A Polymorphism of Bactericidal/Permeability-Increasing Protein Affects Its Neutralization Efficiency towards Lipopolysaccharide
Abstract
1. Introduction
2. Results
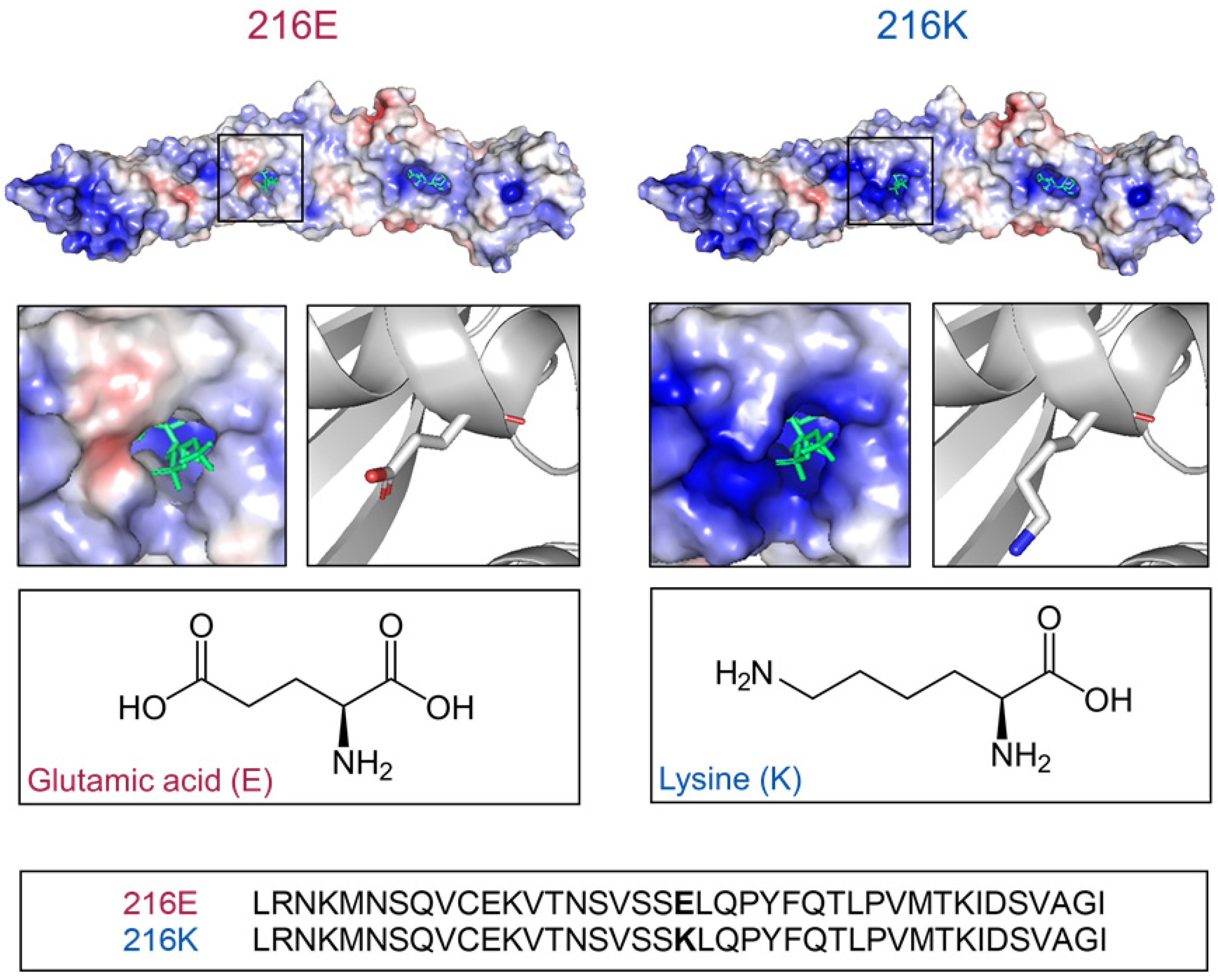
2.1. Models of BPI216E and BPI216K Reveal Different Charge Distributions Proximal to the Lipid Binding Pocket
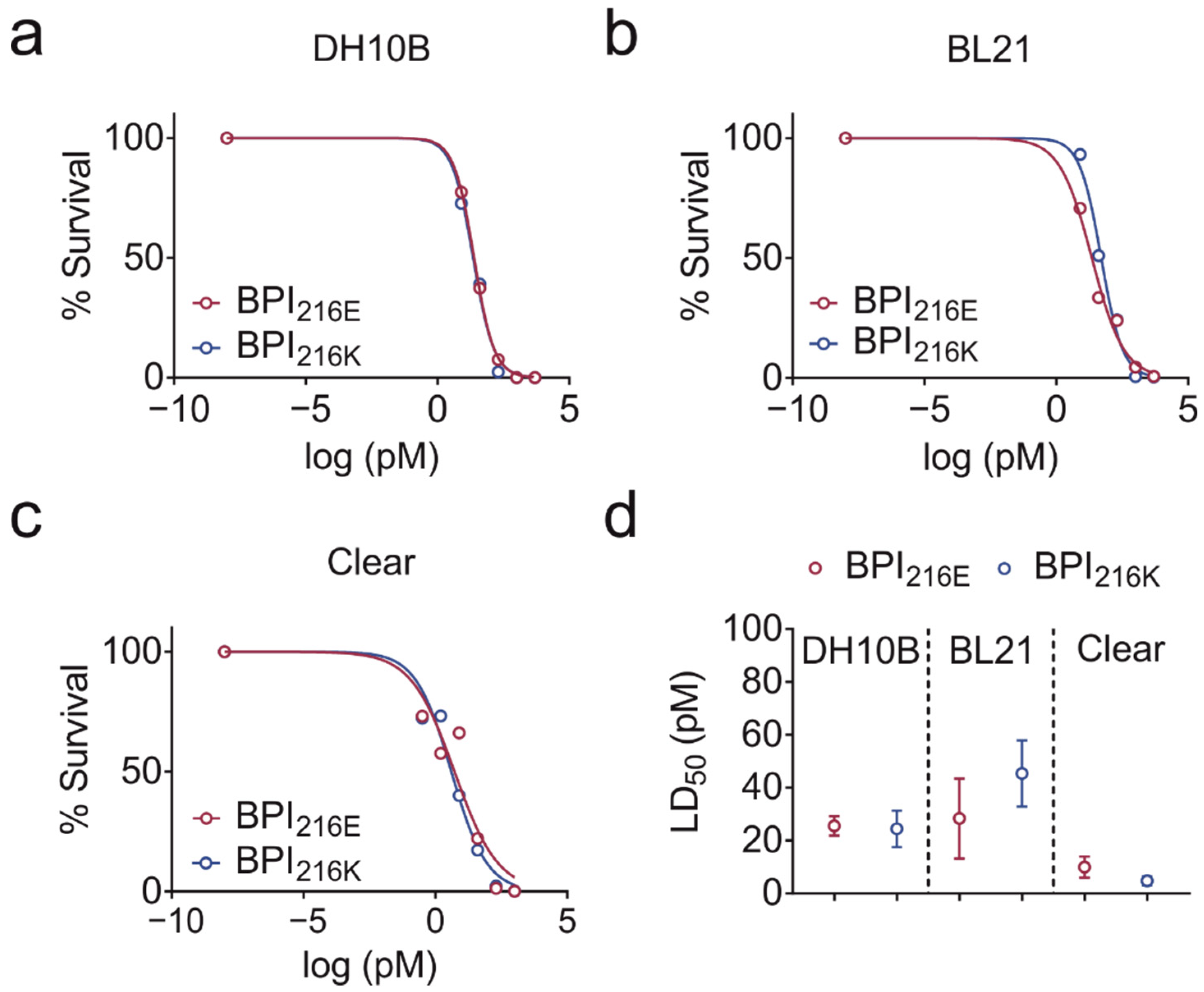
2.2. BPI216E and BPI216K Do Not Differ in Bactericidal Activity
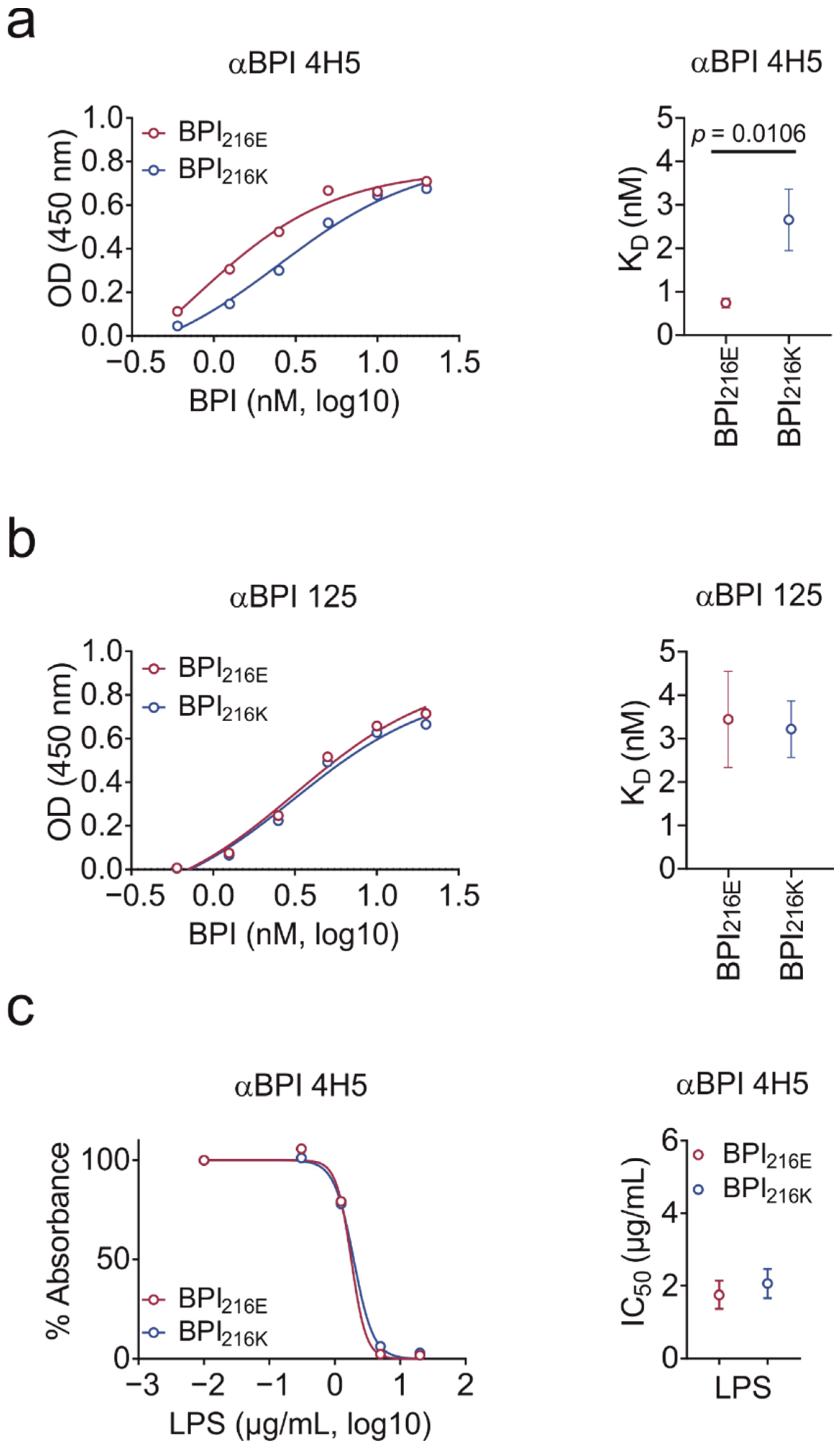
2.3. BPI216K Does Not Exhibit Higher Affinity to LPS as Compared to BPI216E
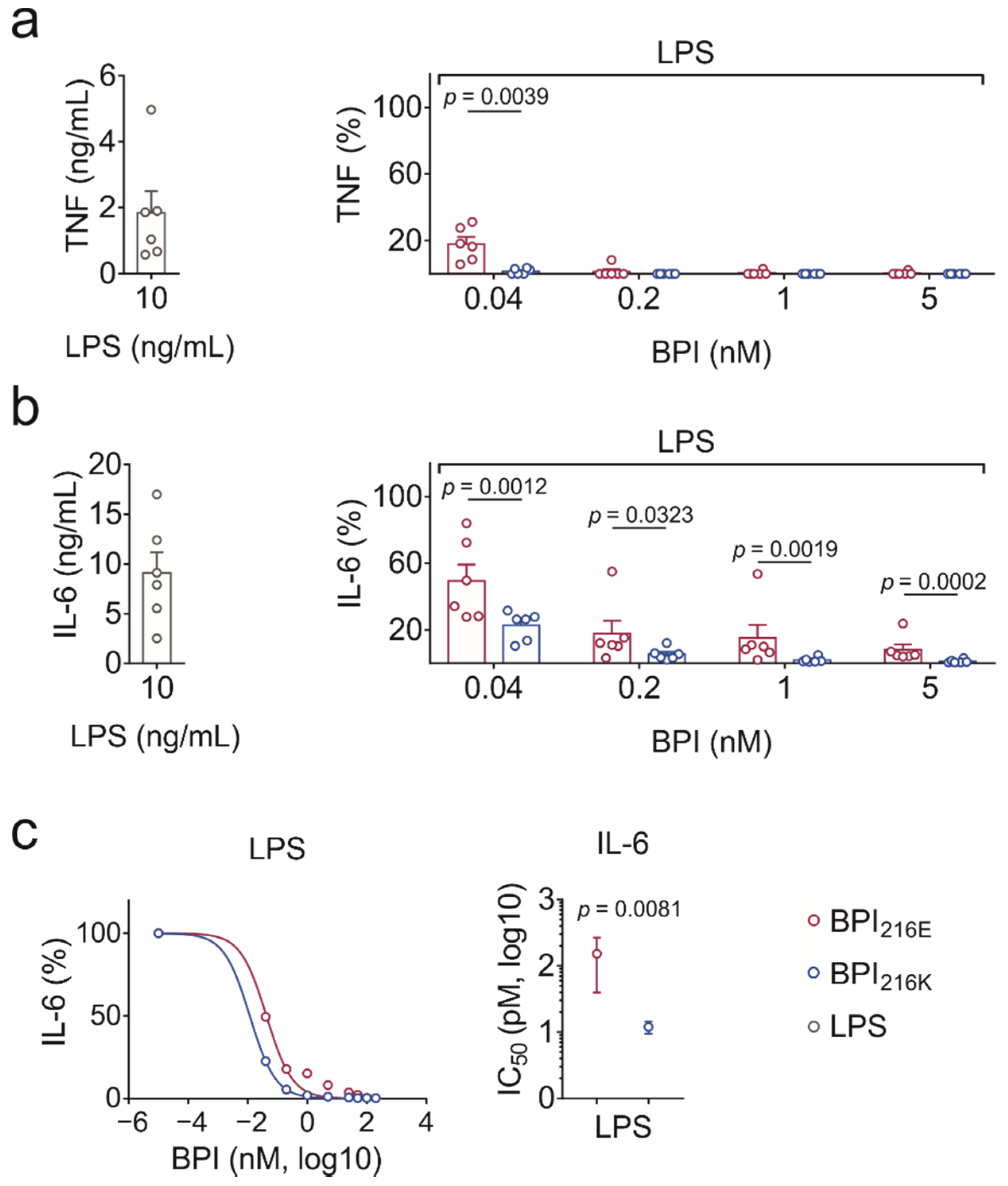
2.4. BPI216K Exceeds BPI216E at Inhibition of LPS-Induced Cytokine Secretion
3. Discussion
4. Materials and Methods
4.1. Generation of Recombinant Human BPI
4.2. Generation and Purification of BPI Antibodies
4.3. Solid-Phase BPI Binding Assay
4.4. Dose Response Experiments for Bactericidal Activity
4.5. Isolation of Human Peripheral Blood Mononuclear Cells
4.6. Quantification of Cytokine Levels
4.7. Structure Modeling, Graphical Depictions, and Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Fleischmann, C.; Reichert, F.; Cassini, A.; Horner, R.; Harder, T.; Markwart, R.; Tröndle, M.; Savova, Y.; Kissoon, N.; Schlattmann, P.; et al. Global incidence and mortality of neonatal sepsis: A systematic review and meta-analysis. Arch. Dis. Child. 2021, 106, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; Scannon, P.J.; Vincent, J.L.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 1999, 180, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Henneke, P.; Dramsi, S.; Mancuso, G.; Chraibi, K.; Pellegrini, E.; Theilacker, C.; Hübner, J.; Santos-Sierra, S.; Teti, G.; Golenbock, D.T.; et al. Lipoproteins are critical TLR2 activating toxins in group B streptococcal sepsis. J. Immunol. 2008, 180, 6149–6158. [Google Scholar] [CrossRef]
- Weiss, J.; Olsson, I. Cellular and subcellular localization of the bactericidal/permeability-increasing protein of neutrophils. Blood 1987, 69, 652–659. [Google Scholar] [CrossRef]
- Skopelja, S.; Hamilton, B.J.; Jones, J.D.; Yang, M.-L.; Mamula, M.; Ashare, A.; Gifford, A.H.; Rigby, W.F. The role for neutrophil extracellular traps in cystic fibrosis autoimmunity. JCI Insight 2016, 1, e88912. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Weiss, J.; Elsbach, P.; Shu, C.; Castillo, J.; Grinna, L.; Horwitz, A.; Theofan, G. Human bactericidal/permeability-increasing protein and a recombinant NH2-terminal fragment cause killing of serum-resistant gram-negative bacteria in whole blood and inhibit tumor necrosis factor release induced by the bacteria. J. Clin. Investig. 1992, 90, 1122–1130. [Google Scholar] [CrossRef]
- Beamer, L.J.; Carroll, S.F.; Eisenberg, D. The BPI/LBP family of proteins: A structural analysis of conserved regions. Protein Sci. 1998, 7, 906–914. [Google Scholar] [CrossRef]
- Beamer, L.J.; Carroll, S.F.; Eisenberg, D. The three-dimensional structure of human bactericidal/permeability-increasing protein. Biochem. Pharmacol. 1999, 57, 225–229. [Google Scholar] [CrossRef]
- Beamer, L.J.; Carroll, S.F.; Eisenberg, D. Crystal structure of human BPI and two bound phospholipids at 2.4 angstrom resolution. Science 1997, 276, 1861–1864. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.E.; Weiss, J.; Doerfler, M.E.; Elsbach, P. Endotoxin-neutralizing properties of the 25 kD N-terminal fragment and a newly isolated 30 kD C-terminal fragment of the 55–60 kD bactericidal/permeability-increasing protein of human neutrophils. J. Exp. Med. 1991, 174, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yang, R.; Marsters, S.; Ashkenazi, A.; Bunting, S.; Marra, M.N.; Scott, R.W.; Baker, J.B. Protection against endotoxic shock by bactericidal/permeability-increasing protein in rats. J. Clin. Investig. 1995, 95, 1947–1952. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Von der Möhlen, M.A.; Kimmings, A.N.; Wedel, N.I.; Mevissen, M.L.; Jansen, J.; Friedmann, N.; Lorenz, T.J.; Nelson, B.J.; White, M.L.; Bauer, R. Inhibition of endotoxin-induced cytokine release and neutrophil activation in humans by use of recombinant bactericidal/permeability-increasing protein. J. Infect. Dis. 1995, 172, 144–151. [Google Scholar] [CrossRef]
- Levin, M.; Quint, P.A.; Goldstein, B.; Barton, P.; Bradley, J.S.; Shemie, S.D.; Yeh, T.; Kim, S.S.; Cafaro, D.P.; Scannon, P.J.; et al. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: A randomised trial. rBPI21 Meningococcal Sepsis Study Group. Lancet 2000, 356, 961–967. [Google Scholar] [CrossRef]
- Esposito, S.; Zampiero, A.; Pugni, L.; Tabano, S.; Pelucchi, C.; Ghirardi, B.; Terranova, L.; Miozzo, M.; Mosca, F.; Principi, N. Genetic polymorphisms and sepsis in premature neonates. PLoS ONE 2014, 9, e101248. [Google Scholar] [CrossRef]
- Michalek, J.; Svetlikova, P.; Fedora, M.; Klimovic, M.; Klapacova, L.; Bartosova, D.; Elbl, L.; Hrstkova, H.; Hubacek, J.A. Bactericidal permeability increasing protein gene variants in children with sepsis. Intensiv. Care Med. 2007, 33, 2158–2164. [Google Scholar] [CrossRef]
- Mamat, U.; Wilke, K.; Bramhill, D.; Schromm, A.B.; Lindner, B.; Kohl, T.A.; Corchero, J.L.; Villaverde, A.; Schaffer, L.; Head, S.R.; et al. Detoxifying Escherichia coli for endotoxin-free production of recombinant proteins. Microb. Cell Fact. 2015, 14, 57. [Google Scholar] [CrossRef]
- Gazzano-Santoro, H.; Parent, J.B.; Conlon, P.J.; Kasler, H.G.; Tsai, C.M.; Lill-Elghanian, D.A.; Hollingsworth, R.I. Characterization of the structural elements in lipid A required for binding of a recombinant fragment of bactericidal/permeability-increasing protein rBPI23. Infect. Immun. 1995, 63, 2201–2205. [Google Scholar] [CrossRef]
- Weiss, J.; Beckerdite-Quagliata, S.; Elsbach, P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: Relation to binding and bacterial lipopolysaccharide structure. J. Clin. Investig. 1980, 65, 619–628. [Google Scholar] [CrossRef]
- Capodici, C.; Chen, S.; Sidorczyk, Z.; Elsbach, P.; Weiss, J. Effect of lipopolysaccharide (LPS) chain length on interactions of bactericidal/permeability-increasing protein and its bioactive 23-kilodalton NH2-terminal fragment with isolated LPS and intact Proteus mirabilis and Escherichia coli. Infect. Immun. 1994, 62, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Guinan, E.C.; Palmer, C.D.; Mancuso, C.J.; Brennan, L.; Stoler-Barak, L.; Kalish, L.A.; Suter, E.E.; Gallington, L.C.; Huhtelin, D.P.; Mansilla, M.; et al. Identification of single nucleotide polymorphisms in hematopoietic cell transplant patients affecting early recognition of, and response to, endotoxin. Innate Immun. 2014, 20, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Wermke, M.; Maiwald, S.; Schmelz, R.; Thiede, C.; Schetelig, J.; Ehninger, G.; Bornhäuser, M.; Wassmuth, R. Genetic variations of interleukin-23R (1143AG) and BPI (A645G), but not of NOD2, are associated with acute graft-versus-host disease after allogeneic transplantation. Biol. Blood Marrow Transplant. 2010, 16, 1718–1727. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hill, G.R.; Crawford, J.M.; Cooke, K.R.; Brinson, Y.S.; Pan, L.; Ferrara, J.L. Total Body Irradiation and Acute Graft-Versus-Host Disease: The Role of Gastrointestinal Damage and Inflammatory Cytokines. Blood 1997, 90, 3204–3213. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.R.; Koyama, M. Cytokines and costimulation in acute graft-versus-host disease. Blood 2020, 136, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Hubacek, J.A.; Stüber, F.; Fröhlich, D.; Book, M.; Wetegrove, S.; Ritter, M.; Rothe, G.; Schmitz, G. Gene variants of the bactericidal/permeability increasing protein and lipopolysaccharide binding protein in sepsis patients: Gender-specific genetic predisposition to sepsis. Crit. Care Med. 2001, 29, 557–561. [Google Scholar] [CrossRef]
- Qing, G.; Howlett, S.; Bortolussi, R. Lipopolysaccharide binding proteins on polymorphonuclear leukocytes: Comparison of adult and neonatal cells. Infect. Immun. 1996, 64, 4638–4642. [Google Scholar] [CrossRef]
- Levy, O.; Martin, S.; Eichenwald, E.; Ganz, T.; Valore, E.; Carroll, S.F.; Lee, K.; Goldmann, D.; Thorne, G.M. Impaired innate immunity in the newborn: Newborn neutrophils are deficient in bactericidal/permeability-increasing protein. Pediatrics 1999, 104, 1327–1333. [Google Scholar] [CrossRef]
- Nupponen, I.; Turunen, R.; Nevalainen, T.; Peuravuori, H.; Pohjavuori, M.; Repo, H.; Andersson, S. Extracellular release of bactericidal/permeability-increasing protein in newborn infants. Pediatr. Res. 2002, 51, 670–674. [Google Scholar] [CrossRef]
- Rosadini, C.V.; Kagan, J.C. Early innate immune responses to bacterial LPS. Curr. Opin. Immunol. 2017, 44, 14–19. [Google Scholar] [CrossRef]
- Reijmerink, N.E.; Bottema, R.W.B.; Kerkhof, M.; Gerritsen, J.; Stelma, F.F.; Thijs, C.; van Schayck, C.P.; Smit, H.A.; Brunekreef, B.; Koppelman, G.H.; et al. TLR-related pathway analysis: Novel gene-gene interactions in the development of asthma and atopy. Allergy 2010, 65, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, J.W.; Whitehead, G.S.; Lin, K.L.; Nakano, H.; Gunn, M.D.; Schwartz, D.A.; Cook, D.N. TLR4 signaling attenuates ongoing allergic inflammation. J. Immunol. 2006, 176, 5856–5862. [Google Scholar] [CrossRef] [PubMed]
- Domingues, M.M.; Lopes, S.C.D.N.; Santos, N.C.; Quintas, A.; Castanho, M.A.R.B. Fold-unfold transitions in the selectivity and mechanism of action of the N-terminal fragment of the bactericidal/permeability-increasing protein (rBPI(21)). Biophys. J. 2009, 96, 987–996. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tobias, P.S.; Soldau, K.; Iovine, N.M.; Elsbach, P.; Weiss, J. Lipopolysaccharide (LPS)-binding proteins BPI and LBP form different types of complexes with LPS. J. Biol. Chem. 1997, 272, 18682–18685. [Google Scholar] [CrossRef]
- Lennartsson, A.; Pieters, K.; Vidovic, K.; Gullberg, U. A murine antibacterial ortholog to human bactericidal/permeability-increasing protein (BPI) is expressed in testis, epididymis, and bone marrow. J. Leukoc. Biol. 2005, 77, 369–377. [Google Scholar] [CrossRef]
- Wittmann, I.; Schönefeld, M.; Aichele, D.; Groer, G.; Gessner, A.; Schnare, M. Murine bactericidal/permeability-increasing protein inhibits the endotoxic activity of lipopolysaccharide and gram-negative bacteria. J. Immunol. 2008, 180, 7546–7552. [Google Scholar] [CrossRef]
- Theprungsirikul, J.; Skopelja-Gardner, S.; Burns, A.S.; Wierzbicki, R.M.; Rigby, W.F.C. Bactericidal/Permeability-Increasing Protein Preeminently Mediates Clearance of Pseudomonas aeruginosa In Vivo via CD18-Dependent Phagocytosis. Front. Immunol. 2021, 12, 659523. [Google Scholar] [CrossRef]
- Kong, Q.; Lv, Z.; Kang, Y.; An, Y.; Liu, Z.; Zhang, J. Bactericidal Permeability Increasing Protein Deficiency Aggravates Acute Colitis in Mice by Increasing the Serum Levels of Lipopolysaccharide. Front. Immunol. 2020, 11, 614169. [Google Scholar] [CrossRef]
- Palmer, C.D.; Guinan, E.C.; Levy, O. Deficient expression of bactericidal/permeability-increasing protein in immunocompromised hosts: Translational potential of replacement therapy. Biochem. Soc. Trans. 2011, 39, 994–999. [Google Scholar] [CrossRef][Green Version]
- Bülow, S.; Zeller, L.; Werner, M.; Toelge, M.; Holzinger, J.; Entzian, C.; Schubert, T.; Waldow, F.; Gisch, N.; Hammerschmidt, S.; et al. Bactericidal/Permeability-Increasing Protein Is an Enhancer of Bacterial Lipoprotein Recognition. Front. Immunol. 2018, 9, 2768. [Google Scholar] [CrossRef]
- Kleiger, G.; Beamer, L.J.; Grothe, R.; Mallick, P.; Eisenberg, D. The 1.7 A crystal structure of BPI: A study of how two dissimilar amino acid sequences can adopt the same fold. J. Mol. Biol. 2000, 299, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ederer, K.U.; Holzinger, J.M.; Maier, K.T.; Zeller, L.; Werner, M.; Toelge, M.; Gessner, A.; Bülow, S. A Polymorphism of Bactericidal/Permeability-Increasing Protein Affects Its Neutralization Efficiency towards Lipopolysaccharide. Int. J. Mol. Sci. 2022, 23, 1324. https://doi.org/10.3390/ijms23031324
Ederer KU, Holzinger JM, Maier KT, Zeller L, Werner M, Toelge M, Gessner A, Bülow S. A Polymorphism of Bactericidal/Permeability-Increasing Protein Affects Its Neutralization Efficiency towards Lipopolysaccharide. International Journal of Molecular Sciences. 2022; 23(3):1324. https://doi.org/10.3390/ijms23031324
Chicago/Turabian StyleEderer, Katharina U., Jonas M. Holzinger, Katharina T. Maier, Lisa Zeller, Maren Werner, Martina Toelge, André Gessner, and Sigrid Bülow. 2022. "A Polymorphism of Bactericidal/Permeability-Increasing Protein Affects Its Neutralization Efficiency towards Lipopolysaccharide" International Journal of Molecular Sciences 23, no. 3: 1324. https://doi.org/10.3390/ijms23031324
APA StyleEderer, K. U., Holzinger, J. M., Maier, K. T., Zeller, L., Werner, M., Toelge, M., Gessner, A., & Bülow, S. (2022). A Polymorphism of Bactericidal/Permeability-Increasing Protein Affects Its Neutralization Efficiency towards Lipopolysaccharide. International Journal of Molecular Sciences, 23(3), 1324. https://doi.org/10.3390/ijms23031324





