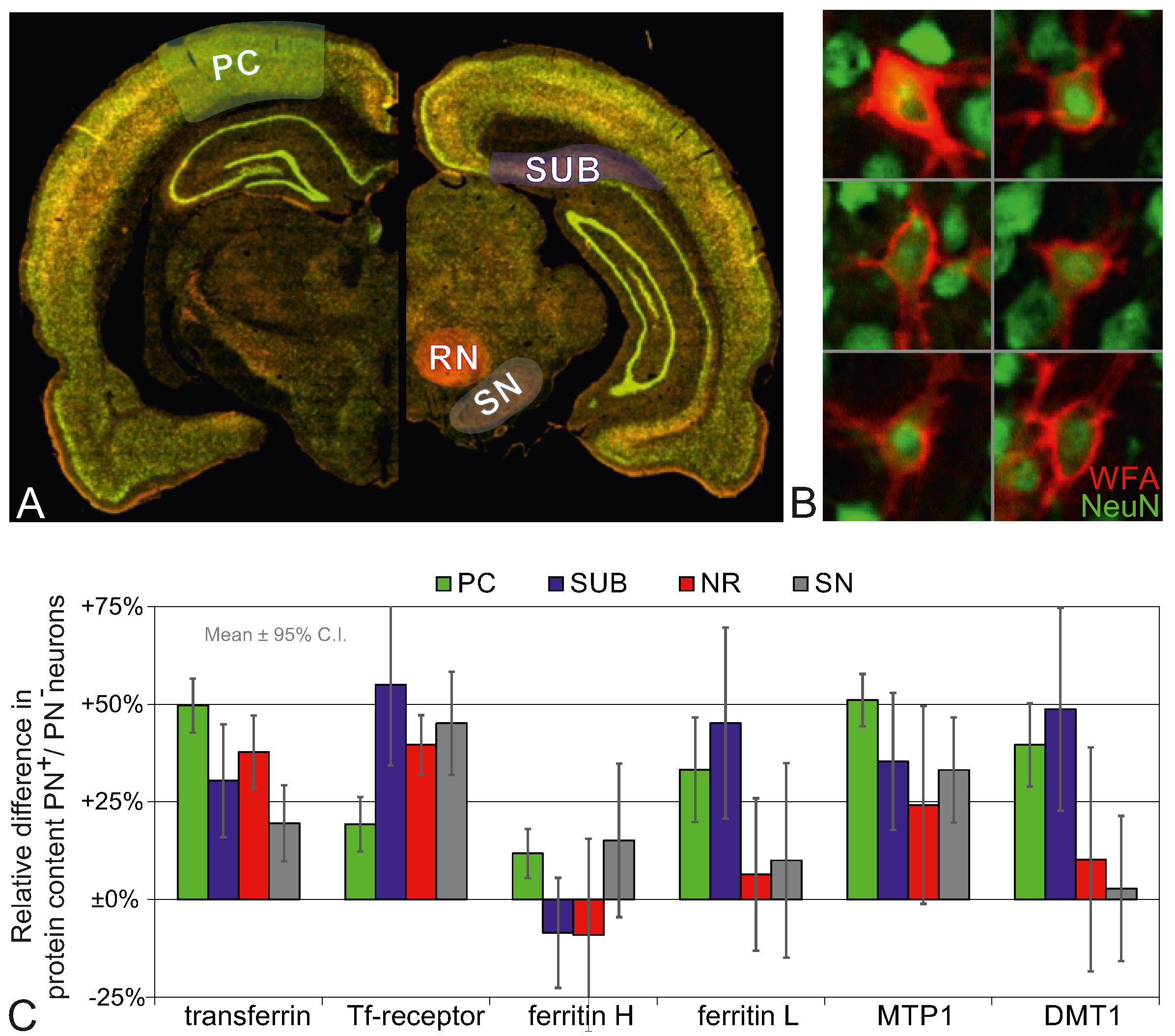
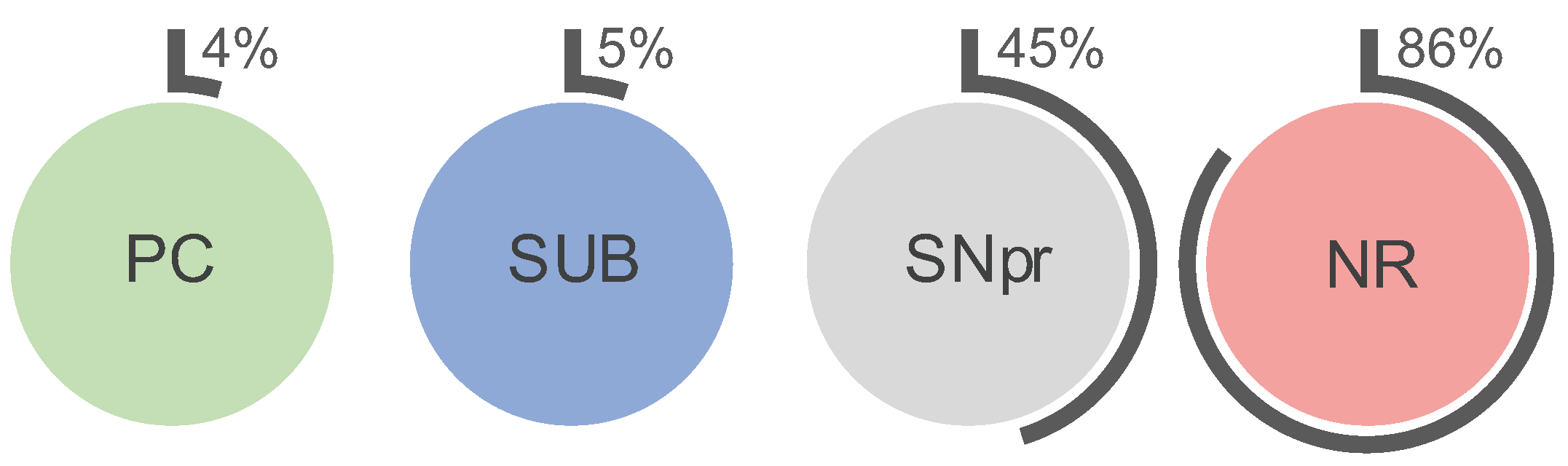
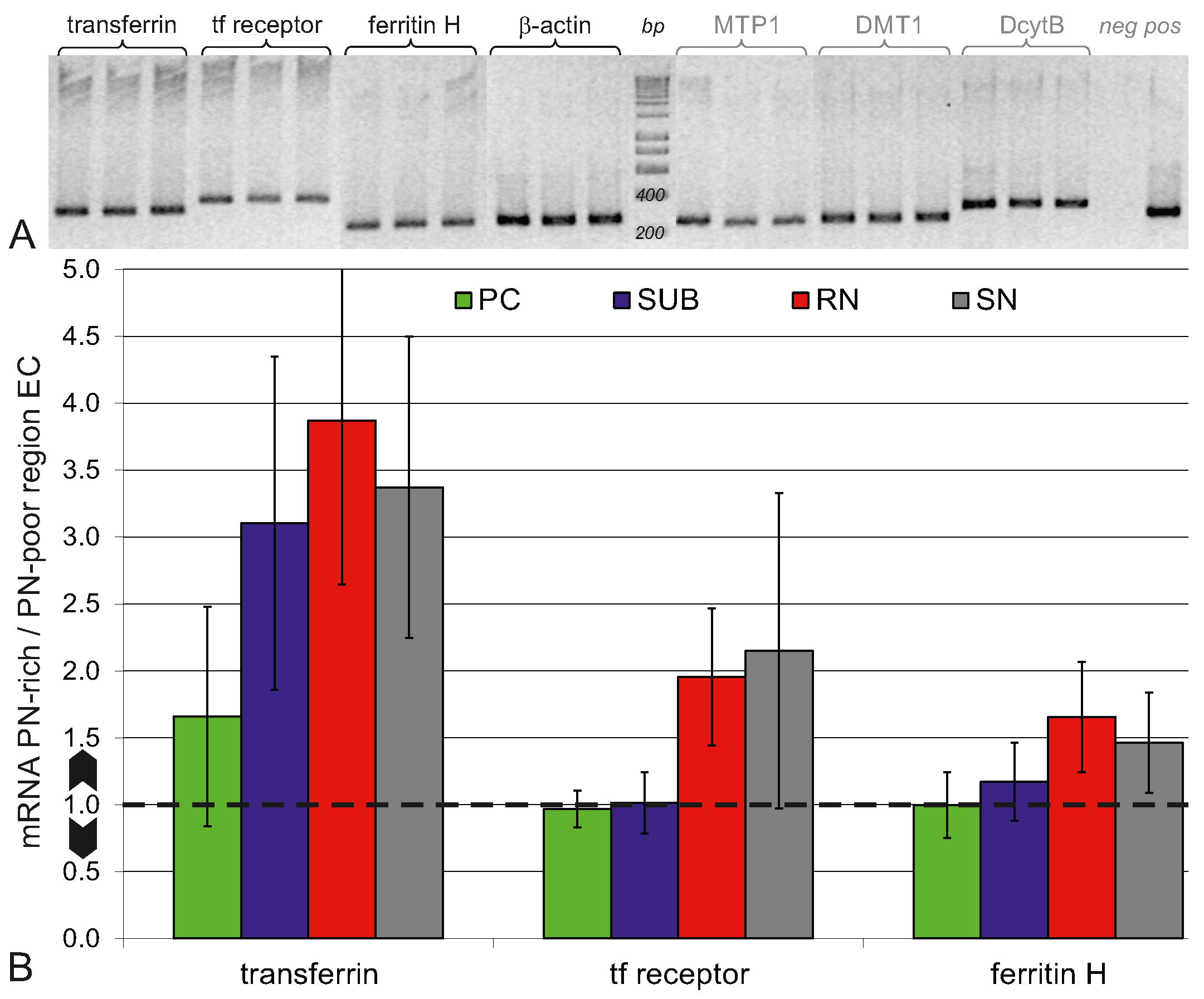
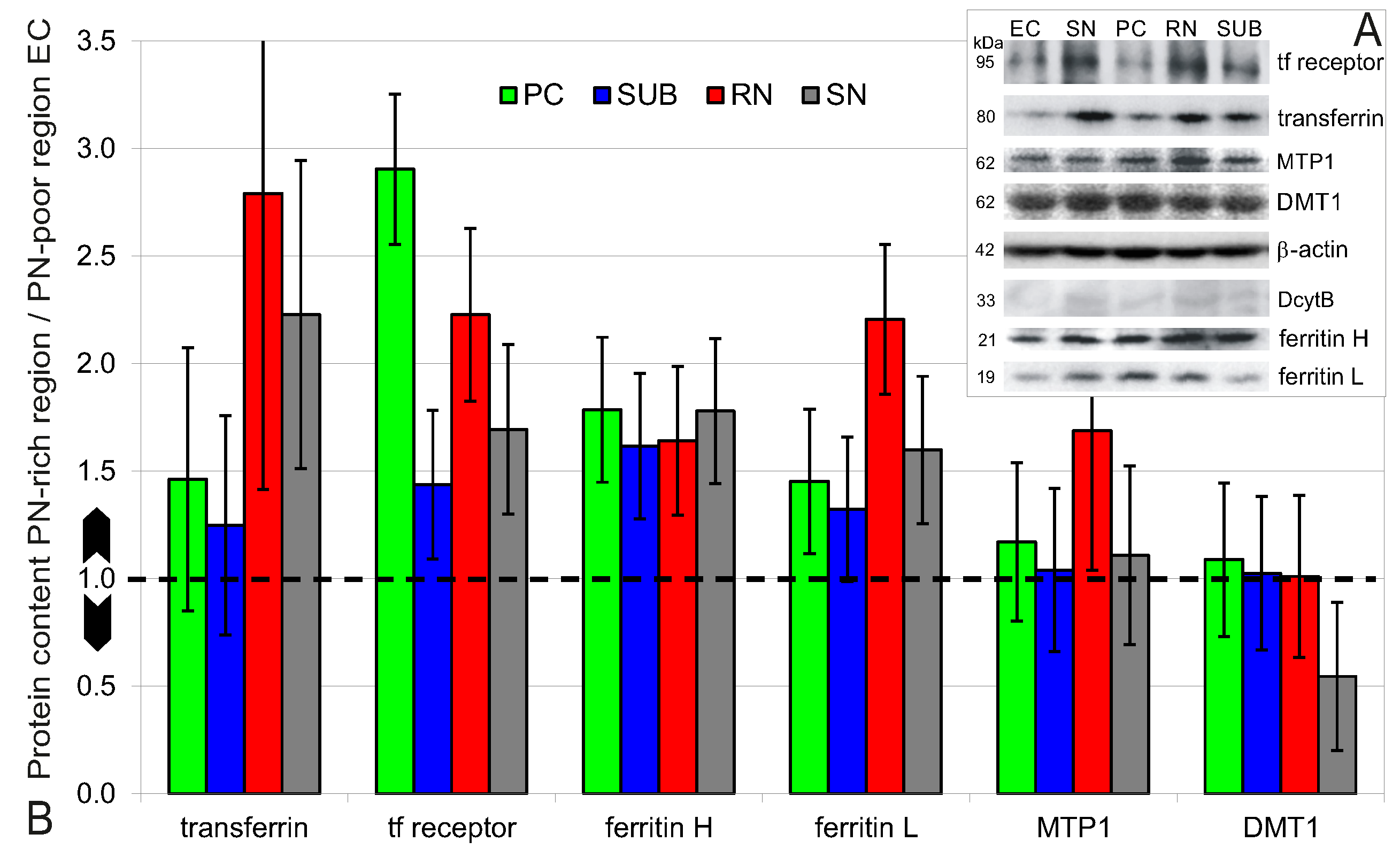
| Cellular: PN+ vs. PN− Neurons | Regional: PN-Rich Region vs. PN-Poor Region (EC) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slide-Based Cytometry | Western Blot | qRT-PCR | |||||||||||||
| Tf | Tf-R | FtH | FtL | MTP1 | DMT1 | Tf | Tf-R | FtH | FtL | MTP1 | DMT1 | Tf | Tf-R | FtH | |
| PC | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ∼ | ↑ | ↑ | ↑ | ∼ | ∼ | ∼ | ∼ | ∼ |
| SUB | ↑ | ↑ | ∼ | ↑ | ↑ | ↑ | ∼ | ↑ | ↑ | ↑ | ∼ | ∼ | ↑ | ∼ | ∼ |
| RN | ↑ | ↑ | ∼ | ∼ | ↑ | ∼ | ↑ | ↑ | ↑ | ↑ | ↑ | ∼ | ↑ | ↑ | ↑ |
| SN | ↑ | ↑ | ∼ | ∼ | ↑ | ∼ | ↑ | ↑ | ↑ | ↑ | ∼ | ↓ | ↑ | ↑ | ↑ |
| 5-Primer-3 | 5-Primer-3 | Size | |
|---|---|---|---|
| Tf | AGATGGAGGTGGAGATGTGG | GAGAGCCGAACAGTTGGAAG | 312 bp |
| TfR | CCTGAGGGTTATGTGGCATT | ATGGGGGAAACTGAGTATGG | 286 bp |
| FtH | CCTGGAGTTGTATGCCTCCT | GTGCACACTCCATTGCATTC | 236 bp |
| DMT1 | CTCCACCATGACTGGAACCT | CAGCCTATTCCGTTGGAGAA | 273 bp |
| MTP1 | GGGTGGATAAGAATGCCAGA | TGCTCCTGTTTTCTCCTGCT | 261 bp |
| DcytB | GTCATGGGCATGATCTTCCT | GGTGGCACCAAAAGTGT | 228 bp |
| -Actin | AGCCATGTACGTAGCCATCC | CTCTCAGCTGTGGTGGTGAA | 280 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinert, A.; Reinert, T.; Arendt, T.; Morawski, M. High Iron and Iron Household Protein Contents in Perineuronal Net-Ensheathed Neurons Ensure Energy Metabolism with Safe Iron Handling. Int. J. Mol. Sci. 2022, 23, 1634. https://doi.org/10.3390/ijms23031634
Reinert A, Reinert T, Arendt T, Morawski M. High Iron and Iron Household Protein Contents in Perineuronal Net-Ensheathed Neurons Ensure Energy Metabolism with Safe Iron Handling. International Journal of Molecular Sciences. 2022; 23(3):1634. https://doi.org/10.3390/ijms23031634
Chicago/Turabian StyleReinert, Anja, Tilo Reinert, Thomas Arendt, and Markus Morawski. 2022. "High Iron and Iron Household Protein Contents in Perineuronal Net-Ensheathed Neurons Ensure Energy Metabolism with Safe Iron Handling" International Journal of Molecular Sciences 23, no. 3: 1634. https://doi.org/10.3390/ijms23031634
APA StyleReinert, A., Reinert, T., Arendt, T., & Morawski, M. (2022). High Iron and Iron Household Protein Contents in Perineuronal Net-Ensheathed Neurons Ensure Energy Metabolism with Safe Iron Handling. International Journal of Molecular Sciences, 23(3), 1634. https://doi.org/10.3390/ijms23031634






