Current Knowledge about the New Drug Firibastat in Arterial Hypertension
Abstract
1. Introduction
2. Arterial Hypertension
2.1. Causes
2.2. Mortality and Prevalence
2.3. Diagnosis
3. Standard Treatment Options for Arterial Hypertension
3.1. Diuretics
3.2. Angiotensin Converting Enzyme (ACE) Inhibitors
3.3. Angiotensin-II-Receptor Blockers
3.4. Calcium Channel Blockers
3.5. Beta-Adrenoceptor Antagonists (BAAs)
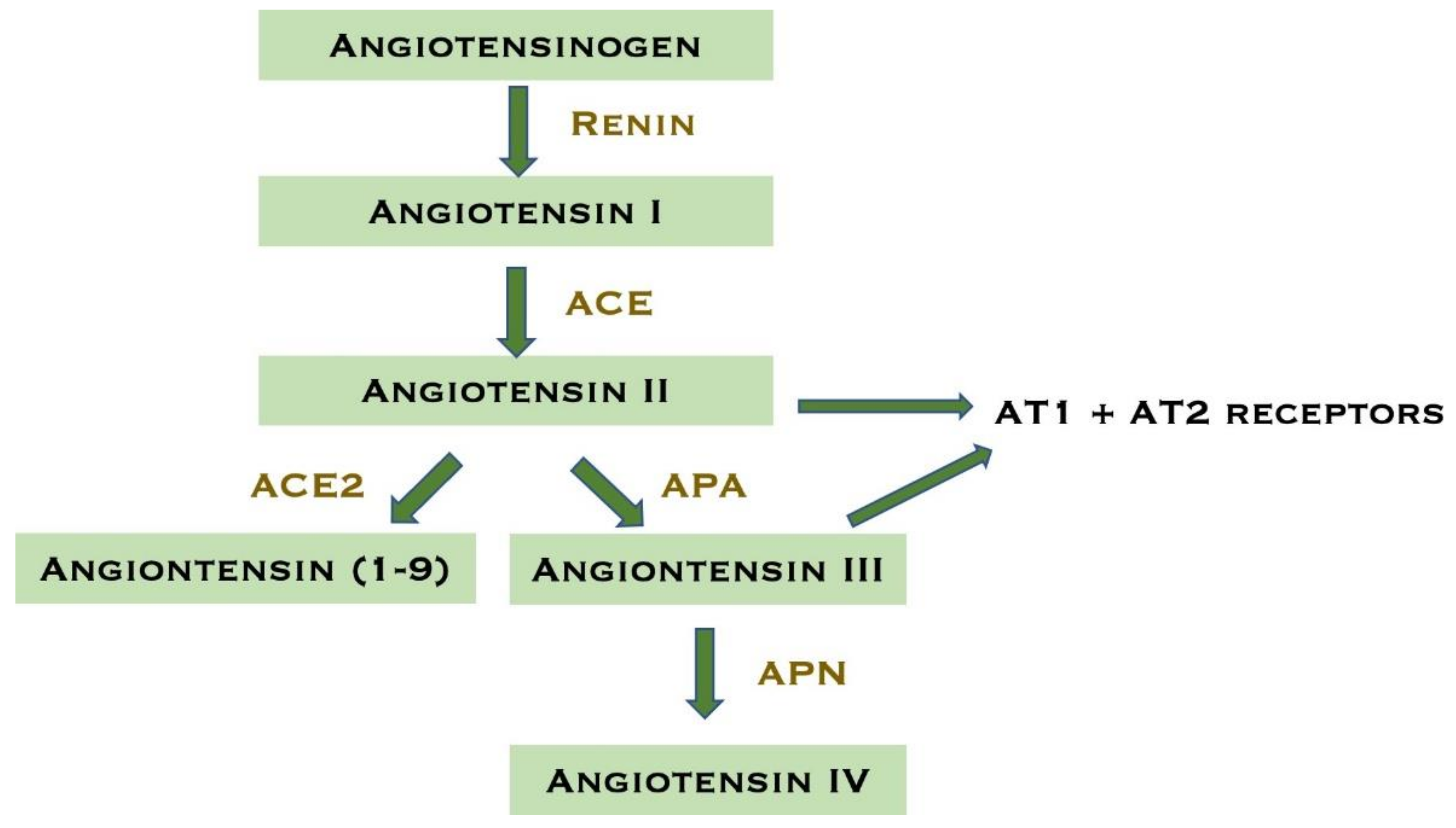
4. Firibastat
4.1. Mechanism of Action
4.2. Indications
4.3. Pharmacokinetics
4.4. Interactions
4.5. Animal Studies
5. Clinical Trials with Firibastat
5.1. Safety and Adverse Effects of Firibastat
5.2. Firibastat vs. Standard Pharmacological Treatment
6. Materials
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABPM | Ambulatory blood pressure measurement |
| ACE | Angiotensin converting enzyme |
| AE(s) | Adverse effect(s) |
| Ang-II | Angiotensin-II |
| Ang-III | Angiotensin III |
| Ang-IV | Angiotensin IV |
| APA | Aminopeptidase A |
| APN | Aminopeptidase IV |
| ARB(s) | Angiotensin-II-receptor blocker(s) |
| AT1 | Angiotensin type 1 receptor |
| AT2 | Angiotensin type 2 receptor |
| AVP | Arginine-vasopressin |
| BP | Blood pressure |
| CVD | Cardiovascular disease |
| DBP | Diastolic blood pressure |
| DOCA | Deoxycorticosterone acetate |
| EH | Essential hypertension |
| HBPM | Home blood pressure measurement |
| HT | Hypertension |
| RAAS | Renin–angiotensin–aldosterone system |
| RAS | Renin–angiotensin system |
| SBP | Systolic blood pressure |
| SH | Secondary hypertension |
| SHR | spontaneously hypertensive rat |
| SNS | Sympathetic nervous system |
| WHO | World Health Organization |
References
- Jordan, J.; Kurschat, C.; Reuter, H. Arterial Hypertension. Dtsch. Arztebl. Int. 2018, 115, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Staff, M.C. Overview: High Blood Pressure (Hypertension). Available online: https://www.mayoclinic.org/diseases-conditions/high-blood-pressure/symptoms-causes/syc-20373410 (accessed on 21 October 2021).
- Oxlund, C.; Jeppesen, J.; Christensen, K.L.; Bang, L.E.; Pareek, M.; Olsen, M.H. Arteriel Hypertension. Available online: https://nbv.cardio.dk/hypertension (accessed on 21 October 2021).
- Balavoine, F.; Azizi, M.; Bergerot, D.; De Mota, N.; Patouret, R.; Roques, B.P.; Llorens-Cortes, C. Randomised, double-blind, placebo-controlled, dose-escalating phase I study of QGC001, a centrally acting aminopeptidase a inhibitor prodrug. Clin. Pharm. 2014, 53, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Organisation, W.H. Noncommunicable Diseases: Hypertension. Available online: https://www.who.int/news-room/q-a-detail/noncommunicable-diseases-hypertension (accessed on 21 October 2021).
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Stergiou, G.S.; Palatini, P.; Parati, G.; O’Brien, E.; Januszewicz, A.; Lurbe, E.; Persu, A.; Mancia, G.; Kreutz, R. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J. Hypertens. 2021, 39, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Wermelt, J.A.; Schunkert, H. Management of arterial hypertension. Herz 2017, 42, 515–526. [Google Scholar] [CrossRef]
- Rogers, J. Understanding the most commonly billed diagnoses in primary care: Hypertension. Nurse Pract. 2020, 45, 50–55. [Google Scholar] [CrossRef]
- Charles, L.; Triscott, J.; Dobbs, B. Secondary Hypertension: Discovering the Underlying Cause. Am. Fam. Physician 2017, 96, 453–461. [Google Scholar]
- WHO. Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 22 October 2021).
- Ostchega, Y.; Fryar, C.D.; Nwankwo, T.; Nguyen, D.T. Hypertension Prevalence among Adults Aged 18 and Over: United States, 2017–2018. NCHS Data Brief 2020, 364, 1–8. [Google Scholar]
- Chockalingam, A. Impact of World Hypertension Day. Can. J. Cardiol. 2007, 23, 517–519. [Google Scholar] [CrossRef]
- Rapsomaniki, E.; Timmis, A.; George, J.; Pujades-Rodriguez, M.; Shah, A.D.; Denaxas, S.; White, I.R.; Caulfield, M.J.; Deanfield, J.E.; Smeeth, L.; et al. Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 2014, 383, 1899–1911. [Google Scholar] [CrossRef]
- Rethy, L.; Shah, N.S.; Paparello, J.J.; Lloyd-Jones, D.M.; Khan, S.S. Trends in Hypertension-Related Cardiovascular Mortality in the United States, 2000 to 2018. Hypertension 2020, 76, e23–e25. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Barzi, F.; Chalmers, J. Mortality patterns in hypertension. J. Hypertens. 2011, 29 (Suppl. 1), S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redón, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef] [PubMed]
- Maass, P.G.; Aydin, A.; Luft, F.C.; Schächterle, C.; Weise, A.; Stricker, S.; Lindschau, C.; Vaegler, M.; Qadri, F.; Toka, H.R.; et al. PDE3A mutations cause autosomal dominant hypertension with brachydactyly. Nat. Genet. 2015, 47, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Raymaekers, V.; Brenard, C.; Hermans, L.; Frederix, I.; Staessen, J.A.; Dendale, P. How to reliably diagnose arterial hypertension: Lessons from 24 h blood pressure monitoring. Blood Press 2019, 28, 93–98. [Google Scholar] [CrossRef]
- Clinic, M. Diuretics. Available online: https://www.mayoclinic.org/diseases-conditions/high-blood-pressure/in-depth/diuretics/art-20048129 (accessed on 30 December 2021).
- National Institute of Diabetes and Digestive and Kidney Diseases. Loop Diuretics. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Akbari, P.; Khorasani-Zadeh, A. Thiazide Diuretics. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. Amiloride. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Messerli, F.H.; Bangalore, S.; Bavishi, C.; Rimoldi, S.F. Angiotensin-Converting Enzyme Inhibitors in Hypertension: To Use or Not to Use? J. Am. Coll. Cardiol. 2018, 71, 1474–1482. [Google Scholar] [CrossRef]
- Hjermitslev, M.; Grimm, D.G.; Wehland, M.; Simonsen, U.; Krüger, M. Azilsartan Medoxomil, an Angiotensin II Receptor Antagonist for the Treatment of Hypertension. Basic Clin. Pharmacol. Toxicol. 2017, 121, 225–233. [Google Scholar] [CrossRef]
- Elliott, W.J.; Ram, C.V. Calcium channel blockers. J. Clin. Hypertens. (Greenwich) 2011, 13, 687–689. [Google Scholar] [CrossRef]
- Fisker, F.Y.; Grimm, D.; Wehland, M. Third-generation beta-adrenoceptor antagonists in the treatment of hypertension and heart failure. Basic Clin. Pharmacol. Toxicol. 2015, 117, 5–14. [Google Scholar] [CrossRef]
- Wehland, M.; Grosse, J.; Simonsen, U.; Infanger, M.; Bauer, J.; Grimm, D. The effects of newer beta-adrenoceptor antagonists on vascular function in cardiovascular disease. Curr. Vasc. Pharmacol. 2012, 10, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Olawi, N.; Krüger, M.; Grimm, D.; Infanger, M.; Wehland, M. Nebivolol in the treatment of arterial hypertension. Basic Clin. Pharmacol. Toxicol. 2019, 125, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Genomics, Q. Science, the BAPAIs. Available online: https://quantum-genomics.com/en/science/the-bapais/ (accessed on 16 November 2021).
- Marc, Y.; Hmazzou, R.; Balavoine, F.; Flahault, A.; Llorens-Cortes, C. Central antihypertensive effects of chronic treatment with RB150: An orally active aminopeptidase A inhibitor in deoxycorticosterone acetate-salt rats. J. Hypertens. 2018, 36, 641–650. [Google Scholar] [CrossRef]
- Lenkei, Z.; Palkovits, M.; Corvol, P.; Llorens-Cortès, C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: A functional neuroanatomical review. Front. Neuroendocrinol. 1997, 18, 383–439. [Google Scholar] [CrossRef] [PubMed]
- Vazeux, G.; Iturrioz, X.; Corvol, P.; Llorens-Cortes, C. A glutamate residue contributes to the exopeptidase specificity in aminopeptidase A. Biochem. J. 1998, 334 Pt 2, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Marc, Y.; Llorens-Cortes, C. The role of the brain renin-angiotensin system in hypertension: Implications for new treatment. Prog. Neurobiol. 2011, 95, 89–103. [Google Scholar] [CrossRef]
- Marc, Y.; Gao, J.; Balavoine, F.; Michaud, A.; Roques, B.P.; Llorens-Cortes, C. Central antihypertensive effects of orally active aminopeptidase A inhibitors in spontaneously hypertensive rats. Hypertension 2012, 60, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Fournie-Zaluski, M.C.; Fassot, C.; Valentin, B.; Djordjijevic, D.; Reaux-Le Goazigo, A.; Corvol, P.; Roques, B.P.; Llorens-Cortes, C. Brain renin-angiotensin system blockade by systemically active aminopeptidase A inhibitors: A potential treatment of salt-dependent hypertension. Proc. Natl. Acad. Sci. USA 2004, 101, 7775–7780. [Google Scholar] [CrossRef]
- Reaux, A.; Fournie-Zaluski, M.C.; David, C.; Zini, S.; Roques, B.P.; Corvol, P.; Llorens-Cortes, C. Aminopeptidase A inhibitors as potential central antihypertensive agents. Proc. Natl. Acad. Sci. USA 1999, 96, 13415–13420. [Google Scholar] [CrossRef]
- Zini, S.; Fournie-Zaluski, M.C.; Chauvel, E.; Roques, B.P.; Corvol, P.; Llorens-Cortes, C. Identification of metabolic pathways of brain angiotensin II and III using specific aminopeptidase inhibitors: Predominant role of angiotensin III in the control of vasopressin release. Proc. Natl. Acad. Sci. USA 1996, 93, 11968–11973. [Google Scholar] [CrossRef]
- Ferrario, C.M. Angiotensin-converting enzyme 2 and angiotensin-(1-7): An evolving story in cardiovascular regulation. Hypertension 2006, 47, 515–521. [Google Scholar] [CrossRef]
- Kokje, R.J.; Wilson, W.L.; Brown, T.E.; Karamyan, V.T.; Wright, J.W.; Speth, R.C. Central pressor actions of aminopeptidase-resistant angiotensin II analogs: Challenging the angiotensin III hypothesis. Hypertension 2007, 49, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xia, H.; Santos, R.A.; Speth, R.; Lazartigues, E. Angiotensin-converting enzyme 2: A new target for neurogenic hypertension. Exp. Physiol. 2010, 95, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Yamazato, M.; Yamazato, Y.; Sun, C.; Diez-Freire, C.; Raizada, M.K. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension 2007, 49, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Ferdinand, K.C.; Balavoine, F.; Besse, B.; Black, H.R.; Desbrandes, S.; Dittrich, H.C.; Nesbitt, S.D. Efficacy and Safety of Firibastat, A First-in-Class Brain Aminopeptidase A Inhibitor, in Hypertensive Overweight Patients of Multiple Ethnic Origins. Circulation 2019, 140, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Boitard, S.E.; Marc, Y.; Keck, M.; Mougenot, N.; Agbulut, O.; Balavoine, F.; Llorens-Cortes, C. Brain renin-angiotensin system blockade with orally active aminopeptidase A inhibitor prevents cardiac dysfunction after myocardial infarction in mice. J. Mol. Cell. Cardiol. 2019, 127, 215–222. [Google Scholar] [CrossRef]
- Aronow, W.S. Approaches for the Management of Resistant Hypertension in 2020. Curr. Hypertens. Rep. 2020, 22, 3. [Google Scholar] [CrossRef]
- Bodineau, L.; Frugière, A.; Marc, Y.; Inguimbert, N.; Fassot, C.; Balavoine, F.; Roques, B.; Llorens-Cortes, C. Orally active aminopeptidase A inhibitors reduce blood pressure: A new strategy for treating hypertension. Hypertension 2008, 51, 1318–1325. [Google Scholar] [CrossRef]
- Azizi, M.; Courand, P.Y.; Denolle, T.; Delsart, P.; Zhygalina, V.; Amar, L.; Lantelme, P.; Mounier-Vehier, C.; De Mota, N.; Balavoine, F.; et al. A pilot double-blind randomized placebo-controlled crossover pharmacodynamic study of the centrally active aminopeptidase A inhibitor, firibastat, in hypertension. J. Hypertens. 2019, 37, 1722–1728. [Google Scholar] [CrossRef]
- Phase IIa Study of the Product QGC001 Compared with Placebo in Patients with Essential Hypertension (2QG1). Available online: https://clinicaltrials.gov/ct2/show/NCT02322450?term=NCT02322450&draw=2&rank=1 (accessed on 30 December 2021).
- Novel Evaluation with QGC001 in Hypertensive Overweight Patients of Multiple Ethnic Origins (NEW-HOPE). Available online: https://clinicaltrials.gov/ct2/show/NCT03198793?term=NCT03198793&draw=2&rank=1 (accessed on 30 December 2021).
- Firibastat in Treatment-Resistant Hypertension (FRESH). Available online: https://clinicaltrials.gov/ct2/show/NCT04277884?term=NCT04277884&draw=2&rank=1 (accessed on 26 November 2021).
- Randomized Study of Extended Treatment with Firibastat in Treatment-Resistant Hypertension (REFRESH). Available online: https://clinicaltrials.gov/ct2/show/NCT04857840?term=NCT04857840&draw=2&rank=1 (accessed on 30 December 2021).
- Kitamura, K.; Aihara, M.; Osawa, J.; Naito, S.; Ikezawa, Z. Sulfhydryl drug-induced eruption: A clinical and histological study. J. Dermatol. 1990, 17, 44–51. [Google Scholar] [CrossRef]
- Jaffe, I.A. Adverse effects profile of sulfhydryl compounds in man. Am. J. Med. 1986, 80, 471–476. [Google Scholar] [CrossRef]
- Chazova, I.E.; Dongre, N.; Vigdorchik, A.V. Real-life safety and effectiveness of amlodipine/valsartan combination in the treatment of hypertension. Adv. Ther. 2011, 28, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Jia, M.; Ran, H.; Tang, H.; Zhang, Y.; Zhang, J.; Wang, X.; Wang, H.; Yang, C.; Zeng, C. Fixed-combination of amlodipine and diuretic chronotherapy in the treatment of essential hypertension: Improved blood pressure control with bedtime dosing-a multicenter, open-label randomized study. Hypertens. Res. 2011, 34, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Andreadis, E.A.; Tsourous, G.I.; Marakomichelakis, G.E.; Katsanou, P.M.; Fotia, M.E.; Vassilopoulos, C.V.; Diamantopoulos, E.J. High-dose monotherapy vs low-dose combination therapy of calcium channel blockers and angiotensin receptor blockers in mild to moderate hypertension. J. Hum. Hypertens. 2005, 19, 491–496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rouette, J.; Yin, H.; Pottegård, A.; Nirantharakumar, K.; Azoulay, L. Use of Hydrochlorothiazide and Risk of Melanoma and Nonmelanoma Skin Cancer. Drug Saf. 2021, 44, 245–254. [Google Scholar] [CrossRef]
- Pedersen, S.A.; Gaist, D.; Schmidt, S.A.J.; Hölmich, L.R.; Friis, S.; Pottegård, A. Hydrochlorothiazide use and risk of nonmelanoma skin cancer: A nationwide case-control study from Denmark. J. Am. Acad. Dermatol. 2018, 78, 673–681.e679. [Google Scholar] [CrossRef]
- Pottegård, A.; Bech, B.H.; Pedersen, S.A.; Christensen, B. Use of hydrochlorothiazide in Denmark following publication of skin cancer risk findings. Pharmacoepidemiol. Drug. Saf. 2021, 30, 1611–1616. [Google Scholar] [CrossRef]
- Bueno, C.T.; Pereira, A.C.; Santos, H.C.; Gómez, L.M.G.; Horimoto, A.; Krieger, E.M.; Krieger, J.E.; Santos, P. Association of the genetic ancestry with resistant hypertension in the ReHOT (Resistant Hypertension Optimal Treatment) randomized study. Sci. Rep. 2020, 10, 1476. [Google Scholar] [CrossRef]
- Marc, Y.; Hmazzou, R.; De Mota, N.; Balavoine, F.; Llorens-Cortes, C. Effects of firibastat in combination with enalapril and hydrochlorothiazide on blood pressure and vasopressin release in hypertensive DOCA-salt rats. Biomed. Pharmacother. 2021, 140, 111682. [Google Scholar] [CrossRef]
- Genomics, Q. Quantum Genomics, Firibastat Phase III to Start by End of the Year. Available online: https://www.edisongroup.com/publication/firibastat-phase-iii-to-start-by-end-of-the-year/25253/ (accessed on 30 December 2021).

| Parameter | Firibastat 125 mg | Firibastat 500 mg | Firibastat 1250 mg |
|---|---|---|---|
| Cmax (ng/mL) | 4.2 | 25.9 | 62.6 |
| tmax (h) | 0.7 | 1.7 | 3 |
| t1/2 (h) | NC | 1.1 | 1.7 |
| CLR (L/h) | 135.3 | 92.3 | 66.7 |
| Ae (%) | 0.6 | 1.2 | 1.6 |
| Parameter | EC33 125 mg | EC33 500 mg | EC33 1250 mg |
| Cmax (ng/mL) | 15.4 | 56.3 | 77.3 |
| tmax (h) | 2.5 | 3 | 4 |
| t1/2 (h) | NC | NC | 2.8 |
| Ae (%) | 0.9 | 1 | 1.1 |
| Title | Design | Results | Conclusions |
|---|---|---|---|
| Phase I Study in Healthy Male Subjects Comparing QGC001 to Placebo NCT01900171 [4] | Randomized Double-blinded Placebo-controlled 56 participants Phase 1 | Single oral doses from 10 to 1250 mg were all well tolerated both clinically and biologically in the 56 healthy male volunteers. RB150 and EC33 both had a peak plasma concentration (Cmax) linear with the dose. The plasma elimination half-life of RB150 was 1.6 h at all doses. No significant changes in SBP, DBP or heart rate were observed in any of the treatment groups AEs: 5 treatment-emergent AEs, only one (mild asymptomatic orthostatic hypotension) was considered related to study treatment | RB150 is well tolerated in healthy volunteers with a single oral administration up to 1250 mg. RB150 does not affect the systemic RAS. In normotensive individuals, single dose of RB150 did not affect SBP, DBP, or heart rate. No severe or life-threatening adverse effects were observed |
| Phase IIa Study of the Product QGC001 Compared with Placebo in Patients with Essential Hypertension (2QG1) NCT02322450 [48,49] | A pilot multicenter double-blind randomized placebo-controlled crossover pharmacodynamic study. 34 participants Phase IIa | Firibastat was studied for 4 weeks in patients with primary mild HT. Daytime ambulatory SBP and office SBP decreased by 2.7 and 4.7 mmHg, respectively, compared to placebo. No major side effects were observed. Firibastat had no effect on heart rate, plasma renin, aldosterone, apelin and copeptin concentrations during the 4 weeks. AEs: 14 patients with 16 reversible adverse events of mild intensity. One case of withdrawal due to rash, vestibular disorder and arthralgia each. | Treatment with firibastat in hypertensive subjects over 4 weeks reduced their SBP relative to placebo. The systemic RAS was not affected. These results allowed larger, longer, and more powerful clinical trials to fully explore the efficacy of firibastat. |
| Novel Evaluation with QGC001 in Hy- pertensive Overweight Patients of Multiple Ethnic Origins (NEW-HOPE) NCT03198793 [44,50] | Open-Label Dose-TitratingSafety and Efficacy Study 256 participants Phase IIb | For 8 weeks, the effect of twice daily administration of oral firibastat among hypertensive overweight/obese people of different races has been investigated. Firibastat reduced office SBP by 9.5 mmHg and office DPB by 4.2 mmHg. Significant de- creases in BP were found in all subgroups. Among obese people, SBP decreased by 10.2 mmHg, 10.5 mmHg among blacks and 8.9 mmHg among non-blacks. AEs: 14.1% related treatment-emergent AEs (headache, skin reactions most common), one serious related AE (erythema multiforme) resulted in discontinuation | Firibastat is effective in lowering the BP, also in the high-risk groups. Firibastat is a possible alternative when it comes to difficult-to-treat patients or resistant hypertension where monotherapy with standard treatment options is no longer effective. |
| Firibastat in Treatment-resistant Hypertension (FRESH) NCT04277884 [51] | Double-blind, Placebo-controlled, Efficacy and Safety Study 502 participants Phase 3 | This study is still recruiting and has not been completed yet. For that reason, the results are missing from this study. The objective of this study is to investigate the effect of firibastat in people ≥18 years with uncontrolled primary hypertension. Administrations of firibastat 500 mg orally twice daily over 12 weeks are compared with placebo. | No conclusions can be drawn since the study is not completed. |
| Randomized Study of Extended Treatment with Firibastat in Treatment-Resistant Hypertension. (REFRESH) NCT04857840 [52] | Double-blind, Placebo-controlled and Open-label Efficacy and Long-term Safety Study 750 participants Phase 3 | This study is still recruiting and has not been completed yet. The objective is to investigate the efficacy and safety of 1000 mg (2 × 500 mg p.o.) firibastat in addition to their chronic antihypertensive therapies for up to 48 weeks in patients with difficult-to-treat/treatment-resistant HTN | No results have been reported yet |
| Keyword | Number of Hits |
|---|---|
| Hypertension | 577,486 |
| Hypertension and treatment | 322,085 |
| Arterial hypertension and treatment | 310,742 |
| APA inhibitor | 643 |
| Brain RAS and hypertension | 421 |
| EC33 | 32 |
| RB150 | 30 |
| APA inhibitor and Ang-III | 25 |
| Firibastat | 23 |
| Quantum Genomics and firibastat | 12 |
| QGC001 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, E.; Grimm, D.; Wehland, M. Current Knowledge about the New Drug Firibastat in Arterial Hypertension. Int. J. Mol. Sci. 2022, 23, 1459. https://doi.org/10.3390/ijms23031459
Hansen E, Grimm D, Wehland M. Current Knowledge about the New Drug Firibastat in Arterial Hypertension. International Journal of Molecular Sciences. 2022; 23(3):1459. https://doi.org/10.3390/ijms23031459
Chicago/Turabian StyleHansen, Emma, Daniela Grimm, and Markus Wehland. 2022. "Current Knowledge about the New Drug Firibastat in Arterial Hypertension" International Journal of Molecular Sciences 23, no. 3: 1459. https://doi.org/10.3390/ijms23031459
APA StyleHansen, E., Grimm, D., & Wehland, M. (2022). Current Knowledge about the New Drug Firibastat in Arterial Hypertension. International Journal of Molecular Sciences, 23(3), 1459. https://doi.org/10.3390/ijms23031459







