C-Reactive Protein as a Biomarker for Major Depressive Disorder?
Abstract
:1. Introduction
2. Material and Methods
2.1. Search Sources and Strategies
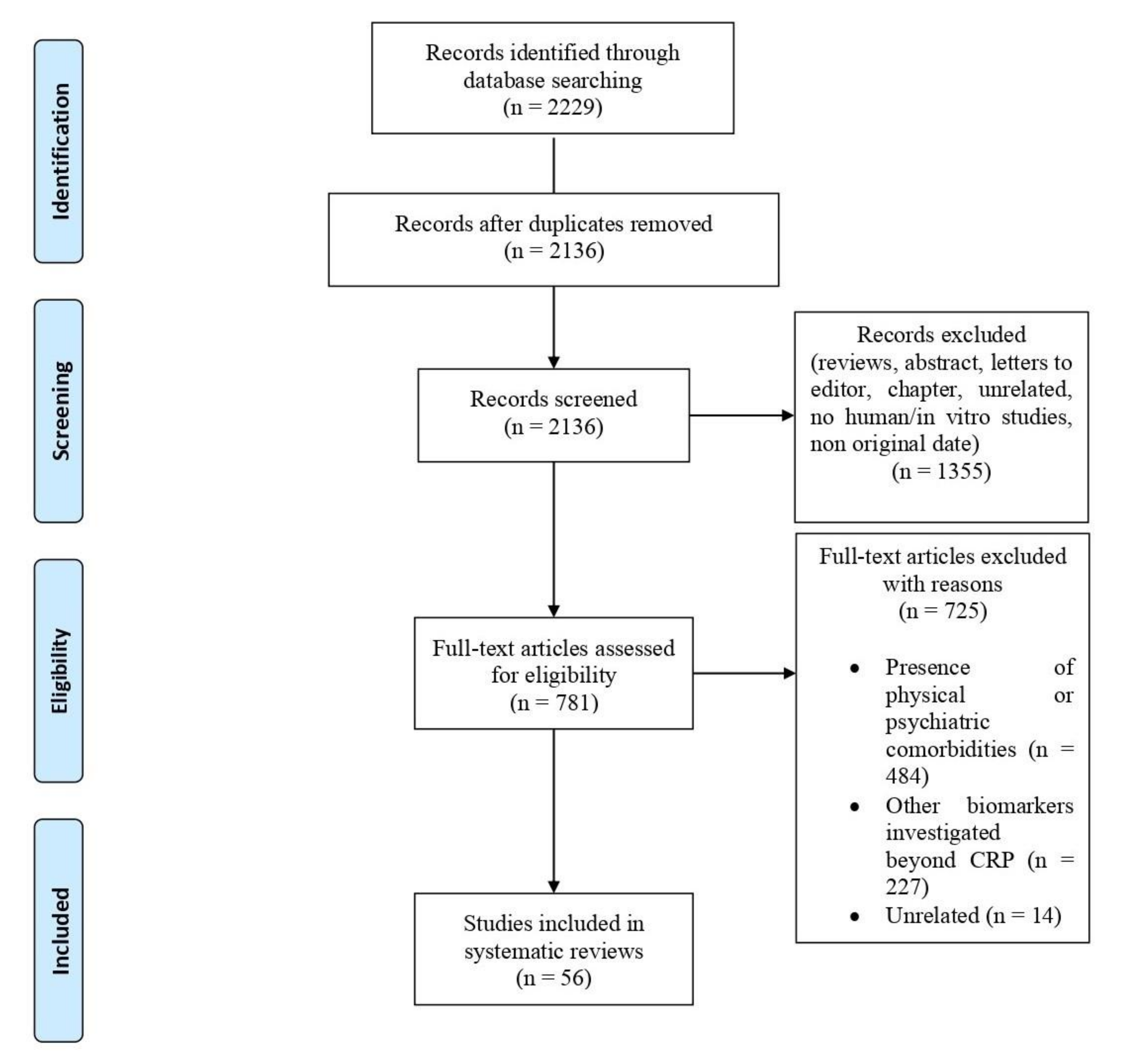
2.2. Study Selection
2.3. Data Extraction and Management
2.4. Characteristics of Included Studies
2.5. Quality Assessment
3. Results
3.1. Studies on the Association between CRP Levels and Depression
3.1.1. Cross-Sectional Studies
3.1.2. Case-Control Studies
3.1.3. Cohort Studies
3.2. Studies on Gender Differences of CRP Levels in Depression
3.2.1. Cross-Sectional Studies
3.2.2. Cohort Studies
3.2.3. RCT Studies
3.3. Studies on Ethnic Differences of CRP Levels in Depression
Cross-Sectional Studies
3.4. Studies on Severity/Specific Cluster Domains in the Association between CRP Levels and Depression
3.4.1. Cross-Sectional Studies
3.4.2. Cohort Studies
3.5. Studies on Genetic Correlation and Single-Nucleotide Polymorphisms (SNPs) in the Association between CRP Levels and Depression
3.5.1. Cross-Sectional Studies
3.5.2. Case-Control Studies
3.6. Studies Investigating the Association between CRP Levels and Antidepressant Treatment
3.6.1. Cross-Sectional Studies
3.6.2. Case-Control Studies
3.6.3. RCT Studies
3.7. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, G.Y.; Tam, W.W.; Lu, Y.; Ho, C.S.; Zhang, M.W.; Ho, R. Prevalence of Depression in the Community from 30 Countries between 1994 and 2014. Sci. Rep. 2018, 8, 2861. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008; Available online: https://apps.who.int/iris/handle/10665/43942 (accessed on 20 December 2021).
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/handle/10665/254610 (accessed on 20 December 2021).
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Kessler, R.C.; Bromet, E.J. The Epidemiology of Depression Across Cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [Green Version]
- Kuehner, C. Why is depression more common among women than among men? Lancet Psychiatry 2017, 4, 146–158. [Google Scholar] [CrossRef]
- Perez-Caballero, L.; Torres-Sanchez, S.; Romero-López-Alberca, C.; González-Saiz, F.; Mico, J.A.; Berrocoso, E. Monoaminergic system and depression. Cell Tissue Res. 2019, 377, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Yirmyia, R.; Noraberg, J.; Brene, S.; Hibbeln, J.; Perini, G.; Kubera, M.; Bob, P.; Lerer, B.; Maj, M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: Leads for future research and new drug developments in depression. Metab. Brain Dis. 2009, 24, 27–53. [Google Scholar] [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M.; Schatzberg, A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef]
- Gałecki, P.; Talarowska, M. Neurodevelopmental theory of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 267–272. [Google Scholar] [CrossRef]
- Uchida, S.; Yamagata, H.; Seki, T.; Watanabe, Y. Epigenetic mechanisms of major depression: Targeting neuronal plasticity. Psychiatry Clin. Neurosci. 2018, 72, 212–227. [Google Scholar] [CrossRef] [Green Version]
- Gałecki, P.; Talarowska, M. Inflammatory theory of depression. Psychiatr. Polska 2018, 52, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Nobis, A.; Zalewski, D.; Waszkiewicz, N. Peripheral Markers of Depression. J. Clin. Med. 2020, 9, 3793. [Google Scholar] [CrossRef] [PubMed]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5166 patients and 5083 controls. Brain Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Siegmann, E.-M.; Müller, H.H.O.; Luecke, C.; Philipsen, A.; Kornhuber, J.; Grömer, T.W. Association of Depression and Anxiety Disorders With Autoimmune Thyroiditis: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 577–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Parra, S.; Daudén, E. Psoriasis and Depression: The Role of Inflammation. Actas Dermo-Sifiliográficas 2019, 110, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Stiernborg, M.; Skott, E.; Söderström, Å.; Giacobini, M.; Lavebratt, C. Proinflammatory mediators and their associations with medication and comorbid traits in children and adults with ADHD. Eur. Neuropsychopharmacol. 2020, 41, 118–131. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Cavanagh, J.; Tindell, A.; Derakhshan, M.; Paterson, C.; Porter, D.; McInnes, I.B.; Siebert, S. Depression and anxiety in an early rheumatoid arthritis inception cohort. associations with demographic, socioeconomic and disease features. RMD Open 2020, 6, e001376. [Google Scholar] [CrossRef]
- Huang, Y.; Su, Y.; Chen, H.; Liu, H.; Hu, J. Serum Levels of CRP are Associated with Depression in a Middle-aged and Elderly Population with Diabetes Mellitus: A Diabetes Mellitus-Stratified Analysis in a Population-Based Study. J. Affect. Disord. 2021, 281, 351–357. [Google Scholar] [CrossRef]
- Pope, J.E.; Choy, E.H. C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin. Arthritis Rheum. 2021, 51, 219–229. [Google Scholar] [CrossRef]
- Byrne, G.; Rosenfeld, G.; Leung, Y.; Qian, H.; Raudzus, J.; Nunez, C.; Bressler, B. Prevalence of Anxiety and Depression in Patients with Inflammatory Bowel Disease. Can. J. Gastroenterol. Hepatol. 2017, 2017, 6496727. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Clinical Application of C-Reactive Protein for Cardiovascular Disease Detection and Prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Benros, M.; Pedersen, M.G.; Rasmussen, H.; Eaton, W.W.; Nordentoft, M.; Mortensen, P.B. A Nationwide Study on the Risk of Autoimmune Diseases in Individuals With a Personal or a Family History of Schizophrenia and Related Psychosis. Am. J. Psychiatry 2014, 171, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine, Sickness Behavior, and Depression. Neurol. Clin. 2006, 24, 441–460. [Google Scholar] [CrossRef] [Green Version]
- McFarland, D.C.; Walsh, L.E.; Saracino, R.; Nelson, C.J.; Breitbart, W.; Rosenfeld, B. The Sickness Behavior Inventory-Revised: Sickness behavior and its associations with depression and inflammation in patients with metastatic lung cancer. Palliat. Support. Care 2020, 19, 312–321. [Google Scholar] [CrossRef]
- Osimo, E.F.; Stochl, J.; Zammit, S.; Lewis, G.; Jones, P.B.; Khandaker, G.M. Longitudinal population subgroups of CRP and risk of depression in the ALSPAC birth cohort. Compr. Psychiatry 2019, 96, 152143. [Google Scholar] [CrossRef]
- Nehring, S.M.; Goyal, A.; Bansal, P.; Patel, B.C. C Reactive Protein. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK441843/ (accessed on 20 December 2021).
- Windgassen, E.B.; Funtowicz, L.; Lunsford, T.N.; Harris, L.A.; Mulvagh, S.L. C-Reactive Protein and High-Sensitivity C-Reactive Protein: An Update for Clinicians. Postgrad. Med. 2011, 123, 114–119. [Google Scholar] [CrossRef]
- Joseph, J.; Depp, C.; Martin, A.S.; Daly, R.E.; Glorioso, D.K.; Palmer, B.; Jeste, D.V. Associations of high sensitivity C-reactive protein levels in schizophrenia and comparison groups. Schizophr. Res. 2015, 168, 456–460. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.-T.; Kim, K.-S.; Kim, E.S.; Lee, S.; Kim, J.; Hoe, H.-S.; Kim, D.-G. Emerging pathogenic role of peripheral blood factors following BBB disruption in neurodegenerative disease. Ageing Res. Rev. 2021, 68, 101333. [Google Scholar] [CrossRef]
- Felger, J.C.; Haroon, E.; Patel, T.A.; Goldsmith, D.R.; Wommack, E.C.; Woolwine, B.J.; Le, N.-A.; Feinberg, R.; Tansey, M.G.; Miller, A.H. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol. Psychiatry 2020, 25, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Carmichael, S.T. Blood−brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr. Opin. Neurol. 2015, 28, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menard, C.; Pfau, M.L.; Hodes, G.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aveleira, C.A.; Lin, C.-M.; Abcouwer, S.F.; Ambrosio, A.F.; Antonetti, D.A. TNF-Signals Through PKC/NF-B to Alter the Tight Junction Complex and Increase Retinal Endothelial Cell Permeability. Diabetes 2010, 59, 2872–2882. [Google Scholar] [CrossRef] [Green Version]
- Horn, S.R.; Long, M.M.; Nelson, B.W.; Allen, N.B.; Fisher, P.A.; Byrne, M.L. Replication and reproducibility issues in the relationship between C-reactive protein and depression: A systematic review and focused meta-analysis. Brain Behav. Immun. 2018, 73, 85–114. [Google Scholar] [CrossRef]
- Kuhlmann, C.R.; Librizzi, L.; Closhen, D.; Pflanzner, T.; Lessmann, V.; Pietrzik, C.U.; de Curtis, M.; Luhmann, H.J. Mechanisms of C-Reactive Protein-Induced Blood–Brain Barrier Disruption. Stroke 2009, 40, 1458–1466. [Google Scholar] [CrossRef] [Green Version]
- D’Mello, C.; Le, T.; Swain, M.G. Cerebral Microglia Recruit Monocytes into the Brain in Response to Tumor Necrosis Factor Signaling during Peripheral Organ Inflammation. J. Neurosci. 2009, 29, 2089–2102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKim, D.B.; Weber, M.D.; Niraula, A.; Sawicki, C.M.; Liu, X.; Jarrett, B.L.; Ramirez-Chan, K.; Wang, Y.; Roeth, R.M.; Sucaldito, A.D.; et al. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatry 2018, 23, 1421–1431. [Google Scholar] [CrossRef] [Green Version]
- Wesselingh, R.; Butzkueven, H.; Buzzard, K.; Tarlinton, D.; O’Brien, T.; Monif, M. Innate Immunity in the Central Nervous System: A Missing Piece of the Autoimmune Encephalitis Puzzle? Front. Immunol. 2019, 10, 2066. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2009; Volume 5. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Almeida, O.P.; Norman, P.E.; Allcock, R.; van Bockxmeer, F.; Hankey, G.J.; Jamrozik, K.; Flicker, L. Polymorphisms of the CRP gene inhibit inflammatory response and increase susceptibility to depression: The Health in Men Study. Int. J. Epidemiol. 2009, 38, 1049–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancelin, M.-L.; Farre, A.; Carrière, I.; Ritchie, K.; Chaudieu, I.; Ryan, J. C-reactive protein gene variants: Independent association with late-life depression and circulating protein levels. Transl. Psychiatry 2015, 5, e499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldirola, D.; Daccò, S.; Cuniberti, F.; Grassi, M.; Lorusso, S.; Diaferia, G.; Perna, G. Elevated C-reactive protein levels across diagnoses: The first comparison among inpatients with major depressive disorder, bipolar disorder, or obsessive–compulsive disorder. J. Psychosom. Res. 2021, 150, 110604. [Google Scholar] [CrossRef]
- Case, S.M.; Stewart, J.C. Race/ethnicity moderates the relationship between depressive symptom severity and C-reactive protein: 2005–2010 NHANES data. Brain Behav. Immun. 2014, 41, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Chaiton, M.; O’Loughlin, J.; Karp, I.; Lambert, M. Depressive Symptoms and C-Reactive Protein Are Not Associated in a Population-Based Sample of Adolescents. Int. J. Behav. Med. 2010, 17, 216–222. [Google Scholar] [CrossRef]
- Chang, H.H.; Lee, I.H.; Gean, P.W.; Lee, S.-Y.; Chi, M.H.; Yang, Y.K.; Lu, R.-B.; Chen, P.S. Treatment response and cognitive impairment in major depression: Association with C-reactive protein. Brain Behav. Immun. 2012, 26, 90–95. [Google Scholar] [CrossRef]
- Chapman, A.; Santos-Lozada, A.R. Racial and ethnic differences in the associations between social integration, C-reactive protein and depressive symptoms. SSM-Popul. Health 2020, 12, 100663. [Google Scholar] [CrossRef]
- Cho, S.H.; Lim, J.-E.; Lee, J.; Lee, J.S.; Jeong, H.-G.; Lee, M.-S.; Ko, Y.-H.; Han, C.; Ham, B.-J.; Han, K.-M. Association between high-sensitivity C-reactive protein levels and depression: Moderation by age, sex, obesity, and aerobic physical activity. J. Affect. Disord. 2021, 291, 375–383. [Google Scholar] [CrossRef]
- De Berardis, D.; Fornaro, M.; Orsolini, L.; Iasevoli, F.; Tomasetti, C.; de Bartolomeis, A.; Serroni, N.; De Lauretis, I.; Girinelli, G.; Mazza, M.; et al. Effect of agomelatine treatment on C-reactive protein levels in patients with major depressive disorder: An exploratory study in «real-world,» everyday clinical practice. CNS Spectr. 2017, 22, 342–347. [Google Scholar] [CrossRef]
- Elovainio, M.; Aalto, A.-M.; Kivimäki, M.; Pirkola, S.; Sundvall, J.; Lönnqvist, J.; Reunanen, A. Depression and C-Reactive Protein: Population-Based Health 2000 Study. Psychosom. Med. 2009, 71, 423–430. [Google Scholar] [CrossRef]
- Ford, D.E.; Erlinger, T.P. Depression and C-reactive protein in US adults: Data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2004, 164, 1010–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mac Giollabhui, N.; Alloy, L.B.; Schweren, L.J.S.; Hartman, C.A. Investigating whether a combination of higher CRP and depression is differentially associated with worse executive functioning in a cohort of 43,896 adults. Brain Behav. Immun. 2021, 96, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Halder, I.; Marsland, A.L.; Cheong, J.; Muldoon, M.F.; Ferrell, R.E.; Manuck, S.B. Polymorphisms in the CRP gene moderate an association between depressive symptoms and circulating levels of C-reactive protein. Brain Behav. Immun. 2010, 24, 160–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickman, R.J.; Khambaty, T.; Stewart, J.C. C-reactive protein is elevated in atypical but not nonatypical depression: Data from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. J. Behav. Med. 2014, 37, 621–629. [Google Scholar] [CrossRef]
- Huang, T.-L.; Lin, F.-C. High-sensitivity C-reactive protein levels in patients with major depressive disorder and bipolar mania. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 370–372. [Google Scholar] [CrossRef]
- Hughes, A.; Kumari, M. Associations of C-reactive protein and psychological distress are modified by antidepressants, supporting an inflammatory depression subtype: Findings from UKHLS. Brain Behav. Immun. 2017, 66, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.-E.; Kang, K.Y. Elevated hs-CRP level is associated with depression in younger adults: Results from the Korean National Health and Nutrition Examination Survey (KNHANES 2016). Psychoneuroendocrinology 2019, 109, 104397. [Google Scholar] [CrossRef]
- Khan, A.; Leonard, D.; Defina, L.; Barlow, C.E.; Willis, B.; Brown, E.S. Association between C reactive protein and depression in a population of healthy adults: The Cooper Center Longitudinal Study. J. Investig. Med. 2020, 68, 1019–1023. [Google Scholar] [CrossRef]
- Köhler-Forsberg, O.; Buttenschøn, H.; Tansey, K.; Maier, W.; Hauser, J.; Dernovsek, M.Z.; Henigsberg, N.; Souery, D.; Farmer, A.; Rietschel, M.; et al. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain, Behav. Immun. 2017, 62, 344–350. [Google Scholar] [CrossRef]
- Lee, S.; Oh, S.; Jang, S.-I.; Park, E.-C. Sex Difference in the Association between High-sensitivity C-reactive Protein and Depression: The 2016 Korea National Health and Nutrition Examination Survey. Sci. Rep. 2019, 9, 1918. [Google Scholar] [CrossRef]
- Li, X.; Sun, N.; Yang, C.; Liu, Z.; Li, X.; Zhang, K. C-Reactive Protein Gene Variants in Depressive Symptoms & Antidepressants Efficacy. Psychiatry Investig. 2019, 16, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Liukkonen, T.; Silvennoinen-Kassinen, S.; Jokelainen, J.; Räsänen, P.; Leinonen, M.; Meyer-Rochow, V.B.; Timonen, M. The Association Between C-Reactive Protein Levels and Depression: Results from the Northern Finland 1966 Birth Cohort Study. Biol. Psychiatry 2006, 60, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Luukinen, H.; Jokelainen, J.; Hedberg, P. The relationships between high-sensitivity C-reactive protein and incident depressed mood among older adults. Scand. J. Clin. Lab. Investig. 2010, 70, 75–79. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, S.T.; de Figueiredo, R.C.; Goulart, A.C.; Nunes, M.A.; Benseñor, I.M.; Viana, M.C.; Barreto, S.M. Lack of association between depression and C-reactive protein level in the baseline of Longitudinal Study of Adult Health (ELSA-Brasil). J. Affect Disord. 2017, 208, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Moriarity, D.P.; van Borkulo, C.; Alloy, L.B. Inflammatory phenotype of depression symptom structure: A network perspective. Brain, Behav. Immun. 2020, 93, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Pikhart, H.; Hubacek, J.A.; Kubinova, R.; Nicholson, A.; Peasey, A.; Capkova, N.; Poledne, R.; Bobak, M. Depressive symptoms and levels of C-reactive protein: A population-based study. Soc Psychiatry Psychiatr. Epidemiol. 2009, 44, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Song, B.M.; Lee, J.-M.; Choi, W.; Youm, Y.; Chu, S.H.; Park, Y.-R.; Kim, H.C. Association between C reactive protein level and depressive symptoms in an elderly Korean population: Korean Social Life, Health and Aging Project. BMJ Open 2015, 5, e006429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabatabaeizadeh, S.-A.; Abdizadeh, M.F.; Meshkat, Z.; Khodashenas, E.; Darroudi, S.; Fazeli, M.; Ferns, G.A.; Avan, A.; Ghayour-Mobarhan, M. There is an association between serum high-sensitivity C-reactive protein (hs-CRP) concentrations and depression score in adolescent girls. Psychoneuroendocrinology 2018, 88, 102–104. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Kivimäki, M.; Jokela, M.; Batty, G.D. Association of inflammation with specific symptoms of depression in a general population of older people: The English Longitudinal Study of Ageing. Brain Behav. Immun. 2017, 61, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Wysokiński, A.; Margulska, A.; Strzelecki, D.; Kłoszewska, I. Levels of C-reactive protein (CRP) in patients with schizophrenia, unipolar depression and bipolar disorder. Nord. J. Psychiatry 2014, 69, 346–353. [Google Scholar] [CrossRef]
- Zavos, H.M.; Zunszain, P.A.; Jayaweera, K.; Powell, T.R.; Chatzivasileiadou, M.; Harber-Aschan, L.; Adikari, A.; Pannala, G.; Siribaddana, S.; Badini, I.; et al. Relationship between CRP and depression: A genetically sensitive study in Sri Lanka. J. Affect. Disord. 2021, 297, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.-L.; Zhang, L.-L.; Guo, L.-L.; Li, H.; Li, D. No association between C-reactive protein and depressive symptoms among the middle-aged and elderly in China: Evidence from the China Health and Retirement Longitudinal Study. Medicine 2018, 97, e12352. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, A.; Fabbri, C.; Rietschel, M.; Hauser, J.; Mors, O.; Maier, W.; Zobel, A.; Farmer, A.; Aitchison, K.J.; McGuffin, P.; et al. Genetic disposition to inflammation and response to antidepressants in major depressive disorder. J. Psychiatr. Res. 2018, 105, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamberlain, S.R.; Cavanagh, J.; de Boer, P.; Mondelli, V.; Jones, D.N.; Drevets, W.C.; Cowen, P.J.; Harrison, N.A.; Pointon, L.; Pariante, C.M.; et al. Treatment-resistant depression and peripheral C-reactive protein. Br. J. Psychiatry 2019, 214, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.H.; Wang, T.-Y.; Lee, I.H.; Lee, S.-Y.; Chen, K.C.; Huang, S.-Y.; Yang, Y.K.; Lu, R.-B.; Chen, P.S. C-reactive protein: A differential biomarker for major depressive disorder and bipolar II disorder. World J. Biol. Psychiatry 2016, 18, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Foley, É.M.; Parkinson, J.T.; Kappelmann, N.; Khandaker, G.M. Clinical phenotypes of depressed patients with evidence of inflammation and somatic symptoms. Compr. Psychoneuroendocrinol. 2021, 8, 100079. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Sardesai, U.; Razdan, R. C-reactive protein level in late-onset depression: A case–control study. Indian J. Psychiatry 2018, 60, 467–471. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Scott, L.V.; Dinan, T. Antidepressant therapy and C-reactive protein levels. Br. J. Psychiatry 2006, 188, 449–452. [Google Scholar] [CrossRef] [Green Version]
- Pitharouli, M.C.; Hagenaars, S.P.; Glanville, K.P.; Coleman, J.R.; Hotopf, M.; Lewis, C.M.; Pariante, C.M. Elevated C-Reactive Protein in Patients With Depression, Independent of Genetic, Health, and Psychosocial Factors: Results From the UK Biobank. Am. J. Psychiatry 2021, 178, 522–529. [Google Scholar] [CrossRef]
- Qiao, J.; Geng, D.; Qian, L.; Zhu, X.; Zhao, H. Correlation of clinical features with hs-CRP in TRD patients. Exp. Ther. Med. 2018, 17, 344–348. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhong, H.; Lu, M.; Song, G.; Zhang, X.; Lin, M.; Yang, S.; Qian, M. Higher Serum C Reactive Protein Determined C Reactive Protein Single-Nucleotide Polymorphisms Are Involved in Inherited Depression. Psychiatry Investig. 2018, 15, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Wysokiński, A.; Socha, K.; Sołtysik, B.K.; Kłoszewska, I.; Sobów, T.; Kostka, T. Levels of C-reactive protein (CRP) in elderly patients with unipolar depression—Case control analysis. Nord. J. Psychiatry 2016, 70, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Yibulaiyin, H.-; Sun, H.; Yang, Y. Depression is associated with CRP SNPs in patients with family history. Transl. Neurosci. 2017, 8, 201–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Au, B.; Smith, K.; Gariépy, G.; Schmitz, N. The longitudinal associations between C-reactive protein and depressive symptoms: Evidence from the English Longitudinal Study of Ageing (ELSA). Int. J. Geriatr. Psychiatry 2014, 30, 976–984. [Google Scholar] [CrossRef]
- Copeland, W.E.; Shanahan, L.; Worthman, C.M.; Angold, A.; Costello, E.J. Cumulative Depression Episodes Predict Later C-Reactive Protein Levels: A Prospective Analysis. Biol. Psychiatry 2012, 71, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Chiriboga, D.E.; Pagoto, S.L.; Rosal, M.C.; Li, W.; Merriam, P.A.; Hébert, J.R.; Whited, M.C.; Ockene, I.S. Association between Depression and C-Reactive Protein. Cardiol. Res. Pract. 2011, 2011, 286509. [Google Scholar] [CrossRef] [Green Version]
- Maas, D.W.; van der Mast, R.C.; de Craen, A.J.M. Increased C-reactive protein is not associated with apathy: The Leiden 85-Plus Study. Int. J. Geriatr. Psychiatry 2009, 24, 1177–1184. [Google Scholar] [CrossRef]
- Matthews, K.A.; Schott, L.L.; Bromberger, J.T.; Cyranowski, J.M.; Everson-Rose, S.A.; Sowers, M. Are there bi-directional associations between depressive symptoms and C-reactive protein in mid-life women? Brain Behav. Immun. 2010, 24, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Pasco, J.A.; Nicholson, G.; Williams, L.; Jacka, F.N.; Henry, A.P.M.J.; Kotowicz, M.; Schneider, H.G.; Leonard, B.E.; Berk, M. Association of high-sensitivity C-reactive protein with de novo major depression. Br. J. Psychiatry 2010, 197, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Sonsin-Diaz, N.; Gottesman, R.; Fracica, E.; Walston, J.; Windham, B.G.; Knopman, D.S.; Walker, K.A. Chronic Systemic Inflammation Is Associated With Symptoms of Late-Life Depression: The ARIC Study. Am. J. Geriatr. Psychiatry 2020, 28, 87–98. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, Y.; Thapa, A.; Fang, J.; Zhao, S.; Shi, W.; Yang, Z.; Li, Y.; Yuan, Y. Baseline serum C-reactive protein levels may predict antidepressant treatment responses in patients with major depressive disorder. J. Affect. Disord. 2019, 250, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Baune, B.T.; Sampson, E.; Louise, J.; Hori, H.; Schubert, K.O.; Clark, S.R.; Mills, N.T.; Fourrier, C. No evidence for clinical efficacy of adjunctive celecoxib with vortioxetine in the treatment of depression: A 6-week double-blind placebo controlled randomized trial. Eur. Neuropsychopharmacol. 2021, 53, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.; Minhajuddin, A.; Chin-Fatt, C.; Greer, T.L.; Carmody, T.J.; Trivedi, M.H. Sex differences in the association of baseline c-reactive protein (CRP) and acute-phase treatment outcomes in major depressive disorder: Findings from the EMBARC study. J. Psychiatr. Res. 2019, 113, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Uher, R.; Tansey, K.; Dew, T.; Maier, W.; Mors, O.; Hauser, J.; Dernovsek, M.Z.; Henigsberg, N.; Souery, D.; Farmer, A.; et al. An Inflammatory Biomarker as a Differential Predictor of Outcome of Depression Treatment With Escitalopram and Nortriptyline. Am. J. Psychiatry 2014, 171, 1278–1286. [Google Scholar] [CrossRef]
- Wells, G.A.; Sher, B.; O’Connel, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessment the quality of nonrandomized studies in meta-analyses. Clin. Epidemiol. 2014. [Google Scholar]
- Franco, F.G.D.M.; Laurinavicius, A.G.; Lotufo, P.A.; Conceição, R.D.; Morita, F.; Katz, M.; Wajngarten, M.; Carvalho, J.A.M.; Bosworth, H.B.; Santos, R.D. Persistent Depressive Symptoms are Independent Predictors of Low-Grade Inflammation Onset Among Healthy Individuals. Arq. Bras. de Cardiol. 2017, 109, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Troubat, R.; Barone, P.; Leman, S.; DeSmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Ramírez, L.A.; Pérez-Padilla, E.A.; García-Oscos, F.; Salgado, H.; Atzori, M.; Pineda, J.C. A new theory of depression based on the serotonin/kynurenine relationship and the hypothalamicpituitary-adrenal axis. Biomedica 2018, 38, 437–450. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G. Editorial: The Kynurenine and Melatonergic Pathways in Psychiatric and CNS Disorders. Curr. Pharm. Des. 2016, 22, 947–948. [Google Scholar] [CrossRef]
- Catena-Dell’Osso, M.; Bellantuono, C.; Consoli, G.; Baroni, S.; Rotella, F.; Marazziti, D. Inflammatory and neurodegenerative pathways in depression: A new avenue for antidepressant development? Curr. Med. Chem. 2011, 18, 245–255. [Google Scholar] [CrossRef]
- Gałecki, P.; Talarowska, M.; Bobińska, K.; Zajączkowska, M.; Su, K.-P.; Maes, M. Impact of oxidative/nitrosative stress and inflammation on cognitive functions in patients with recurrent depressive disorders. Med Sci. Monit. 2014, 20, 110–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-K.; Paik, J.-W.; Lee, S.-W.; Yoon, D.; Han, C.; Lee, B.-H. Increased plasma nitric oxide level associated with suicide attempt in depressive patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 1091–1096. [Google Scholar] [CrossRef]
- Gałecki, P.; Szemraj, J.; Bieńkiewicz, M.; Florkowski, A.; Gałecka, E. Lipid peroxidation and antioxidant protection in patients during acute depressive episodes and in remission after fluoxetine treatment. Pharmacol. Rep. 2009, 61, 436–447. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; De Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, S.; Swatton, J.E.; Ryan, M.M.; Huffaker, S.J.; Huang, J.T.-J.; Griffin, J.L.; Wayland, M.; Freeman, T.; Dudbridge, F.; Lilley, K.S.; et al. Mitochondrial dysfunction in schizophrenia: Evidence for compromised brain metabolism and oxidative stress. Mol. Psychiatry 2004, 9, 684–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, M.; Berk, M.; Goehler, L.; Song, C.; Anderson, G.; Gałecki, P.; Leonard, B. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012, 10, 66. [Google Scholar] [CrossRef] [Green Version]
- Machado, A.K.; Pan, A.Y.; da Silva, T.M.; Duong, A.; Andreazza, A.C. Upstream Pathways Controlling Mitochondrial Function in Major Psychosis: A Focus on Bipolar Disorder. Can. J. Psychiatry 2016, 61, 446–456. [Google Scholar] [CrossRef] [Green Version]
- Talarowska, M.; Gałecki, P.; Maes, M.; Gardner, A.; Chamielec, M.; Orzechowska, A.; Bobińska, K.; Kowalczyk, E. Malondialdehyde plasma concentration correlates with declarative and working memory in patients with recurrent depressive disorder. Mol. Biol. Rep. 2011, 39, 5359–5366. [Google Scholar] [CrossRef]
- Gałecki, P.; Talarowska, M.; Bobińska, K.; Kowalczyk, E.; Gałecka, E.; Lewiński, A. Thiol protein groups correlate with cognitive impairment in patients with recurrent depressive disorder. Neuro Endocrinol. Lett. 2013, 34, 780–786. [Google Scholar]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappaB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [Green Version]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of Depression With C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valkanova, V.; Ebmeier, K.P.; Allan, C.L. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013, 150, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1β, IL-6, TNF-α and CRP in elderly patients with depression or Alzheimer’s disease: Systematic review and meta-analysis. Sci. Rep. 2018, 8, 12050. [Google Scholar] [CrossRef] [PubMed]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimäki, M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wium-Andersen, M.K.; Ørsted, D.D.; Nielsen, S.F.; Nordestgaard, B.G. Elevated C-Reactive Protein Levels, Psychological Distress, and Depression in 73 131 Individuals. JAMA Psychiatry 2013, 70, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Khandaker, G.M.; Pearson, R.M.; Zammit, S.; Lewis, G.; Jones, P.B. Association of Serum Interleukin 6 and C-Reactive Protein in Childhood With Depression and Psychosis in Young Adult Life: A Population-Based Longitudinal Study. JAMA Psychiatry 2014, 71, 1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osimo, E.F.; Baxter, L.J.; Lewis, G.; Jones, P.B.; Khandaker, G.M. Prevalence of low-grade inflammation in depression: A systematic review and meta-analysis of CRP levels. Psychol. Med. 2019, 49, 1958–1970. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Scully, P.; Fitzgerald, P.; Scott, L.V.; Dinan, T.G. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J. Psychiatr. Res. 2007, 41, 326–331. [Google Scholar] [CrossRef]
- Yang, C.; Wardenaar, K.J.; Bosker, F.J.; Li, J.; Schoevers, R.A. Inflammatory markers and treatment outcome in treatment resistant depression: A systematic review. J. Affect. Disord. 2019, 257, 640–649. [Google Scholar] [CrossRef]
- Tuglu, C.; Kara, S.H.; Caliyurt, O.; Vardar, E.; Abay, E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology 2003, 170, 429–433. [Google Scholar] [CrossRef]
- Mosiołek, A.; Pięta, A.; Jakima, S.; Zborowska, N.; Mosiołek, J.; Szulc, A. Effects of Antidepressant Treatment on Peripheral Biomarkers in Patients with Major Depressive Disorder (MDD). J. Clin. Med. 2021, 10, 1706. [Google Scholar] [CrossRef] [PubMed]
- Köhler-Forsberg, O.; Lydholm, C.N.; Hjorthøj, C.; Nordentoft, M.; Mors, O.; Benros, M.E. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: Meta-analysis of clinical trials. Acta Psychiatr. Scand. 2019, 139, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Więdłocha, M.; Marcinowicz, P.; Krupa, R.; Janoska-Jaździk, M.; Janus, M.; Debowska, W.; Mosiołek, A.; Waszkiewicz, N.; Szulc, A. Effect of antidepressant treatment on peripheral inflammation markers—A meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Rutherford, R.E.; Woolwine, B.J.; Shuo, C.; Schettler, P.; Drake, D.F.; Haroon, E.; Miller, A.H. A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant Depression: The Role of Baseline Inflammatory Biomarkers. JAMA Psychiatry 2013, 70, 31. [Google Scholar] [CrossRef]
- Köhler, O.; Benros, M.E.; Nordentoft, M.; Farkouh, M.E.; Iyengar, R.L.; Mors, O.; Krogh, J. Effect of Anti-inflammatory Treatment on Depression, Depressive Symptoms, and Adverse Effects: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Psychiatry 2014, 71, 1381. [Google Scholar] [CrossRef] [PubMed]
- Eyre, H.A.; Air, T.; Proctor, S.; Rositano, S.; Baune, B.T. A critical review of the efficacy of non-steroidal anti-inflammatory drugs in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 57, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Kappelmann, N.; Lewis, G.; Dantzer, R.; Jones, P.; Khandaker, G. Antidepressant activity of anti-cytokine treatment: A systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry 2016, 23, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Bekhbat, M.; Chu, K.; Le, N.-A.; Woolwine, B.J.; Haroon, E.; Miller, A.H.; Felger, J.C. Glucose and lipid-related biomarkers and the antidepressant response to infliximab in patients with treatment-resistant depression. Psychoneuroendocrinology 2018, 98, 222–229. [Google Scholar] [CrossRef]
- Bavaresco, D.V.; Uggioni, M.L.R.; Ferraz, S.D.; Marques, R.M.M.; Simon, C.S.; Dagostin, V.S.; Grande, A.J.; da Rosa, M.I. Efficacy of infliximab in treatment-resistant depression: A systematic review and meta-analysis. Pharmacol. Biochem. Behav. 2020, 188, 172838. [Google Scholar] [CrossRef]
- Arteaga-Henríquez, G.; Simon, M.S.; Burger, B.; Weidinger, E.; Wijkhuijs, A.; Arolt, V.; Birkenhager, T.K.; Musil, R.; Müller, N.; Drexhage, H.A. Low-Grade Inflammation as a Predictor of Antidepressant and Anti-Inflammatory Therapy Response in MDD Patients: A Systematic Review of the Literature in Combination With an Analysis of Experimental Data Collected in the EU-MOODINFLAME Consortium. Front. Psychiatry 2019, 10, 458. [Google Scholar] [CrossRef]
- Zhu, C.-B.; Blakely, R.D.; Hewlett, W.A. The Proinflammatory Cytokines Interleukin-1beta and Tumor Necrosis Factor-Alpha Activate Serotonin Transporters. Neuropsychopharmacology 2006, 31, 2121–2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geerlings, M.I.; Bouter, L.M.; Schoevers, R.A.; Beekman, A.T.F.; Jonker, C.E.E.S.; Deeg, D.J.H.; Schmand, B. Depression and risk of cognitive decline and Alzheimer’s disease. Results of two prospective community-based studies in The Netherlands. Br. J. Psychiatry 2000, 176, 568–575. [Google Scholar] [CrossRef]
- Ozawa, K.; Hashimoto, K.; Kishimoto, T.; Shimizu, E.; Ishikura, H.; Iyo, M. Immune Activation During Pregnancy in Mice Leads to Dopaminergic Hyperfunction and Cognitive Impairment in the Offspring: A Neurodevelopmental Animal Model of Schizophrenia. Biol. Psychiatry 2006, 59, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Miller, A.H. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol. Ther. 2011, 130, 226–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, K.; Yoshino, H.; Ogawa, Y.; Yamamuro, K.; Kimoto, S.; Noriyama, Y.; Makinodan, M.; Yamashita, M.; Saito, Y.; Kishimoto, T. Maternal Immune Activation Affects Hippocampal Excitatory and Inhibitory Synaptic Transmission in Offspring From an Early Developmental Period to Adulthood. Front. Cell. Neurosci. 2020, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Chun, W.; Lee, H.J.; Kim, S.-M.; Min, J.-H.; Kim, D.-Y.; Kim, M.-O.; Ryu, H.W.; Lee, S.U. The Role of Microglia in the Development of Neurodegenerative Diseases. Biomedicines 2021, 9, 1449. [Google Scholar] [CrossRef]
| Study | Study Design | Primary and Secondary Outcomes | Participants Characteristics | Assessment | Main Findings | Is There an Association between CRP Levels and Depression? |
|---|---|---|---|---|---|---|
| [45] | Cross-sectional study (Australia) | To determine if polymorphisms of SNPs rs1130864 and rs1205 are associated with prevalent depression | 3700 men aged > or = 70 years |
|
|
|
| [46] | Cross-sectional study (France) | Association between variants in the CRP gene that influence protein levels and depression | 990 people aged at least 65 years |
|
|
|
| [47] | Cross-sectional study (Italy) | To compare CRP (i.e., serum CRP > 3 and ≤10 mg/L) in patients with MDD, BD and OCD | 388 inpatients, (156 MDD, 135 BD, 97 OCD) |
|
|
|
| [48] | Cross-sectional study (USA) | To evaluate whether specific symptoms clusters are strongly associated with CRP levels and if race/ethnicity may affect this association | 10,149 adults who participated to the NHANES |
|
|
|
| [49] | Cross-sectional study (Canada) | To examine the association between hs-CRP concentrations and depressive symptoms in youth | 1535 adolescents (aged 13–16) |
|
|
|
| [50] | Cross-sectional study (China) | To evaluate the association between CRP, depressive symptoms and cognitive impairment in MDD patients treated with antidepressants (venlafaxine and fluoxetine) for 6 weeks | 149 MDD subjects (M = 42 F = 107) |
|
|
|
| [51] | Cross-sectional study (USA) | Association between social integration, race/ethnicity, inflammation, and depressive symptoms | 5634 participants aged 40 and older from the NHANES |
|
|
|
| [52] | Cross-sectional study (Republic of Korea) | Examine the association between serum hs-CRP levels and depressive symptoms in adults and explore the potential moderating effects of age, sex, BMI, and aerobic physical activity on the association between hsCRP levels and depression | 10,702 Adults (≥19 years) (M = 4746; F = 5956) |
|
|
|
| [53] | Cross-sectional study (Italy) | To investigate the effects of agomelatine on CRP levels in MDD patients and whether CRP variations are associated with clinical improvement | 30 adult MDD outpatients (M = 12 F = 18) |
|
|
|
| [54] | Cross sectional study (Finland) | To evaluate whether depression is independently associated with elevated CRP levels | 6000 Finns aged > 30 years (M = 2784F = 3257) |
|
|
|
| [55] | Cross-sectional study (USA) | To determine the association between MDD and elevated CRP levels in a nationally representative US cohort | 6914 noninstitutionalized (M = 3154 F = 3760) aged 18–39 yy |
|
|
|
| [56] | Cross-sectional study (USA) | To investigate whether the combination between CRP levels and depression is associated with worse executive functioning | 43,896 adults aged 44.13 years |
|
|
|
| [57] | Cross-sectional study (USA) | To explore whether plasma CRP levels may covary with depressive symptomatology as a function of allelic variation in the CRP gene | 868 healthy community volunteers |
|
|
|
| [58] | Cross-sectional study (US) | To evaluate the association between depression subtypes with inflammatory state | 19 atypical MDD patients, 93 non-atypical MDD, 1682 without MDD |
|
|
|
| [59] | Cross-sectional study (China) | To assess the difference in serum hs-CRP levels between BD-I and MDD | 23 MDD13 BD-I (manic episodes)31 healthy controls |
|
|
|
| [60] | Cross-sectional study (UK) | Associations of CRP and psychological distress mediated by antidepressants, supporting an inflammatory depression subtype | 10,363 UK adults aged 16–98 |
|
|
|
| [61] | Cross-sectional study (Republic of Korea) | To examine the association between hs-CRP levels and depression | 5447 participants coming from KNHANES VII-1 study |
|
|
|
| [62] | Cross-sectional study (USA) | To determine the association between hs-CRP levels and depression in a large sample of healthy adults | 26,638 healthy adults |
|
|
|
| [63] | Cross-sectional study (Germany) | To evaluate the association between CRP levels and depression severity, including specific depressive symptoms | 231 MDD patients (F = 142 M = 89) recruited from GENDEP study |
|
|
|
| [64] | Cross-sectional study | To evaluate the sex difference in the relationship between CRP and depression | 5483 Korean adults (2373 men and 3110 women) recruited from KNHANES |
|
|
|
| [65] | Cross-sectional study (China) | To explore whether CRP SNPs are related to depressive symptoms and antidepressants efficacy | 440 patients with first-episode depression |
|
|
|
| [66] | Cross-sectional study (Finland) | To investigate whether depressive episodes are associated in both genders with hs-CRP levels | 5269 participants (M = 2641 F = 2828) |
|
|
|
| [67] | Cross-sectional study (Finland) | Association between hs-CRP levels and depressed mood among the elderly | 764 subjects aged 70 years or older |
|
|
|
| [68] | Cross-sectional study (Brazil) | To investigate relationship between serum CRP levels and depression | 14,821 participants recruited from ELSA-Brazil study |
|
|
|
| [69] | Cross-sectional study (USA) | To investigate the possible association between inflammation and a specific phenotype of depression | 4157 participants from NHANES (F = 51.3%) with mean age of 47.59 |
|
|
|
| [70] | Cross-sectional study (Czech Republic) | To confirm the possible association between depression and CRP levels | 6126 individuals (45–69 yy) (M = 2829; F = 3297) |
|
|
|
| [71] | Cross-sectional study (Republic of Korea) | Association between CRP levels and depressive symptoms in an elderly Korean population | 569 (M = 224 F = 345) recruited from Korean Social Life, Health and Aging Project Health Examination Cohort aged 60 or over |
|
|
|
| [72] | Cross-sectional study (Iran) | Association between serum hs-CRP levels and depression score in adolescent girls | 563 adolescent girls aged 12–18 years |
|
|
|
| [73] | Cross-sectional study (USA) | To find a possible association between inflammation and specific depressive symptoms | 5909 patients recruited from ELSA |
|
|
|
| [74] | Cross-sectional study (Poland) | To determine whether there are differences in CRP levels between different psychiatric disorder | 458 schizophrenia patients 319 unipolar depression 146 BD 114 BD depression 32 BD mania |
|
|
|
| [75] | Cross-sectional study (Sri Lanka) | To consider the extent to which shared genetic and environmental factors may contribute to the association between CRP levels and depression | 2577 twins and 899 singletons |
|
|
|
| [76] | Cross-sectional study (China) | Possible associations between CRP levels and depressive symptoms among the middle-aged and elderly in China | 9459 Chinese middle-aged and elderly individuals (M = 4404F = 5055 selected on the CHARLS |
|
|
|
| [77] | Cross-sectional study (UK) | Association between CRP levels and a worse response to escitalopram and better response to nortriptyline in consideration of genetic disposition to inflammation | 755 unrelated individuals |
|
|
|
| [78] | Case-control study (UK) | To explore CRP levels in MDD and its phenotypic associations | 102 TRD patients with MDD currently experiencing depression, 48 treatment-responsive patients with MDD not currently experiencing depression, 48 patients with depression who were not receiving medication, and 54 healthy volunteers |
|
|
|
| [79] | Case-control study (China) | To examine whether CRP levels could be used to differentiate between MDD and BD II | 96 healthy controls, 88 BD-II and 72 MDD drug-naïve patients in their major depressive episode |
|
|
|
| [80] | Case-control Study (UK) | To identify a distinct phenotypic profile of depression associated with inflammation | 84 depressed patient divided in two group: with inflammation (CRP ≥ 3 mg/L) (N = 40) and without inflammation (CRP < 3 mg/L) (N = 44) |
|
|
|
| [81] | Case-control study (India) | To compare CRP levels in late-onset depression compared with age-matched healthy controls and evaluate whether (any) association between CRP levels and depressive symptoms severity | 25 patients aged ≥ 55 years with a first depressive episode and 27 age matched healthy controls |
|
|
|
| [82] | Case-control study (USA) | To examine CRP levels in depressive disorders and evaluate the impact of SSRI | A two-part study:1–32 patients with history of depression (20 currently depressed, 12 euthymic) treated with SSRI and 20 healthy comparison group2-CRP measured in 20 MDD patients both before and after SSRI treatment |
|
|
|
| [83] | Case-control study (UK) | To assess the inflammation in MDD subjects through CRP levels and the possible association with genetic, lifestyle, and phenotypic factors | 26,894 MDD patients and 59,000 healthy controls |
|
|
|
| [84] | Case-control study (China) | Correlation of clinical features with hs-CRP levels in TRD patients | 103 TRD and 103 non-TRD patients |
|
|
|
| [85] | Case-control study (China) | Considering CRP SNPs could regulate plasma CRP levels, the study hypothesized that inherited CRP allelic variations may covary with depressive symptomatology | 60 depression patients with family depression history and 60 healthy control volunteers |
|
|
|
| [86] | Case-control study (Poland) | To determine differences regarding CRP levels between elderly patients with unipolar depression and healthy controls | 404 patients (202 with unipolar depression202 healthy controls) |
|
|
|
| [87] | Case-control study (China) | To investigate whether inherited CRP allelic variations may co-vary with depressive symptoms | 200 patients (100 MDD, with or without family depression history and 100 healthy controls) |
|
|
|
| [88] | Cohort study | Association between CRP levels and depressive symptomatology among older adults | 3397 participants from the English Longitudinal Study of Ageing |
|
|
|
| [89] | Cohort study(USA) | To compare the effect of current depression with the effect of cumulative episodes of depression on the CRP levels | 1334 children, adolescents, and young adults |
|
|
|
| [40] | Cohort study (Brazil) | To evaluate the association between persistent depressive symptoms and the onset of low-grade inflammation | 1508 young individuals (134 with persistent depressive symptoms and 1374 negative at BDI) |
|
|
|
| [90] | Cohort study (USA) | Association between depression and hs-CRP levels | 508 healthy adults (F = 49%, mean age 48.5 yy) |
|
|
|
| [91] | Cohort study (The Netherlands) | To assess whether depression and apathy had different etiologiesin the elderly | 599 elderly subjects assessed annually form age 85 to 90 |
|
|
|
| [92] | Cohort study (USA) | Association between depressive symptoms and CRP levels in mid-life women | 3302 pre- and early perimenopausal women |
|
|
|
| [29] | Cohort Study (UK) | To evaluate if increasing levels of CRP in childhood and/or early-adulthood is associated with the risk of depression in early-adulthood | 1561 participants (M = 770; F = 791) |
|
|
|
| [93] | Cohort study (Australia) | Association between CRP levels and increased risk of de novo MDD | 1494 randomly selected women |
|
|
|
| [94] | Cohort Study | To examine long-term patterns of systemic inflammation in aging adults and determined whether individuals with chronic elevations in inflammation were at increased risk for having symptoms of depression as older adults | 4476 participants (mean age: 75.5(SD = 5.1)) M = 1775; F = 2701 |
|
|
|
| [95] | Cohort study (China) | To test whether baseline serum CRP levels could predict antidepressant treatment responses | 75 adult inpatients (M = 26 F = 49) with major MDD |
|
|
|
| [96] | RCT (Australia) | To measure the efficacy of anti-inflammatory augmentation of antidepressant treatment in MDD patients and whether treatment response was dependent on baseline inflammation levels | 119 MDD |
|
|
|
| [97] | RCT (USA) | To evaluate the sex differences in the association between CRP levels and the response to antidepressant treatments | 220 individuals (M = 75 F = 145) from EMBARC study |
|
|
|
| [98] | RCT | To test the hypothesis that CRP predicts differential response to escitalopram and nortriptyline | 241 MDD |
|
|
|
| Study | Selection | Comparability | Outcome | Overall | ||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the sample | Sample size | Non-respondents | Ascertainment of depression | Based on design and analysis | Assessment of the outcome | Statistical test | ||
| [70] | * | / | / | * | * | ** | * | 6 |
| [61] | * | * | / | * | ** | ** | * | 8 |
| [52] | * | / | * | * | ** | ** | * | 8 |
| [58] | * | / | / | ** | ** | ** | * | 7 |
| [47] | / | / | / | * | ** | ** | * | 6 |
| [59] | * | / | / | ** | * | ** | * | 7 |
| [74] | * | / | / | * | * | ** | * | 6 |
| [49] | * | / | / | * | ** | ** | * | 7 |
| [50] | / | / | * | * | / | ** | * | 5 |
| [68] | * | / | / | * | ** | ** | * | 7 |
| [76] | * | / | * | * | ** | ** | * | 8 |
| [62] | * | * | / | * | ** | ** | * | 8 |
| [75] | * | / | / | ** | ** | ** | * | 8 |
| [54] | * | * | / | * | ** | ** | * | 8 |
| [64] | * | * | / | * | ** | ** | * | 8 |
| [71] | * | / | / | * | ** | ** | * | 7 |
| [55] | * | * | / | * | ** | ** | * | 8 |
| [66] | * | * | * | * | ** | ** | * | 9 |
| [67] | * | / | / | * | * | ** | * | 6 |
| [63] | * | / | / | * | ** | ** | * | 7 |
| [72] | * | / | / | * | ** | ** | * | 7 |
| [51] | * | * | / | * | ** | ** | * | 7 |
| [48] | * | / | / | * | ** | ** | * | 7 |
| [73] | * | / | / | * | ** | ** | * | 7 |
| [69] | * | / | / | * | / | ** | * | 5 |
| [56] | * | * | / | * | ** | ** | * | 9 |
| [57] | * | / | / | * | ** | ** | * | 7 |
| [65] | * | / | / | * | ** | ** | * | 7 |
| [45] | * | / | / | * | ** | ** | * | 7 |
| [46] | * | / | / | * | ** | ** | * | 7 |
| [60] | * | * | / | * | ** | ** | * | 8 |
| [53] | * | / | / | * | ** | ** | * | 7 |
| [77] | * | / | / | * | * | ** | * | 6 |
| Study | Selection | Comparability | Exposure | Overall | |||||
|---|---|---|---|---|---|---|---|---|---|
| Is the case definition adequate? | Representativeness of the case | Selection of controls | Definition of controls | Based on design or analysis | Ascertainment of exposure | Same method ascertainment | Non- response rate | ||
| [80] | * | * | / | / | * | * | * | / | 5 |
| [83] | * | * | * | * | ** | * | * | / | 8 |
| [81] | * | * | * | * | * | * | * | / | 7 |
| [79] | * | / | * | / | * | * | * | / | 5 |
| [86] | * | / | * | * | * | * | * | / | 6 |
| [87] | * | / | / | * | * | * | * | / | 5 |
| [85] | * | / | * | * | * | * | * | / | 6 |
| [84] | * | * | * | / | * | * | * | / | 6 |
| [78] | * | / | * | * | ** | * | * | / | 7 |
| [82] | * | / | / | * | * | / | * | / | 5 |
| Study | Selection | Comparability | Outcome | Overall | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome was not present at start | Based on design and analysis | Assessment of outcome | Enough follow-up | Adequacy of follow-up | ||
| [29] | * | * | * | / | ** | * | * | * | 8 |
| [94] | * | * | * | / | ** | * | * | * | 8 |
| [100] | / | * | * | * | ** | * | * | / | 7 |
| [89] | / | * | * | / | ** | * | * | * | 7 |
| [88] | * | * | * | / | ** | * | * | * | 8 |
| [90] | / | * | * | / | ** | * | * | * | 7 |
| [92] | * | * | * | / | ** | * | * | * | 8 |
| [93] | * | * | * | / | ** | * | * | * | 8 |
| [91] | * | * | * | / | / | + | * | * | 6 |
| [95] | / | * | * | / | * | * | / | * | 5 |
| Study | Selection | Comparability | Outcome | Overall | |||||
|---|---|---|---|---|---|---|---|---|---|
| Is the case definition adequate? | Representativeness of the case | Selection of control | Definition of control | Based on design and analysis | Assessment of exposure | Same method ascertainment | Non- response rate | ||
| [97] | * | * | * | * | ** | * | * | / | 8 |
| [98] | * | * | * | * | * | * | * | / | 7 |
| [96] | * | * | * | * | * | * | * | / | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orsolini, L.; Pompili, S.; Tempia Valenta, S.; Salvi, V.; Volpe, U. C-Reactive Protein as a Biomarker for Major Depressive Disorder? Int. J. Mol. Sci. 2022, 23, 1616. https://doi.org/10.3390/ijms23031616
Orsolini L, Pompili S, Tempia Valenta S, Salvi V, Volpe U. C-Reactive Protein as a Biomarker for Major Depressive Disorder? International Journal of Molecular Sciences. 2022; 23(3):1616. https://doi.org/10.3390/ijms23031616
Chicago/Turabian StyleOrsolini, Laura, Simone Pompili, Silvia Tempia Valenta, Virginio Salvi, and Umberto Volpe. 2022. "C-Reactive Protein as a Biomarker for Major Depressive Disorder?" International Journal of Molecular Sciences 23, no. 3: 1616. https://doi.org/10.3390/ijms23031616
APA StyleOrsolini, L., Pompili, S., Tempia Valenta, S., Salvi, V., & Volpe, U. (2022). C-Reactive Protein as a Biomarker for Major Depressive Disorder? International Journal of Molecular Sciences, 23(3), 1616. https://doi.org/10.3390/ijms23031616








