Nanoapatites Doped and Co-Doped with Noble Metal Ions as Modern Antibiofilm Materials for Biomedical Applications against Drug-Resistant Clinical Strains of Enterococcus faecalis VRE and Staphylococcus aureus MRSA
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Properties of Obtained Apatites
2.2. Microbiological Analysis of Nanoapatites
- At the beginning, our purpose was to select clinical drug-resistant strains of Enterococcus faecalis and Staphylococcus aureus with the strongest intensity of biofilm production. Among tested strains, E. faecalis VRE 200 and S. aureus MRSA P19 were selected and used in the next stages of our research.
- Futher research was focused on evaluation of basic antimicrobial properties (MIC, MBC, and FICI values) as well as antibiofilm properties of nanoparticles against these strains. Experiments testing the release of ions from nanoparticles via the ICP-OES method were also perfomed. The antibiofilm assays included qualitative and quantitative analysis of the impact of nanoparticles on bacterial cell adhesion and biofilm formation. These measurements were performed using fluorescence microscopy and SEM (scanning electron microscopy) together with computational analysis of the obtained pictures.
- The last stage was focused on measurements of potential toxicy of nanoparticles. For this purpose, MTT assays, adhesion of Balb/3T3 mouse embryonic fibroblasts on nanoparticles, as well as influence of bacterial biofilm on the viability of fibroblast cells on nanoapatites were performed. Fluorescence microscopy and computational analysis of images were used to obtain the results.
2.2.1. Selection of Bacterial Strains Based on Their Biofilm Production
2.2.2. Evaluation of MIC and MBC of Nanoapatites against Selected Strains
2.2.3. Evaluation of Fractional Inhibitory Concentration Index of Nanoapatites Composition
2.2.4. Release of Metal Ions in the TSB Medium by (ICP-OES)
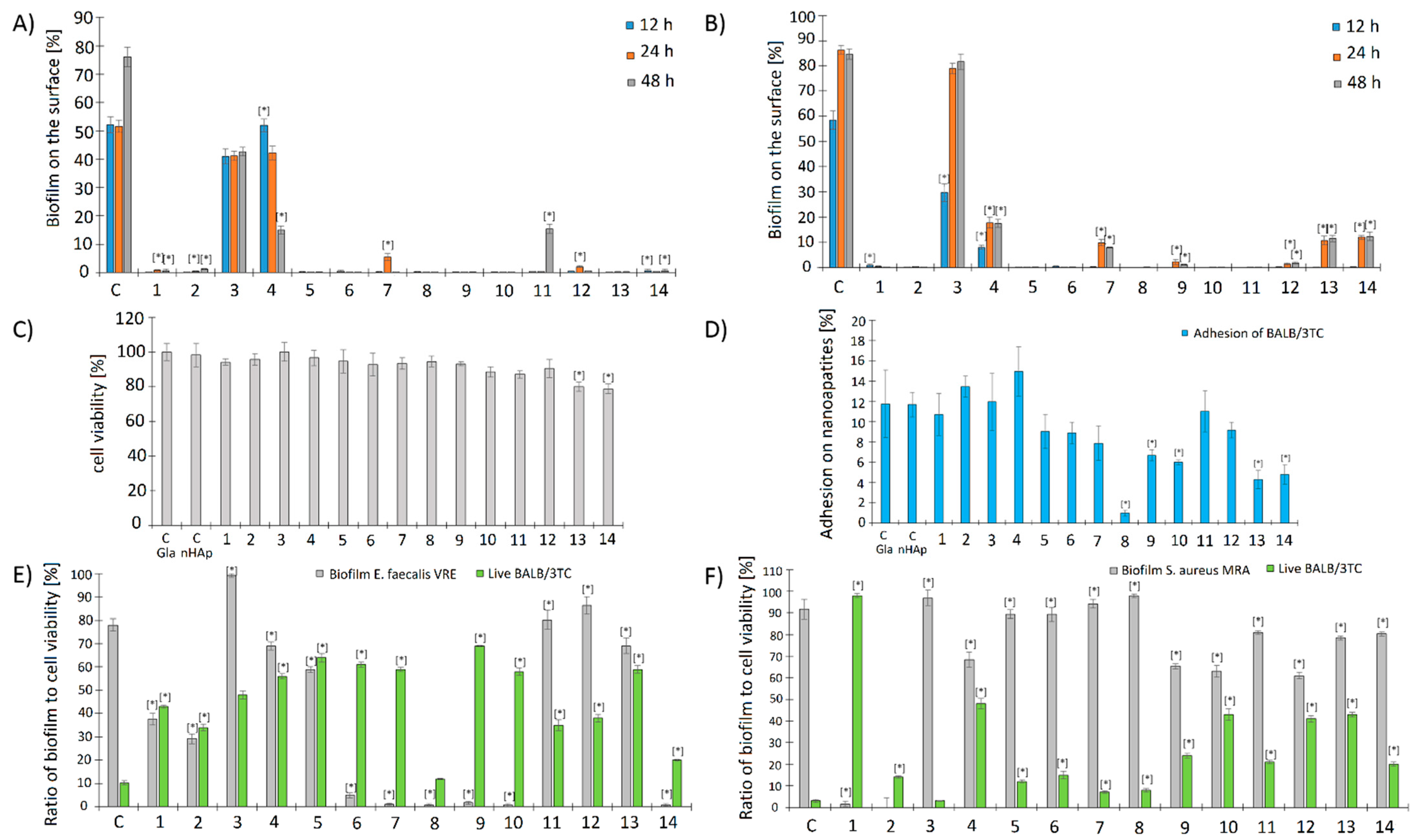
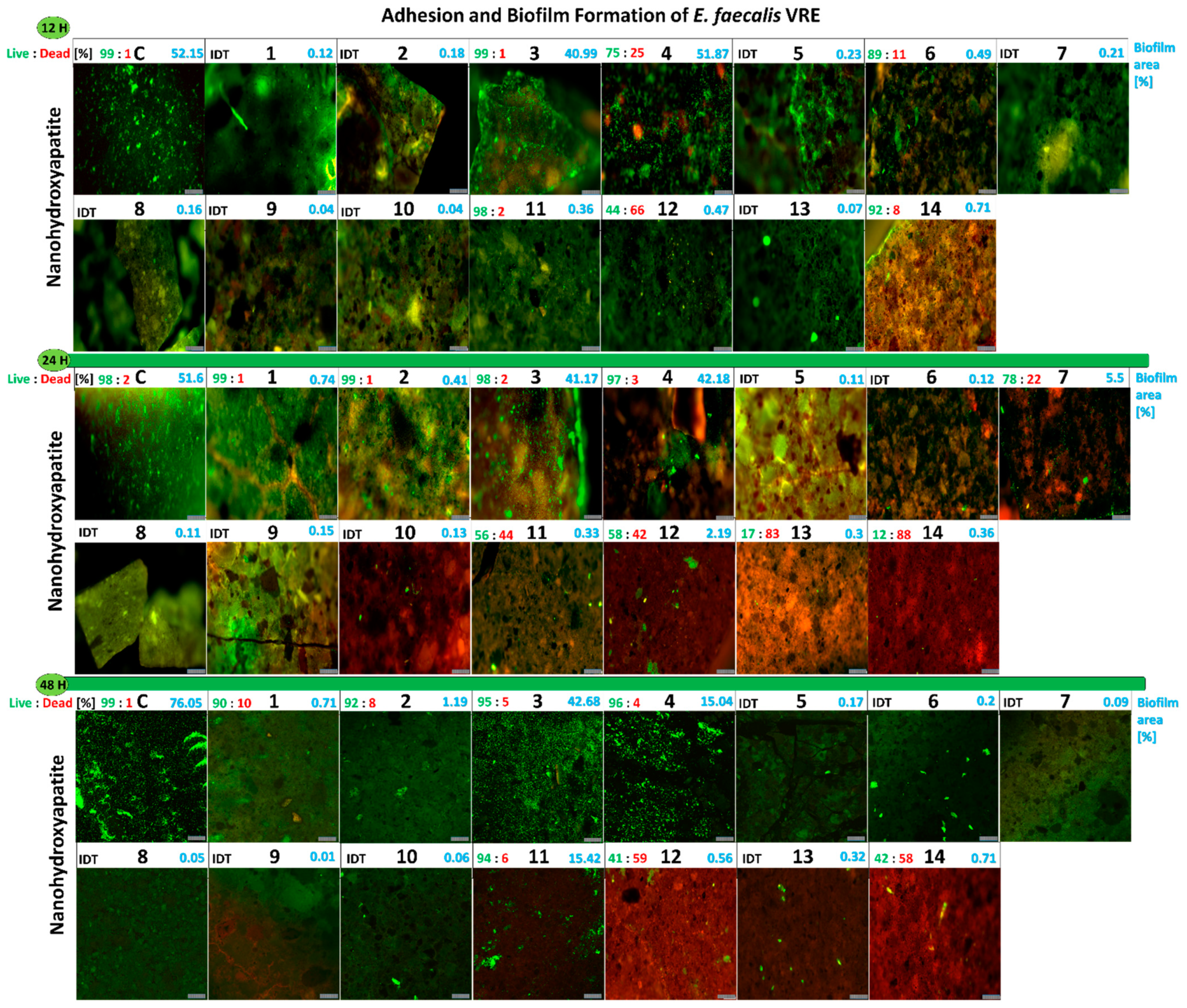
2.2.5. Influence of the Studied Nanoapatites on the Adhesion and Biofilm Formation by Drug-Resistant E. faecalis VRE 200
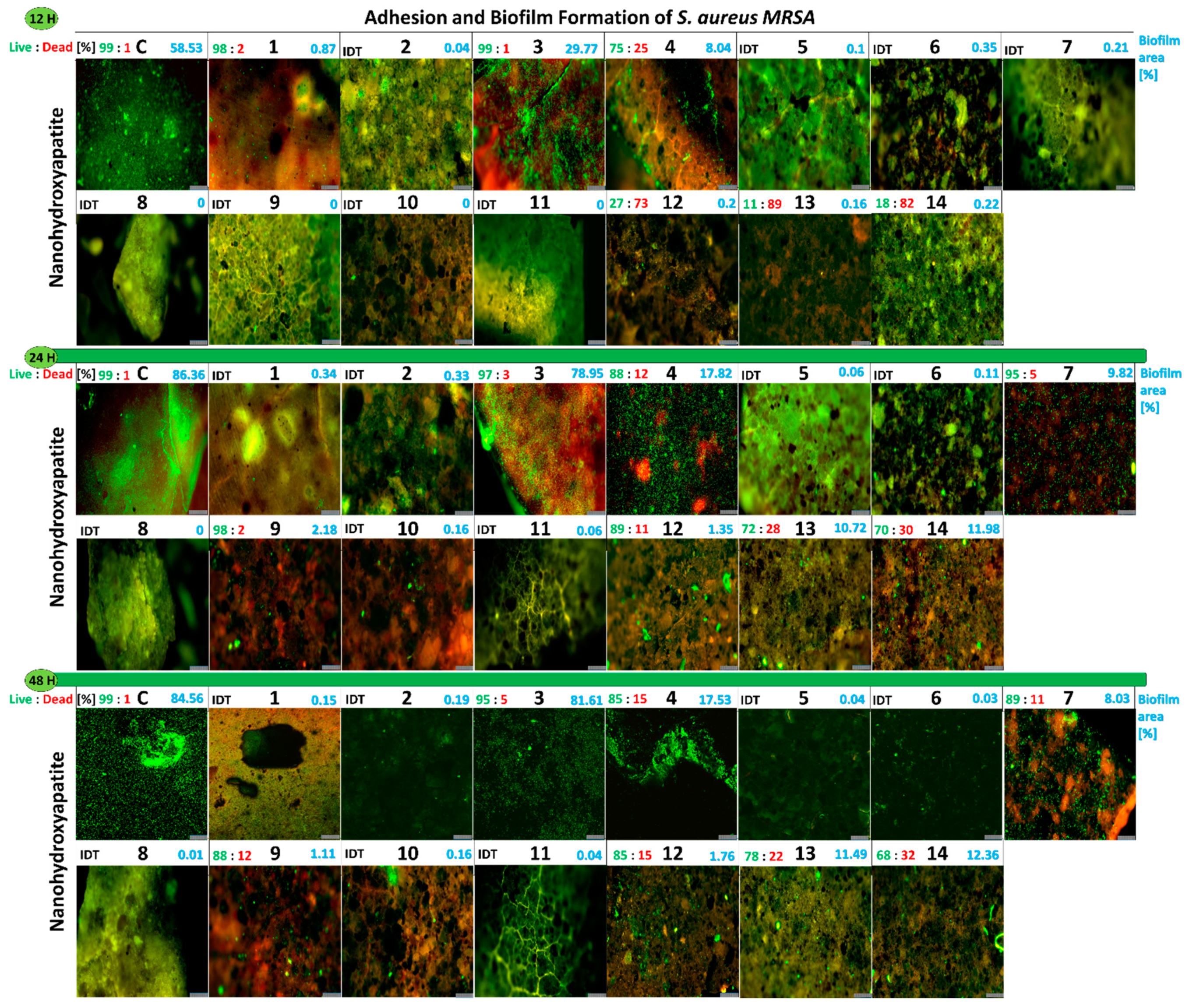
2.2.6. Influence of the Studied Nanoapatites on the Adhesion and Biofilm Formation by Drug-Resistant S. aureus MRSA P19
2.2.7. Cytotoxicity of the Studied Biomaterials on Balb/3T3 Fibroblasts (MTT)
2.2.8. Adhesion of Balb/3T3 Fibroblasts to Surface Tested Nanoapatites
2.2.9. Influence of the Biofilm Produced by E. faecalis VRE 200 on the Viability of Balb/3T3 Fibroblasts
2.2.10. Influence of the Biofilm Produced by S. aureus MRSA P19 on the Viability of Balb/3T3 Fibroblasts
2.2.11. Viability of E. faecalis VRE 200 Biofilm on the Surface of Nanoapatites
2.2.12. Viability of Biofilm S. aureus MRSA P19 on the Surface of Nanoapatites
2.2.13. Adhesion and Biofilm Formation of Drug-Resistant Clinical Strains of E. faecalis VRE 200 and S. aureus MRSA P19 Observed by Scanning Electron Microscopy (SEM)
3. Discussion
4. Materials and Methods
4.1. Synthesis of Nanoapatites
4.2. Characterization of Obtained Materials
4.3. Microbiological Analysis of Nanoapatites
4.3.1. Strains and Growth Conditions of Bacteria
4.3.2. Assessment of Biofilm Production Ability
4.3.3. Minimal Inhibitory and Bactericidal Concentrations (MIC and MBC)
4.3.4. Fractional Inhibitory Concentration Index (FICI)
4.3.5. Release of Metal Ions in the TSB Medium by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
4.3.6. Adhesion and Biofilm Formation of Bacterial Strains on Nanoapatites
4.3.7. Fibroblast Cell Culture
4.3.8. Cell Proliferation Assays (MTT Test)
4.3.9. Adhesion Assay
4.3.10. Influence of Bacterial Biofilm on the Viability of Fibroblast Cells on Nanoapatites
4.3.11. Fluorescence Microscopy (FM) and Computational Analysis of Pictures
4.3.12. Scanning Electron Microscope (SEM)
4.3.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.W.; Phillips, K.S.; Gu, H.; Kazemzadeh-Narbat, M.; Ren, D. How microbes read the map: Effects of implant topography on bacterial adhesion and biofilm formation. Biomaterials 2021, 268, 120595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Yang, C.; Ma, C.; Tang, J. Enzyme-inorganic hybrid nanoflowers: Classification, synthesis, functionalization and potential applications. Chem. Eng. J. 2021, 415, 129075. [Google Scholar] [CrossRef]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-C.; Wang, C.; Han, M.-F.; Tong, Z.; Hu, X.-R.; Lin, Y.-T.; Zhao, X. Reduction of biofilm adhesion strength by adjusting the characteristics of biofilms through enzymatic quorum quenching. Chemosphere 2022, 288, 132465. [Google Scholar] [CrossRef] [PubMed]
- Petroll, K.; Kopp, D.; Care, A.; Bergquist, P.L.; Sunna, A. Tools and strategies for constructing cell-free enzyme pathways. Biotechnol. Adv. 2019, 37, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, Q.; Zhang, D.; Yan, W. Mechanisms and control strategies of antibiotic resistance in pathological biofilms. J. Microbiol. Biotechnol. 2021, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hrynyshyn, A.; Simões, M.; Borges, A. Biofilms in surgical site infections: Recent advances and novel prevention and eradication strategies. Antibiotics 2022, 11, 69. [Google Scholar] [CrossRef]
- Wood, T.K. The secret lives of single cells. Microb. Biotechnol. 2021, 15, 13–17. [Google Scholar] [CrossRef]
- Conwell, M.; Dooley, J.S.G.; Naughton, P.J. Enterococcal biofilm–A nidus for antibiotic resistance transfer? J. Appl. Microbiol. 2022, 1–17. [Google Scholar] [CrossRef]
- Paluch, E.; Piecuch, A.; Obłąk, E.; Lamch, Ł.; Wilk, K.A. Antifungal activity of newly synthesized chemodegradable dicephalic-type cationic surfactants. Colloids Surf. B Biointerfaces 2018, 164, 31–41. [Google Scholar] [CrossRef]
- Przekora, A. Current trends in fabrication of biomaterials for bone and cartilage regeneration: Materials modifications and biophysical stimulations. Int. J. Mol. Sci. 2019, 20, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. WHO R & D Recommendations for Priority Microbiological Research on Multi-Drug-Resistant bac-Terial Strains. Available online: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 23 December 2021).
- Otari, S.V.; Patel, S.K.S.; Jeong, J.-H.; Lee, J.H.; Lee, J.-K. A green chemistry approach for synthesizing thermostable antimicrobial peptide-coated gold nanoparticles immobilized in an alginate biohydrogel. RSC Adv. 2016, 6, 86808–86816. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, A.; Katas, H.; Samsudin, S.N.; Zin, N.M. Regioselective sequential modification of chitosan via azide-alkyne click reaction: Synthesis, characterization, and antimicrobial activity of chitosan derivatives and nanoparticles. PLoS ONE 2015, 10, e0123084. [Google Scholar] [CrossRef] [Green Version]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Dorovskikh, S.I.; Vikulova, E.S.; Chepeleva, E.V.; Vasilieva, M.B.; Nasimov, D.A.; Maksimovskii, E.A.; Tsygankova, A.R.; Basova, T.V.; Sergeevichev, D.S.; Morozova, N.B. Noble metals for modern implant materials: MOCVD of film structures and cytotoxical, antibacterial, and histological studies. Biomedicines 2021, 9, 851. [Google Scholar] [CrossRef]
- Murthy, S.K. Nanoparticles in modern medicine: State of the art and future challenges. Int. J. Nanomed. 2007, 2, 129–141. [Google Scholar]
- Das, P.; Karankar, V.S. New avenues of controlling microbial infections through anti-microbial and anti-biofilm potentials of green mono-and multi-metallic nanoparticles: A review. J. Microbiol. Methods 2019, 167, 105766. [Google Scholar] [CrossRef]
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic Nanoparticles for Antimicrobial Applications. Front. Chem. 2020, 8, 412. [Google Scholar] [CrossRef]
- Silver, S. Bacterial silver resistance: Molecular biology and uses and misuses of silver compounds. FEMS Microbiol. Rev. 2003, 27, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Silver, S.; Phung, L.T.; Silver, G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J. Ind. Microbiol. Biotechnol. 2006, 33, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.L.J.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.H.; Barrick, J.E. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front. Genet. 2015, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Malankowska, A.; Mikołajczyk, A.; Mȩdrzycka, J.; Wysocka, I.; Nowaczyk, G.; Jarek, M.; Puzyn, T.; Mulkiewicz, E. The effect of Ag, Au, Pt, and Pd on the surface properties, photocatalytic activity and toxicity of multicomponent TiO2-based nanomaterials. Environ. Sci. Nano 2020, 7, 3557–3574. [Google Scholar] [CrossRef]
- Li, T.; Albee, B.; Alemayehu, M.; Diaz, R.; Ingham, L.; Kamal, S.; Rodriguez, M.; Bishnoi, S.W. Comparative toxicity study of Ag, Au, and Ag-Au bimetallic nanoparticles on Daphnia magna. Anal. Bioanal. Chem. 2010, 398, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Sobierajska, P.; Pozniak, B.; Tikhomirov, M.; Miller, J.; Mrowczynska, L.; Piecuch, A.; Rewak-Soroczynska, J.; Dorotkiewicz-Jach, A.; Drulis-Kawa, Z.; Wiglusz, R.J. Multifunctionality of nanosized calcium apatite dual-doped with Li(+)/Eu(3+) ions related to cell culture studies and cytotoxicity evaluation in vitro. Biomolecules 2021, 11, 1388. [Google Scholar] [CrossRef]
- Sudarsanan, K.; Young, R.A. Significant precision in crystal structural details. Holly Springs hydroxyapatite. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 1534–1543. [Google Scholar] [CrossRef]
- Hendricks, S.B.; Jefferson, M.E.; Mosley, V.M. The crystal structures of some natural and synthetic apatite-like substances. Z. Für Krist. Cryst. Mater. 1932, 81, 352–369. [Google Scholar] [CrossRef]
- Swathi, S.; Arun, K.; Dzubinska, A.; Reiffers, M.; Nagalakshmi, R. Systematic investigations on the magnetic properties of moderate heavy Fermion CeAg0.68Si1.32 alloy. Phys. B Condens. Matter 2019, 575, 411679. [Google Scholar] [CrossRef]
- Owen, E.A. XLI. Precision measurements of crystal parameters. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1933, 15, 472–488. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, M.; Liu, M.; Xiao, Y.; Li, Z.; Chen, J.; Sun, Y.; Zhao, J.; Fang, S.; Jia, D.; et al. Homogeneous Pd nanoparticles produced in direct reactions: Green synthesis, formation mechanism and catalysis properties. J. Mater. Chem. A 2014, 2, 1369–1374. [Google Scholar] [CrossRef]
- Zenou, V.Y.; Fowler, D.E.; Gautier, R.; Barnett, S.A.; Poeppelmeier, K.R.; Marks, L.D. Redox and phase behavior of Pd-substituted (La,Sr)CrO3 perovskite solid oxide fuel cell anodes. Solid State Ionics 2016, 296, 90–105. [Google Scholar] [CrossRef] [Green Version]
- Sobierajska, P.; Nowak, N.; Rewak-Soroczynska, J.; Targonska, S.; Lewińska, A.; Grosman, L.; Wiglusz, R.J. Investigation of topography effect on antibacterial properties and biocompatibility of nanohydroxyapatites activated with zinc and copper ions: In vitro study of colloids, hydrogel scaffolds and pellets. Mater. Sci. Eng. C 2021, 112547. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial Gold Nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Ramkumar, V.S.; Archunan, G.; Chaiyasut, C.; Suganthy, N. Biogenic synthesis of silver palladium bimetallic nanoparticles from fruit extract of Terminalia chebula—In vitro evaluation of anticancer and antimicrobial activity. J. Drug Deliv. Sci. Technol. 2019, 51, 139–151. [Google Scholar] [CrossRef]
- WHO Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 23 December 2021).
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [Green Version]
- Lansdown, A.B. Silver I: Its antibacterial properties and mechanism of action. J. Wound Care 2002, 11, 125–130. [Google Scholar] [CrossRef]
- Basova, T.V.; Vikulova, E.S.; Dorovskikh, S.I.; Hassan, A.; Morozova, N.B. The use of noble metal coatings and nanoparticles for the modification of medical implant materials. Mater. Des. 2021, 204, 109672. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Deng, H.; McShan, D.; Zhang, Y.; Sinha, S.S.; Arslan, Z.; Ray, P.C.; Yu, H. Mechanistic study of the synergistic antibacterial activity of combined silver nanoparticles and common antibiotics. Environ. Sci. Technol. 2016, 50, 8840–8848. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Lu, F.; Ye, H.; Yu, K.; Lu, B.; Bao, R.; Xiao, Y.; Dai, F.; Lan, G. Silver inlaid with gold nanoparticles: Enhanced antibacterial ability coupled with the ability to visualize antibacterial efficacy. ACS Sustain. Chem. Eng. 2018, 6, 9813–9821. [Google Scholar] [CrossRef]
- Li, Q.; Lu, F.; Zhou, G.; Yu, K.; Lu, B.; Xiao, Y.; Dai, F.; Wu, D.; Lan, G. Silver inlaid with gold nanoparticle/chitosan wound dressing enhances antibacterial activity and porosity, and promotes wound healing. Biomacromolecules 2017, 18, 3766–3775. [Google Scholar] [CrossRef] [PubMed]
- Shareena Dasari, T.P.; Zhang, Y.; Yu, H. Antibacterial activity and cytotoxicity of gold (I) and (III) ions and gold nanoparticles. Biochem. Pharmacol. Open Access 2015, 4, 199. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Dasari, T.P.S.; Deng, H.; Yu, H. Antimicrobial activity of gold nanoparticles and ionic gold. J. Environ. Sci. Health Part C 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef] [PubMed]
- Avellan, A.; Simonin, M.; McGivney, E.; Bossa, N.; Spielman-Sun, E.; Rocca, J.D.; Bernhardt, E.S.; Geitner, N.K.; Unrine, J.M.; Wiesner, M.R.; et al. Gold nanoparticle biodissolution by a freshwater macrophyte and its associated microbiome. Nat. Nanotechnol. 2018, 13, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Al-khafaji, M.H.; Hashim, M.H. The synergistic effect of biosynthesized gold nanoparticles with antibiotic against clinical isolates. J. Biotechnol. Res. Cent. 2019, 13, 58–62. [Google Scholar] [CrossRef]
- Chlumsky, O.; Purkrtova, S.; Michova, H.; Sykorova, H.; Slepicka, P.; Fajstavr, D.; Ulbrich, P.; Viktorova, J.; Demnerova, K. Antimicrobial properties of palladium and platinum nanoparticles: A new tool for combating food-borne pathogens. Int. J. Mol. Sci. 2021, 22, 7892. [Google Scholar] [CrossRef]
- Umemura, T.; Sato, K.; Kusaka, Y.; Satoh, H. Chapter 49-Palladium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1113–1123. ISBN 978-0-444-59453-2. [Google Scholar]
- Nirmala, R.; Park, H.-M.; Kalpana, D.; Kang, H.-S.; Navamathavan, R.; Lee, Y.-S.; Kim, H.Y. Bactericidal activity and in vitro cytotoxicity assessment of hydroxyapatite containing gold nanoparticles. J. Biomed. Nanotechnol. 2011, 7, 342–350. [Google Scholar] [CrossRef]
- Fang, G.; Li, W.; Shen, X.; Perez-Aguilar, J.M.; Chong, Y.; Gao, X.; Chai, Z.; Chen, C.; Ge, C.; Zhou, R. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat. Commun. 2018, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Silva-Holguín, P.N.; Reyes-López, S.Y. Synthesis of hydroxyapatite-Ag composite as antimicrobial agent. Dose. Response. 2020, 18, 1559325820951342. [Google Scholar] [CrossRef] [PubMed]
- Ahmadvand, S.; Elahifard, M.; Jabbarzadeh, M.; Mirzanejad, A.; Pflughoeft, K.; Abbasi, B.; Abbasi, B. Bacteriostatic effects of apatite-covered Ag/AgBr/TiO2 nanocomposite in the dark: Anomaly in bacterial motility. J. Phys. Chem. B 2019, 123, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Karwowska, E. Antibacterial potential of nanocomposite-based materials-A short review. Nanotechnol. Rev. 2017, 6, 243–254. [Google Scholar] [CrossRef]
- Zhang, Q.; Yue, C.; Zhang, Y.; Lü, Y.; Hao, Y.; Miao, Y.; Li, J.; Liu, Z. Six metal-organic frameworks assembled from asymmetric triazole carboxylate ligands: Synthesis, crystal structures, photoluminescence properties and antibacterial activities. Inorganica Chim. Acta 2018, 473, 112–120. [Google Scholar] [CrossRef]
- Patel, M.N.; Dosi, P.A.; Bhatt, B.S. Antibacterial, DNA interaction and superoxide dismutase activity of drug based copper(II) coordination compounds. Polyhedron 2010, 29, 3238–3245. [Google Scholar] [CrossRef]
- Psomas, G.; Dendrinou-Samara, C.; Philippakopoulos, P.; Tangoulis, V.; Raptopoulou, C.P.; Samaras, E.; Kessissoglou, D.P. CuII-herbicide complexes: Structure and bioactivity. Inorg. Chim. Acta 1998, 272, 24–32. [Google Scholar] [CrossRef]
- Hao, Y.; Yue, C.; Jin, B.; Lv, Y.; Zhang, Q.; Li, J.; Liu, Z.; Hou, H. Syntheses, structures, luminescent properties and antibacterial activities of seven polymers based on an asymmetric triazole dicarboxylate ligand. Polyhedron 2018, 139, 296–307. [Google Scholar] [CrossRef]
- Villa-García, L.D.; Márquez-Preciado, R.; Ortiz-Magdaleno, M.; Patrón-Soberano, O.A.; Álvarez-Pérez, M.A.; Pozos-Guillén, A.; Sánchez-Vargas, L.O. Antimicrobial effect of gold nanoparticles in the formation of the Staphylococcus aureus biofilm on a polyethylene surface. Braz. J. Microbiol. 2021, 52, 619–625. [Google Scholar] [CrossRef]
- Prakash, J.; Prema, D.; Venkataprasanna, K.S.; Balagangadharan, K.; Selvamurugan, N.; Venkatasubbu, G.D. Nanocomposite chitosan film containing graphene oxide/hydroxyapatite/gold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 154, 62–71. [Google Scholar] [CrossRef]
- Tran, H.A.; Tran, P.A. Immobilization-enhanced eradication of bacterial biofilms and in situ antimicrobial coating of implant material surface–An in vitro study. Int. J. Nanomed. 2019, 14, 9351–9360. [Google Scholar] [CrossRef] [Green Version]
- Jacquart, S.; Siadous, R.; Henocq-Pigasse, C.; Bareille, R.; Roques, C.; Rey, C.; Combes, C. Composition and properties of silver-containing calcium carbonate-calcium phosphate bone cement. J. Mater. Sci. Mater. Med. 2013, 24, 2665–2675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Zhang, S.; Pan, F.; Chen, S.; Zhong, L.; Wang, J.; Pei, X. Nanomaterial-based ROS-mediated strategies for combating bacteria and biofilms. J. Mater. Res. 2021, 36, 822–845. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent advances in metal decorated nanomaterials and their various biological applications: A review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Naidi, S.N.; Khan, F.; Tan, A.L.; Harunsani, M.H.; Kim, Y.-M.; Khan, M.M. Green synthesis of CeO2 and Zr/Sn-dual doped CeO2 nanoparticles with photoantioxidant and antibiofilm activities. Biomater. Sci. 2021, 9, 4854–4869. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Anu; Kumar, K.; Kumar, A. Effect of (Ag, Zn) co-doping on structural, optical and bactericidal properties of CuO nanoparticles synthesized by a microwave-assisted method. Dalt. Trans. 2021, 50, 6188–6203. [Google Scholar] [CrossRef] [PubMed]
- Zeller, B.; Stöckli, S.; Zaugg, L.K.; Astasov-Frauenhoffer, M.; Hauser-Gerspach, I.; Waltimo, T.; Zitzmann, N.U. Biofilm formation on metal alloys, zirconia and polyetherketoneketone as implant materials in vivo. Clin. Oral Implants Res. 2020, 31, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Chahales, P.; Thanassi, D.G. Structure, function, and assembly of adhesive organelles by uropathogenic bacteria. Microbiol. Spectr. 2015, 3, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.J.; Eaton, J.W.; Ugarova, T.P.; Tang, L. Molecular basis of biomaterial-mediated foreign body reactions. Blood 2001, 98, 1231–1238. [Google Scholar] [CrossRef] [Green Version]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Keivani, F.; Shokrollahi, P.; Zandi, M.; Irani, S.; Shokrolahi, F.; Khorasani, S.C. Engineered electrospun poly (caprolactone)/polycaprolactone-g-hydroxyapatite nano-fibrous scaffold promotes human fibroblasts adhesion and proliferation. Mater. Sci. Eng. C 2016, 68, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Czajkowska, J.; Junka, A.; Hoppe, J.; Toporkiewicz, M.; Pawlak, A.; Migdał, P.; Oleksy-Wawrzyniak, M.; Fijałkowski, K.; Śmiglak, M.; Markowska-Szczupak, A. The co-culture of staphylococcal biofilm and fibroblast cell line: The correlation of biological phenomena with metabolic NMR1 footprint. Int. J. Mol. Sci. 2021, 22, 5826. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P. M07-A9 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; ISBN 1562387839. [Google Scholar]
- Khan, M.S.A.; Ahmad, I. Antifungal activity of essential oils and their synergy with fluconazole against drug-resistant strains of Aspergillus fumigatus and Trichophyton rubrum. Appl. Microbiol. Biotechnol. 2011, 90, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Migdał, P.; Paluch, E.; Karwańska, M.; Wieliczko, A.; Gościniak, G. Myricetin as an antivirulence compound interfering with a morphological transformation into coccoid forms and potentiating activity of antibiotics against Helicobacter pylori. Int. J. Mol. Sci. 2021, 22, 2695. [Google Scholar] [CrossRef] [PubMed]
- Szymczycha-Madeja, A.; Welna, M.; Pohl, P. Determination of essential and non-essential elements in green and black teas by FAAS and ICP OES simplified–Multivariate classification of different tea products. Microchem. J. 2015, 121, 122–129. [Google Scholar] [CrossRef]
- Ahmadian, S.; Barar, J.; Saei, A.A.; Fakhree, M.A.A.; Omidi, Y. Cellular toxicity of nanogenomedicine in MCF-7 cell line: MTT assay. J. Vis. Exp. 2009, 26, 1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paluch, E.; Okińczyc, P.; Zwyrzykowska-Wodzińska, A.; Szperlik, J.; Żarowska, B.; Duda-Madej, A.; Bąbelewski, P.; Włodarczyk, M.; Wojtasik, W.; Kupczyński, R.; et al. Composition and antimicrobial activity of Ilex leaves water extracts. Molecules 2021, 26, 7442. [Google Scholar] [CrossRef]
- Paluch, E.; Szperlik, J.; Lamch, Ł.; Wilk, K.A.; Obłąk, E. Biofilm eradication and antifungal mechanism of action against Candida albicans of cationic dicephalic surfactants with a labile linker. Sci. Rep. 2021, 11, 8896. [Google Scholar] [CrossRef]

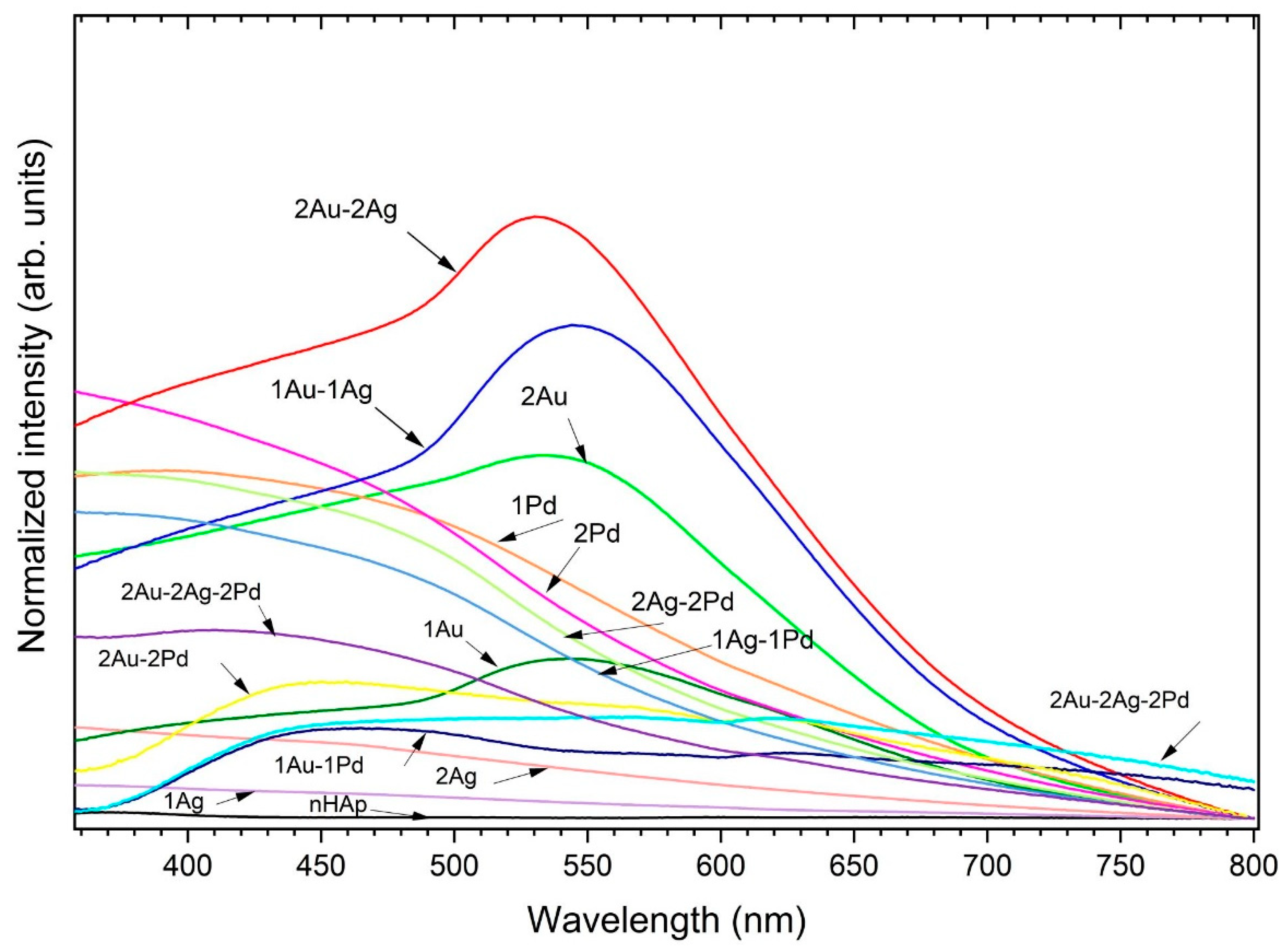
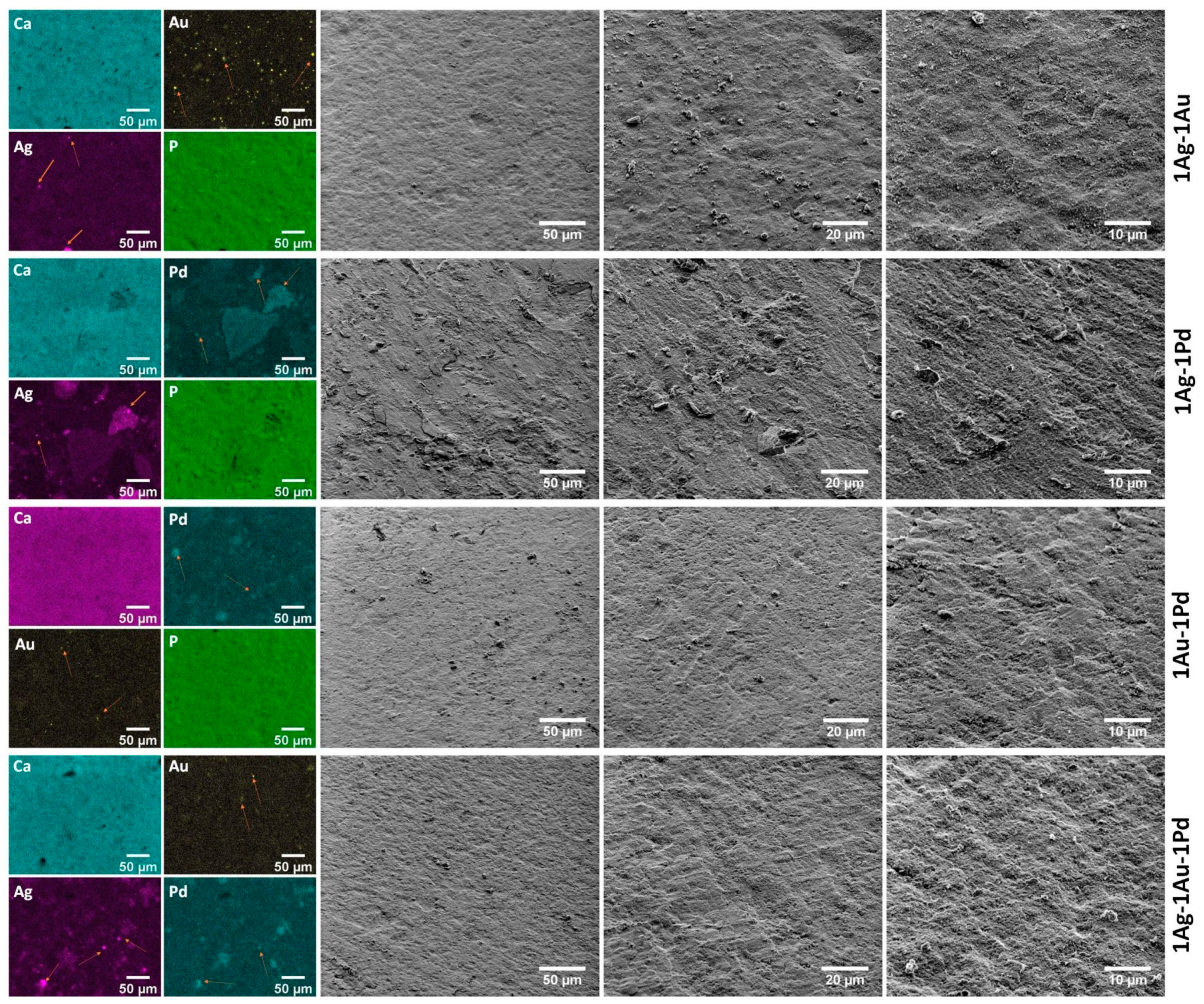
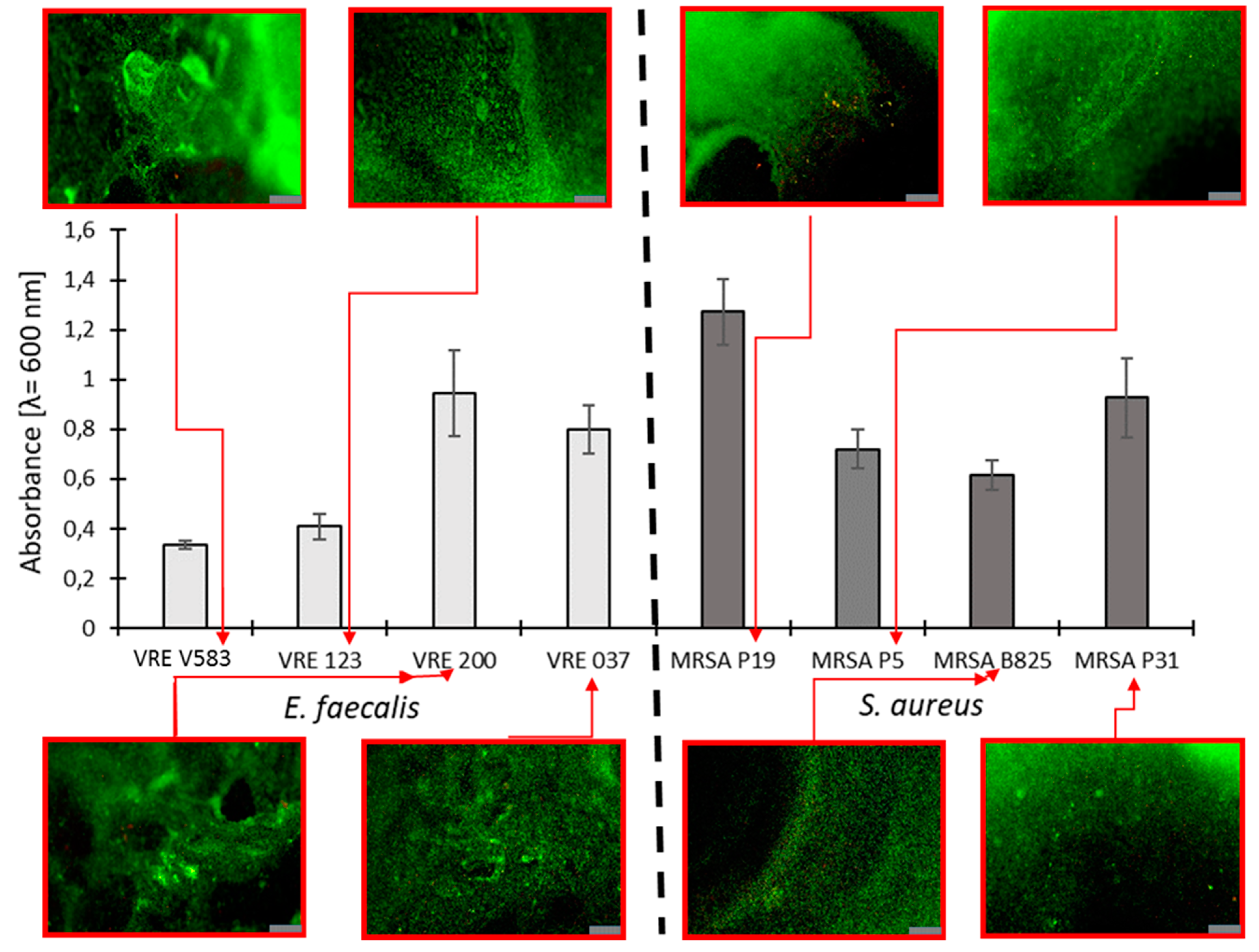
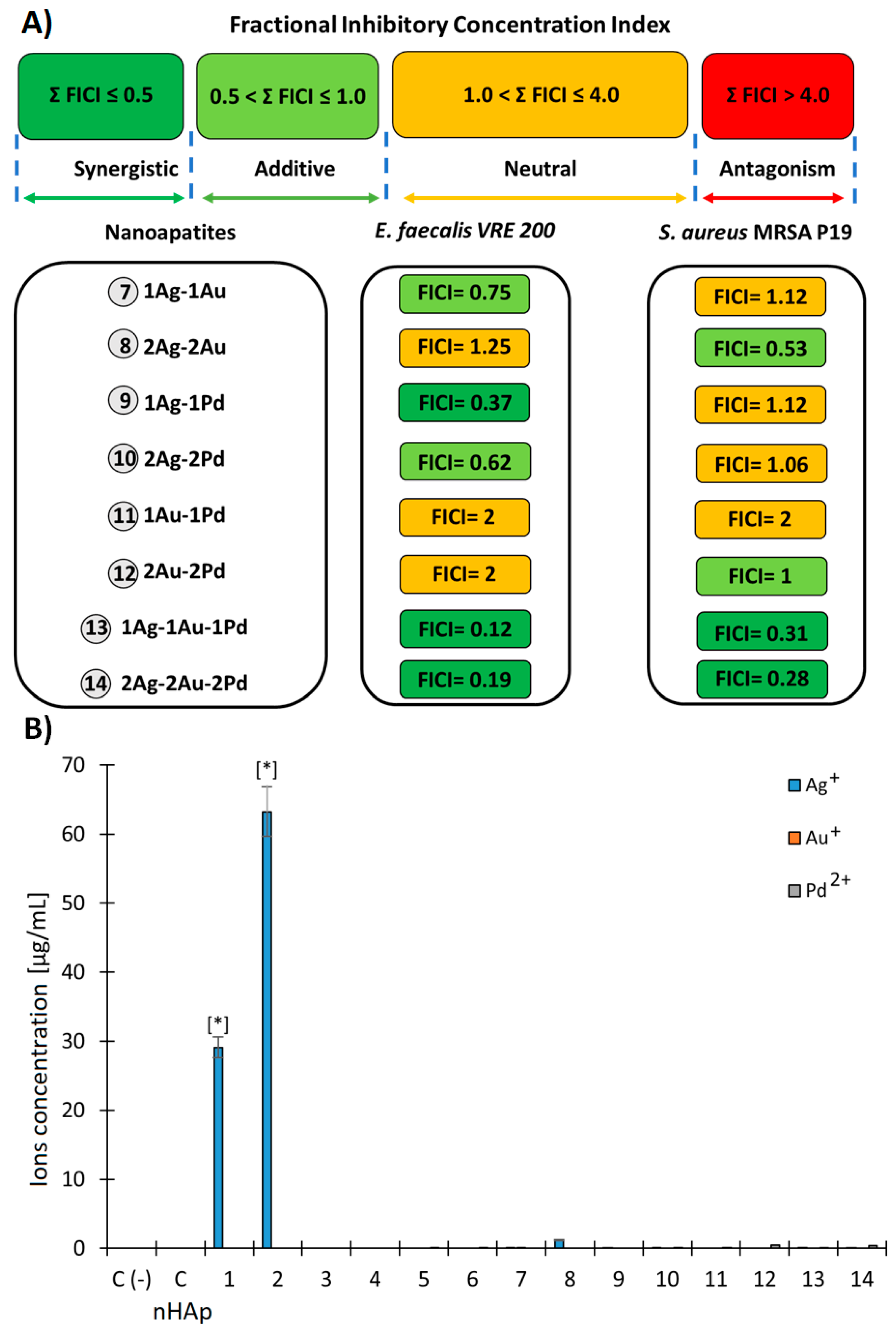

| Abb. | Sample | Ag (mol%) | Au (mol%) | Pd (mol%) | Cl/(Cl + OH) × 100% | cat./P |
|---|---|---|---|---|---|---|
| C | nHAp | - | - | - | - | 1.64 |
| 1 | 1Ag | 1.1 | - | - | - | 1.68 |
| 2 | 2Ag | 1.8 | - | - | - | 1.62 |
| 3 | 1Au | - | 1.0 | - | 25 | 1.71 |
| 4 | 2Au | - | 1.3 | - | 49 | 1.71 |
| 5 | 1Pd | - | - | 1.3 | 13 | 1.68 |
| 6 | 2Pd | - | - | 2.3 | 23 | 1.70 |
| 7 | 1Ag-1Au | 0.8 | 0.9 | - | 43 | 1.69 |
| 8 | 2Ag-2Au | 1.2 | 1.9 | - | 58 | 1.63 |
| 9 | 1Ag-1Pd | 0.6 | - | 0.9 | 14 | 1.65 |
| 10 | 2Ag-2Pd | 1.6 | - | 1.7 | 40 | 1.68 |
| 11 | 1Au-1Pd | - | 1.0 | 0.5 | 73 | 1.69 |
| 12 | 2Au-2Pd | - | 2.3 | 1.8 | 85 | 1.70 |
| 13 | 1Ag-1Au-1Pd | 0.5 | 0.9 | 0.8 | 90 | 1.69 |
| 14 | 2Ag-2Au-2Pd | 1.5 | 1.9 | 1.8 | 79 | 1.72 |
| Abb. | Sample | E. faecalis VRE 200 | S. aureus MRSA P19 | ||
|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | ||
| C | nHAp | >8192 | >8192 | >8192 | >8192 |
| 1 | 1Ag | 2048 | 2048 | 1024 | 1024 |
| 2 | 2Ag | 1024 | 1024 | 512 | 512 |
| 3 | 1Au | 4096 | 8192 | 8192 | >8192 |
| 4 | 2Au | 4096 | 8192 | 8192 | >8192 |
| 5 | 1Pd | 4096 | 8192 | 8192 | >8192 |
| 6 | 2Pd | 4096 | 8192 | 8192 | >8192 |
| 7 | 1Ag-1Au | 1024 | 1024 | 1024 | 1024 |
| 8 | 2Ag-2Au | 1024 | 1024 | 256 | 256 |
| 9 | 1Ag-1Pd | 512 | 512 | 1024 | 1024 |
| 10 | 2Ag-2Pd | 512 | 512 | 512 | 512 |
| 11 | 1Au-1Pd | 4096 | 8192 | 8192 | >8192 |
| 12 | 2Au-2Pd | 4096 | 8192 | 4096 | >8192 |
| 13 | 1Ag-1Au-1Pd | 128 | 128 | 256 | 256 |
| 14 | 2Ag-2Au-2Pd | 128 | 128 | 128 | 128 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paluch, E.; Sobierajska, P.; Okińczyc, P.; Widelski, J.; Duda-Madej, A.; Krzyżanowska, B.; Krzyżek, P.; Ogórek, R.; Szperlik, J.; Chmielowiec, J.; et al. Nanoapatites Doped and Co-Doped with Noble Metal Ions as Modern Antibiofilm Materials for Biomedical Applications against Drug-Resistant Clinical Strains of Enterococcus faecalis VRE and Staphylococcus aureus MRSA. Int. J. Mol. Sci. 2022, 23, 1533. https://doi.org/10.3390/ijms23031533
Paluch E, Sobierajska P, Okińczyc P, Widelski J, Duda-Madej A, Krzyżanowska B, Krzyżek P, Ogórek R, Szperlik J, Chmielowiec J, et al. Nanoapatites Doped and Co-Doped with Noble Metal Ions as Modern Antibiofilm Materials for Biomedical Applications against Drug-Resistant Clinical Strains of Enterococcus faecalis VRE and Staphylococcus aureus MRSA. International Journal of Molecular Sciences. 2022; 23(3):1533. https://doi.org/10.3390/ijms23031533
Chicago/Turabian StylePaluch, Emil, Paulina Sobierajska, Piotr Okińczyc, Jarosław Widelski, Anna Duda-Madej, Barbara Krzyżanowska, Paweł Krzyżek, Rafał Ogórek, Jakub Szperlik, Jacek Chmielowiec, and et al. 2022. "Nanoapatites Doped and Co-Doped with Noble Metal Ions as Modern Antibiofilm Materials for Biomedical Applications against Drug-Resistant Clinical Strains of Enterococcus faecalis VRE and Staphylococcus aureus MRSA" International Journal of Molecular Sciences 23, no. 3: 1533. https://doi.org/10.3390/ijms23031533
APA StylePaluch, E., Sobierajska, P., Okińczyc, P., Widelski, J., Duda-Madej, A., Krzyżanowska, B., Krzyżek, P., Ogórek, R., Szperlik, J., Chmielowiec, J., Gościniak, G., & Wiglusz, R. J. (2022). Nanoapatites Doped and Co-Doped with Noble Metal Ions as Modern Antibiofilm Materials for Biomedical Applications against Drug-Resistant Clinical Strains of Enterococcus faecalis VRE and Staphylococcus aureus MRSA. International Journal of Molecular Sciences, 23(3), 1533. https://doi.org/10.3390/ijms23031533










