The Enhanced Cytotoxic Effects in B-Cell Leukemia and Lymphoma Following Activation of Prostaglandin EP4 Receptor and Targeting of CD20 Antigen by Monoclonal Antibodies
Abstract
:1. Introduction
2. Results
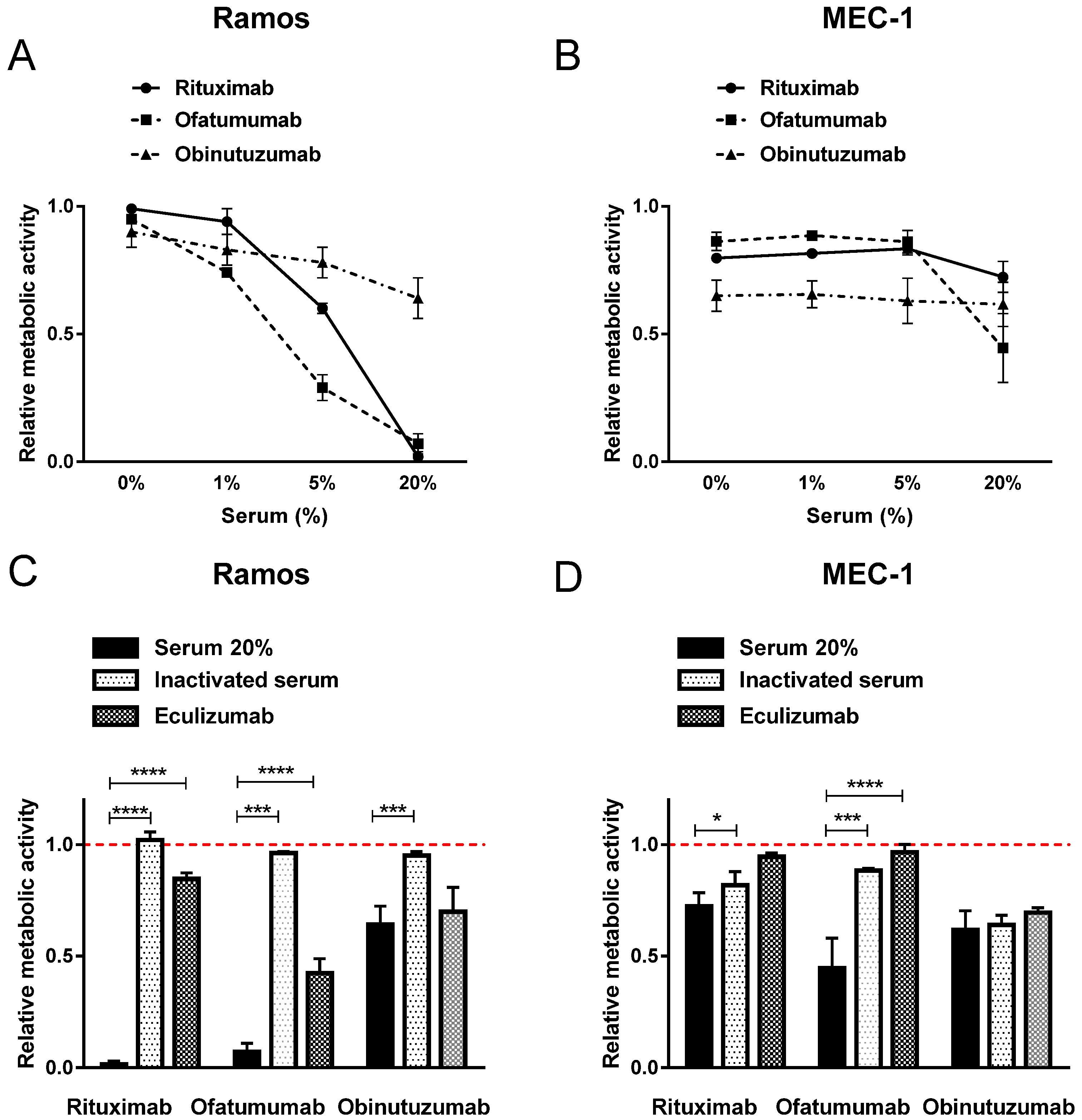
2.1. Diverse Induction of Cell Death by Anti-CD20 MAbs
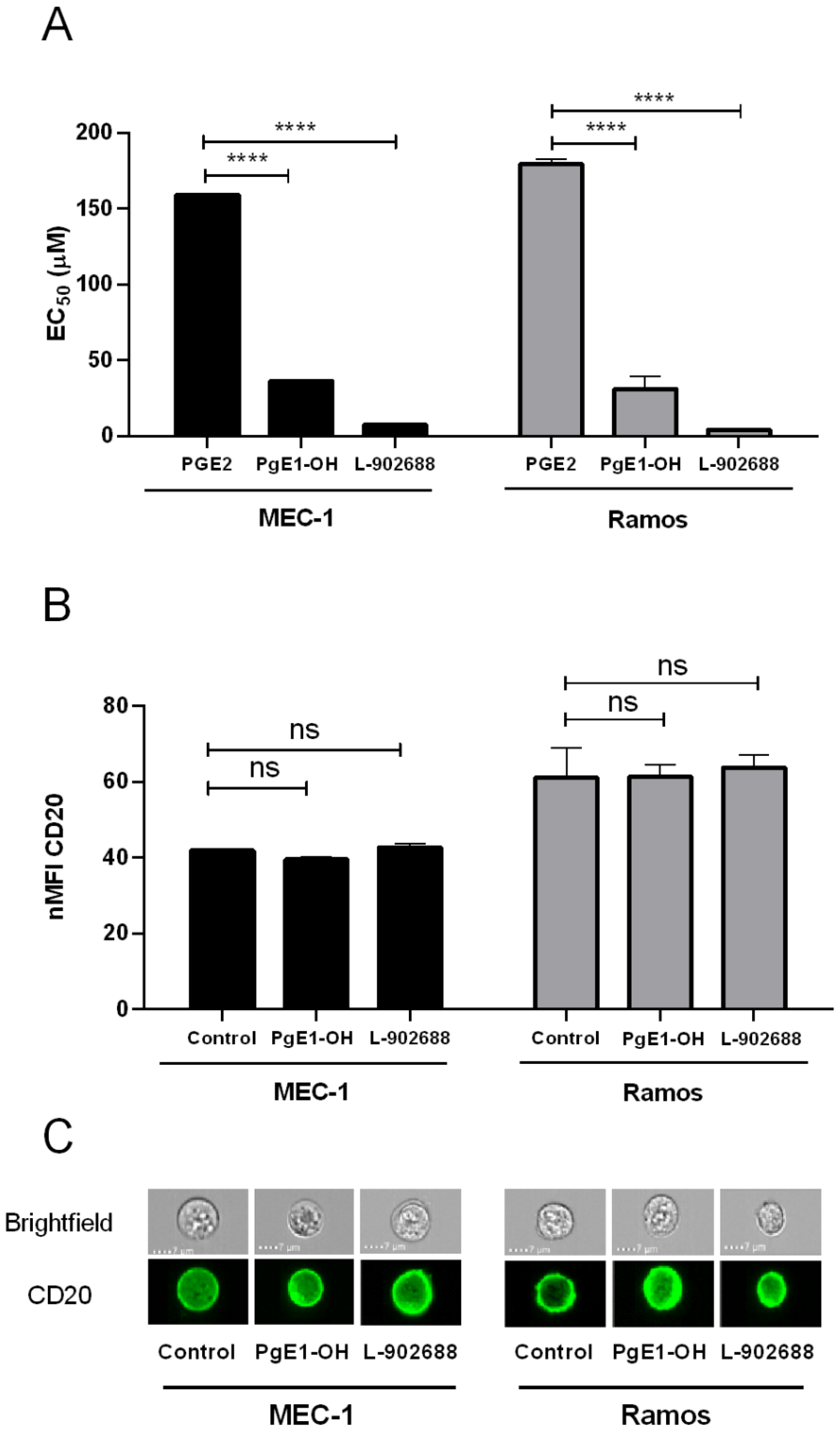
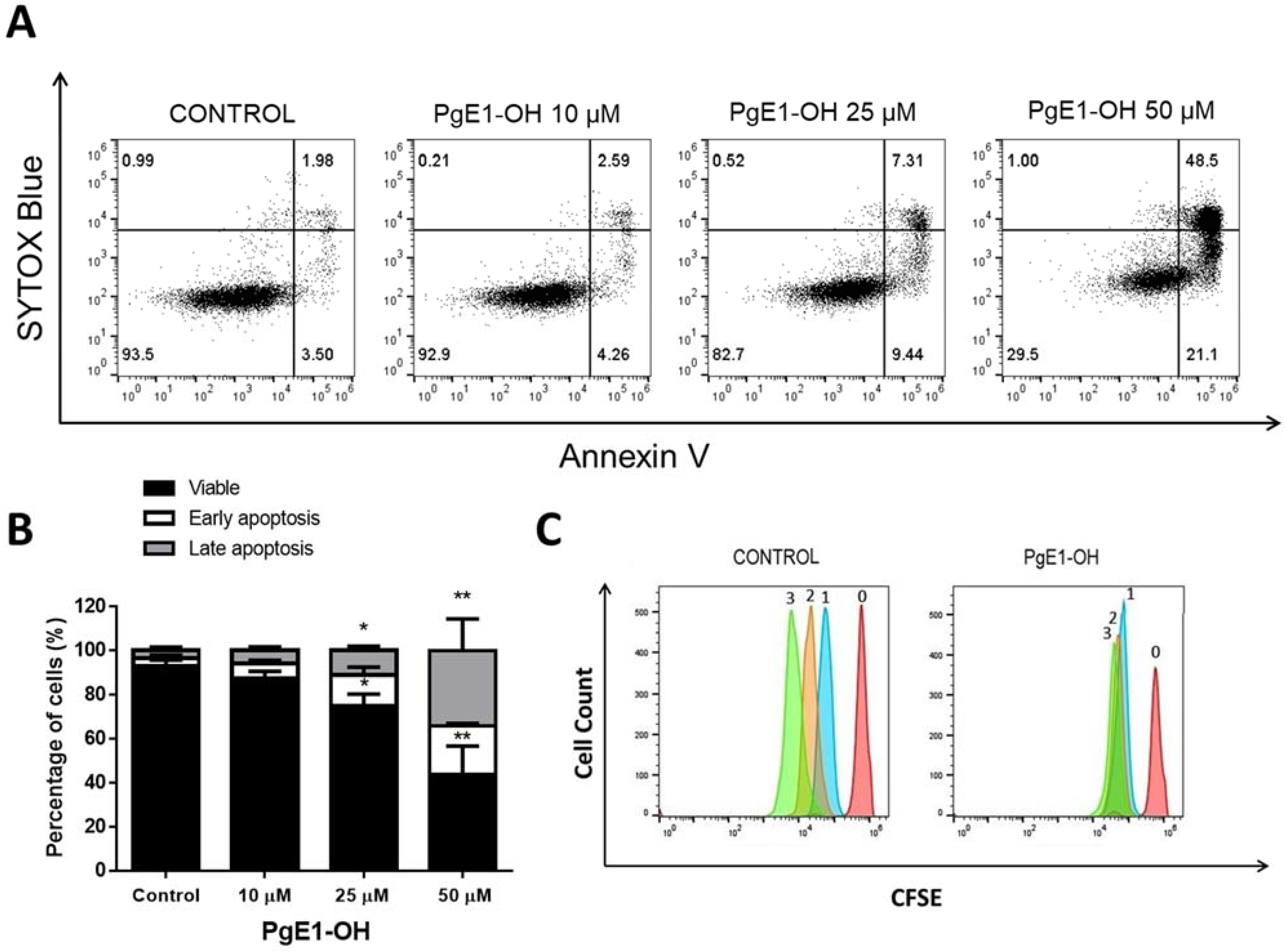
2.2. EP4 Receptor Agonists PgE1-OH and L-902688 Induce Time- and Concentration-Dependent Cytotoxic Effects via EP4 Receptor Activation
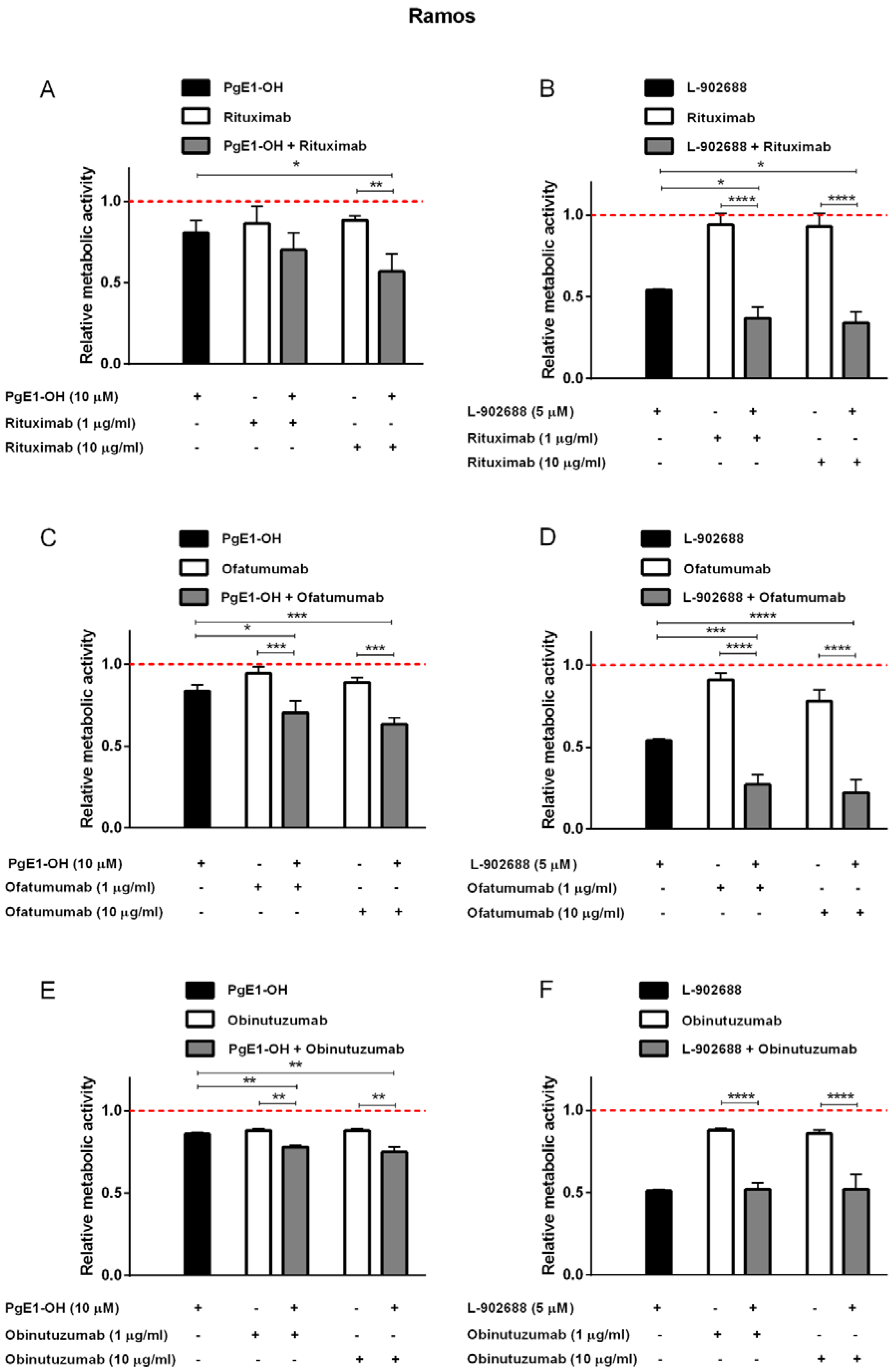
2.3. EP4 Receptor Agonists PgE1-OH and L-902688 Enhanced the Cytotoxic Potential of Anti-CD20 MAbs Rituximab and Ofatumumab in Burkitt Lymphoma Cells Ramos
2.4. EP4 Receptor Agonists PgE1-OH and L-902688 Enhanced the Cytotoxic Potential of Anti-CD20 MAbs in CLL Cells MEC-1
2.5. Anti-CD20 MAbs and PgE1-OH Act Synergistically Cytotoxic in Primary CLL Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Metabolic Activity Assay
4.4. Viability Assessment Using Propidium Iodide (PI Exclusion Assay)
4.5. CFSE Assay
4.6. Analysis of Apoptosis with Annexin V/Sytox Blue Staining
4.7. CDC Assay
4.8. Evaluation of CD20 Antigen Expression
4.9. Combination Index Calculation
4.10. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, O.; Cosimo, E.; Dobbin, E.; McCaig, A.M.; Clarke, C.L.; Brant, A.M.; Leach, M.; Michie, A.; Wheadon, H. Complement deficiencies limit CD20 monoclonal antibody treatment efficacy in CLL. Leukemia 2015, 29, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrd, J.C.; Stilgenbauer, S.; Flinn, I.W. Chronic Lymphocytic Leukemia. Hematology 2004, 2004, 163–183. [Google Scholar] [CrossRef] [PubMed]
- Glennie, M.J.; French, R.R.; Cragg, M.S.; Taylor, R.P. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol. Immunol. 2007, 44, 3823–3837. [Google Scholar] [CrossRef]
- Hallek, M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 2019, 94, 1266–1287. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.H.; Beers, S.A.; French, R.R.; Johnson, P.W.; Glennie, M.J.; Cragg, M.S. Anti-CD20 monoclonal antibodies: Historical and future perspectives. Haematologica 2010, 95, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Okroj, M.; Österborg, A.; Blom, A.M. Effector mechanisms of anti-CD20 monoclonal antibodies in B cell malignancies. Cancer Treat. Rev. 2013, 39, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Luan, C.; Chen, B. Clinical application of obinutuzumab for treating chronic lymphocytic leukemia. Drug Des. Dev. Ther. 2019, 13, 2899–2909. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, P.; Grillo-López, A.J.; Link, B.K.; Levy, R.; Czuczman, M.S.; Williams, M.E.; Heyman, M.R.; Bence-Bruckler, I.; White, C.A.; Cabanillas, F.; et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998, 16, 2825–2833. [Google Scholar] [CrossRef]
- Davis, T.A.; Grillo-López, A.J.; White, C.A.; McLaughlin, P.; Czuczman, M.S.; Link, B.K.; Maloney, D.G.; Weaver, R.L.; Rosenberg, J.; Levy, R. Rituximab Anti-CD20 Monoclonal Antibody Therapy in Non-Hodgkin’s Lymphoma: Safety and Efficacy of Re-Treatment. J. Clin. Oncol. 2000, 18, 3135–3143. [Google Scholar] [CrossRef] [PubMed]
- Bonavida, B. Rituximab-induced inhibition of antiapoptotic cell survival pathways: Implications in chemo/immunoresistance, rituximab unresponsiveness, prognostic and novel therapeutic interventions. Oncogene 2007, 26, 3629–3636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezvani, A.R.; Maloney, D.G. Rituximab resistance. Best Pract. Res. Clin. Haematol. 2011, 24, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Tomita, A. Genetic and Epigenetic Modulation of CD20 Expression in B-Cell Malignancies: Molecular Mechanisms and Significance to Rituximab Resistance. J. Clin. Exp. Hematop. 2016, 56, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Prijatelj, M.; Celhar, T.; Gobec, M.; Mlinaric-Rascan, I. EP4 receptor signalling in immature B cells involves cAMP and NF-κB dependent pathways. J. Pharm. Pharmacol. 2012, 64, 1090–1098. [Google Scholar] [CrossRef]
- Prijatelj, M.; Celhar, T.; Mlinarič-Raščan, I. Prostaglandin EP4 receptor enhances BCR-induced apoptosis of immature B cells. Prostaglandins Other Lipid Mediat. 2011, 95, 19–26. [Google Scholar] [CrossRef]
- Markovič, T.; Jakopin, Ž.; Dolenc, M.S.; Mlinarič-Raščan, I. Structural features of subtype-selective EP receptor modulators. Drug Discov. Today 2017, 22, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Gobec, M.; Prijatelj, M.; Delic, J.; Markovic, T.; Mlinarič-Raščan, I. Chemo-sensitizing effects of EP4 receptor-induced inactivation of nuclear factor-κB. Eur. J. Pharmacol. 2014, 742, 81–88. [Google Scholar] [CrossRef]
- Nabergoj, S.; Markovič, T.; Avsec, D.; Gobec, M.; Podgornik, H.; Jakopin, Ž.; Mlinarič-Raščan, I. EP4 receptor agonist L-902688 augments cytotoxic activities of ibrutinib, idelalisib, and venetoclax against chronic lymphocytic leukemia cells. Biochem. Pharmacol. 2021, 183, 114352. [Google Scholar] [CrossRef]
- Konya, V.; Marsche, G.; Schuligoi, R.; Heinemann, A. E-type prostanoid receptor 4 (EP4) in disease and therapy. Pharmacol. Ther. 2013, 138, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, P.; Copas, J.L.; Schneider, C.; Collier, H. 2-Decarboxy-2-hydroxymethyl prostaglandin E1 (TR4161), a prostaglandin bronchodilator of low tracheobronchial irritancy. Prostaglandins 1980, 19, 349–370. [Google Scholar] [CrossRef]
- Nizankowska, E.; Sheridan, A.Q.; Maile, M.H.; Cross, C.J.; Niżankowski, R.; Prochowska, K.; Szczeklik, A. Bronchodilatory properties of 2-decarboxy-2-hydroxymethyl prostaglandin E1. Prostaglandins 1985, 29, 349–362. [Google Scholar] [CrossRef]
- Young, R.N.; Billot, X.; Han, Y.; Slipetz, D.A.; Chauret, N.; Belley, M.; Metters, K.; Mathieu, M.-C.; Greig, G.M.; Denis, D.; et al. Discovery and Synthesis of a Potent, Selective and Orally Bioavailable EP4 Receptor Agonist. Heterocycles 2004, 64, 437. [Google Scholar] [CrossRef]
- Akram, A.; Gibson, C.L.; Grubb, B.D. Neuroprotection mediated by the EP4 receptor avoids the detrimental side effects of COX-2 inhibitors following ischaemic injury. Neuropharmacology 2013, 65, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Demars, K.M.; McCrea, A.O.; Siwarski, D.M.; Sanz, B.D.; Yang, C.; Candelario-Jalil, E. Protective Effects of L-902,688, a Prostanoid EP4 Receptor Agonist, against Acute Blood-Brain Barrier Damage in Experimental Ischemic Stroke. Front. Neurosci. 2018, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Foudi, N.; Kotelevets, L.; Louedec, L.; Leséche, G.; Henin, D.; Chastre, E.; Norel, X. Vasorelaxation induced by prostaglandin E2in human pulmonary vein: Role of the EP4receptor subtype. Br. J. Pharmacol. 2008, 154, 1631–1639. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-H.; Hsu, H.-H.; Chang, G.-J.; Chen, I.-C.; Ho, W.-J.; Hsu, P.-C.; Chen, W.-J.; Pang, J.-H.S.; Huang, C.-C.; Lai, Y.-J. Prostanoid EP4 agonist L-902,688 activates PPARγ and attenuates pulmonary arterial hypertension. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L349–L359. [Google Scholar] [CrossRef] [Green Version]
- Ozen, G.; Benyahia, C.; Mani, S.; Boukais, K.; Silverstein, A.M.; Bayles, R.; Nelsen, A.C.; Castier, Y.; Danel, C.; Mal, H.; et al. Bronchodilation induced by PGE 2 is impaired in Group III pulmonary hypertension. Br. J. Pharmacol. 2020, 177, 161–174. [Google Scholar] [CrossRef]
- Benyahia, C.; Gomez, I.; Kanyinda, L.; Boukais, K.; Danel, C.; Lesèche, G.; Longrois, D.; Norel, X. PGE2 receptor (EP4) agonists: Potent dilators of human bronchi and future asthma therapy? Pulm. Pharmacol. Ther. 2012, 25, 115–118. [Google Scholar] [CrossRef]
- Murn, J.; Mlinaric-Rascan, I.; Vaigot, P.; Alibert, O.; Frouin, V.; Gidrol, X. A Myc-regulated transcriptional network controls B-cell fate in response to BCR triggering. BMC Genom. 2009, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Okroj, M.; Eriksson, I.; Österborg, A.; Blom, A. Killing of CLL and NHL cells by rituximab and ofatumumab under limited availability of complement. Med. Oncol. 2013, 30, 759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.; Zhang, X.; Mohamed, N.; Inghirami, G.; Takeshita, K.; Pecora, A.; Nardone, L.L.; Pincus, S.E.; Casey, L.S.; Spitalny, G.L. A DeImmunized chimeric anti-C3b/iC3b monoclonal antibody enhances rituximab-mediated killing in NHL and CLL cells via complement activation. Cancer Immunol. Immunother. 2005, 54, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- VanDerMeid, K.R.; Elliott, M.; Baran, A.; Barr, P.M.; Chu, C.C.; Zent, C.S. Cellular Cytotoxicity of Next-Generation CD20 Monoclonal Antibodies. Cancer Immunol. Res. 2018, 6, 1150–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawluczkowycz, A.W.; Beurskens, F.J.; Beum, P.V.; Lindorfer, M.A.; Van De Winkel, J.G.J.; Parren, P.W.H.I.; Taylor, R.P. Binding of Submaximal C1q Promotes Complement-Dependent Cytotoxicity (CDC) of B Cells Opsonized with Anti-CD20 mAbs Ofatumumab (OFA) or Rituximab (RTX): Considerably Higher Levels of CDC Are Induced by OFA than by RTX. J. Immunol. 2009, 183, 749–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Nagasaki, E.; Kan, S.; Ito, M.; Kamata, Y.; Homma, S.; Aiba, K. Gemcitabine enhances rituximab-mediated complement-dependent cytotoxicity to B cell lymphoma by CD20 upregulation. Cancer Sci. 2016, 107, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Skarzynski, M.; Niemann, C.; Lee, Y.S.; Martyr, S.; Maric, I.; Salem, D.; Stetler-Stevenson, M.; Marti, G.E.; Calvo, K.R.; Yuan, C.; et al. Interactions between Ibrutinib and Anti-CD20 Antibodies: Competing Effects on the Outcome of Combination Therapy. Clin. Cancer Res. 2016, 22, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illidge, T.; Klein, C.; Sehn, L.H.; Davies, A.; Salles, G.; Cartron, G. Obinutuzumab in hematologic malignancies: Lessons learned to date. Cancer Treat. Rev. 2015, 41, 784–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crombie, J.L.; Brown, J.R. The future of antibody therapy in chronic lymphocytic leukemia. Expert Opin. Emerg. Drugs 2021, 26, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]


| EP4 Receptor Agonist | Cell Line | Metabolic Activity Assay | Propidium Iodide | ||
|---|---|---|---|---|---|
| IC50 (µM) 24 h | IC50 (µM) 48 h | IC50 (µM) 24 h | IC50 (µM) 48 h | ||
| PgE1-OH | MEC-1 | 36.3 | 21.5 | 35.6 | 25.6 |
| Ramos | 31.1 | 16.5 | 57.2 | 25.0 | |
| L-902688 | MEC-1 | 7.3 | 5.2 | 7.0 | 5.6 |
| Ramos | 4.0 | 3.5 | 8.4 | 4.7 | |
| EP4 Receptor Agonist | Mab (µg/mL) | Concentration | CI (Ramos) | CI (MEC-1) |
|---|---|---|---|---|
| PgE1-OH | Rituximab | 1 µg/mL | 0.52 | 0.58 |
| 10 µg/mL | 0.46 | 0.58 | ||
| Ofatmumab | 1 µg/mL | 0.53 | 0.37 | |
| 10 µg/mL | 0.46 | 0.50 | ||
| Obinutuzumab | 1 µg/mL | 0.97 | 0.10 | |
| 10 µg/mL | 0.94 | 0.08 | ||
| L-902688 | Rituximab | 1 µg/mL | 1.17 | 0.85 |
| 10 µg/mL | 1.12 | 0.89 | ||
| Ofatmumab | 1 µg/mL | 1.00 | 0.91 | |
| 10 µg/mL | 0.92 | 1.02 | ||
| Obinutuzumab | 1 µg/mL | 1.40 | 0.38 | |
| 10 µg/mL | 1.40 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markovič, T.; Podgornik, H.; Avsec, D.; Nabergoj, S.; Mlinarič-Raščan, I. The Enhanced Cytotoxic Effects in B-Cell Leukemia and Lymphoma Following Activation of Prostaglandin EP4 Receptor and Targeting of CD20 Antigen by Monoclonal Antibodies. Int. J. Mol. Sci. 2022, 23, 1599. https://doi.org/10.3390/ijms23031599
Markovič T, Podgornik H, Avsec D, Nabergoj S, Mlinarič-Raščan I. The Enhanced Cytotoxic Effects in B-Cell Leukemia and Lymphoma Following Activation of Prostaglandin EP4 Receptor and Targeting of CD20 Antigen by Monoclonal Antibodies. International Journal of Molecular Sciences. 2022; 23(3):1599. https://doi.org/10.3390/ijms23031599
Chicago/Turabian StyleMarkovič, Tijana, Helena Podgornik, Damjan Avsec, Sanja Nabergoj, and Irena Mlinarič-Raščan. 2022. "The Enhanced Cytotoxic Effects in B-Cell Leukemia and Lymphoma Following Activation of Prostaglandin EP4 Receptor and Targeting of CD20 Antigen by Monoclonal Antibodies" International Journal of Molecular Sciences 23, no. 3: 1599. https://doi.org/10.3390/ijms23031599
APA StyleMarkovič, T., Podgornik, H., Avsec, D., Nabergoj, S., & Mlinarič-Raščan, I. (2022). The Enhanced Cytotoxic Effects in B-Cell Leukemia and Lymphoma Following Activation of Prostaglandin EP4 Receptor and Targeting of CD20 Antigen by Monoclonal Antibodies. International Journal of Molecular Sciences, 23(3), 1599. https://doi.org/10.3390/ijms23031599






