Abstract
The greenhouse gas nitrous oxide (N2O) has strong potential to drive climate change. Soils are a major source of N2O, with microbial nitrification and denitrification being the primary processes involved in such emissions. The soybean endosymbiont Bradyrhizobium diazoefficiens is a model microorganism to study denitrification, a process that depends on a set of reductases, encoded by the napEDABC, nirK, norCBQD, and nosRZDYFLX genes, which sequentially reduce nitrate (NO3−) to nitrite (NO2−), nitric oxide (NO), N2O, and dinitrogen (N2). In this bacterium, the regulatory network and environmental cues governing the expression of denitrification genes rely on the FixK2 and NnrR transcriptional regulators. To understand the role of FixK2 and NnrR proteins in N2O turnover, we monitored real-time kinetics of NO3−, NO2−, NO, N2O, N2, and oxygen (O2) in a fixK2 and nnrR mutant using a robotized incubation system. We confirmed that FixK2 and NnrR are regulatory determinants essential for NO3− respiration and N2O reduction. Furthermore, we demonstrated that N2O reduction by B. diazoefficiens is independent of canonical inducers of denitrification, such as the nitrogen oxide NO3−, and it is negatively affected by acidic and alkaline conditions. These findings advance the understanding of how specific environmental conditions and two single regulators modulate N2O turnover in B. diazoefficiens.
1. Introduction
Under shortage of oxygen, bacteria can adapt and thrive by two ATP-generating mechanisms: (i) induction of dedicated high-affinity terminal oxidases that permit bacteria to respire oxygen at very low concentrations or (ii) making use of inorganic terminal electron acceptors such as nitrate (NO3−), which can be reduced through the denitrification pathway to dinitrogen (N2) or through dissimilatory nitrate reduction to ammonium (DNRA). Although such anaerobic respiration generates less ATP per mol electron than aerobic respiration, it allows bacteria to grow and persist in the absence of oxygen (O2) [1]. Denitrification has been defined as the sequential reduction of NO3− or nitrite (NO2−) to nitric oxide (NO), nitrous oxide (N2O), and N2 [2]. This process is catalyzed by the periplasmic (Nap) or membrane-bound (Nar) nitrate reductase, nitrite reductases (NirK/NirS), nitric oxide reductases (cNor, qNor, or CuANor), and nitrous oxide reductase (N2OR) encoded by nap/nar, nirK/nirS, nor, and nos genes, respectively [2,3,4]. In addition to denitrification, multiple pathways for N2O generation have been reported, including nitrification, nitrifier denitrification, nitrite oxidation, ammonia oxidation, heterotrophic denitrification, anaerobic ammonium oxidation (anammox), and DNRA [3,5]. N2OR is the only known enzyme catalyzing the reduction of N2O to N2 [6]. Accordingly, expression and activity of N2OR are considered natural targets to mitigate N2O emissions from agricultural soils [7].
Given the impact of N2O as a powerful greenhouse gas in global warming and in depletion of the ozone layer [5,7], understanding its dynamics of production/reduction in soils and aquatic environments has become a priority. In fact, the application of synthetic nitrogen (N) fertilizers to agricultural soils, as well as local oxygen concentrations, water content, carbon availability, and pH, greatly affect N2O emissions from soils and aquatic ecosystems [7,8,9].
Many legumes stablish symbiotic associations with soil bacteria, collectively termed “rhizobia”, which fix nitrogen in so-called root nodules on legume roots and on the stems of some aquatic legumes. Following invasion of the plant cells via a complex signaling pathway between bacteria and plant, rhizobia stop dividing and undergo differentiation into nitrogen-fixing bacteroids, at which point the nitrogenase complex reduces atmospheric N2 into biologically useful forms in a process called “Biological N2 Fixation”. Consequently, cultivation of legumes can reduce the need for environmentally polluting synthetic nitrogen fertilizers, thus decreasing N2O emissions as well as protecting ground water from toxicity while improving soil fertility. However, legume crops can also contribute to N2O emissions in several ways: (i) by biologically fixed N2 being converted to NO and N2O through nitrification and denitrification [10]; (ii) by providing N-rich residues for decomposition [11], and (iii) directly by some rhizobia that can denitrify under free-living conditions or in symbiotic association with legumes [12,13]. In this context, one strategy to reduce N2O emissions from legume crops is to use as inoculants rhizobia strains with high N2OR activity. In fact, it has been shown that N2O emissions from soybean crops can be reduced by inoculating legumes with strains of the soybean endosymbiont Bradyrhizobium diazoefficiens that overexpress N2OR [14,15].
B. diazoefficiens is considered a model bacterium to study denitrification in rhizobia, since it is the only rhizobial species that, in addition to fixing N2, has the ability to grow under anoxic conditions by reducing NO3− through the complete denitrification pathway, a process widely studied in this bacterium both in free-living conditions and in symbiosis with soybeans [12,13,16]. B. diazoefficiens possesses the complete set of napEDABC, nirK, norCBQD, and nosRZDFYLX denitrification genes [12], which encode the periplasmic nitrate reductase (Nap), copper-containing nitrite reductase (NirK), nitric oxide reductase type c (cNor), and nitrous oxide reductase (N2OR), respectively (Figure 1). Like many other denitrifiers, expression of denitrification genes in B. diazoefficiens requires both oxygen limitation and the presence of NO3− or a nitrogen oxide (NOx) derived from its reduction. The response to low oxygen (≤0.5% O2 in the gas phase, i.e., ≤5–10 µM O2) is mediated by the FixLJ-FixK2-NnrR regulatory cascade [3], in which the response regulator FixJ in its active phosphorylated form induces the expression of several genes, including fixK2, which encodes the transcriptional regulator FixK2 (Figure 1). This protein induces the expression of more than 300 genes, including genes associated with microoxic metabolism (fixNOQP), denitrification genes (napEDABC, nirK, norCBQD, and nosRZDFYLX), and regulatory genes (rpoN1, fixK1, and nnrR) [12,17,18]. It has also been demonstrated that expression of napEDABC, nirK, and nosRZDFYLX genes requires microoxic conditions and directly depends on FixK2, while expression of norCBQD genes relies on NO, being the transcriptional regulator NnrR the candidate that directly interacts with norCBQD promoter [19,20] (Figure 1).
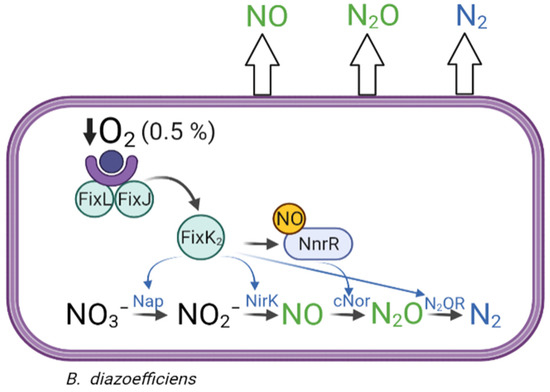
Figure 1.
Schematic representation of the denitrification process and its regulation in Bradyrhizobium diazoefficiens. B. diazoefficiens can reduce nitrate (NO3−) to nitrite (NO2−), nitric oxide (NO), nitrous oxide (N2O), and dinitrogen (N2) by the periplasmic nitrate reductase (Nap), copper-containing nitrite reductase (NirK), nitric oxide reductase type c (cNor), and nitrous oxide reductase (N2OR) enzymes, respectively. In B. diazoefficiens, expression of denitrification enzymes is tightly regulated by the FixLJ, FixK2, and NnrR regulatory proteins (see Introduction for further details). However, despite the coordinated activation of each reductase, environmental unfriendly gases such as NO and N2O can leak from denitrification and be released to the atmosphere.
Although much is known about the role of FixK2 and NnrR in the regulation of denitrification in B. diazoefficiens, this knowledge needs to be extended to include relevant physiological conditions that this bacterium is expected to meet in nature. In the present work, we investigated the dynamics of N2O balance by FixK2 and NnrR as well as the influence of specific environmental conditions such as the presence of nitrogen oxides, O2 concentration, pH, and the redox state of C-sources.
2. Results
2.1. N2O Emissions by B. diazoefficiens 110spc4 Depend on the FixK2 and NnrR Regulatory Proteins
B. diazoefficiens strains were raised oxically under vigorous stirring, and aliquots were inoculated into culture vials to an initial OD600 of 0.01 (8 × 106 cells mL−1). Next, 2% O2 and 10 mM NO3− were added as oxic and anoxic electron acceptors, respectively. Figure 2 and Figure S1 show the O2, NO3−, NO2−, NO, N2O, and N2 concentrations throughout the 120 h incubation of B. diazoefficiens 110spc4 (wild type) (Figure 2A and Figure S1A) and fixK2 (Figure 2B) and nnrR (Figure 2C and Figure S1B) mutant strains. B. diazoefficiens wild type consumed O2 within 28 h, and bacterial OD600 increased following O2 depletion.
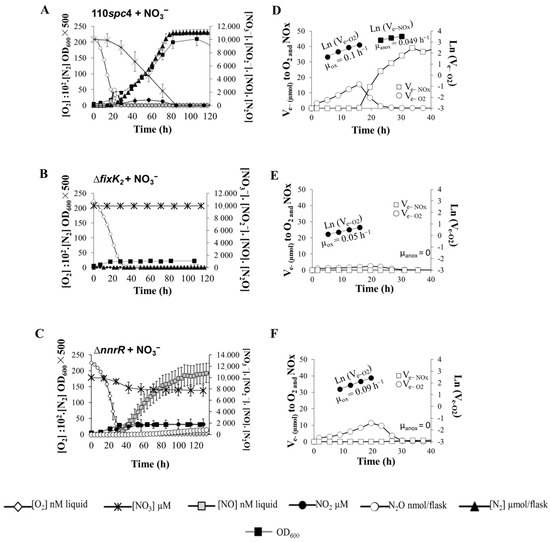
Figure 2.
Denitrification phenotypes of the parental strain B. diazoefficiens 110spc4 (A) and the two mutant strains ∆fixK2 (B) and ∆nnrR (C). (A–C) measurement of O2 and NO3− respiration, concentrations of denitrifying intermediaries (NO2−, NO, N2O, N2), and bacterial growth (OD600) yielded from such dynamics. (D–F) electron flow rates to O2 and nitrogen oxides (NOx). Cells were incubated with 2% O2 and 10 mM NO3− as oxic and anoxic respiratory substrates, respectively. O2, NOx concentrations, and bacterial growth were monitored by automatic sampling from headspace and liquid phase. See Figure S1 to visualize individual gases’ dynamics from (A,C). Data are the means and standard deviations of at least three different cultures.
Rates of O2 consumption for each time increment between two samplings were taken to calculate electron (e−) flow rates to oxygen (Ve−O2). As shown in Figure 2D, Ve−O2 increased exponentially in the wild type during the first 16 h and declined gradually in response to diminishing O2 concentrations. The increase in electron flow can be taken as an indirect measure of growth (µox) [21]. Thus, the apparent µox estimated by linear regression of ln (Ve−O2) against time was 0.10 (±0.03) h−1 (Figure 2D, Table 1A). Final OD600 resulting from the consumption of 2% O2 was 0.080 (±0.005) (6.40 × 107 cells mL−1, Table 1B), equivalent to a yield of 13.3 (±1.1) cells pmol−1 e− to O2 (Table 1A). Remarkably, in contrast to the fast depletion of O2 observed in the parental strain, the capacity to consume O2 in the fixK2 and nnrR mutant strains was slightly reduced (Figure 2A–C). In the case of the fixK2 mutant, Ve−O2 increased exponentially throughout the first 19 h and then declined gradually (Figure 2E). As shown in Table 1A, the apparent µox was 0.055 (± 0.008) (Figure 2E, Table 1A). The final OD600 from O2 respiration was 0.044 (±0.003) (Table 1B), resulting in a yield of 6.6 (±0.3) cells pmol−1 e− to O2 (Table 1A). In the nnrR mutant, electron flow to O2 increased exponentially throughout the first 19 h with an apparent µox of 0.090 (±0.004) and then decreased slowly (Figure 2F). The final OD600 during oxic phase was 0.079 (±0.001) (Table 1B), with a subsequent yield of 13.1 (±0.6) cells pmol−1 e− to O2 (Table 1A).

Table 1.
Summary of growth parameters from O2 (oxic growth phase) and NO3− respiration (anoxic growth phase) in the B. diazoefficiens 110spc4 parental and fixK2 and nnrR mutant strains (A) and other parameters observed through anoxic NO3− respiration (B).
Initiation of denitrification in the parental strain, hallmarked by the reduction of NO3− and transient emissions of NO and N2O (Figure 2A and Figure S1A), was observed at O2 concentrations of ≤5 (±0.3) μM O2 (Figure 2A and Figure S1A and Table 1B) after 17 h of incubation. Rapidly, N2 production was detected as individual final product from NO3− denitrification, with 100% of NO3− being converted to N2 within 80 h of growth. NO2− accumulated for a longer period than NO and N2O; however, its concentration was maintained at low levels until it was totally reduced to its depletion (Figure S1A).
As shown in Figure 2A, growth of B. diazoefficiens increased proportionally with NO3− respiration. Interestingly, the parental strain was able to derive electrons to NO3− reduction during the oxic phase before O2 was depleted, thus securing the transition from aerobic to anaerobic respiration and avoiding anaerobic entrapment (Figure 2D). Electron flow to NO3− increased exponentially during the anoxic phase, with an estimated growth rate (µanox) of 0.049 (±0.004) h−1 (Figure 2D; Table 1A). The final OD600 was 0.40 (±0.05) (Table 1B) and cell yield resulting from NO3− respiration (5.1 (±0.8) cells pmol−1 e− to NO3−) (Table 1A) was around 2.6-fold lower than that observed during oxic respiration.
In contrast to the competent transition from aerobic to anaerobic NO3− respiration by the parental strain, the fixK2 mutant strain was unable to shift to anaerobic respiration (Figure 2B), and following the oxygen depletion, the electron flow dropped drastically to zero (Figure 2E). Remarkably, ∆nnrR was able to initiate denitrification at O2 concentrations of ≤3.3 μM (±2.3) after 31 h incubation but was unable to consume NO derived from NO2− reduction, and consequently, NO accumulated in the headspace of the incubation medium (Figure 2C and Figure S1B and Table 1B). This accumulation of NO probably inhibited NO3− reduction and concomitant growth.
2.2. N2O Reduction by B. diazoefficiens 110spc4 Relies on the FixK2 and NnrR Regulatory Proteins in a Nitrogen-Oxides-Independent Manner
Transient detection of N2O in B. diazoefficiens wild type and inhibition of the denitrification process in ∆fixK2 and ∆nnrR strains precluded comparison of their N2O reduction capacities. Thus, to specifically assess the capacity of B. diazoefficiens wild type and fixK2 and nnrR mutant strains to consume N2O, we undertook a complementary approach. We supplied B. diazoefficiens bacterial cells with artificial N2O and analyzed their capacity to consume it. N2O reduction and subsequent N2 production were monitored in vials containing 5% N2O injected into the headspace. In addition, to study the impact that the presence of nitrogen oxides (NOx) might exert on N2O reduction, we also examined B. diazoefficiens’ capacity to consume N2O in the absence (Figure 3A,C,E) and in the presence (Figure 3B,D,F) of NO3−. In addition to N2O, 0.5% O2 was also added to the headspace as aerobic respiratory substrate due to the incapacity of B. diazoefficiens to initiate growth in the total absence of O2 (data not shown). Regardless of the presence of NO3−, externally supplied N2O was rapidly reduced to N2 by the parental strain until its complete depletion (Figure 3A,B). The final OD600 (Table 2B) and yield (Table 2A) of B. diazoefficiens parental cells were also monitored upon N2O consumption, and we found that both growth parameters were significantly enhanced when the bacterium was simultaneously incubated with both alternative electron acceptors, N2O and NO3− (Table 2A,B).
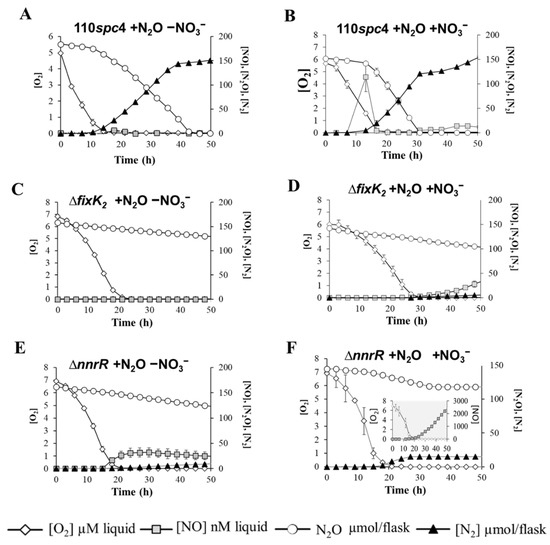
Figure 3.
Impact of FixK2 and NnrR inactivation on N2O consumption. Measurement of O2 and N2O respiration and concentrations of NO and N2. B. diazoefficiens 110spc4 parental strain (A,B) and fixK2 (C,D) and nnrR (E,F) mutant strains were incubated in vials containing 0.5% O2 and 5% N2O as oxic respiratory and anoxic respiratory substrates, respectively. In addition, a second set of vials were also supplemented with 10 mM NO3− (B,D,F) as anoxic respiratory substrate. The gradual decline in N2O concentration in (C,D,E) corresponds to dilution of headspace gases due to sampling. Data are the means and standard deviations of at least three different cultures.

Table 2.
Summary of growth parameters from N2O consumption in the B. diazoefficiens 110spc4 parental and fixK2 and nnrR mutant strains (A) and other parameters observed through the incubations, depending on the presence or absence of NO3− (B).
In the absence of NO3−, N2O reduction was initiated at O2 concentrations of ≤0.66 (±0.05) µM in the parental strain (Figure 3A; Table 2B). Under these conditions, electron flow to N2O increased with an apparent growth rate (µN2O) of 0.028 (±0.002) h−1 estimated by linear regression of ln (Ve−N2O) against time (Figure S2A, Table 2A). Electron flow rates to N2O remained unnoticeable during the first 5 h of oxic respiration; however, they increased exponentially after 8 h when electron flow to O2 was high. Similar to that which was previously observed during anaerobic NO3− respiration, this premature induction of the N2OR in the presence of O2 might be a mechanism to elude anoxia entrapment during the transition from oxic to anoxic conditions.
When NO3− was present, initiation of denitrification, hallmarked by a transient emission of NO (113 nM ± 30), took place after 7 h incubation under nearly anaerobic conditions (O2 concentrations of 1.5 (±0.15) μM) (Table 2B and Figure 3B, respectively), and it preceded induction of N2O consumption. In fact, N2O reduction was initiated at lower O2 concentrations of ≤0.15 (±0.05) µM (Figure 3B; Table 2B), indicating the B. diazoefficiens’ preference for NO3− as terminal electron acceptor. Estimated anaerobic growth rate supported by N2O in the presence of NO3− was µN2O = 0.046 (±0.003) h−1 (Figure S2B, Table 2A). Equivalently to that which was observed in the absence of NO3−, electron flow to N2O reduction occurred during active O2 respiration after 5 h incubation in the presence of NO3− (Figure S2B).
Strikingly, B. diazoefficiens strains lacking the regulatory transcriptional factors FixK2 or NnrR were severely impaired in N2O consumption capacity and growth (Figure 3C–F, Table 2A,B). Despite ∆fixK2 being unable to reduce N2O and grow either in the absence or the presence of NO3−, traces of NO gas were detected after 40 h incubation with NO3− (Figure 3D), but such residual respiratory activity was not coupled to growth. A mutant strain defective in the nnrR gene was significantly defective in its capacity to reduce N2O when incubated without NO3− (only 8 (±0.5)% of N2O was reduced to N2), likely due to its incapacity to detoxify NO, which permanently accumulated in the medium up to 32.2 (±8.8) nM (Figure 3E, Table 2B). The presence of NO3− slightly induced N2O reduction by the nnrR mutant (12.5 (±2.1)% of N2O was reduced to N2) at O2 concentrations of 0.8 (±0.3) μM after 13 h incubation (Figure 3F, Table 2B). However, under these conditions, NO3− in the medium was further reduced to NO, which was accumulated after 20 h incubation reaching levels up to ~2 µM after 50 h incubation (Figure 3F, insert, Table 2B).
Our results explain that B. diazoefficiens can co-respire NO3− and N2O and that activation of the N2O reductase relies on the FixK2 and NnrR regulatory proteins, independently of the presence of nitrogen oxides. Lastly, we also found that N2O reductase activity in B. diazoefficiens is highly sensitive to accumulation of endogenous NO derived from NO3− respiration, further supporting the importance of coordinated activation of denitrifying reductases by the FixK2 and NnrR regulators.
2.3. Acidic and Alkaline pHs Impair N2O Reduction by B. diazoefficiens 110spc4
To further elucidate how environmental cues prevailing in B. diazoefficiens niches might modulate N2O reduction, we monitored the expression of nosRZDFYLX genes and the capacity of B. diazoefficiens to reduce N2O in the presence of C-substrates commonly encountered in a plant’s rhizosphere [22], such as succinate, which generates 2 mol e− per C-mol oxidized, and butyrate, which generates 5 mol e− per C-mol oxidized. Interestingly, such C-sources did not affect expression of the nos operon (Figure S3A). Next, we analyzed N2O consumption by B. diazoefficiens determined as changes in N2O concentration in the headspace of vials containing 0.5% O2 plus 5% N2O inoculated with aerobically raised bacterial cells. Monitoring O2 uptake by B. diazoefficiens during the oxic phase also allowed us to evaluate any effect of C-source on bacterial metabolism/energetic that could subsequently alter N2O respiration. Despite N2O consumption was delayed around 20 h in the presence of butyrate compared to succinate (Figure 4A,B), such impairment could be attributed to a general metabolic defect, as oxygen consumption during the first hours of growth also was attenuated in that C-source. Further metabolic analyses are required to shed light on this respiratory inhibition induced by reduced C-sources.
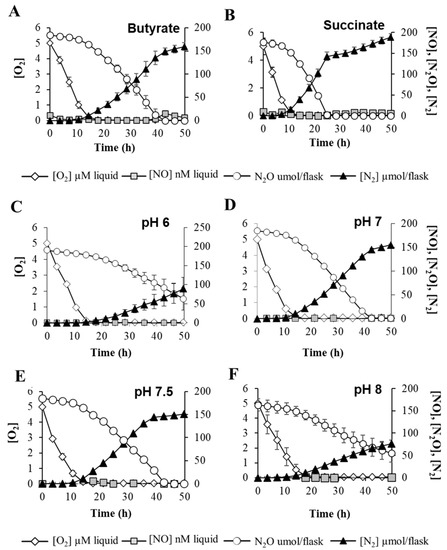
Figure 4.
Impact of C-source and pH on N2O consumption. Measurement of O2 and N2O respiration and concentrations of NO and N2. B. diazoefficiens 110spc4 parental strain was incubated in vials containing 0.5% O2, 5% N2O, and 10 mM NO3− as substrates for aerobic and anaerobic respiration, respectively. C-sources (A,B) and pH (C–F) of the growth medium were modified as shown on the graphs (see Material and Methods for further details). O2 and NOx concentrations were monitored by automatic sampling from headspace phase. Data are the means and standard deviations of at least three different cultures.
To understand if local changes in soil pH might affect N2O emissions from B. diazoefficiens, we also examined N2OR gene expression and N2O consumption in cells incubated at different pHs. As shown in Figure S3B, nos expression levels after 20 and 30 h incubation were not affected by different pH levels. Interestingly, while O2 consumption was similar at different pH levels, N2O reduction was strongly diminished at pH 6 and 8 (Figure 4C–F). These findings imply that, in addition to the impact of FixK2 and NnrR regulatory proteins on N2O reduction, relevant environmental factors such as pH importantly influence dynamics of N2O reduction by B. diazoefficiens.
3. Discussion
Given the damaging effect of N2O on climate, strategies to mitigate N2O emissions arising from intensive agricultural practices must be developed. These strategies include: (i) management of soil chemistry and microbiology to ensure that bacterial denitrification proceeds to completion, forming N2; (ii) promotion of sustainable agriculture, i.e., obtaining higher output from the same cultivated area of land; (iii) a better understanding of the environmental and regulatory factors that contribute to the generation and consumption of biological N2O; and (iv) reducing the dependence on fertilizers by using engineered crops that fix dinitrogen themselves or, alternatively, through application of nitrogen-fixing bacteria to legume crops. Despite the latter being one of the most promising alternatives to reduce N2O emissions, denitrification within endosymbiotic and free-living rhizobia released from nodules also contributes to the emission of N2O [10,13,16,23,24]; therefore, a better knowledge of the environmental and cellular factors controlling rhizobial denitrification is required.
Environmental cues (oxygen tensions and nitrogen oxides) and regulatory proteins (FixK2 and NnrR) governing denitrification in B. diazoefficiens are well-known [19,20,25] (Figure 1). In this work, we have validated, under physiological conditions, the importance of the FixK2 and NnrR transcription factors in real-time N2O dynamics using a robotized incubation system. Hence, we were able to simultaneously monitor changes in O2, NO3−, NO2− NO, N2O, and N2 concentration during the transition from aerobic to anaerobic respiration in B. diazoefficiens wild type and fixK2 and nnrR regulatory mutants. In addition, we also performed precise estimations of growth parameters (i.e., μ, yield) and defined accurately the O2 concentrations in which each step of the denitrification process is triggered. Therefore, we were able to determine that the denitrification process in B. diazoefficiens occurs at O2 concentrations of ≤5 (±0.3) μM. This concomitant induction of the denitrifying machinery with oxic respiration ensures a smooth and efficient transition from aerobic to anaerobic respiration, avoiding depression of electron flow when O2 is scarce (Figure 2A,D). A similar scenario was previously observed in the plant pathogen Agrobacterium tumefaciens [26]. In contrast to the early induction of the denitrification process found in these plant-interacting bacteria, Paracoccus denitrificans initiates transcription of nitrite reductase very late, resulting in entrapment of the majority of cells in anoxia [27].
We have also demonstrated, for the first time, that B. diazoefficiens 110spc4 is an efficient denitrifier, as it is able to transform 100% of NO3− to N2 (Figure 2A and Figure S1A). Interestingly, emission of N2O was detected at an early peak in O2 concentration of ≤5 μM (Figure 2A and Figure S1A and Table 2) during the transition from aerobic to anaerobic respiration, but the bacteria rapidly reduced its concentration, keeping it under very low levels (~2 ppm in headspace; 40 nM in the liquid). Collectively, these results reveal that denitrification in B. diazoefficiens 110spc4 emits marginal amounts of N2O, implying, as demonstrated by Mania et al., (2020) [28] and by Gao et al., (2021) [29], that bradyrhizobia can constitute a strong sink of the N2O released by neighboring organisms in the soil. Such denitrifying activity depends on coordinated activities of FixK2 and NnrR regulatory proteins. The tight control on emission of N2O and other denitrifying gases has been previously described in diverse bacterial species [26,27,28,29].
Although NO is a key signal molecule for the regulation of many processes, at high concentrations it exerts toxicity at different cellular levels [30,31,32]. Consequently, bacteria employ dedicated regulatory systems to keep NO at very low concentrations. Strikingly, we found that NO levels in B. diazoefficiens cultures reached very high concentrations (~600 nM) (Table 1B). Similarly, A. tumefaciens also accumulates large amounts of NO; however, those NO concentrations were not detrimental for this closely related rhizobium [26]. Conversely, P. denitrificans, Pseudomonas aerofaciens, and strains from the genus Thauera present a relatively tight control of NO production, maintaining NO concentration lower than 10–50 nM [21,27,33]. Although the reason for these differences in control of and tolerance to NO concentrations is unknown, it might arise from differential selective pressures exhibited by their ecological niches. Hence, while P. denitrificans, P. aerofaciens, and Thauera genus comprise bacteria that exist under free-living conditions, A. tumefaciens and B. diazoefficiens are bacteria that can interact with plants establishing pathogenic and symbiotic relationship, respectively. During its interaction with plants, A. tumefaciens might face diverse host defense systems such as NO production. Thus, a high tolerance to NO might confer a certain fitness advantage in respect to other soil competitors. NO is also known to be produced by plants in early stages during its interaction with nitrogen-fixing bacteria, as well as within the mature nodule [34,35,36,37]. In this context, symbiotic bacteria might require higher tolerance to NO to establish a productive symbiotic interaction with the plant.
In contrast to the efficient denitrifying capacity of B. diazoefficens wild type, we found that the fixK2 mutant was unable to initiate NO3− reduction. On the contrary, nnrR mutant cells were able to initiate the reduction of NO3− to NO2− and to NO; however, they were entrapped into anoxia due to accumulation of toxic concentrations of NO (Figure 2B,C). This disparate response of fixK2 and nnrR mutants confirms previous results in vitro, where we demonstrated that FixK2 directly controls the expression of napEDABC, nirK, and nosRZDFYLX genes in response to microoxic conditions and NnrR is the regulator that directly interacts with norCBQD promoter in response to NO [19,20]. Similar denitrification phenotypes were observed in P. denitrificans mutants deficient in the O2 and NO sensors FnrP and NNR, respectively [38].
Since NO3− reduction in ∆fixK2 and ∆nnrR was abrogated, we could not valuate their capacity to produce or consume N2O resulting from NO3− reduction. To achieve this goal, we incubated the cells in the presence of N2O, and we analyzed N2O and N2 fluxes. In the parental strain B. diazoefficiens 110spc4, N2O reduction was initiated at O2 concentrations of 0.15 (±0.05) and 0.66 (±0.05) μM in the presence and in the absence of NO3−, respectively. In contrast to the low O2 concentration required to trigger N2O consumption in B. diazoefficiens, in other rhizobia species such as Ensifer meliloti strain 1021, N2O consumption was initiated at O2 concentrations of 8 μM [39], indicating that B. diazoefficiens presents a N2OR more sensitive to O2 than other closely related rhizobial species.
In addition to microoxia, the nitrogen oxide NO3− and its reduction products NO2− or NO are considered essential inducers of denitrification in B. diazoefficiens [3,20]. Remarkably, we demonstrated in this work that N2O reduction in this bacterium was triggered in the absence of NO3−. Supporting our observations, it has been previously reported that microoxia is the main signal of expression of B. diazoefficiens nosRZDYFLX genes and N2OR activity [19]. This independence from NO3− was also reported in E. meliloti [39] and P. denitrificans [33].
When N2O was externally supplied, the parental strain reduced 100% of N2O to N2. In contrast, the N2O-reducing capacity of the fixK2 mutant was totally abolished in a medium without or with NO3−. However, nnrR mutant cells were able to reduce some N2O to N2 in the absence or in the presence of NO3− (8 (±0.5)% and 12.5 (±2.1)%, respectively) (Table 2B). These results confirm previous reports that propose FixK2 but not NnrR as the main transcriptional activator of the nosRZDYFLX genes [19]. In contrast to the disparate contribution of FixK2 and NnrR observed in our studies, it has been proposed that the homologous regulators of P. denitrificans FnrP and NNR contribute equally to N2OR induction [38,40,41]. Interestingly, cultures from ∆nnrR, with or without nitrate, showed a weak N2OR activity. In contrast to the transient accumulation of NO detected in cultures from the WT strain with NO3− (Figure 3B), the ∆nnrR mutant seems to be unable to detoxify NO, which remains permanently in the medium throughout the incubation (Figure 3F, insert). This long-lasting accumulation of NO was also observed when the medium was not supplemented with nitrate (Figure 3E). This NO may arise from traces of nitrate present in this medium (~50–100 µM, data not shown). The permanent accumulation of NO (32 nM) in ∆nnrR cells incubated without nitrate or when they were incubated with nitrate (~2 µM) might impair N2O reduction of the B. diazoefficiens ∆nnrR mutant.
An optimal management of soils is crucial to induce N2OR activity. In this context, it has been reported that maintaining soil pH at high ranges promotes N2OR activity. This strategy is based on the reported sensitivity of the N2O reductase activity to low pH in denitrifying bacteria [33,39], in bacterial communities extracted from soils and in intact soils [42]. Carbon availability also has an important role in N2O emissions from soils [43]. However, how specific forms of reductants might affect expression and activity of N2OR is largely unexplored. To study ecologically relevant environmental factors that could influence B. diazoefficiens N2OR expression and activity, we analyzed the expression of a nosR-lacZ transcriptional fusion as well as N2OR activity by monitoring N2O consumption, in the presence of reduced or oxidized C-sources such as butyrate or succinate and at different pH values. Despite the fact that expression of the nos genes was not affected by any of the conditions tested, N2OR activity was significantly attenuated when B. diazoefficiens cells were incubated under acidic and alkaline pHs (i.e., pH 6 and pH 8). Moreover, N2OR activity was also negatively affected when cells were incubated with reduced C-sources. However, reduced C-sources also affected oxygen consumption, which may indicate a general defect in bacterial metabolism when using such a C-source.
Confirming these observations, low pH had little effect on the transcription of the nosZ gene in P. denitrificans [33]. Instead, the enzymatic rate of N2O reduction was significantly attenuated at low pH levels, suggesting that environmental pH may have a direct posttranslational effect on the assembly and/or activity of the N2OR holoenzyme. Consistent with these findings, pH did not affect gene expression of Marinobacter hydrocarbonoclasticus N2OR genes; however, the amount of N2O reductase isolated from cells grown at pH 6.5 was lower than that at pH 7.5 and 8.5, pointing to a post-transcriptional regulation [44]. Indeed, biochemical studies of the M. hydrocarbonoclasticus N2OR revealed that redox properties of its catalytic site are significantly altered by changes in pH values ranging from 6.5 to 8.5 [44]. Similarly, as observed in B. diazoefficiens, an inhibitory effect of reduced carbon sources such as butyrate or low pH on N2OR activity was already observed in E. meliloti [39]. In contrast to E. meliloti [39] and M. hydrocarbonoclasticus [44], in our work, B. diazoefficiens N2OR was also inhibited at a high pH, buttressing the importance of controlling’ soils pH regarding N2O emissions. Such sensitivity of B. diazoefficiens N2OR to high pH is currently under investigation.
Altogether, these observations expand the knowledge of the regulatory and environmental factors that control N2O emissions by bacterial species associated with legumes. This information should be taken into consideration when developing new programs to manage N2O emissions from legume crops.
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
Bradyrhizobium diazoefficiens 110spc4 [45] and ΔfixK2::Ω and ΔnnrR:: aphII mutant strains [20] were used in this work. To analyze expression of the nosRZDYFLX genes, a B. diazoefficiens strain (110spc4-BG0306) containing a chromosomally integrated transcriptional fusion within the nosRZDYFLX genes promoter and the lacZ reporter gene was used [19]. B. diazoefficiens strains were firstly grown aerobically in 120 mL serum vials each containing a magnetic stirring bar and 50 mL of Peptone-Salts-Yeast extract (PSY) complete medium [46] at 30 °C. To analyze anaerobic growth from B. diazoefficiens, aliquots from aerobic cultures raised under vigorous stirring to avoid anoxic microzones by cells aggregation were transferred to vials with minimal defined Bergersen’s medium [47]. Oxygen from vials was removed by 6 cycles of air evacuation for 360 s and helium (He) filling for 40 s. Influence of pH on N2O consumption was analyzed by cultivating B. diazoefficiens under N2O respiring conditions in minimally defined medium buffered with 50 mM phosphate buffer at pH 6, 7, 7.5, and 8. In all the treatments, the headspace was filled with an initial concentration of O2 of 0.5 or 2% (6 or 24 µM dissolved O2 at 30 °C, respectively). To study the N2O consumption by the bacterium, vials were also supplemented with N2O 5% (1.2 mM). A concentration of 10 mM KNO3− was also added to the cultures as alternative respiratory substrate as indicated in the text. When needed, antibiotics were used at the following concentrations (in µg/mL): kanamycin, 30; spectinomycin, 25; streptomycin, 25; tetracycline, 10.
4.2. Gas Measurements
After transferring aerobically grown bacteria into anaerobic vials, they were placed together with blanks and gas standards in a thermostatic water incubator at 30 °C. Cells dispersion and equal distribution of gases throughout the vial liquid and headspace was achieved by continuous stirring at 700 rpm. Emission of gases (O2, NO, N2O, and N2) resulting from bacterial aerobic and anaerobic metabolism were monitored by automatic gas sampling. Gas measurements were analyzed as described by Bueno at al., 2015, and Molstad et al., 2007 [39,48]. Briefly, the gas samples were drawn from each bacterial culture, and with each sampling an equal volume of He was pumped back into the vials to maintain gas pressure at 1 atm. Sampling and gases’ measurements were performed as previously described in detail [39].
4.3. Determination of Bacterial Growth and NO3− and NO2− Concentrations
To measure bacterial growth and NO3− and NO2− concentrations, aliquots from the liquid phase of vials were withdrawn manually by using sterile syringes. Bacterial growth was determined by measuring cell density at 600 nm (OD600). Concentrations of NO3− and NO2− were determined as described by Bueno at al., 2015 [39].
4.4. Kinetic Analysis from Aerobic and Anaerobic Respiration
Aerobic and anaerobic respiration kinetics were determined as described by Bueno et al., 2015 [39]. To determine O2 and NO concentrations in the liquid, we considered the pressure of the gases, their solubilities, and their transport coefficients among headspace and liquid. O2 dissolved in liquid was also calculated considering O2 respiration rate during bacterial growth (see Molstad et al., 2007 for details). We analyzed N2O concentrations as µmol N2O vial−1, while N2 was estimated as net production of N2. Growth rates (µox) and reduction of NOx during the anoxic phase (µanox) were determined by regression [ln (Ve−) against time] for the phases with exponentially increasing rates. Determination of cells yield (cells pmol−1 e−) was estimated considering the number of biomass produced per pmol electron consumed by the transport electron chain to reduce O2 to H2O in the oxic phase (Yieldox) or by the denitrifying machinery during the anoxic phase (Yieldanox). Vmax tells us about the specific efficiency for O2 and NOx respiration per cell. For further details regarding these calculations, see Molstad et al. (2007) [48] and Nadeem et al. (2013) [27].
4.5. Determination β-Galactosidase Activity
β-galactosidase activity to investigate gene expression was analyzed as previously described [49]. In brief, 5 mL of cells incubated for 20 and 30 h under the conditions detailed in the text were collected, centrifuged, and resuspended in 500 µL of growth medium. In total, 25 µL of this culture was mixed with 20 µL of freshly prepared SDS 0.1%, 25 µL chloroform, and 100 µL of Z-buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, and 50 mM β-mercaptoethanol). Next, 20 µL of ONPG (4 mg/mL) was added to initiate the reaction. Reaction mix was incubated at room temperature before the reaction was terminated by addition of 75 µL of 1 M Na2CO3. Supernatant was collected and absorbance at OD420 and OD550 used to determine β-galactosidase specific activity in Miller units.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms23031486/s1.
Author Contributions
Conceptualization, E.B., Å.F., L.R.B. and M.J.D.; methodology, E.B., D.M., Å.F., L.R.B. and M.J.D.; validation, E.B., Å.F., L.R.B. and M.J.D.; formal analysis, E.B., Å.F., L.R.B. and M.J.D.; investigation, E.B.; resources, Å.F., L.R.B., S.M. and M.J.D.; writing—original draft preparation, E.B., writing—review and editing, E.B., Å.F., L.R.B., E.J.B., S.M. and M.J.D.; visualization, E.B., Å.F., L.R.B. and M.J.D.; supervision, E.B., Å.F., L.R.B. and M.J.D.; project administration, E.B., Å.F., L.R.B. and M.J.D.; funding acquisition, E.B., Å.F., L.R.B., S.M. and M.J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MCIN/AEI/10.13039/501100011033, “ERDF A way of making Europe”, grant AGL2017-85676-R to María J Delgado, grants AGL2015-63651-P and PID2020-114330GB-100 to Socorro Mesa, and also Junta de Andalucía, grant P18-RT-1401 to María J Delgado and Socorro Mesa. EB was supported by a personal visiting researcher grant–IS-MOBIL (Oslo University, Norway) and the CSIC JAE-DOC Program co-financed by ESF.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We acknowledge the continuous support from Junta de Andalucía. We also acknowledge the support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Simon, J.; van Spanning, R.J.M.; Richardson, D.J. The organisation of proton motive and non-proton motive redox loops in prokaryotic respiratory systems. Biochim. Biophys. Acta-Bioenerg. 2008, 1777, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997, 61, 533–616. [Google Scholar] [PubMed]
- Torres, M.J.; Simon, J.; Rowley, G.; Bedmar, E.J.; Richardson, D.J.; Gates, A.J.; Delgado, M.J. Nitrous Oxide Metabolism in Nitrate-Reducing Bacteria: Physiology and Regulatory Mechanisms. Adv. Microb. Physiol. 2016, 68, 353–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Spanning, R.J.M.; Richardson, D.J.; Ferguson, S.J. Introduction to the biochemistry and molecular biology of denitrification. In Biology of the Nitrogen Cycle, 1st ed.; Bothe, H., Ferguson, S.J., Newton, W.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 3–20. [Google Scholar]
- Conrad, R. Metabolism of Nitric Oxide in Soil and Soil Microorganisms and Regulation of Flux into the Atmosphere. Microbiol. Atmos. Trace Gases 1996, 60, 167–203. [Google Scholar] [CrossRef]
- Zumft, W.G.; Kroneck, P.M.H. Respiratory transformation of nitrous oxide (N2O) to dinitrogen by Bacteria and Archaea. Adv. Microb. Physiol. 2007, 52, 107–227. [Google Scholar] [CrossRef]
- Richardson, D.; Felgate, H.; Watmough, N.; Thomson, A.; Baggs, E. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle-could enzymic regulation hold the key? Trends Biotechnol. 2009, 27, 388–397. [Google Scholar] [CrossRef]
- Lazcano, C.; Zhu-Barker, X.; Decock, C. Effects of organic fertilizers on the soil microorganisms responsible for N2O emissions: A review. Microorganisms 2021, 9, 983. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 1621. [Google Scholar] [CrossRef]
- Inaba, S.; Ikenishi, F.; Itakura, M.; Kikuchi, M.; Eda, S.; Chiba, N.; Katsuyama, C.; Suwa, Y.; Mitsui, H.; Minamisawa, K. N2O emission from degraded soybean nodules depends on denitrification by Bradyrhizobium japonicum and other microbes in the rhizosphere. Microbes Environ. 2012, 27, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Baggs, E.; Rees, R.; Smith, K.; Vinten, A.J. Crop Residues. Biomass Energ. 1983, 163–236. [Google Scholar] [CrossRef]
- Bedmar, E.J.; Robles, E.F.; Delgado, M.J. The complete denitrification pathway of the symbiotic, nitrogen-fixing bacterium Bradyrhizobium japonicum. Biochem. Soc. Trans. 2005, 33, 141–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, M.J.; Casella, S.; Bedmar, E.J. Denitrification in rhizobia-legume symbiosis. In Biology of the Nitrogen Cycle; Bothe, H., Ferguson, S.J., Newton, W.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 1, pp. 83–93. [Google Scholar] [CrossRef]
- Itakura, M.; Uchida, Y.; Akiyama, H.; Hoshino, Y.T.; Shimomura, Y.; Morimoto, S.; Tago, K.; Wang, Y.; Hayakawa, C.; Uetake, Y.; et al. Mitigation of nitrous oxide emissions from soils by Bradyrhizobium japonicum inoculation. Nat. Clim. Chang. 2013, 3, 208–212. [Google Scholar] [CrossRef]
- Akiyama, H.; Hoshino, Y.T.; Itakura, M.; Shimomura, Y.; Wang, Y.; Yamamoto, A.; Tago, K.; Nakajima, Y.; Minamisawa, K.; Hayatsu, M. Mitigation of soil N2O emission by inoculation with a mixed culture of indigenous Bradyrhizobium diazoefficiens. Sci. Rep. 2016, 6, 32869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedmar, E.; Bueno, E.; Correa, D.; Torres, M.; Delgado, M.; Mesa, S. Ecology of Denitrification in Soils and Plant-Associated Bacteria. Benef. Plant-Microb. Interact. Ecol. Appl. 2013, 146–182. [Google Scholar] [CrossRef]
- Delgado, M.J.; Bonnard, N.; Tresierra-Ayala, A.; Bedmar, E.J.; Müller, P. The Bradyrhizobium japonicum napEDABC genes encoding the periplasmic nitrate reductase are essential for nitrate respiration. Microbiology 2003, 149, 3395–3403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesa, S.; Ucurum, Z.; Hennecke, H.; Fischer, H.M. Transcription activation in vitro by the Bradyrhizobium japonicum regulatory protein FixK2. J. Bacteriol. 2005, 187, 3329–3338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, M.J.; Bueno, E.; Jiménez-Leiva, A.; Cabrera, J.J.; Bedmar, E.J.; Mesa, S.; Delgado, M.J. FixK2 is the main transcriptional activator of Bradyrhizobium diazoefficiens nosRZDYFLX genes in response to low oxygen. Front. Microbiol. 2017, 8, 1621. [Google Scholar] [CrossRef] [Green Version]
- Bueno, E.; Robles, E.F.; Torres, M.J.; Krell, T.; Bedmar, E.J.; Delgado, M.J.; Mesa, S. Disparate response to microoxia and nitrogen oxides of the Bradyrhizobium japonicum napEDABC, nirK and norCBQD denitrification genes. Nitric Oxide-Biol. Chem. 2017, 68, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Mao, Y.; Bergaust, L.; Bakken, L.R.; Frostegård, Å. Strains in the genus Thauera exhibit remarkably different denitrification regulatory phenotypes. Environ. Microbiol. 2013, 15, 2816–2828. [Google Scholar] [CrossRef]
- Koo, B.J.; Adriano, D.C.; Bolan, N.S.; Barton, C.D. Root exudates and microorganisms. Encycl. Soils Environ. 2005, 421–428. [Google Scholar] [CrossRef]
- Tortosa, G.; Pacheco, P.J.; Hidalgo-García, A.; Granados, A.; Delgado, A.; Mesa, S.; Bedmar, E.J.; Delgado, M.J. Copper modulates nitrous oxide emissions from soybean root nodules. Environ. Exp. Bot. 2020, 180, 104262. [Google Scholar] [CrossRef]
- Tortosa, G.; Hidalgo, A.; Salas, A.; Bedmar, E.J.; Mesa, S.; Delgado, M.J. Nitrate and flooding induce N2O emissions from soybean nodules. Symbiosis 2015, 67, 125–133. [Google Scholar] [CrossRef]
- Jiménez-Leiva, A.; Cabrera, J.J.; Bueno, E.; Torres, M.J.; Salazar, S.; Bedmar, E.J.; Delgado, M.J.; Mesa, S. Expanding the regulon of the Bradyrhizobium diazoefficiens NnrR transcription factor: New insights into the denitrification pathway. Front. Microbiol. 2019, 10, 1926. [Google Scholar] [CrossRef] [Green Version]
- Bergaust, L.; Shapleigh, J.; Frostegård, Å.; Bakken, L. Transcription and activities of NOx reductases in Agrobacterium tumefaciens: The influence of nitrate, nitrite and oxygen availability. Environ. Microbiol. 2008, 10, 3070–3081. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.; Dörsch, P.; Bakken, L.R. Autoxidation and acetylene-accelerated oxidation of NO in a 2-phase system: Implications for the expression of denitrification in ex situ experiments. Soil Biol. Biochem. 2013, 57, 606–614. [Google Scholar] [CrossRef] [Green Version]
- Mania, D.; Woliy, K.; Degefu, T.; Frostegård, Å. A common mechanism for efficient N2O reduction in diverse isolates of nodule-forming bradyrhizobia. Environ. Microbiol. 2020, 22, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Mania, D.; Mousavi, S.A.; Lycus, P.; Arntzen, M.; Woliy, K.; Lindström, K.; Shapleigh, J.P.; Bakken, L.R.; Frostegård, Å. Competition for electrons favours N2O reduction in denitrifying Bradyrhizobium isolates. Environ. Microbiol. 2021, 23, 2244–2259. [Google Scholar] [CrossRef]
- Stern, A.M.; Zhu, J. An Introduction to nitric oxide sensing and response in bacteria. Adv. Appl. Microbiol. 2014, 87, 187–220. [Google Scholar] [CrossRef]
- Toledo, J.C.; Augusto, O. Connecting the chemical and biological properties of nitric oxide. Chem. Res. Toxicol. 2012, 25, 975–989. [Google Scholar] [CrossRef]
- Möller, M.N.; Rios, N.; Trujillo, M.; Radi, R.; Denicola, A.; Alvarez, B. Detection and quantification of nitric oxide-derived oxidants in biological systems. J. Biol. Chem. 2019, 294, 14776–14802. [Google Scholar] [CrossRef] [Green Version]
- Bergaust, L.; Mao, Y.; Bakken, L.R.; Frostegård, Å. Denitrification response patterns during the transition to anoxic respiration and posttranscriptional effects of suboptimal pH on nitrogen oxide reductase in Paracoccus denitrificans. Appl. Environ. Microbiol. 2010, 76, 6387–6396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoda, Y.; Nagata, M.; Suzuki, A.; Abe, M.; Sato, S.; Kato, T.; Tabata, S.; Higashi, S.; Uchiumi, T. Symbiotic rhizobium and nitric oxide induce gene expression of non-symbiotic hemoglobin in Lotus japonicus. Plant Cell Physiol. 2005, 46, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ferrarini, A.; De Stefano, M.; Baudouin, E.; Pucciariello, C.; Polverari, A.; Puppo, A.; Delledonne, M. Expression of Medicago truncatula genes responsive to nitric oxide in pathogenic and symbiotic conditions. Mol. Plant-Microbe Interact. 2008, 21, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Pii, Y.; Crimi, M.; Cremonese, G.; Spena, A.; Pandolfini, T. Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biol. 2007, 7, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, C.; Gates, A.J.; Meakin, G.E.; Uchiumi, T.; Girard, L.; Richardson, D.J.; Bedmar, E.J.; Delgado, M.J. Production of nitric oxide and nitrosylleghemoglobin complexes in soybean nodules in response to flooding. Mol. Plant-Microbe Interact. 2010, 23, 702–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergaust, L.; van Spanning, R.J.M.; Frostegård, Å.; Bakken, L.R. Expression of nitrous oxide reductase in Paracoccus denitrificans is regulated by oxygen and nitric oxide through FnrP and NNR. Microbiology 2012, 158, 826–834. [Google Scholar] [CrossRef] [Green Version]
- Bueno, E.; Mania, D.; Frostegard, Å.; Bedmar, E.J.; Bakken, L.R.; Delgado, M.J. Anoxic growth of Ensifer meliloti 1021 by N2O-reduction, a potential mitigation strategy. Front. Microbiol. 2015, 6, 537. [Google Scholar] [CrossRef] [Green Version]
- Veldman, R.; Reijnders, W.N.M.; Van Spanning, R.J.M. Specificity of FNR-type regulators in Paracoccus denitrificans. Biochem. Soc. Trans. 2006, 34, 94–96. [Google Scholar] [CrossRef]
- Bouchal, P.; Struhárová, I.; Budinská, E.; Šedo, O.; Vyhlídalová, T.; Zdráhal, Z.; van Spanning, R.; Kučera, I. Unraveling an FNR based regulatory circuit in Paracoccus denitrificans using a proteomics-based approach. Biochim. Biophys. Acta-Proteins Proteom. 2010, 1804, 1350–1358. [Google Scholar] [CrossRef]
- Liu, B.; Frostegård, Å.; Bakken, L. Impaired Reduction of N2O to N2 in acid soils is due to a posttranscriptional interference with the expression of nosZ. mBio 2014, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Murray, P.J.; Hatch, D.J.; Dixon, E.R.; Stevens, R.J.; Laughlin, R.J.; Jarvis, S.C. Denitrification potential in a grassland subsoil: Effect of carbon substrates. Soil Biol. Biochem. 2004, 36, 545–547. [Google Scholar] [CrossRef]
- Carreira, C.; Nunes, R.F.; Mestre, O.; Moura, I.; Pauleta, S.R. The effect of pH on Marinobacter hydrocarbonoclasticus denitrification pathway and nitrous oxide reductase. J. Biol. Inorg. Chem. 2020, 25, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Regensburger, B.; Hennecke, H. RNA polymerase from Rhizobium japonicum. Arch. Microbiol. 1983, 135, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Mesa, S.; Hauser, F.; Friberg, M.; Malaguti, E.; Fischer, H.M.; Hennecke, H. Comprehensive assessment of the regulons con-trolled by the FixLJ-FixK2-FixK1 cascade in Bradyrhizobium japonicum. J. Bacteriol. 2008, 190, 6568–6579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergersen, F.J. A Treatise on Dinitrogen Fixation. In Biology, Section III; Hardy, R.W., Silver, W., Eds.; Willey: New York, NY, USA, 1977. [Google Scholar]
- Molstad, L.; Dörsch, P.; Bakken, L.R. Robotized incubation system for monitoring gases (O2, NO, N2O N2) in denitrifying cultures. J. Microbiol. Methods 2007, 71, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory: New York, NY, USA, 1972. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).