Organ-Specific Endothelial Cell Differentiation and Impact of Microenvironmental Cues on Endothelial Heterogeneity
Abstract
1. Introduction
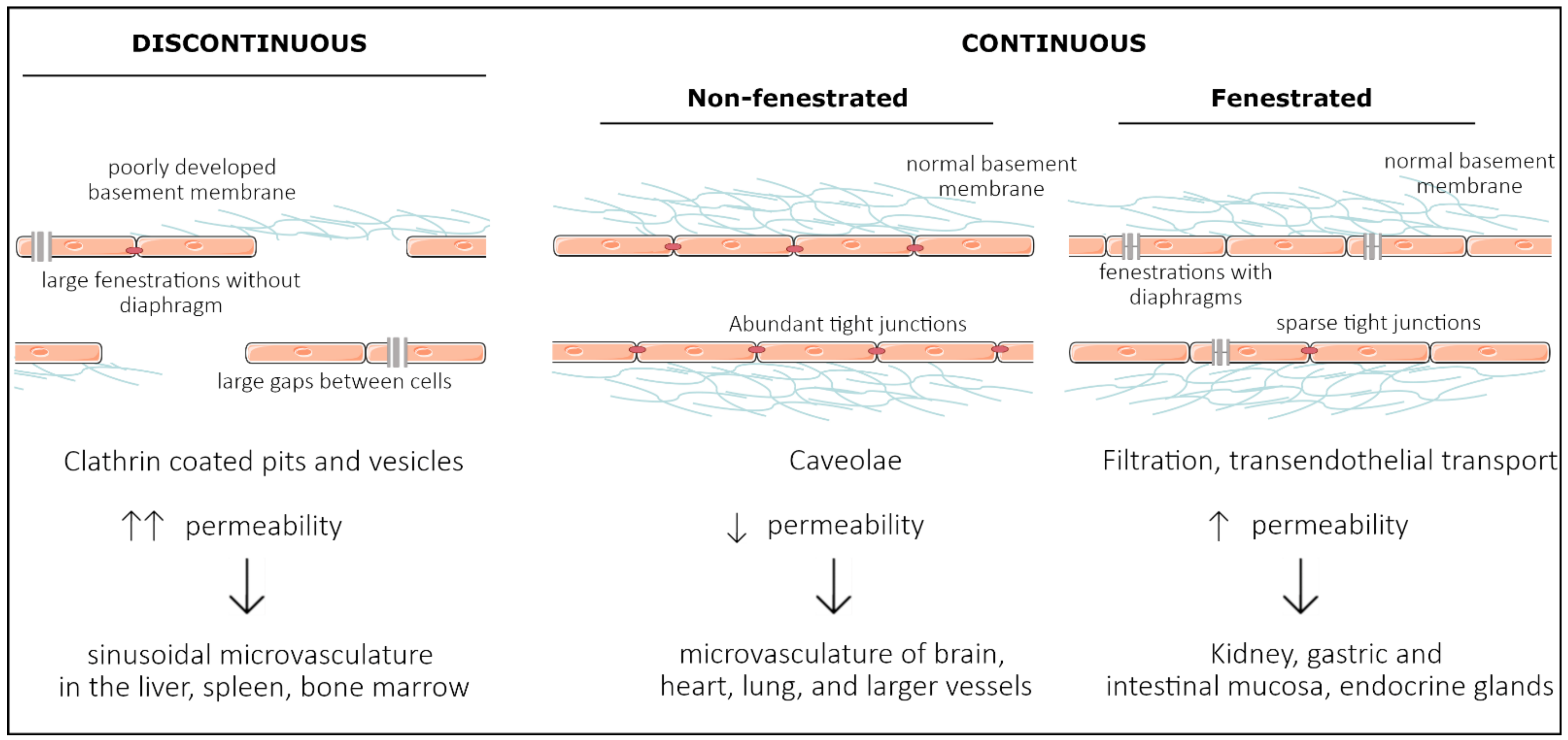
2. Development of Organ Specificity among Endothelial Cells
2.1. Vasculogenesis—Intrinsic Versus Extrinsic Factors

2.2. Large Conduit Vessel Differentiation
2.3. Organ-Specific Vascular Development
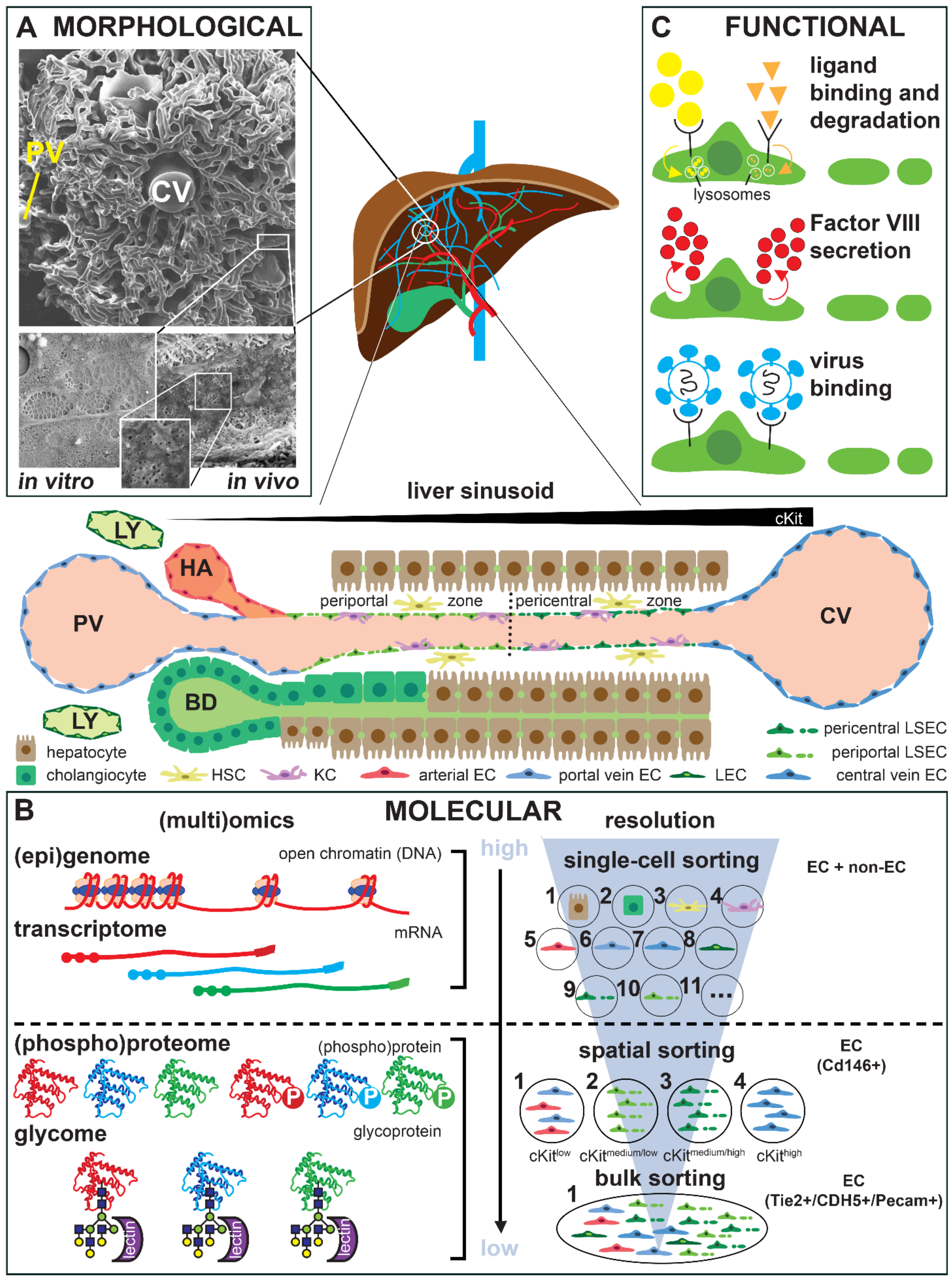
3. Technological Progress in Assessing Endothelial Cell Heterogeneity
4. Organ-Specific Endothelial Cell Culture and Phenotypic Drift
5. Role of the Tissue Microenvironment in Adult Endothelial Cell Heterogeneity
5.1. Mechanical Cues Determining Endothelial Cell Heterogeneity
Organ-Specific Responses to Tissue Stiffness, Shear Stress, and Cellular Stretch
5.2. Biochemical Cues Determining Endothelial Cell Heterogeneity
Organ-Specific Responses to Specific Biochemical Cues
6. Impact of Endothelial Cell Heterogeneity on Drug Development
| Organ/ Tissue | Cell Type | Pass. Nr. | Co-Culture | Tissue Mimicking | Characterization Technique | Time in Culture | Refs | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mechanical | Biochemical | Genetics | Morphology | Function | ||||||
| Brain | human brain microvascular (mv) ECs (HBMECs) (C) | P2–P3 | human astrocytes | 6.2 dynes/cm2; PP hollow fibers | FN | RNA microarray | - | TEER; glucose consumption and lactate production | 30 d | [118] |
| P4–P7 | - | 10–20, 40 dynes/cm2; silicone | FN + Astrocyte conditioned medium | - | IF: CD31, ZO-1 and CLDN-5; WB: Transport markers P-gp and GLUT1 | Src/ERK pathway activation | 4 d | [161] | ||
| bovine primary BMECs (F) | P1–P7 | Glial cells (astrocytes >95%) (F) | - | Col solution | - | IF: CLDN, OCLN, ZO, β-cat, p120cat, actin cytoskeleton | Permeability assays | 14 d | [129] | |
| mouse primary BMECs (F) | P1 | - | - | Matrigel | RNA-seq, transcriptome | IF: CLDN-5, OCLN, ZO-1, ZO-2, JAM-A, VE-cad & β-cat | TEER | 7 d | [130] | |
| mouse primary BECs (F) | P1 | - | - | Col I | RNA-seq and ATAC-seq | IF: CD31 | - | 10 d | [131] | |
| iPSCs-derived HBMECs & human umbilical vein ECs (HUVECs) (D) | P1–P7 | - | ~4 dynes/cm2; cylindrical 150 μm Ø channel Col hydrogel | Col I | GLUT1 and P-gp expression | IF: ZO-1, CLDN-5 and OCLN | Permeability assays | 6 d | [133] | |
| iPSCs-derived HBMECs (D) | P2 | - | - | Genipin-crosslinked Col I gels, with FN and Col IV | - | IF: ZO-1 and CLDN-5 | TEER, microvessel formation | 7 d | [135] | |
| hESCs-derived ECs (D) | - | hESCs-derived cortical organoids | Perfusion tests | cortical organoids | TJ & nutrient transporter expression; single-cell map vhCOs | - | TEER | 120 d | [134] | |
| Immortalized mouse BMECs (bEnd3) (C) | - | patient-derived glioblastoma cells | Alginate fibers | thiolated sodium hyaluronate | qPCR | IF | VEGF release | 14 d | [119] | |
| Immortalized HBMECs; HUVECs (C) | - | - | 8, 12, 16 dynes/cm2 | - | - | IF: F-actin and ZO-1; WB: β-catenin and ZO-1; cell alignment | - | 36 h | [120] | |
| Lung | human pulmonary artery ECs (HPAECs) (C) | P6–P8 | - | flexible-bottomed BioFlex plates; 5 and 18% elongation cyclic stretch | Col I | gene profiling | IF: F-actin; stress fiber & actin alignment; WB: pathway factors | cytoskeletal rearrang. & TEER | 2 d | [121] |
| P7–P10 | - | 1, 3, 8 dynes/cm2; glass | Gelatin | - | IF: MitoTracker and caveolin-1 | Real-time imaging: mit. ATP levels; Ca2+ influx | few min | [122] | ||
| human pulmonary mv ECs (HPMECs) (C) | P4–P7 | - | silicon chamber; 10, 20, 30% stretch strains | FN | qPCR: TRPV-2, TRPV-4 | IF: Tie-2, CD31, F-actin | Stretch-activated Ca2+ influx | few min | [123] | |
| mouse primary PMECs & cardiac mv ECs (both E4ORF1) (C) | - | - | 4 dynes/cm2; PS slides | FN | - | FC: CD31, CD144; cell alignment & area; AFM: cell stiff. | - | 12 h | [124] | |
| - | - | 2 dynes/cm2; PDMS (500 kPa) and PS (2–3 GPa) slides | Cardiac & lung ECM vs. FN | - | cell alignment and area; FC: integrins αv and β3 | - | 12 h | [125] | ||
| Heart | bovine primary aortic ECs (F) | - | - | 12 dynes/cm2; glass; 100 pN pulsatile & 10 pN continuous forces | FN or Col I | - | WB: RhoA, ph-CREB, ph-PKA, PKA, ph-serine; IF: actin, vinculin, β-cat, HUTS-4, VE-cad | Bead displacement by pulsatile force; cAMP; integrin activation | 30 min | [169] |
| HPMEC-ST1.6R (F) | - | Adipose tissue-derived stromal cells | Left ventricle-, mitral valve-, aorta-derived hydrogels (3, 3, 7 kPa) | Left ventricle, mitral valve & aorta ECM | - | IF: SM22α, actin, CD31 | Vascular network formation | 7 d | [132] | |
| Liver | HUVECs (F) | - | fetal liver cells | perfusion at 0.5 mL/min; liver decellularized scaffolds | Liver decellular. ECMs; matrigel | - | IF: vWF, eNOS, Ki67, TUNEL | Vascular network formation; prolif.; platelet deposition | 7 d | [138] |
| Unspecific | HUVECs (C) | - | - | 20 dynes/cm2; ibidi slides | - | qPCR: Wnt ligands | cell polarity & orientation; IF: Cleaved Caspase-3, Col IV, Erg1/2/3, GM130, Golph4, ICAM2, Lef1, NG2; FC: CD31, CD45 | - | 4 h | [139] |
| HUVECs (C) | P6–P10 | THP1 cells | FITC-conjugated dextran flow | 15(S)-hydroxyeicosatetraenoic acid | - | IF: ZO-1, OCLN | Barrier permeability & disruption; THP1 transmigration | 8 h | [199] | |
| bovine aorta ECs & HUVECs (C) | P6–P10 | - | 6, 12, 18 & 22 dynes/cm2; 100 Pa, 2.5, 3, 10 & 30 kPa PAA gels | FN | - | cell alignment & area; IF: actin, NF-κB | TNF-α induced NF-κB transloc. to nucleus | 24 h | [128] | |
| human pulmonary artery ECs (HPAECs) (C) | P5–P9 | - | 1.1 & 40 kPa hydrogels, or glass (~50 GPa) | FN or Col IV | - | IF: VE-cad, paxillin, actin | Magnetic twisting cytometry for VE-cad receptor perturbation & displacement; Monolayer stress microscopy | 5 d | [200] | |
| immortalized human mv ECs (HMEC-1) & HUVECs (C) | P4–P8 | - | 3, 35 & 70 kPa PAA gels | Col I | Transcriptom. and qPCR | IF: pMLC & actin & WB | Traction force microscopy | 2 h | [156] | |
| human umbilical artery ECs (HUAECs) & HUVECs (C) | - | - | - | Col I; hypoxia | qPCR: β-actin, HPRT1 | FC: VE-cad, CD31, KDR, CD146, PDGFRβ; IF & WB: Col I, Col IV, FN, laminin, actin | Hypoxia & conditioned ECM deposition | 7 d | [140] | |
| Diverse | fetal human primary kidney, lung, liver & heart ECs (F) | P2–P5 | rat primary hepatocytes | Gravity-driven flow; cells in Col microfluidic channels | Col I | RNAseq of freshly isolated vs. cultured ECs | IF: CD31, CD144, vWF, PV1 & Caveolin 1 | TEER, spheroid sprouting, metabolic assays | 5 d | [106] |
| human primary mv dermal, lung, renal glomerular, brain & liver ECs; large vessel coronary artery ECs & HUVECs (C) | P2–P8 | - | - | Dilutions of TTP/sporadic HUS patients’ plasma | qPCR: Fas transcripts | FC: annexin II | Apoptosis: Cdc2 kinase assay, procoagulant activities | 16-18 h | [126] | |
| human primary mv cardiac, dermal, lung & uterine ECs; aortic, cardiac artery, iliac ECs, HPAECs & HUVECs (C) | P2–P6 | - | - | Hypoxia | Gene expression microarray; qPCR: HIF1A, HIF-2a, 18S, TBP | WB: HIF-1a, HIF-2a; β-actin | Hypoxia effects in transcriptome | 2 d | [127] | |
| human adipose-derived endothelial cells & HUVECs (F); human mv cardiac, aortic, pulmonary and dermal ECs (C); ETV2-transduced. | P5–P10 | Colorect. cancer, colon & small intestine organoids;pancreat. islets | Gravity-driven perfusion tests in microfluidic devices | Matrigel or mixture of laminin, entactin & col IV | single-cell transcriptom. & epigenetics | IF: VE-cad, CD31, PDGFRβ; FC: CD31, CD45; WB: RAP1, ETV2, ETS1, p-AKT; vessel area | Vascular tube formation; glucose-responsive insulin-secreting (islets); intestine & organoid vascularization | 12 w | [136] | |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Cleaver, O.; Melton, D.A. Endothelial signaling during development. Nat. Med. 2003, 9, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Jourde-Chiche, N.; Fakhouri, F.; Dou, L.; Bellien, J.; Burtey, S.; Frimat, M.; Jarrot, P.A.; Kaplanski, G.; Le Quintrec, M.; Pernin, V.; et al. Endothelium structure and function in kidney health and disease. Nat. Rev. Nephrol. 2019, 15, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S. New aspects in fenestrated capillary and tissue dynamics in the sensory circumventricular organs of adult brains. Front. Neurosci. 2015, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. Getting Leukocytes to the Site of Inflammation. Vet. Pathol. 2013, 50, 7. [Google Scholar] [CrossRef]
- Parkar, N.S.; Akpa, B.S.; Nitsche, L.C.; Wedgewood, L.E.; Place, A.T.; Sverdlov, M.S.; Chaga, O.; Minshall, R.D. Vesicle Formation and Endocytosis: Function, Machinery, Mechanisms, and Modeling. Antioxid. Redox Signal. 2009, 11, 1301. [Google Scholar] [CrossRef]
- Milici, A.J.; Furie, M.B.; Carley, W.W. The formation of fenestrations and channels by capillary endothelium in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 6181–6185. [Google Scholar] [CrossRef]
- Rubin, L.L.; Hall, D.E.; Porter, S.; Barbu, K.; Cannon, C.; Horner, H.C.; Janatpour, M.; Liaw, C.W.; Manning, K.; Morales, J.; et al. A cell culture model of the blood-brain barrier. J. Cell Biol. 1991, 115, 1725–1735. [Google Scholar] [CrossRef]
- Chi, J.T.; Chang, H.Y.; Haraldsen, G.; Jahnsen, F.L.; Troyanskaya, O.G.; Chang, D.S.; Wang, Z.; Rockson, S.G.; Van De Rijn, M.; Botstein, D.; et al. Endothelial cell diversity revealed by global expression profiling. Proc. Natl. Acad. Sci. USA 2003, 100, 10623–10628. [Google Scholar] [CrossRef]
- Burridge, K.A.; Friedman, M.H. Environment and vascular bed origin influence differences in endothelial transcriptional profiles of coronary and iliac arteries. Am. J. Physiol. Hear. Circ. Physiol. 2010, 299, H837–H846. [Google Scholar] [CrossRef]
- Coultas, L.; Chawengsaksophak, K.; Rossant, J. Endothelial cells and VEGF in vascular development. Nature 2005, 438, 937–945. [Google Scholar] [CrossRef]
- Yamaguchi, T.P.; Dumont, D.J.; Conlon, R.A.; Breitman, M.L.; Rossant, J. Flk-1, an fit-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 1993, 118, 489–498. [Google Scholar] [CrossRef]
- Drake, C.J.; Fleming, P.A. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood 2000, 95, 1671–1679. [Google Scholar] [CrossRef]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O’Shea, K.S.; Powell-Braxton, L.; Hillan, K.J.; Moore, M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [Google Scholar] [CrossRef]
- Shalaby, F.; Janet, R.; Yamaguchi, T.P.; Gertsenstein, M.; Wu, X.F.; Breitman, M.L.; Schuh, A.C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995, 376, 62–66. [Google Scholar] [CrossRef]
- Fong, G.H.; Rossant, J.; Gertsenstein, M.; Breitman, M.L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995, 376, 66–70. [Google Scholar] [CrossRef]
- De Val, S.; Anderson, J.P.; Heidt, A.B.; Khiem, D.; Xu, S.M.; Black, B.L. Mef2c is activated directly by Ets transcription factors through an evolutionarily conserved endothelial cell-specific enhancer. Dev. Biol. 2004, 275, 424–434. [Google Scholar] [CrossRef]
- Ferdous, A.; Caprioli, A.; Iacovino, M.; Martin, C.M.; Morris, J.; Richardson, J.A.; Latif, S.; Hammer, R.E.; Harvey, R.P.; Olson, E.N.; et al. Nkx2–5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc. Natl. Acad. Sci. USA 2009, 106, 814–819. [Google Scholar] [CrossRef]
- Lee, D.; Park, C.; Lee, H.; Lugus, J.J.; Kim, S.H.; Arentson, E.; Chung, Y.S.; Gomez, G.; Kyba, M.; Lin, S.; et al. ER71 Acts Downstream of BMP, Notch, and Wnt Signaling in Blood and Vessel Progenitor Specification. Cell Stem Cell 2008, 2, 497–507. [Google Scholar] [CrossRef]
- Greenberg, J.M.; Thompson, F.Y.; Brooks, S.K.; Shannon, J.M.; McCormick-Shannon, K.; Cameron, J.E.; Mallory, B.P.; Akeson, A.L. Mesenchymal expression of vascular endothelial growth factors D and A defines vascular patterning in developing lung. Dev. Dyn. 2002, 224, 144–153. [Google Scholar] [CrossRef]
- Yang, J.; Hernandez, B.J.; Alanis, D.M.; del Pilar, O.N.; Vila-Ellis, L.; Akiyama, H.; Evans, S.E.; Ostrin, E.J.; Chen, J. The development and plasticity of alveolar type 1 cells. Development 2016, 143, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Parera, M.C.; Van Dooren, M.; Van Kempen, M.; De Krijger, R.; Grosveld, F.; Tibboel, D.; Rottier, R. Distal angiogenesis: A new concept for lung vascular morphogenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.L.; Walker, D.J.; Cwiklinski, E.; Tait, C.; Tee, A.R.; Land, S.C. Control of HIF-1α and vascular signaling in fetal lung involves cross talk between mTORC1 and the FGF-10/FGFR2b/Spry2 airway branching periodicity clock. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, 455–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- White, A.C.; Lavine, K.J.; Ornitz, D.M. FGF9 and SHH regulate mesenchymal Vegfa expression and development of the pulmonary capillary network. Development 2007, 134, 3743–3752. [Google Scholar] [CrossRef]
- Giordano, F.J.; Gerber, H.P.; Williams, S.P.; Vanbruggen, N.; Bunting, S.; Ruiz-Lozano, P.; Gu, Y.; Nath, A.K.; Huang, Y.; Hickey, R.; et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc. Natl. Acad. Sci. USA 2001, 98, 5780–5785. [Google Scholar] [CrossRef]
- Winnier, G.; Blessing, M.; Labosky, P.A.; Hogan, B.L.M. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995, 9, 2105–2116. [Google Scholar] [CrossRef]
- Chang, H.; Huylebroeck, D.; Verschueren, K.; Guo, Q.; Matzuk, M.M.; Zwijsen, A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development 1999, 126, 1631–1642. [Google Scholar] [CrossRef]
- Sirard, C.; De La Pompa, J.L.; Elia, A.; Itie, A.; Mirtsos, C.; Cheung, A.; Hahn, S.; Wakeham, A.; Schwartz, L.; Kern, S.E.; et al. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev. 1998, 12, 107–119. [Google Scholar] [CrossRef]
- Yang, X.; Li, C.; Xu, X.; Deng, C. The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc. Natl. Acad. Sci. USA 1998, 95, 3667–3672. [Google Scholar] [CrossRef]
- Kelly, M.A.; Hirschi, K.K. Signaling hierarchy regulating human endothelial cell development. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 718–724. [Google Scholar] [CrossRef]
- Daems, M.; Peacock, H.M.; Jones, E.A.V. Fluid flow as a driver of embryonic morphogenesis. Development 2020, 147, 1–40. [Google Scholar] [CrossRef]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef]
- Lucitti, J.L.; Jones, E.A.V.; Huang, C.; Chen, J.; Fraser, S.E.; Dickinson, M.E. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 2007, 134, 3317–3326. [Google Scholar] [CrossRef]
- Chouinard-Pelletier, G.; Jahnsen, E.D.; Jones, E.A. V Increased shear stress inhibits angiogenesis in veins and not arteries during vascular development. Angiogenesis 2013, 16, 71–83. [Google Scholar] [CrossRef]
- le Noble, F.; Moyon, D.; Pardanaud, L.; Yuan, L.; Djonov, V.; Matthijsen, R.; Bréant, C.; Fleury, V.; Eichmann, A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development 2004, 131, 361–375. [Google Scholar] [CrossRef]
- Chong, D.C.; Koo, Y.; Xu, K.; Fu, S.; Cleaver, O. Stepwise arteriovenous fate acquisition during mammalian vasculogenesis. Dev. Dyn. 2011, 240, 2153–2165. [Google Scholar] [CrossRef]
- Buschmann, I.; Pries, A.; Styp-Rekowska, B.; Hillmeister, P.; Loufrani, L.; Henrion, D.; Shi, Y.; Duelsner, A.; Hoefer, I.; Gatzke, N.; et al. Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development 2010, 137, 2187–2196. [Google Scholar] [CrossRef]
- Stenman, J.M.; Rajagopal, J.; Carroll, T.J.; Ishibashi, M.; McMahon, J.; McMahon, A.P. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 2008, 322, 1247–1250. [Google Scholar] [CrossRef]
- Daneman, R.; Agalliu, D.; Zhou, L.; Kuhnert, F.; Kuo, C.J.; Barres, B.A. Wnt/β-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 641–646. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Tischfield, M.; Williams, J.; Smallwood, P.M.; Rattner, A.; Taketo, M.M.; Nathans, J. Canonical WNT signaling components in vascular development and barrier formation. J. Clin. Investig. 2014, 124, 3825–3846. [Google Scholar] [CrossRef]
- He, L.; Vanlandewijck, M.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. Data Descriptor: Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci. Data 2018, 5, 180160. [Google Scholar] [CrossRef]
- Kuhnert, F.; Mancuso, M.R.; Shamloo, A.; Wang, H.T.; Choksi, V.; Florek, M.; Su, H.; Fruttiger, M.; Young, W.L.; Heilshorn, S.C.; et al. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science 2010, 330, 985–989. [Google Scholar] [CrossRef]
- Anderson, K.D.; Pan, L.; Yang, X.M.; Hughes, V.C.; Walls, J.R.; Dominguez, M.G.; Simmons, M.V.; Burfeind, P.; Xue, Y.; Wei, Y.; et al. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 2807–2812. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonnière, L.; Bernard, M.; et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef]
- Géraud, C.; Koch, P.S.; Zierow, J.; Klapproth, K.; Busch, K.; Olsavszky, V.; Leibing, T.; Demory, A.; Ulbrich, F.; Diett, M.; et al. GATA4-dependent organ-specific endothelial differentiation controls liver development and embryonic hematopoiesis. J. Clin. Investig. 2017, 127, 1099–1114. [Google Scholar] [CrossRef] [PubMed]
- Desroches-Castan, A.; Tillet, E.; Ricard, N.; Ouarné, M.; Mallet, C.; Belmudes, L.; Couté, Y.; Boillot, O.; Scoazec, J.-Y.; Bailly, S.; et al. Bone Morphogenetic Protein 9 Is a Paracrine Factor Controlling Liver Sinusoidal Endothelial Cell Fenestration and Protecting Against Hepatic Fibrosis. Hepatology 2019, 70, 1392–1408. [Google Scholar] [CrossRef] [PubMed]
- Boström, K.I.; Yao, J.; Wu, X.; Yao, Y. Endothelial Cells May Have Tissue-Specific Origins. J. Cell Biol. Histol. 2018, 1, 104. [Google Scholar]
- Goldman, O.; Han, S.; Hamou, W.; Jodon De Villeroche, V.; Uzan, G.; Lickert, H.; Gouon-Evans, V. Endoderm Generates Endothelial Cells during Liver Development. Stem Cell Rep. 2014, 3, 556–565. [Google Scholar] [CrossRef]
- Lotto, J.; Drissler, S.; Cullum, R.; Wei, W.; Setty, M.; Bell, E.M.; Boutet, S.C.; Nowotschin, S.; Kuo, Y.Y.; Garg, V.; et al. Single-Cell Transcriptomics Reveals Early Emergence of Liver Parenchymal and Non-parenchymal Cell Lineages. Cell 2020, 183, 702–716.e14. [Google Scholar] [CrossRef]
- Little, D.R.; Gerner-Mauro, K.N.; Flodby, P.; Crandall, E.D.; Borok, Z.; Akiyama, H.; Kimura, S.; Ostrin, E.J.; Chen, J. Transcriptional control of lung alveolar type 1 cell development and maintenance by NK homeobox 2–1. Proc. Natl. Acad. Sci. USA 2019, 116, 20545–20555. [Google Scholar] [CrossRef]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ. Res. 2007, 100, 174–190. [Google Scholar] [CrossRef]
- Hennigs, J.K.; Matuszcak, C.; Trepel, M.; Körbelin, J. Vascular endothelial cells: Heterogeneity and targeting approaches. Cells 2021, 10, 2712. [Google Scholar] [CrossRef]
- Chavkin, N.W.; Hirschi, K.K. Single Cell Analysis in Vascular Biology. Front. Cardiovasc. Med. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Den Braanker, H.; van Stigt, A.C.; Kok, M.R.; Lubberts, E.; Bisoendial, R.J. Single-cell RNA sequencing reveals heterogeneity and functional diversity of lymphatic endothelial cells. Int. J. Mol. Sci. 2021, 22, 11976. [Google Scholar] [CrossRef]
- Gomez-Salinero, J.M.; Itkin, T.; Rafii, S. Developmental angiocrine diversification of endothelial cells for organotypic regeneration. Dev. Cell 2021, 56, 3042–3051. [Google Scholar] [CrossRef]
- Minami, T.; Muramatsu, M.; Kume, T. Organ/tissue-specific vascular endothelial cell heterogeneity in health and disease. Biol. Pharm. Bull. 2019, 42, 1609–1619. [Google Scholar] [CrossRef]
- Koch, P.S.; Lee, K.H.; Goerdt, S.; Augustin, H.G. Angiodiversity and organotypic functions of sinusoidal endothelial cells. Angiogenesis 2021, 24, 289–310. [Google Scholar] [CrossRef]
- Nolan, D.J.; Ginsberg, M.; Israely, E.; Palikuqi, B.; Poulos, M.G.; James, D.; Ding, B.S.; Schachterle, W.; Liu, Y.; Rosenwaks, Z.; et al. Molecular Signatures of Tissue-Specific Microvascular Endothelial Cell Heterogeneity in Organ Maintenance and Regeneration. Dev. Cell 2013, 26, 204–219. [Google Scholar] [CrossRef]
- Sørensen, K.K.; Simon-Santamaria, J.; McCuskey, R.S.; Smedsrød, B. Liver sinusoidal endothelial cells. Compr. Physiol. 2015, 5, 1751–1774. [Google Scholar] [CrossRef]
- Wisse, E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J. Ultrasructure Res. 1970, 31, 125–150. [Google Scholar] [CrossRef]
- Debbaut, C.; Segers, P.; Cornillie, P.; Casteleyn, C.; Dierick, M.; Laleman, W.; Monbaliu, D. Analyzing the human liver vascular architecture by combining vascular corrosion casting and micro-CT scanning: A feasibility study. J. Anat. 2014, 224, 509–517. [Google Scholar] [CrossRef]
- Kardon, R.H.; Kessel, R.G. Three-dimensional organization of the hepatic microcirculation in the rodent as observed by scanning electron microscopy of corrosion casts. Gastroenterology 1980, 79, 72–81. [Google Scholar] [CrossRef]
- Jiřík, M.; Tonar, Z.; Králíčková, A.; Eberlová, L.; Mírka, H.; Kochová, P.; Gregor, T.; Hošek, P.; Svobodová, M.; Rohan, E.; et al. Stereological quantification of microvessels using semiautomated evaluation of X-ray microtomography of hepatic vascular corrosion casts. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 1803–1819. [Google Scholar] [CrossRef]
- de Haan, W.; Dheedene, W.; Apelt, K.; Décombas-Deschamps, S.; Vinckier, S.; Verhulst, S.; Conidi, A.; Deffieux, T.; Staring, M.W.; Vandervoort, P.; et al. Endothelial Zeb2 preserves the hepatic angioarchitecture and protects against liver fibrosis. Cardiovasc. Res. 2021, 1–14. [Google Scholar] [CrossRef]
- Vidal-Vanaclocha, F.; Barberá-Guillem, E. Fenestration patterns in endothelial cells of rat liver sinusoids. J. Ultrastruct. Res. Mol. Struct. Res. 1985, 90, 115–123. [Google Scholar] [CrossRef]
- Barberá-Guillem, E.; Rocha, M.; Alvarez, A.; Vidal-Vanaclocha, F. Differences in the lectin-binding patterns of the periportal and perivenous endothelial domains in the liver sinusoids. Hepatology 1991, 14, 131–139. [Google Scholar] [CrossRef]
- Wisse, E.; de Zanger, R.B.; Charels, K.; van der Smissen, P.; McCuskey, R.S. The liver sieve: Considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of disse. Hepatology 1985, 5, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Coppiello, G.; Collantes, M.; Sirerol-Piquer, M.S.; Vandenwijngaert, S.; Schoors, S.; Swinnen, M.; Vandersmissen, I.; Herijgers, P.; Topal, B.; Van Loon, J.; et al. Meox2/Tcf15 heterodimers program the heart capillary endothelium for cardiac fatty acid uptake. Circulation 2015, 131, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Zhou, L.; Agalliu, D.; Cahoy, J.D.; Kaushal, A.; Barres, B.A. The Mouse Blood-Brain Barrier Transcriptome: A New Resource for Understanding the Development and Function of Brain Endothelial Cells. PLoS ONE 2010, 5, e13741. [Google Scholar] [CrossRef] [PubMed]
- Géraud, C.; Schledzewski, K.; Demory, A.; Klein, D.; Kaus, M.; Peyre, F.; Sticht, C.; Evdokimov, K.; Lu, S.; Schmieder, A.; et al. Liver sinusoidal endothelium: A microenvironment-dependent differentiation program in rat including the novel junctional protein liver endothelial differentiation-associated protein-1. Hepatology 2010, 52, 313–326. [Google Scholar] [CrossRef]
- Khan, S.; Taverna, F.; Rohlenova, K.; Treps, L.; Geldhof, V.; De Rooij, L.; Sokol, L.; Pircher, A.; Conradi, L.C.; Kalucka, J.; et al. EndoDB: A database of endothelial cell transcriptomics data. Nucleic Acids Res. 2019, 47, D736–D744. [Google Scholar] [CrossRef]
- Elvevold, K.; Smedsrød, B.; Martinez, I. The liver sinusoidal endothelial cell: A cell type of controversial and confusing identity. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G391–G400. [Google Scholar] [CrossRef]
- Lalor, P.F.; Lai, W.K.; Curbishley, S.M.; Shetty, S.; Adams, D.H. Human hepatic sinusoidal endothelial cells can be distinguished by expression of phenotypic markers related to their specialised functions in vivo. World J. Gastroenterol. 2006, 12, 5429–5439. [Google Scholar] [CrossRef]
- Ramachandran, P.; Dobie, R.; Wilson-Kanamori, J.R.; Dora, E.F.; Henderson, B.E.P.; Luu, N.T.; Portman, J.R.; Matchett, K.P.; Brice, M.; Marwick, J.A.; et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 2019, 575, 512–518. [Google Scholar] [CrossRef]
- Miller, S.; Walker, S.W.; Arthur, J.R.; Lewin, M.H.; Pickard, K.; Nicol, F.; Howie, A.F.; Beckett, G.J. Selenoprotein expression in endothelial cells from different human vasculature and species. Biochim. Biophys. Acta Mol. Basis Dis. 2002, 1588, 85–93. [Google Scholar] [CrossRef][Green Version]
- Soroush, F.; Tang, Y.; Mustafa, O.; Sun, S.; Yang, Q.; Kilpatrick, L.E.; Kiani, M.F. Neutrophil-endothelial interactions of murine cells is not a good predictor of their interactions in human cells. FASEB J. 2020, 34, 2691–2702. [Google Scholar] [CrossRef]
- Ramachandran, P.; Matchett, K.P.; Dobie, R.; Wilson-Kanamori, J.R.; Henderson, N.C. Single-cell technologies in hepatology: New insights into liver biology and disease pathogenesis. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 457–472. [Google Scholar] [CrossRef]
- Kalucka, J.; de Rooij, L.P.M.H.; Goveia, J.; Rohlenova, K.; Dumas, S.J.; Meta, E.; Conchinha, N.V.; Taverna, F.; Teuwen, L.A.; Veys, K.; et al. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell 2020, 180, 764.e20–779.e20. [Google Scholar] [CrossRef]
- Vanlandewijck, M.; He, L.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef]
- Aizarani, N.; Saviano, A.; Sagar; Mailly, L.; Durand, S.; Herman, J.S.; Pessaux, P.; Baumert, T.F.; Grün, D. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature 2019, 572, 199–204. [Google Scholar] [CrossRef]
- Halpern, K.B.; Shenhav, R.; Massalha, H.; Toth, B.; Egozi, A.; Massasa, E.E.; Medgalia, C.; David, E.; Giladi, A.; Moor, A.E.; et al. Paired-cell sequencing enables spatial gene expression mapping of liver endothelial cells. Nat. Biotechnol. 2018, 36, 962. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Yang, Y.; Lai, S.; Jeong, J.; Jung, Y.; McConnell, M.; Utsumi, T.; Iwakiri, Y. Single-Cell Transcriptomics Reveals Zone-Specific Alterations of Liver Sinusoidal Endothelial Cells in Cirrhosis. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1139–1161. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Kuang, H.; Ansari, S.; Liu, T.; Gong, J.; Wang, S.; Zhao, X.-Y.; Ji, Y.; Li, C.; Guo, L.; et al. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Mol. Cell 2019, 75, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.; Paik, D.T.; Yang, J.Y.; Nagelberg, D.; Williams, I.; Tian, L.; Roth, R.; Chandy, M.; Ban, J.; Belbachir, N.; et al. Endocardial/endothelial angiocrines regulate cardiomyocyte development and maturation and induce features of ventricular non-compaction. Eur. Heart J. 2021, 42, 4264–4276. [Google Scholar] [CrossRef]
- Jiron Tamburini, B.A.; Finlon, J.M.; Gillen, A.E.; Kriss, M.S.; Riemondy, K.A.; Fu, R.; Schuyler, R.P.; Hesselberth, J.R.; Rosen, H.R.; Burchill, M.A. Chronic liver disease in humans causes expansion and differentiation of liver lymphatic endothelial cells. Front. Immunol. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Verhulst, S.; van Os, E.A.; De Smet, V.; Eysackers, N.; Mannaerts, I.; van Grunsven, L.A. Gene Signatures Detect Damaged Liver Sinusoidal Endothelial Cells in Chronic Liver Diseases. Front. Med. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Burchill, M.A.; Finlon, J.M.; Goldberg, A.R.; Gillen, A.E.; Dahms, P.A.; McMahan, R.H.; Tye, A.; Winter, A.B.; Reisz, J.A.; Bohrnsen, E.; et al. Oxidized Low-Density Lipoprotein Drives Dysfunction of the Liver Lymphatic System. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 573–595. [Google Scholar] [CrossRef]
- Koenitzer, J.R.; Wu, H.; Atkinson, J.J.; Brody, S.L.; Humphreys, B.D. Single-nucleus RNA-sequencing profiling of mouse lung reduced dissociation bias and improved rare cell-type detection compared with single-cell RNA sequencing. Am. J. Respir. Cell Mol. Biol. 2020, 63, 739–747. [Google Scholar] [CrossRef]
- Wolfien, M.; Galow, A.M.; Müller, P.; Bartsch, M.; Brunner, R.M.; Goldammer, T.; Wolkenhauer, O.; Hoeflich, A.; David, R. Single nuclei sequencing of entire mammalian hearts: Strain-dependent cell-type composition and velocity. Cardiovasc. Res. 2020, 116, 1249–1251. [Google Scholar] [CrossRef]
- Wu, H.; Kirita, Y.; Donnelly, E.L.; Humphreys, B.D. Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: Rare cell types and novel cell states revealed in fibrosis. J. Am. Soc. Nephrol. 2019, 30, 23–32. [Google Scholar] [CrossRef]
- Yamada, S.; Nomura, S. Review of single-cell RNA sequencing in the heart. Int. J. Mol. Sci. 2020, 21, 8345. [Google Scholar] [CrossRef]
- Cavalli, M.; Diamanti, K.; Pan, G.; Spalinskas, R.; Kumar, C.; Deshmukh, A.S.; Mann, M.; Sahlén, P.; Komorowski, J.; Wadelius, C. A Multi-Omics Approach to Liver Diseases: Integration of Single Nuclei Transcriptomics with Proteomics and HiCap Bulk Data in Human Liver. Omi. A J. Integr. Biol. 2020, 24, 180–194. [Google Scholar] [CrossRef]
- Guilliams, M.; Bonnardel, J.; Haest, B.; Vanderborght, B.; Wagner, C.; Remmerie, A.; Bujko, A.; Martens, L.; Thoné, T.; Browaeys, R.; et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell 2022, 185, 1–18. [Google Scholar] [CrossRef]
- Moncada, R.; Barkley, D.; Wagner, F.; Chiodin, M.; Devlin, J.C.; Baron, M.; Hajdu, C.H.; Simeone, D.M.; Yanai, I. Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nat. Biotechnol. 2020, 38, 333–342. [Google Scholar] [CrossRef]
- Hou, X.; Yang, Y.; Li, P.; Zeng, Z.; Hu, W.; Zhe, R.; Liu, X.; Tang, D.; Ou, M.; Dai, Y. Integrating Spatial Transcriptomics and Single-Cell RNA-seq Reveals the Gene Expression Profling of the Human Embryonic Liver. Front. Cell Dev. Biol. 2021, 9, 1–14. [Google Scholar] [CrossRef]
- Inverso, D.; Shi, J.; Lee, K.H.; Jakab, M.; Ben-Moshe, S.; Kulkarni, S.R.; Schneider, M.; Wang, G.; Komeili, M.; Vélez, P.A.; et al. A spatial vascular transcriptomic, proteomic, and phosphoproteomic atlas unveils an angiocrine Tie–Wnt signaling axis in the liver. Dev. Cell 2021, 56, 1677.e10–1693.e10. [Google Scholar] [CrossRef]
- Andueza, A.; Kumar, S.; Kim, J.; Kang, D.-W.; Mumme, H.L.; Perez, J.I.; Villa-Roel, N.; Jo, H. Endothelial Reprogramming by Disturbed Flow Revealed by Single-Cell RNA and Chromatin Accessibility Study. Cell Rep. 2020, 33, 108491. [Google Scholar] [CrossRef]
- Cao, J.; Cusanovich, D.A.; Ramani, V.; Aghamirzaie, D.; Pliner, A.; Hill, H.; Daza, A.J.; McFaline-Figueroa, R.M.; Packer, J.L.; Christiansen, S.; et al. Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science 2018, 361, 1380–1385. [Google Scholar] [CrossRef]
- Muto, Y.; Wilson, P.C.; Ledru, N.; Wu, H.; Dimke, H.; Waikar, S.S.; Humphreys, B.D. Single cell transcriptional and chromatin accessibility profiling redefine cellular heterogeneity in the adult human kidney. Nat. Commun. 2021, 12, 2190. [Google Scholar] [CrossRef]
- Sabbagh, M.F.; Heng, J.S.; Luo, C.; Castanon, R.G.; Nery, J.R.; Rattner, A.; Goff, L.A.; Ecker, J.R.; Nathans, J. Transcriptional and epigenomic landscapes of CNS and non-CNS vascular endothelial cells. eLife 2018, 7, 1–44. [Google Scholar] [CrossRef]
- Schlereth, K.; Weichenhan, D.; Bauer, T.; Heumann, T.; Giannakouri, E.; Lipka, D.; Jaeger, S.; Schlesner, M.; Aloy, P.; Eils, R.; et al. The transcriptomic and epigenetic map of vascular quiescence in the continuous lung endothelium. eLife 2018, 7, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Oh, S.; Gregory, S.; Shen, X.; Diehl, A.M. Single-cell omics analysis reveals functional diversification of hepatocytes during liver regeneration. JCI Insight 2020, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, M.; Shah, A.M.; Tan, W.; Liu, N.; Duby, B.-R.; Olson, E.N. Cell-Type-Specific Gene Regulatory Networks Underlying Murine Neonatal Heart Regeneration at Single-Cell Resolution. Cell Rep. 2020, 33, 108472. [Google Scholar] [CrossRef]
- Marx, V. A dream of single-cell proteomics. Nat. Methods 2019, 16, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Powell, D.R.; Curtis, D.J.; Wong, N.C. From reads to insight: A hitchhiker’s guide to ATAC-seq data analysis. Genome Biol. 2020, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Marcu, R.; Choi, Y.J.; Xue, J.; Fortin, C.L.; Wang, Y.; Nagao, R.J.; Xu, J.; MacDonald, J.W.; Bammler, T.K.; Murry, C.E.; et al. Human Organ-Specific Endothelial Cell Heterogeneity. iScience 2018, 4, 20–35. [Google Scholar] [CrossRef] [PubMed]
- De Haan, W.; Øie, C.; Benkheil, M.; Dheedene, W.; Vinckier, S.; Coppiello, G.; Aranguren, X.L.; Beerens, M.; Jaekers, J.; Topal, B.; et al. Unraveling the transcriptional determinants of liver sinusoidal endothelial cell specialization. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G803–G815. [Google Scholar] [CrossRef] [PubMed]
- Jambusaria, A.; Hong, Z.; Zhang, L.; Srivastava, S.; Jana, A.; Toth, P.T.; Dai, Y.; Malik, A.B.; Rehman, J. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. eLife 2020, 9, 1–32. [Google Scholar] [CrossRef]
- Arai, T.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Iinuma, N.; Iesato, Y.; Koyama, T.; Yoshizawa, T.; Uetake, R.; Yamauchi, A.; et al. Induction of LYVE-1/stabilin-2-positive liver sinusoidal endothelial-like cells from embryoid bodies by modulation of adrenomedullin-RAMP2 signaling. Peptides 2011, 32, 1855–1865. [Google Scholar] [CrossRef]
- Gage, B.K.; Liu, J.C.; Innes, B.T.; MacParland, S.A.; McGilvray, I.D.; Bader, G.D.; Keller, G.M. Generation of Functional Liver Sinusoidal Endothelial Cells from Human Pluripotent Stem-Cell-Derived Venous Angioblasts. Cell Stem Cell 2020, 27, 254–269.e9. [Google Scholar] [CrossRef]
- Nonaka, H.; Watabe, T.; Saito, S.; Miyazono, K.; Miyajima, A. Development of stabilin2+ endothelial cells from mouse embryonic stem cells by inhibition of TGFβ/activin signaling. Biochem. Biophys. Res. Commun. 2008, 375, 256–260. [Google Scholar] [CrossRef]
- De Smedt, J.; van Os, E.A.; Talon, I.; Ghosh, S.; Toprakhisar, B.; Furtado Madeiro Da Costa, R.; Zaunz, S.; Vazquez, M.A.; Boon, R.; Baatsen, P.; et al. PU.1 drives specification of pluripotent stem cell-derived endothelial cells to LSEC-like cells. Cell Death Dis. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Shahani, T.; Covens, K.; Lavend’homme, R.; Jazouli, N.; Sokal, E.; Peerlinck, K.; Jacquemin, M. Human liver sinusoidal endothelial cells but not hepatocytes contain factor VIII. J. Thromb. Haemost. 2014, 12, 36–42. [Google Scholar] [CrossRef]
- Zapotoczny, B.; Szafranska, K.; Kus, E.; Braet, F.; Wisse, E.; Chlopicki, S.; Szymonski, M. Tracking Fenestrae Dynamics in Live Murine Liver Sinusoidal Endothelial Cells. Hepatology 2019, 69, 876–888. [Google Scholar] [CrossRef]
- Wijesekara, P.; Ng, W.H.; Feng, M.; Ren, X. Bioengineering the innate vasculature of complex organs: What have we learned so far. Curr. Opin. Organ Transplant. 2018, 23, 657–663. [Google Scholar] [CrossRef]
- Vargas-Valderrama, A.; Messina, A.; Mitjavila-Garcia, M.T.; Guenou, H. The endothelium, a key actor in organ development and hPSC-derived organoid vascularization. J. Biomed. Sci. 2020, 27, 1–13. [Google Scholar] [CrossRef]
- Tambe, D.T.; Croutelle, U.; Trepat, X.; Park, C.Y.; Kim, J.H.; Millet, E.; Butler, J.P.; Fredberg, J.J. Monolayer Stress Microscopy: Limitations, Artifacts, and Accuracy of Recovered Intercellular Stresses. PLoS ONE 2013, 8, e55172. [Google Scholar] [CrossRef]
- Cucullo, L.; Hossain, M.; Puvenna, V.; Marchi, N.; Janigro, D. The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neurosci. 2011, 12, 40. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Sinha, S.; Peterson, A.; Grant, G.A.; Yang, F. Mimicking brain tumor-vasculature microanatomical architecture via co-culture of brain tumor and endothelial cells in 3D hydrogels. Biomaterials 2019, 202, 35–44. [Google Scholar] [CrossRef]
- Reinitz, A.; DeStefano, J.; Ye, M.; Wong, A.D.; Searson, P.C. Human brain microvascular endothelial cells resist elongation due to shear stress. Microvasc. Res. 2015, 99, 8–18. [Google Scholar] [CrossRef]
- Birukov, K.G.; Jacobson, J.R.; Flores, A.A.; Ye, S.Q.; Birukova, A.A.; Verin, A.D.; Garcia, J.G.N. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2003, 285, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Imamura, H.; Ando, J. Shear stress augments mitochondrial atp generation that triggers atp release and Ca2+ signaling in vascular endothelial cells. Am. J. Physiol.—Hear. Circ. Physiol. 2018, 315, H1477–H1485. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Suki, B.; Kume, H.; Numaguchi, Y.; Ishii, M.; Iwaki, M.; Kondo, M.; Naruse, K.; Hasegawa, Y.; Sokabe, M. Actin cytoskeleton regulates stretch-activated Ca2+ influx in human pulmonary microvascular endothelial cells. Am. J. Respir. Cell Mol. Biol. 2010, 43, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Merna, N.; Wong, A.K.; Barahona, V.; Llanos, P.; Kunar, B.; Palikuqi, B.; Ginsberg, M.; Rafii, S.; Rabbany, S.Y. Laminar shear stress modulates endothelial luminal surface stiffness in a tissue-specific manner. Microcirculation 2018, 25, e12455. [Google Scholar] [CrossRef]
- Bacci, C.; Wong, V.; Barahona, V.; Merna, N. Cardiac and lung endothelial cells in response to fluid shear stress on physiological matrix stiffness and composition. Microcirculation 2021, 28, e12659. [Google Scholar] [CrossRef]
- Mitra, D.; Jaffe, E.A.; Weksler, B.; Hajjar, K.A.; Soderland, C.; Laurence, J. Thrombotic thrombocytopenic purpura and sporadic hemolytic-uremic syndrome plasmas induce apoptosis in restricted lineages of human microvascular endothelial cells. Blood 1997, 89, 1224–1234. [Google Scholar] [CrossRef]
- Bartoszewski, R.; Moszyńska, A.; Serocki, M.; Cabaj, A.; Polten, A.; Ochocka, R.; Dell’Italia, L.; Bartoszewska, S.; Króliczewski, J.; Dąbrowski, M.; et al. Primary endothelial-specific regulation of hypoxiainducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia. FASEB J. 2019, 33, 7929–7941. [Google Scholar] [CrossRef]
- Galie, P.A.; Van Oosten, A.; Chen, C.S.; Janmey, P.A. Application of multiple levels of fluid shear stress to endothelial cells plated on polyacrylamide gels. Lab Chip 2015, 15, 1205–1212. [Google Scholar] [CrossRef]
- Hamm, S.; Dehouck, B.; Kraus, J.; Wolburg-Buchholz, K.; Wolburg, H.; Risau, W.; Cecchelli, R.; Engelhardt, B.; Dehouck, M.P. Astrocyte mediated modulation of blood-brain barrier permeability does not correlate with a loss of tight junction proteins from the cellular contacts. Cell Tissue Res. 2004, 315, 157–166. [Google Scholar] [CrossRef][Green Version]
- Castro Dias, M.; Coisne, C.; Lazarevic, I.; Baden, P.; Hata, M.; Iwamoto, N.; Francisco, D.M.F.; Vanlandewijck, M.; He, L.; Baier, F.A.; et al. Claudin-3-deficient C57BL/6J mice display intact brain barriers. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Sabbagh, M.F.; Nathans, J. A genome-wide view of the dedifferentiation of central nervous system endothelial cells in culture. eLife 2020, 9, e51276. [Google Scholar] [CrossRef]
- Liguori, G.R.; Liguori, T.T.A.; de Moraes, S.R.; Sinkunas, V.; Terlizzi, V.; van Dongen, J.A.; Sharma, P.K.; Moreira, L.F.P.; Harmsen, M.C. Molecular and Biomechanical Clues from Cardiac Tissue Decellularized Extracellular Matrix Drive Stromal Cell Plasticity. Front. Bioeng. Biotechnol. 2020, 8, 520. [Google Scholar] [CrossRef]
- Linville, R.M.; DeStefano, J.G.; Sklar, M.B.; Xu, Z.; Farrell, A.M.; Bogorad, M.I.; Chu, C.; Walczak, P.; Cheng, L.; Mahairaki, V.; et al. Human iPSC-derived blood-brain barrier microvessels: Validation of barrier function and endothelial cell behavior. Biomaterials 2019, 190–191, 24–37. [Google Scholar] [CrossRef]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.S.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef]
- Katt, M.E.; Linville, R.M.; Mayo, L.N.; Xu, Z.S.; Searson, P.C. Functional brain-specific microvessels from iPSC-derived human brain microvascular endothelial cells: The role of matrix composition on monolayer formation. Fluids Barriers CNS 2018, 15, 1–12. [Google Scholar] [CrossRef]
- Palikuqi, B.; Nguyen, D.H.T.; Li, G.; Schreiner, R.; Pellegata, A.F.; Liu, Y.; Redmond, D.; Geng, F.; Lin, Y.; Gómez-Salinero, J.M.; et al. Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature 2020, 585, 426–432. [Google Scholar] [CrossRef]
- Kocherova, I.; Bryja, A.; Mozdziak, P.; Volponi, A.A.; Dyszkiewicz-Konwińska, M.; Piotrowska-Kempisty, H.; Antosik, P.; Bukowska, D.; Bruska, M.; Iżycki, D.; et al. Human umbilical vein endothelial cells (HUVECs) co-culture with osteogenic cells: From molecular communication to engineering prevascularised bone grafts. J. Clin. Med. 2019, 8, 1602. [Google Scholar] [CrossRef]
- Baptista, P.M.; Siddiqui, M.M.; Lozier, G.; Rodriguez, S.R.; Atala, A.; Soker, S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 2011, 53, 604–617. [Google Scholar] [CrossRef]
- Franco, C.A.; Jones, M.L.; Bernabeu, M.O.; Vion, A.C.; Barbacena, P.; Fan, J.; Mathivet, T.; Fonseca, C.G.; Ragab, A.; Yamaguchi, T.P.; et al. Non-canonical wnt signalling modulates the endothelial shear stress flow sensor in vascular remodelling. eLife 2016, 5, 1–22. [Google Scholar] [CrossRef]
- Kusuma, S.; Zhao, S.; Gerecht, S. The extracellular matrix is a novel attribute of endothelial progenitors and of hypoxic mature endothelial cells. FASEB J. 2012, 26, 4925–4936. [Google Scholar] [CrossRef]
- Pappalardo, A.; Herron, L.; Alvarez Cespedes, D.E.; Abaci, H.E. Quantitative Evaluation of Human Umbilical Vein and Induced Pluripotent Stem Cell-Derived Endothelial Cells as an Alternative Cell Source to Skin-Specific Endothelial Cells in Engineered Skin Grafts. Adv. Wound Care 2021, 10, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Lacorre, D.A.; Baekkevold, E.S.; Garrido, I.; Brandtzaeg, P.; Haraldsen, G.; Amalric, F.; Girard, J.P. Plasticity of endothelial cells: Rapid dedifferentiation of freshly isolated high endothelial venule endothelial cells outside the lymphoid tissue microenvironment. Blood 2004, 103, 4164–4172. [Google Scholar] [CrossRef] [PubMed]
- Ricard, N.; Bailly, S.; Guignabert, C.; Simons, M. The quiescent endothelium: Signalling pathways regulating organ-specific endothelial normalcy. Nat. Rev. Cardiol. 2021, 18, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Molema, G. Heterogeneity in endothelial responsiveness to cytokines, molecular causes, and pharmacological consequences. Semin. Thromb. Hemost. 2010, 36, 246–264. [Google Scholar] [CrossRef]
- Dessalles, C.A.; Leclech, C.; Castagnino, A.; Barakat, A.I. Integration of substrate- and flow-derived stresses in endothelial cell mechanobiology. Commun. Biol. 2021, 4, 764. [Google Scholar] [CrossRef] [PubMed]
- Iskratsch, T.; Wolfenson, H.; Sheetz, M.P. Appreciating force and shape-the rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 2014, 15, 825–833. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Weickenmeier, J.; de Rooij, R.; Budday, S.; Steinmann, P.; Ovaert, T.C.; Kuhl, E. Brain stiffness increases with myelin content. Acta Biomater. 2016, 42, 265–272. [Google Scholar] [CrossRef]
- Ford, A.J.; Jain, G.; Rajagopalan, P. Designing a fibrotic microenvironment to investigate changes in human liver sinusoidal endothelial cell function. Acta Biomater. 2015, 24, 220–227. [Google Scholar] [CrossRef]
- Bhana, B.; Iyer, R.K.; Chen, W.L.K.; Zhao, R.; Sider, K.L.; Likhitpanichkul, M.; Simmons, C.A.; Radisic, M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol. Bioeng. 2010, 105, 1148–1160. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Fraser, R.; Bowler, L.M.; Day, W.A.; Dobbs, B.; Johnson, H.D.; Lee, D. High perfusion pressure damages the sieving ability of sinusoidal endothelium in rat livers. Br. J. Exp. Pathol. 1980, 61, 222–228. [Google Scholar]
- Shah, V.; Haddad, F.G.; Garcia-Cardena, G.; Frangos, J.A.; Mennone, A.; Groszmann, R.J.; Sessa, W.C. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J. Clin. Investig. 1997, 100, 2923–2930. [Google Scholar] [CrossRef]
- Rockey, D.C.; Chung, J.J. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: Endothelial dysfunction in portal hypertension. Gastroenterology 1998, 114, 344–351. [Google Scholar] [CrossRef]
- Kohn, J.C.; Zhou, D.W.; Bordeleau, F.; Zhou, A.L.; Mason, B.N.; Mitchell, M.J.; King, M.R.; Reinhart-King, C.A. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys. J. 2015, 108, 471–478. [Google Scholar] [CrossRef]
- Bastounis, E.E.; Yeh, Y.T.; Theriot, J.A. Subendothelial stiffness alters endothelial cell traction force generation while exerting a minimal effect on the transcriptome. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Sewell-Loftin, M.K.; Brown, C.B.; Baldwin, H.S.; Merryman, W.D. A novel technique for quantifying mouse heart valve leaflet stiffness with atomic force microscopy. J. Heart Valve Dis. 2012, 21, 513–520. [Google Scholar]
- Huebsch, N.; Arany, P.R.; Mao, A.S.; Shvartsman, D.; Ali, O.A.; Bencherif, S.A.; Rivera-Feliciano, J.; Mooney, D.J. Harnessing Traction-Mediated Manipulation of the Cell-Matrix Interface to Control Stem Cell Fate. Nat. Mater. 2010, 9, 518–526. [Google Scholar] [CrossRef]
- Tyler, W.J. The mechanobiology of brain function. Nat. Rev. Neurosci. 2012, 13, 867–878. [Google Scholar] [CrossRef]
- Potjewyd, G.; Kellett, K.A.B.; Hooper, N.M. 3D hydrogel models of the neurovascular unit to investigate blood–brain barrier dysfunction. Neuronal Signal. 2021, 5, 1–24. [Google Scholar] [CrossRef]
- Garcia-Polite, F.; Martorell, J.; Del Rey-Puech, P.; Melgar-Lesmes, P.; O’Brien, C.C.; Roquer, J.; Ois, A.; Principe, A.; Edelman, E.R.; Balcells, M. Pulsatility and high shear stress deteriorate barrier phenotype in brain microvascular endothelium. J. Cereb. Blood Flow Metab. 2017, 37, 2614–2625. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, A.M.; Kim, H.; O’Grady, K.P.; Richter, I.; Lee, L.; O’Grady, B.J.; Lippmann, E.S. Influence of Substrate Stiffness on Barrier Function in an iPSC-Derived In Vitro Blood-Brain Barrier Model. Cell. Mol. Bioeng. 2022, 15, 31–42. [Google Scholar] [CrossRef]
- Mahmoud, M.; Cancel, L.; Tarbell, J.M. Matrix Stiffness Affects Glycocalyx Expression in Cultured Endothelial Cells. Front. Cell Dev. Biol. 2021, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Novak, C.; Ballinger, M.N.; Ghadiali, S. Mechanobiology of Pulmonary Diseases: A Review of Engineering Tools to Understand Lung Mechanotransduction. J. Biomech. Eng. 2021, 143, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, M.; Ito, S.; Morioka, M.; Iwata, S.; Numaguchi, Y.; Ishii, M.; Kondo, M.; Kume, H.; Naruse, K.; Sokabe, M.; et al. Mechanical stretch enhances IL-8 production in pulmonary microvascular endothelial cells. Biochem. Biophys. Res. Commun. 2009, 389, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Mechanisms of endothelial cell heterogeneity in health and disease. Circ. Res. 2006, 98, 159–162. [Google Scholar] [CrossRef]
- Merna, N.; Fung, K.M.; Wang, J.J.; King, C.R.; Hansen, K.C.; Christman, K.L.; George, S.C. Differential β3 Integrin Expression Regulates the Response of Human Lung and Cardiac Fibroblasts to Extracellular Matrix and Its Components. Tissue Eng. Part A 2015, 21, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Shyy, J.Y.J.; Chien, S. Role of integrins in endothelial mechanosensing of shear stress. Circ. Res. 2002, 91, 769–775. [Google Scholar] [CrossRef]
- Collins, C.; Osborne, L.D.; Guilluy, C.; Chen, Z.; O’Brien, E.T., III; Reader, J.S.; Burridge, K.; Superfine, R.; Tzima, E. Haemodynamic and extracellular matrix cues regulate the mechanical phenotype and stiffness of aortic endothelial cells. Nat. Commun. 2014, 5, 3984. [Google Scholar] [CrossRef]
- Rickel, A.P.; Sanyour, H.J.; Leyda, N.A.; Hong, Z. Extracellular Matrix Proteins and Substrate Stiffness Synergistically Regulate Vascular Smooth Muscle Cell Migration and Cortical Cytoskeleton Organization. ACS Appl. Bio Mater. 2020, 3, 2360–2369. [Google Scholar] [CrossRef]
- Taha, I.N.; Naba, A. Exploring the extracellular matrix in health and disease using proteomics. Essays Biochem. 2019, 63, 417–432. [Google Scholar] [CrossRef]
- Byron, A.; Humphries, J.D.; Humphries, M.J. Defining the extracellular matrix using proteomics. Int. J. Exp. Pathol. 2013, 94, 75–92. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Shao, X.; Taha, I.N.; Clauser, K.R.; Gao, Y.; Naba, A. MatrisomeDB: The ECM-protein knowledge database. Nucleic Acids Res. 2020, 48, D1136–D1144. [Google Scholar] [CrossRef]
- Naba, A.; Gao, Y. MatrisomeDB. Available online: http://matrisomedb.pepchem.org/ (accessed on 19 December 2021).
- Choudhury, D.; Tun, H.W.; Wang, T.; Naing, M.W. Organ-Derived Decellularized Extracellular Matrix: A Game Changer for Bioink Manufacturing? Trends Biotechnol. 2018, 36, 787–805. [Google Scholar] [CrossRef]
- Randles, M.J.; Humphries, M.J.; Lennon, R. Proteomic definitions of basement membrane composition in health and disease. Matrix Biol. 2017, 57–58, 12–28. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Bharadwaj, S.; Hammam, N.; Carnagey, K.; Myers, R.; Atala, A.; Van Dyke, M. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 2009, 30, 4021–4028. [Google Scholar] [CrossRef]
- Su, J.; Satchell, S.C.; Shah, R.N.; Wertheim, J.A. Kidney decellularized extracellular matrix hydrogels: Rheological characterization and human glomerular endothelial cell response to encapsulation. J. Biomed. Mater. Res. Part A 2018, 106, 2448–2462. [Google Scholar] [CrossRef]
- Simsa, R.; Rothenbücher, T.; Gürbüz, H.; Ghosheh, N.; Emneus, J.; Jenndahl, L.; Kaplan, D.L.; Bergh, N.; Serrano, A.M.; Fogelstrand, P. Brain organoid formation on decellularized porcine brain ECM hydrogels. PLoS ONE 2021, 16, 1–22. [Google Scholar] [CrossRef]
- Reginensi, D.; Ortiz, D.; Pravia, A.; Burillo, A.; Morales, F.; Morgan, C.; Jimenez, L.; Dave, K.R.; Perez-Pinzon, M.A.; Gittens, R.A. Role of Region-Specific Brain Decellularized Extracellular Matrix on in Vitro Neuronal Maturation. Tissue Eng. Part A 2020, 26, 964–978. [Google Scholar] [CrossRef]
- Zhang, W.; Du, A.; Liu, S.; Lv, M.; Chen, S. Research progress in decellularized extracellular matrix-derived hydrogels. Regen. Ther. 2021, 18, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Baldea, I.; Teacoe, I.; Olteanu, D.E.; Vaida-Voievod, C.; Clichici, A.; Sirbu, A.; Filip, G.A.; Clichici, S. Effects of different hypoxia degrees on endothelial cell cultures—Time course study. Mech. Ageing Dev. 2018, 172, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.T.; Nguyen, D.; Stephens, D.; Pamuk, F.; Fernandes, D.; Hasturk, H.; Van Dyke, T.E.; Kantarci, A. Hypoxia-induced endothelial cell responses-possible roles during periodontal disease. Clin. Exp. Dent. Res. 2018, 4, 241–248. [Google Scholar] [CrossRef]
- Halder, S.K.; Milner, R. Mild hypoxia triggers transient blood–brain barrier disruption: A fundamental protective role for microglia. Acta Neuropathol. Commun. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Li, L.; Welser, J.V.; Milner, R. Absence of the αvβ3 integrin dictates the time-course of angiogenesis in the hypoxic central nervous system: Accelerated endothelial proliferation correlates with compensatory increases in α5Β1 integrin expression. J. Cereb. Blood Flow Metab. 2010, 30, 1031–1043. [Google Scholar] [CrossRef]
- Koh, M.Y.; Powis, G. Passing the baton: The HIF switch Mei. Trends Biochem. Sci. 2012, 37, 364–372. [Google Scholar] [CrossRef]
- Conway, E.M.; Carmeliet, P. The diversity of endothelial cells: A challenge for therapeutic angiogenesis. Genome Biol. 2004, 5, 207. [Google Scholar] [CrossRef]
- Xing-Fei Deng, D.; Tsalenko, A.; Vailaya, A.; Ben-Dor, A.; Kundu, R.; Estay, I.; Tabibiazar, R.; Kincaid, R.; Yakhini, Z.; Bruhn, L.; et al. Differences in vascular bed disease susceptibility reflect differences in gene expression response to atherogenic stimuli. Circ. Res. 2006, 98, 200–208. [Google Scholar] [CrossRef]
- Yang, Q.; Wijerathne, H.; Langston, J.C.; Kiani, M.F.; Kilpatrick, L.E. Emerging approaches to understanding microvascular endothelial heterogeneity: A roadmap for developing anti-inflammatory therapeutics. Int. J. Mol. Sci. 2021, 22, 7770. [Google Scholar] [CrossRef]
- Hida, K.; Maishi, N.; Sakurai, Y.; Hida, Y.; Harashima, H. Heterogeneity of tumor endothelial cells and drug delivery. Adv. Drug Deliv. Rev. 2016, 99, 140–147. [Google Scholar] [CrossRef]
- Liang, J.; Wang, S.; Zhang, G.; He, B.; Bie, Q.; Zhang, B. A New Antitumor Direction: Tumor-Specific Endothelial Cells. Front. Oncol. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Molema, G. Design of vascular endothelium-specific drug-targeting strategies for the treatment of cancer. Acta Biochim. Pol. 2005, 52, 301–310. [Google Scholar] [CrossRef]
- Morofuji, Y.; Nakagawa, S. Drug Development for Central Nervous System Diseases Using In vitro Blood-brain Barrier Models and Drug Repositioning. Curr. Pharm. Des. 2020, 26, 1466–1485. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Tachibana, K.; Kondoh, M. Tight junction modulators for drug delivery to the central nervous system. Drug Discov. Today 2020, 25, 1477–1486. [Google Scholar] [CrossRef]
- Ian, M.; Williams, W.J.C. Generation of endothelial cells from human pluripotent stem cells: Methods, considerations, and applications. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1317–1329. [Google Scholar] [CrossRef]
- Glassman, P.M.; Myerson, J.W.; Ferguson, L.T.; Kiseleva, R.Y.; Shuvaev, V.V.; Brenner, J.S.; Muzykantov, V.R. Targeting drug delivery in the vascular system: Focus on endothelium. Adv. Drug Deliv. Rev. 2020, 157, 96–117. [Google Scholar] [CrossRef]
- Chattopadhyay, R.; Dyukova, E.; Singh, N.K.; Ohba, M.; Mobley, J.A.; Rao, G.N. Vascular endothelial tight junctions and barrier function are disrupted by 15(S)-Hydroxyeicosatetraenoic acid partly via Protein Kinase C e-mediated Zona Occludens-1 Phosphorylation at Threonine 770/772. J. Biol. Chem. 2014, 289, 3148–3163. [Google Scholar] [CrossRef]
- Andresen Eguiluz, R.C.; Kaylan, K.B.; Underhill, G.H.; Leckband, D.E. Substrate stiffness and VE-cadherin mechano-transduction coordinate to regulate endothelial monolayer integrity. Biomaterials 2017, 140, 45–57. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gifre-Renom, L.; Daems, M.; Luttun, A.; Jones, E.A.V. Organ-Specific Endothelial Cell Differentiation and Impact of Microenvironmental Cues on Endothelial Heterogeneity. Int. J. Mol. Sci. 2022, 23, 1477. https://doi.org/10.3390/ijms23031477
Gifre-Renom L, Daems M, Luttun A, Jones EAV. Organ-Specific Endothelial Cell Differentiation and Impact of Microenvironmental Cues on Endothelial Heterogeneity. International Journal of Molecular Sciences. 2022; 23(3):1477. https://doi.org/10.3390/ijms23031477
Chicago/Turabian StyleGifre-Renom, Laia, Margo Daems, Aernout Luttun, and Elizabeth A. V. Jones. 2022. "Organ-Specific Endothelial Cell Differentiation and Impact of Microenvironmental Cues on Endothelial Heterogeneity" International Journal of Molecular Sciences 23, no. 3: 1477. https://doi.org/10.3390/ijms23031477
APA StyleGifre-Renom, L., Daems, M., Luttun, A., & Jones, E. A. V. (2022). Organ-Specific Endothelial Cell Differentiation and Impact of Microenvironmental Cues on Endothelial Heterogeneity. International Journal of Molecular Sciences, 23(3), 1477. https://doi.org/10.3390/ijms23031477






