Methods of Measuring Mitochondrial Potassium Channels: A Critical Assessment
Abstract
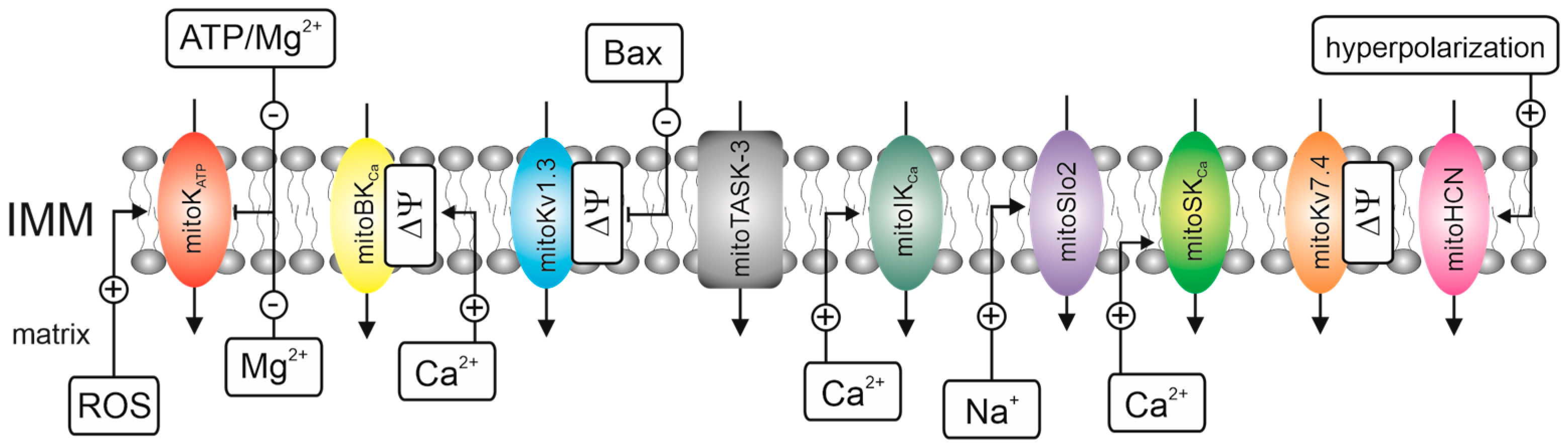
1. Introduction
- -
- Electrophysiological techniques, i.e., the planar lipid bilayer (PLB) technique and patch-clamp technique;
- -
- Flux K+ measurements that use fluorescent probes, i.e., small molecule probes, genetically encoded probes, and thallium (Tl+)-sensitive indicators. We omit flux measurements that use rubidium cations as a tracer for K+. The use of radioactive 86Rb+ and 87Rb+ to study mitochondrial potassium channels is uncommon [42] despite the widespread use of 86Rb+ to study plasma membrane and intracellular potassium channels [43];
- -
- Biochemical techniques, i.e., the use of unique mitochondrial properties such as swelling, respiration, and high membrane potential to track potassium fluxes via inner membrane channel proteins.
2. Reconstitution of Mitochondrial K+ Channels into Planar Lipid Bilayers
3. Patch-Clamp of the Inner Mitochondrial Membrane
4. K+-Specific Fluorescent Indicators in Mitochondrial Potassium Channel Studies
5. Biochemical Methods for Studying Mitochondrial K+ Channels
6. Future Membrane Models: Cubic Phases and Solid Supported Membranes
6.1. Lipid Cubic Mesophases as Membranes for Embedding Potassium Channels
6.2. Solid-Supported and Tethered-Lipid Membranes
- (a)
- They should be easily and reproducibly prepared and stable over time;
- (b)
- They should have a lipid bilayer in the liquid crystalline state to allow lateral mobility;
- (c)
- They should have a water reservoir (or, at the very least, a highly hydrated hydrophilic spacer region) between the electrode and the lipid bilayer.
7. Final Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Xu, H.; Martinoia, E.; Szabo, I. Organellar channels and transporters. Cell Calcium 2015, 58, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, T. Functional Role of Mitochondrial and Nuclear BK Channels. Int. Rev. Neurobiol. 2016, 128, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Stefani, E.; Toro, L. Intracellular BK(Ca) (iBK(Ca)) channels. J. Physiol. 2012, 590, 5937–5947. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Valm, A.; Lippincott-Schwartz, J. Interacting organelles. Curr. Opin. Cell Biol. 2018, 53, 84–91. [Google Scholar] [CrossRef]
- Peruzzo, R.; Biasutto, L.; Szabo, I.; Leanza, L. Impact of intracellular ion channels on cancer development and progression. Eur. Biophys. J. Biophys. Lett. 2016, 45, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.; Schulz, R.; Girao, H.; Kwak, B.; De Stefani, D.; Rizzuto, R.; Bernardi, P.; Di Lisa, F. Mitochondrial ion channels as targets for cardioprotection. J. Cell. Mol. Med. 2020, 24, 7102–7114. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, J. Ion channels as potential redox sensors in lysosomes. Channels 2019, 13, 477–482. [Google Scholar] [CrossRef]
- Szewczyk, A.; Jarmuszkiewicz, W.; Kunz, W.S. Mitochondrial potassium channels. IUBMB Life 2009, 61, 134–143. [Google Scholar] [CrossRef]
- Laskowski, M.; Augustynek, B.; Kulawiak, B.; Koprowski, P.; Bednarczyk, P.; Jarmuszkiewicz, W.; Szewczyk, A. What do we not know about mitochondrial potassium channels? Biochim. Biophys. Acta-Bioenerg. 2016, 1857, 1247–1257. [Google Scholar] [CrossRef]
- Leanza, L.; Checchetto, V.; Biasutto, L.; Rossa, A.; Costa, R.; Bachmann, M.; Zoratti, M.; Szabo, I. Pharmacological modulation of mitochondrial ion channels. Br. J. Pharmacol. 2019, 176, 4258–4283. [Google Scholar] [CrossRef]
- Olszewska, A.; Szewczyk, A. Mitochondria as a pharmacological target: Magnum overview. IUBMB Life 2013, 65, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, A.; Augustynek, B.; Żochowska, M.; Szewczyk, A. Mitochondrial Potassium Channels as Druggable Targets. Biomolecules 2020, 10, 1200. [Google Scholar] [CrossRef] [PubMed]
- Madeira, V. Overview of Mitochondrial Bioenergetics. Methods Mol. Biol. 2018, 1782, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Szabo, I.; Zoratti, M. Mitochondrial channels: Ion fluxes and more. Physiol. Rev. 2014, 94, 519–608. [Google Scholar] [CrossRef]
- Vothknecht, U.; Szabo, I. Channels and transporters for inorganic ions in plant mitochondria: Prediction and facts. Mitochondrion 2020, 53, 224–233. [Google Scholar] [CrossRef]
- Checchetto, V.; Szabo, I. Novel Channels of the Outer Membrane of Mitochondria: Recent Discoveries Change Our View. Bioessays 2018, 40, e1700232. [Google Scholar] [CrossRef]
- Inoue, I.; Nagase, H.; Kishi, K.; Higuti, T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature 1991, 352, 244–247. [Google Scholar] [CrossRef]
- Paucek, P.; Mironova, G.; Mahdi, F.; Beavis, A.D.; Woldegiorgis, G.; Garlid, K.D. Reconstitution and partial purification of the glibenclamide-sensitive, ATP-dependent K+ channel from rat liver and beef heart mitochondria. J. Biol. Chem. 1992, 267, 26062–26069. [Google Scholar] [CrossRef]
- De Marchi, U.; Checchetto, V.; Zanetti, M.; Teardo, E.; Soccio, M.; Formentin, E.; Giacometti, G.M.; Pastore, D.; Zoratti, M.; Szabò, I. ATP-sensitive cation-channel in wheat (Triticum durum Desf.): Identification and characterization of a plant mitochondrial channel by patch-clamp. Cell. Physiol. Biochem. 2010, 26, 975–982. [Google Scholar] [CrossRef]
- Choma, K.; Bednarczyk, P.; Koszela-Piotrowska, I.; Kulawiak, B.; Kudin, A.; Kunz, W.S.; Dołowy, K.; Szewczyk, A. Single channel studies of the ATP-regulated potassium channel in brain mitochondria. J. Bioenerg. Biomembr. 2009, 41, 323–334. [Google Scholar] [CrossRef]
- Siemen, D.; Loupatatzis, C.; Borecky, J.; Gulbins, E.; Lang, F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem. Biophys. Res. Commun. 1999, 257, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Koszela-Piotrowska, I.; Matkovic, K.; Szewczyk, A.; Jarmuszkiewicz, W. A large-conductance calcium-activated potassium channel in potato (Solanum tuberosum) tuber mitochondria. Biochem. J. 2009, 424, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, P.; Koziel, A.; Jarmuszkiewicz, W.; Szewczyk, A. Large-conductance Ca2+-activated potassium channel in mitochondria of endothelial EA.hy926 cells. Am. J. Physiol. Circ. Physiol. 2013, 304, H1415–H1427. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, U.; Sassi, N.; Fioretti, B.; Catacuzzeno, L.; Cereghetti, G.M.; Szabò, I.; Zoratti, M.; Szabo, I.; Zoratti, M. Intermediate conductance Ca2+-activated potassium channel (K(Ca)3.1) in the inner mitochondrial membrane of human colon cancer cells. Cell Calcium 2009, 45, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Sassi, N.; De Marchi, U.; Fioretti, B.; Biasutto, L.; Gulbins, E.; Franciolini, F.; Szabò, I.; Zoratti, M. An investigation of the occurrence and properties of the mitochondrial intermediate-conductance Ca2+-activated K+ channel mtKCa3.1. Biochim. Biophys. Acta-Bioenerg. 2010, 1797, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Stowe, D.F.; Gadicherla, A.K.; Zhou, Y.; Aldakkak, M.; Cheng, Q.; Kwok, W.-M.; Jiang, M.T.; Heisner, J.S.; Yang, M.; Camara, A.K.S. Protection against cardiac injury by small Ca2+-sensitive K+ channels identified in guinea pig cardiac inner mitochondrial membrane. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 427–442. [Google Scholar] [CrossRef]
- Yang, M.; Camara, A.K.S.; Aldakkak, M.; Kwok, W.-M.; Stowe, D.F. Identity and function of a cardiac mitochondrial small conductance Ca2+-activated K+ channel splice variant. Biochim. Biophys. Acta-Bioenerg. 2017, 1858, 442–458. [Google Scholar] [CrossRef]
- Smith, C.; Wang, Y.; Nadtochiy, S.; Miller, J.; Jonas, E.; Dirksen, R.; Nehrke, K.; Brookes, P. Cardiac metabolic effects of K Na 1.2 channel deletion and evidence for its mitochondrial localization. FASEB J. 2018, 32, 6135–6149. [Google Scholar] [CrossRef]
- Szabò, I.; Bock, J.; Jekle, A.; Soddemann, M.; Adams, C.; Lang, F.; Zoratti, M.; Gulbins, E. A novel potassium channel in lymphocyte mitochondria. J. Biol. Chem. 2005, 280, 12790–12798. [Google Scholar] [CrossRef]
- Testai, L.; Barrese, V.; Soldovieri, M.V.; Ambrosino, P.; Martelli, A.; Vinciguerra, I.; Miceli, F.; Greenwood, I.A.; Curtis, M.J.; Breschi, M.C.; et al. Expression and function of Kv7.4 channels in rat cardiac mitochondria: Possible targets for cardioprotection. Cardiovasc. Res. 2016, 110, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Rusznák, Z.; Bakondi, G.; Kosztka, L.; Pocsai, K.; Dienes, B.; Fodor, J.; Telek, A.; Gönczi, M.; Szucs, G.; Csernoch, L. Mitochondrial expression of the two-pore domain TASK-3 channels in malignantly transformed and non-malignant human cells. Virchows Arch. 2008, 452, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; He, H.; Kang, C.; Hu, K. TASK-1 regulates mitochondrial function under hypoxia. Biochem. Biophys. Res. Commun. 2021, 578, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Szabo, I.; Leanza, L.; Gulbins, E.; Zoratti, M. Physiology of potassium channels in the inner membrane of mitochondria. Pflugers Arch. J. Physiol. 2012, 463, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Rotko, D.; Bednarczyk, P.; Koprowski, P.; Kunz, W.S.; Szewczyk, A.; Kulawiak, B. Heme is required for carbon monoxide activation of mitochondrial BKCa channel. Eur. J. Pharmacol. 2020, 881. [Google Scholar] [CrossRef] [PubMed]
- Checchetto, V.; Leanza, L.; De Stefani, D.; Rizzuto, R.; Gulbins, E.; Szabo, I. Mitochondrial K+ channels and their implications for disease mechanisms. Pharmacol. Ther. 2021, 227, 107874. [Google Scholar] [CrossRef] [PubMed]
- Paggio, A.; Checchetto, V.; Campo, A.; Menabò, R.; Di Marco, G.; Di Lisa, F.; Szabo, I.; Rizzuto, R.; De Stefani, D. Identification of an ATP-sensitive potassium channel in mitochondria. Nature 2019, 572, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Lu, R.; Bopassa, J.C.; Meredith, A.L.; Stefani, E.; Toro, L. mitoBKCa is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc. Natl. Acad. Sci. USA 2013, 110, 10836. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Wieckowski, M.R.; Broszkiewicz, M.; Skowronek, K.; Siemen, D.; Szewczyk, A. Putative Structural and Functional Coupling of the Mitochondrial BKCa Channel to the Respiratory Chain. PLoS ONE 2013, 8, e68125. [Google Scholar] [CrossRef]
- Szewczyk, A.; Skalska, J.; Głab, M.; Kulawiak, B.; Malińska, D.; Koszela-Piotrowska, I.; Kunz, W.S. Mitochondrial potassium channels: From pharmacology to function. Biochim. Biophys. Acta-Bioenerg. 2006, 1757, 715–720. [Google Scholar] [CrossRef]
- Szewczyk, A.; Kajma, A.; Malinska, D.; Wrzosek, A.; Bednarczyk, P.; Zabłocka, B.; Dołowy, K. Pharmacology of mitochondrial potassium channels: Dark side of the field. FEBS Lett. 2010, 584, 2063–2069. [Google Scholar] [CrossRef]
- Augustynek, B.; Kunz, W.S.; Szewczyk, A. Guide to the Pharmacology of Mitochondrial Potassium Channels. Handb. Exp. Pharmacol. 2016, 240, 103–127. [Google Scholar] [CrossRef]
- Castrejón, V.; Peña, A.; Peña, P.; Uribe, S. Closure of the Yeast Mitochondria Unspecific Channel (YMUC) Unmasks a Mg2+ and Quinine Sensitive K+ Uptake Pathway in Saccharomyces cerevisiae. J. Bioenerg. Biomembr. 2002, 34, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Lobanov, N.A.; Szewczyk, A.; Nowotny, M.; Nal, M.J.; Wójcik, G.; Nowotny, M.; Nałęcz, M.J. Effects of K+ channel inhibitors on potassium transport in bovine adrenal chromaffin granules. Biochem. End Mol. Biol. 1997, 41, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Zakharian, E. Ion channel reconstitution in lipid bilayers. Methods Enzymol. 2021, 652, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Kulawiak, B.; Bednarczyk, P. Reconstitution of brain mitochondria inner membrane into planar lipid bilayer. Acta. Neurobiol. Exp. 2005, 65, 271–276. [Google Scholar]
- Bednarczyk, P.; Dołowy, K.; Szewczyk, A. Matrix Mg2+ regulates mitochondrial ATP-dependent potassium channel from heart. FEBS Lett. 2005, 579, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, P.; Kicińska, A.; Kominkova, V.; Ondrias, K.; Dolowy, K.; Szewczyk, A. Quinine inhibits mitochondrial ATP-regulated potassium channel from bovine heart. J. Membr. Biol. 2004, 199, 63–72. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Dołowy, K.; Szewczyk, A. New properties of mitochondrial ATP-regulated potassium channels. J. Bioenerg. Biomembr. 2008, 40, 325–335. [Google Scholar] [CrossRef]
- Jiang, M.T.; Ljubkovic, M.; Nakae, Y.; Shi, Y.; Kwok, W.M.; Stowe, D.F.; Bosnjak, Z.J. Characterization of human cardiac mitochondrial ATP-sensitive potassium channel and its regulation by phorbol ester in vitro. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1770–H1776. [Google Scholar] [CrossRef]
- Krajewska, M.; Koprowski, P. Solubilization, purification, and functional reconstitution of human ROMK potassium channel in copolymer styrene-maleic acid (SMA) nanodiscs. Biochim. Biophys. Acta-Biomembr. 2021, 1863. [Google Scholar] [CrossRef]
- Petronilli, V.; Szabò, I.; Zoratti, M. The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Lett. 1989, 259, 137–143. [Google Scholar] [CrossRef]
- Sek, A.; Kampa, R.P.; Kulawiak, B.; Szewczyk, A.; Bednarczyk, P. molecules Identification of the Large-Conductance Ca2+-Regulated Potassium Channel in Mitochondria of Human Bronchial Epithelial Cells. Molecules 2021, 26, 3233. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, P.; Kicinska, A.; Laskowski, M.; Kulawiak, B.; Kampa, R.; Walewska, A.; Krajewska, M.; Jarmuszkiewicz, W.; Szewczyk, A. Evidence for a mitochondrial ATP-regulated potassium channel in human dermal fibroblasts. Biochim. Biophys. Acta-Bioenerg. 2018, 1859, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Frankenreiter, S.; Bednarczyk, P.; Kniess, A.; Bork, N.I.; Straubinger, J.; Koprowski, P.; Wrzosek, A.; Mohr, E.; Logan, A.; Murphy, M.P.; et al. cGMP-Elevating Compounds and Ischemic Conditioning Provide Cardioprotection Against Ischemia and Reperfusion Injury via Cardiomyocyte-Specific BK Channels. Circulation 2017, 136, 2337–2355. [Google Scholar] [CrossRef]
- Toczyłowska-Mamińska, R.; Olszewska, A.; Laskowski, M.; Bednarczyk, P.; Skowronek, K.; Szewczyk, A. Potassium channel in the mitochondria of human keratinocytes. J. Investig. Dermatol. 2014, 134, 764–772. [Google Scholar] [CrossRef]
- Laskowski, M.; Augustynek, B.; Bednarczyk, P.; Żochowska, M.; Kalisz, J.; O’rourke, B.; Szewczyk, A.; Kulawiak, B. Single-channel properties of the ROMK-pore-forming subunit of the mitochondrial ATP-sensitive potassium channel. Int. J. Mol. Sci. 2019, 20, 5323. [Google Scholar] [CrossRef]
- Dahlem, Y.A.; Horn, T.F.; Buntinas, L.; Gonoi, T.; Wolf, G.; Siemen, D. The human mitochondrial KATP channel is modulated by calcium and nitric oxide: A patch-clamp approach. Biochim. Biophys. Acta-Bioenerg. 2004, 1656, 46–56. [Google Scholar] [CrossRef]
- Yin, J.; Hu, Y.; Yoon, J. Fluorescent probes and bioimaging: Alkali metals, alkaline earth metals and pH. Chem. Soc. Rev. 2015, 44, 4619–4644. [Google Scholar] [CrossRef]
- Sambath, K.; Liu, X.; Wan, Z.; Hutnik, L.; Belfield, K.D.; Zhang, Y. Potassium Ion Fluorescence Probes: Structures, Properties and Bioimaging. ChemPhotoChem 2021, 5, 317–325. [Google Scholar] [CrossRef]
- Jezek, P.; Mahdi, F.; Garlid, K.D. Reconstitution of the beef heart and rat liver mitochondrial K+/H+ (Na+/H+) antiporter. Quantitation of K+ transport with the novel fluorescent probe, PBFI. J. Biol. Chem. 1990, 265, 10522–10526. [Google Scholar] [CrossRef]
- Rimmele, T.S.; Chatton, J.Y. A novel optical intracellular imaging approach for potassium dynamics in astrocytes. PLoS ONE 2014, 9, e109243. [Google Scholar] [CrossRef] [PubMed]
- Garlid, K.D.; Paucek, P.; Yarov-Yarovoy, V.; Sun, X.; Schindler, P.A. The mitochondrial KATP channel as a receptor for potassium channel openers. J. Biol. Chem. 1996, 271, 8796–8799. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.A.; Cortassa, S.; Wei, A.C.; Grunnet, M.; O’Rourke, B. Energetic performance is improved by specific activation of K+ fluxes through KCa channels in heart mitochondria. Biochim. Biophys. Acta-Bioenerg. 2010, 1797, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Zoeteweij, J.P.; Van De Water, B.; De Bont, H.J.; Nagelkerke, J.F. Mitochondrial K+ as modulator of Ca(2+)-dependent cytotoxicity in hepatocytes. Novel application of the K(+)-sensitive dye PBFI (K(+)-binding benzofuran isophthalate) to assess free mitochondrial K+ concentrations. Biochem. J. 1994, 299 Pt 2, 539–543. [Google Scholar] [CrossRef]
- Weaver, C.D.; Harden, D.; Dworetzky, S.I.; Robertson, B.; Knox, R.J. A Thallium-Sensitive, Fluorescence-Based Assay for Detecting and Characterizing Potassium Channel Modulators in Mammalian Cells. J. Biomol. Screen. 2016, 9, 671–677. [Google Scholar] [CrossRef]
- Wojtovich, A.P.; Williams, D.M.; Karcz, M.K.; Lopes, C.M.B.; Gray, D.A.; Nehrke, K.W.; Brookes, P.S. A Novel Mitochondrial KATP Channel Assay. Circ. Res. 2010, 106, 1190–1196. [Google Scholar] [CrossRef]
- Wojtovich, A.P.; Sherman, T.A.; Nadtochiy, S.M.; Urciuoli, W.R.; Brookes, P.S.; Nehrke, K. Slo-2 is cytoprotective and contributes to mitochondrial potassium transport. PLoS ONE 2011, 6, e28287. [Google Scholar] [CrossRef]
- Foster, D.B.; Ho, A.S.; Rucker, J.; Garlid, A.O.; Chen, L.; Sidor, A.; Garlid, K.D.; O’Rourke, B. Mitochondrial ROMK Channel Is a Molecular Component of MitoK(ATP). Circ. Res. 2012, 111, 446–454. [Google Scholar] [CrossRef]
- Lewis, L.; Bhave, G.; Chauder, B.; Banerjee, S.; Lornsen, K.; Redha, R.; Fallen, K.; Lindsley, C.; Weaver, C.; Denton, J. High-throughput screening reveals a small-molecule inhibitor of the renal outer medullary potassium channel and Kir7.1. Mol. Pharmacol. 2009, 76, 1094–1103. [Google Scholar] [CrossRef]
- Beacham, D.W.; Blackmer, T.; Grady, M.O.; Hanson, G.T. Cell-Based Potassium Ion Channel Screening Using the FluxORTM Assay. J. Biomol. Screen. 2010, 15, 441–446. [Google Scholar] [CrossRef]
- Bischof, H.; Rehberg, M.; Stryeck, S.; Artinger, K.; Eroglu, E.; Waldeck-Weiermair, M.; Gottschalk, B.; Rost, R.; Deak, A.T.; Niedrist, T.; et al. Novel genetically encoded fluorescent probes enable real-time detection of potassium in vitro and in vivo. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bajgar, R.; Seetharaman, S.; Kowaltowski, A.J.; Garlid, K.D.; Paucek, P. Identification and properties of a novel intracellular (mitochondrial) ATP-sensitive potassium channel in brain. J. Biol. Chem. 2001, 276, 33369–33374. [Google Scholar] [CrossRef]
- Costa, A.D.T.; Garlid, K.D. Intramitochondrial signaling: Interactions among mitoKATP, PKCε, ROS, and MPT. Am. J. Physiol. Circ. Physiol. 2008, 295, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Kicinska, A.; Augustynek, B.; Kulawiak, B.; Jarmuszkiewicz, W.; Szewczyk, A.; Bednarczyk, P. A large-conductance calcium-regulated K+ channel in human dermal fibroblast mitochondria. Biochem. J. 2016, 473, 4457–4471. [Google Scholar] [CrossRef] [PubMed]
- Kicinska, A.; Swida, A.; Bednarczyk, P.; Koszela-Piotrowska, I.; Choma, K.; Dolowy, K.; Szewczyk, A.; Jarmuszkiewicz, W. ATP-sensitive potassium channel in mitochondria of the eukaryotic microorganism Acanthamoeba castellanii. J. Biol. Chem. 2007, 282, 17433–17441. [Google Scholar] [CrossRef]
- Laskowski, M.; Kicinska, A.; Szewczyk, A.; Jarmuszkiewicz, W. Mitochondrial large-conductance potassium channel from Dictyostelium discoideum. Int. J. Biochem. Cell Biol. 2015, 60, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Dolga, A.M.; Netter, M.F.; Perocchi, F.; Doti, N.; Meissner, L.; Tobaben, S.; Grohm, J.; Zischka, H.; Plesnila, N.; Decher, N.; et al. Mitochondrial small conductance sk2 channels prevent glutamate-induced oxytosis and mitochondrial dysfunction. J. Biol. Chem. 2013, 288, 10792–10804. [Google Scholar] [CrossRef] [PubMed]
- Mueller, P.; Rudin, D.O.; Ti Tien, H.; Wescott, W.C. Reconstitution of Cell Membrane Structure in vitro and its Transformation into an Excitable System. Nature 1962, 194, 979–980. [Google Scholar] [CrossRef]
- Daum, G.; Vance, J.E. Import of lipids into mitochondria. Prog. Lipid Res. 1997, 36, 103–130. [Google Scholar] [CrossRef]
- Urbani, A.; Giorgio, V.; Carrer, A.; Franchin, C.; Arrigoni, G.; Jiko, C.; Abe, K.; Maeda, S.; Shinzawa-Itoh, K.; Bogers, J.; et al. Purified F-ATP synthase forms a Ca2+-dependent high-conductance channel matching the mitochondrial permeability transition pore. Nat. Commun. 2019, 10, 4341. [Google Scholar] [CrossRef]
- Mnatsakanyan, N.; Llaguno, M.C.; Yang, Y.; Yan, Y.; Weber, J.; Sigworth, F.J.; Jonas, E.A. A mitochondrial megachannel resides in monomeric F1FO ATP synthase. Nat. Commun. 2019, 10, 5823. [Google Scholar] [CrossRef] [PubMed]
- Overduin, M.; Esmaili, M. Memtein: The fundamental unit of membrane-protein structure and function. Chem. Phys. Lipids 2019, 218, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Denisov, I.G.; Grinkova, Y.V.; Lazarides, A.A.; Sligar, S.G. Directed Self-Assembly of Monodisperse Phospholipid Bilayer Nanodiscs with Controlled Size. J. Am. Chem. Soc. 2004, 126, 3477–3487. [Google Scholar] [CrossRef] [PubMed]
- Dörr, J.M.; Scheidelaar, S.; Koorengevel, M.C.; Dominguez, J.J.; Schäfer, M.; van Walree, C.A.; Killian, J.A. The styrene–maleic acid copolymer: A versatile tool in membrane research. Eur. Biophys. J. 2016, 45, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.L.; Young, J.W.; Zhao, Z.; Fabre, L.; Jun, D.; Li, J.; Li, J.; Dhupar, H.S.; Wason, I.; Mills, A.T.; et al. The peptidisc, a simple method for stabilizing membrane proteins in detergent-free solution. eLife 2018, 7, e34085. [Google Scholar] [CrossRef] [PubMed]
- Angiulli, G.; Dhupar, H.; Suzuki, H.; Wason, I.; Van Hoa, F.D.; Walz, T. New approach for membrane protein reconstitution into peptidiscs and basis for their adaptability to different proteins. eLife 2020, 9, e53530. [Google Scholar] [CrossRef]
- Bao, H.; Duong, F.; Chan, C. A step-by-step method for the reconstitution of an ABC transporter into nanodisc lipid particles. J. Vis. Exp. 2012, 66, e3910. [Google Scholar] [CrossRef]
- Winterstein, L.L.M.; Kukovetz, K.; Rauh, O.; Turman, D.L.D.; Braun, C.; Moroni, A.; Schroeder, I.; Thiel, G. Reconstitution and functional characterization of ion channels from nanodiscs in lipid bilayers. J. Gen. Physiol. 2018, 150, 637–646. [Google Scholar] [CrossRef]
- Overduin, M.; Esmaili, M. Native Nanodiscs and the Convergence of Lipidomics, Metabolomics, Interactomics and Proteomics. Appl. Sci. 2019, 9, 1230. [Google Scholar] [CrossRef]
- Smirnova, I.A.; Sjöstrand, D.; Li, F.; Björck, M.; Schäfer, J.; Östbye, H.; Högbom, M.; von Ballmoos, C.; Lander, G.C.; Ädelroth, P.; et al. Isolation of yeast complex IV in native lipid nanodiscs. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 2984–2992. [Google Scholar] [CrossRef]
- Janson, K.; Zierath, J.; Kyrilis, F.L.; Semchonok, D.A.; Hamdi, F.; Skalidis, I.; Kopf, A.H.; Das, M.; Kolar, C.; Rasche, M.; et al. Solubilization of artificial mitochondrial membranes by amphiphilic copolymers of different charge. Biochim. Biophys. Acta-Biomembr. 2021, 1863, 183725. [Google Scholar] [CrossRef]
- Neher, E.; Sakmann, B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 1976, 260, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Neher, E.; Sakmann, B.; Steinbach, J.H. The extracellular patch clamp: A method for resolving currents through individual open channels in biological membranes. Pflügers Arch. Eur. J. Physiol. 1978, 375, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Segev, A.; Garcia-Oscos, F.; Kourrich, S. Whole-cell Patch-clamp Recordings in Brain Slices. J. Vis. Exp 2016, 112, e54024. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Zhong, X.Z.; Zou, Y.; Zhang, Z.; Toro, L.; Dong, X.-P. BK Channels Alleviate Lysosomal Storage Diseases by Providing Positive Feedback Regulation of Lysosomal Ca2+ Release. Dev. Cell 2015, 33, 427–441. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, Z.; Li, Q.; Tan, Z. Lysosomal Potassium Channels: Potential Roles in Lysosomal Function and Neurodegenerative Diseases. CNS Neurol. Disord. Drug Targets 2018, 17, 261–266. [Google Scholar] [CrossRef]
- Sorgato, M.C.; Keller, B.U.; Stühmer, W. Patch-clamping of the inner mitochondrial membrane reveals a voltage-dependent ion channel. Nature 1987, 330, 498–500. [Google Scholar] [CrossRef]
- Bustamante, J.; Varanda, W. Patch-clamping macromolecular translocation along nuclear pores. Brazilian J. Med. Biol. Res. 1998, 31, 333–354. [Google Scholar] [CrossRef][Green Version]
- Mak, D.-O.D.; Vais, H.; Cheung, K.-H.; Foskett, J.K. Isolating Nuclei from Cultured Cells for Patch-Clamp Electrophysiology of Intracellular Ca2+ Channels. Cold Spring Harb. Protoc. 2013, 2013, 880–884. [Google Scholar] [CrossRef]
- Martinac, B.; Buechner, M.; Delcour, A.H.; Adler, J.; Kung, C. Pressure -sensitive ion channel in Escherichia coli. Proc. Natl. Acad. Sci. USA 1987, 84, 2297–2301. [Google Scholar] [CrossRef]
- Grajkowski, W.; Kubalski, A.; Koprowski, P. Surface changes of the mechanosensitive channel MscS upon its activation, inactivation, and closing. Biophys. J. 2005, 88, 3050–3059. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szabó, I.; Zoratti, M. The mitochondrial megachannel is the permeability transition pore. J. Bioenerg. Biomembr. 1992, 24, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Walewska, A.; Kulawiak, B.; Szewczyk, A.; Koprowski, P. Mechanosensitivity of mitochondrial large-conductance calcium-activated potassium channels. Biochim. Biophys. Acta-Bioenerg. 2018, 1859, 797–805. [Google Scholar] [CrossRef]
- Kampa, R.P.; Kicinska, A.; Jarmuszkiewicz, W.; Pasikowska-Piwko, M.; Dolegowska, B.; Debowska, R.; Szewczyk, A.; Bednarczyk, P. Naringenin as an opener of mitochondrial potassium channels in dermal fibroblasts. Exp. Dermatol. 2019, 28, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Kampa, R.; Sęk, A.; Szewczyk, A.; Bednarczyk, P. Cytoprotective effects of the flavonoid quercetin by activating mitochondrial BK Ca channels in endothelial cells. Biomed. Pharmacother. 2021, 142, 112039. [Google Scholar] [CrossRef] [PubMed]
- Jonas, E.A.; Knox, R.J.; Kaczmarek, L.K. Giga-Ohm Seals on Intracellular Membranes: A Technique for Studying Intracellular Ion Channels in Intact Cells. Neuron 1997, 19, 7–13. [Google Scholar] [CrossRef]
- Jonas, E.A.; Mnatsakanyan, N. Examination of Mitochondrial Ion Conductance by Patch Clamp in Intact Neurons and Mitochondrial Membrane Preparations. Neuromethods 2017, 123, 211–238. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Kampa, R.P.; Gałecka, S.; Se, A.; Walewska, A.; Koprowski, P. Patch-Clamp Recording of the Activity of Ion Channels. In Mitochondrial Medicine; Springer: New York, NY, USA, 2021. [Google Scholar]
- Kirichok, Y.; Krapivinsky, G.; Clapham, D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004, 427, 360–364. [Google Scholar] [CrossRef]
- King, C.; Sengupta, P.; Seo, A.Y.; Lippincott-Schwartz, J. ER membranes exhibit phase behavior at sites of organelle contact. Proc. Natl. Acad. Sci. USA 2020, 117, 7225–7235. [Google Scholar] [CrossRef]
- Jakob, R.; Beutner, G.; Sharma, V.K.; Duan, Y.; Gross, R.A.; Hurst, S.; Jhun, B.S.; O-Uchi, J.; Sheu, S.S. Molecular and functional identification of a mitochondrial ryanodine receptor in neurons. Neurosci. Lett. 2014, 575, 7–12. [Google Scholar] [CrossRef]
- Ryu, S.Y.; Beutner, G.; Kinnally, K.W.; Dirksen, R.T.; Sheu, S.S. Single Channel Characterization of the Mitochondrial Ryanodine Receptor in Heart Mitoplasts. J. Biol. Chem. 2011, 286, 21324–21329. [Google Scholar] [CrossRef] [PubMed]
- Noterman, M.F.; Chaubey, K.; Lin-Rahardja, K.; Rajadhyaksha, A.M.; Pieper, A.A.; Taylor, E.B. Dual-process brain mitochondria isolation preserves function and clarifies protein composition. Proc. Natl. Acad. Sci. USA 2021, 118, e2019046118. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Suzuki, J.; Paranjpe, I.; Unsulangi, T.; Boyman, L.; Milescu, L.; Lederer, W.; Kirichok, Y. The mechanism of MICU-dependent gating of the mitochondrial Ca2+ uniporter. eLife 2021, 10, e69312. [Google Scholar] [CrossRef] [PubMed]
- Szabó, I.; Bernardi, P.; Zoratti, M. Modulation of the mitochondrial megachannel by divalent cations and protons. J. Biol. Chem. 1992, 267, 2940–2946. [Google Scholar] [CrossRef]
- Martinucci, S.; Szabò, I.; Tombola, F.; Zoratti, M. Ca2+-reversible inhibition of the mitochondrial megachannel by ubiquinone analogues. FEBS Lett. 2000, 480, 89–94. [Google Scholar] [CrossRef]
- Giorgio, V.; von Stockum, S.; Antoniel, M.; Fabbro, A.; Fogolari, F.; Forte, M.; Glick, G.D.; Petronilli, V.; Zoratti, M.; Szabó, I.; et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. USA 2013, 110, 5887–5892. [Google Scholar] [CrossRef]
- Carraro, M.; Carrer, A.; Urbani, A.; Bernardi, P. Molecular nature and regulation of the mitochondrial permeability transition pore(s), drug target(s) in cardioprotection. J. Mol. Cell. Cardiol. 2020, 144, 76–86. [Google Scholar] [CrossRef]
- Kinnally, K.; Zorov, D.; Antonenko, Y.; Perini, S. Calcium modulation of mitochondrial inner membrane channel activity. Biochem. Biophys. Res. Commun. 1991, 176, 1183–1188. [Google Scholar] [CrossRef]
- Linley, J. Perforated whole-cell patch-clamp recording. Methods Mol. Biol. 2013, 998, 149–157. [Google Scholar] [CrossRef]
- Liu, D.; Slevin, J.R.; Lu, C.; Chan, S.L.; Hansson, M.; Elmér, E.; Mattson, M.P. Involvement of mitochondrial K+ release and cellular efflux in ischemic and apoptotic neuronal death. J. Neurochem. 2003, 86, 966–979. [Google Scholar] [CrossRef]
- Kong, X.; Su, F.; Zhang, L.; Yaron, J.; Lee, F.; Shi, Z.; Tian, Y.; Meldrum, D.R. A Highly Selective Mitochondria-Targeting Fluorescent K+ Sensor. Angew. Chemie Int. Ed. 2015, 54, 12053–12057. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Tian, Y. Development of a new simple mitochondria-targeted fluorescent K+ sensor and the application in high-throughput monitoring K+ fluxes. Sens. Actuators B Chem. 2020, 307, 127659. [Google Scholar] [CrossRef]
- Song, G.; Jiang, D.; Wang, L.; Ning, J.; Sun, X.; Su, F.; Chen, M.; Tian, Y. A mitochondria-targeting NIR fluorescent potassium ion sensor: Real-time investigation of the mitochondrial K+ regulation of apoptosis in situ. Chem. Commun. 2020, 56, 5405–5408. [Google Scholar] [CrossRef] [PubMed]
- Hille, B. Potassium channels in myelinated nerve. Selective permeability to small cations. J. Gen. Physiol. 1973, 61, 669–686. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, S.; Rancic, V.; Aggarwal, A.; Qian, Y.; Miyashita, S.; Ballanyi, K.; Campbell, R.; Dong, M. Genetically encoded fluorescent indicators for imaging intracellular potassium ion concentration. Commun. Biol. 2019, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Garlid, K.D.; Paucek, P. Mitochondrial potassium transport: The K+ cycle. Biochim. Biophys. Acta-Bioenergetics 2003, 1606, 23–41. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Grav, H.J.; Lindberg, O. Mitochondria from Hamster Brown-Adipose Tissue. Eur. J. Biochem. 1972, 31, 526–533. [Google Scholar] [CrossRef]
- Halestrap, A.P. The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism. Biochim. Biophys. Acta 1989, 973, 355–382. [Google Scholar] [CrossRef]
- Riess, M.L.; Costa, A.D.; Carlson, R.; Garlid, K.D.; Heinen, A.; Stowe, D.F. Differential increase of mitochondrial matrix volume by sevoflurane in isolated cardiac mitochondria. Anesth. Analg. 2008, 106, 1049–1055. [Google Scholar] [CrossRef]
- Javadov, S.; Chapa-Dubocq, X.; Makarov, V. Different approaches to modeling analysis of mitochondrial swelling. Mitochondrion 2018, 38, 58–70. [Google Scholar] [CrossRef]
- Kulawiak, B.; Kudin, A.P.; Szewczyk, A.; Kunz, W.S. BK channel openers inhibit ROS production of isolated rat brain mitochondria. Exp. Neurol. 2008, 212, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Czyz, A.; Szewczyk, A.; Nalecz, M.J.; Wojtczak, L. The role of mitochondrial potassium fluxes in controlling the protonmotive force in energized mitochondria. Biochem. Biophys. Res. Commun. 1995, 210, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Höfer, N.; Aragão, D.; Caffrey, M. Crystallizing Transmembrane Peptides in Lipidic Mesophases. Biophys. J. 2010, 99, L23–L25. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, E.; Bilewicz, R. Catalytic activity of oxidases hosted in lipidic cubic phases on electrodes. Bioelectrochemistry 2007, 71, 8–14. [Google Scholar] [CrossRef]
- Landau, E.; Rosenbusch, J. Lipidic cubic phases: A novel concept for the crystallization of membrane proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 14532–14535. [Google Scholar] [CrossRef]
- Li, D.; Caffrey, M. Lipid cubic phase as a membrane mimetic for integral membrane protein enzymes. Proc. Natl. Acad. Sci. USA 2011, 108, 8639–8644. [Google Scholar] [CrossRef]
- Caffrey, M. IUCr A comprehensive review of the lipid cubic phase or in meso method for crystallizing membrane and soluble proteins and complexes. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71, 3–18. [Google Scholar] [CrossRef]
- Tiefenbrunn, T.; Liu, W.; Chen, Y.; Katritch, V.; Stout, C.D.; Fee, J.A.; Cherezov, V. High Resolution Structure of the ba3 Cytochrome c Oxidase from Thermus thermophilus in a Lipidic Environment. PLoS ONE 2011, 6, e22348. [Google Scholar] [CrossRef]
- Santos, J.S.; Asmar-Rovira, G.A.; Han, G.W.; Liu, W.; Syeda, R.; Cherezov, V.; Baker, K.A.; Stevens, R.C.; Montal, M. Crystal Structure of a Voltage-gated K+ Channel Pore Module in a Closed State in Lipid Membranes. J. Biol. Chem. 2012, 287, 43063–43070. [Google Scholar] [CrossRef]
- Zabara, A.; Chong, J.T.Y.; Martiel, I.; Stark, L.; Cromer, B.A.; Speziale, C.; Drummond, C.J.; Mezzenga, R. Design of ultra-swollen lipidic mesophases for the crystallization of membrane proteins with large extracellular domains. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Cherezov, V. Lipidic cubic phase technologies for membrane protein structural studies. Curr. Opin. Struct. Biol. 2011, 21, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Weichert, D.; Aleksandrov, L.A.; Jensen, T.J.; Riordan, J.R.; Liu, X.; Kobilka, B.K.; Caffrey, M. The cubicon method for concentrating membrane proteins in the cubic mesophase. Nat. Protoc. 2017, 12, 1745–1762. [Google Scholar] [CrossRef] [PubMed]
- Belrhali, H.; Nollert, P.; Royant, A.; Menzel, C.; Rosenbusch, J.P.; Landau, E.M.; Pebay-Peyroula, E. Protein, lipid and water organization in bacteriorhodopsin crystals: A molecular view of the purple membrane at 1.9 Å resolution. Structure 1999, 7, 909–917. [Google Scholar] [CrossRef]
- Nazaruk, E.; Szlęzak, M.; Górecka, E.; Bilewicz, R.; Osornio, Y.M.; Uebelhart, P.; Landau, E.M. Design and Assembly of pH-Sensitive Lipidic Cubic Phase Matrices for Drug Release. Langmuir 2014, 30, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Vallooran, J.J.; Fong, W.-K.; Mezzenga, R. Lyotropic Liquid Crystalline Cubic Phases as Versatile Host Matrices for Membrane-Bound Enzymes. J. Phys. Chem. Lett. 2016, 7, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Zatloukalová, M.; Nazaruk, E.; Novák, D.; Vacek, J.; Bilewicz, R. Lipidic liquid crystalline cubic phases for preparation of ATP-hydrolysing enzyme electrodes. Biosens. Bioelectron. 2018, 100, 437–444. [Google Scholar] [CrossRef]
- Speziale, C.; Manni, L.S.; Manatschal, C.; Landau, E.M.; Mezzenga, R. A macroscopic H+ and Cl− ions pump via reconstitution of EcClC membrane proteins in lipidic cubic mesophases. Proc. Natl. Acad. Sci. USA 2016, 113, 7491–7496. [Google Scholar] [CrossRef]
- Speziale, C.; Zabara, A.F.; Drummond, C.J.; Mezzenga, R. Active Gating, Molecular Pumping, and Turnover Determination in Biomimetic Lipidic Cubic Mesophases with Reconstituted Membrane Proteins. ACS Nano 2017, 11, 11687–11693. [Google Scholar] [CrossRef]
- Conn, C.E.; Drummond, C.J. Nanostructured bicontinuous cubic lipid self-assembly materials as matrices for protein encapsulation. Soft Matter 2013, 9, 3449–3464. [Google Scholar] [CrossRef]
- Li, D.; Caffrey, M. Structure and Functional Characterization of Membrane Integral Proteins in the Lipid Cubic Phase. J. Mol. Biol. 2020, 432, 5104–5123. [Google Scholar] [CrossRef]
- Meikle, T.G.; Conn, C.E.; Separovic, F.; Drummond, C.J. Exploring the structural relationship between encapsulated antimicrobial peptides and the bilayer membrane mimetic lipidic cubic phase: Studies with gramicidin A’. RSC Adv. 2016, 6, 68685–68694. [Google Scholar] [CrossRef]
- Buta, A.; Nazaruk, E.; Dziubak, D.; Szewczyk, A.; Bilewicz, R. Properties of electrode-supported lipid cubic mesophase films with embedded gramicidin A: Structure and ion-transport studies. Bioelectrochemistry 2022, 144, 108042. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, E.; Michota, A.; Bukowska, J.; Shleev, S.; Gorton, L.; Bilewicz, R. Properties of native and hydrophobic laccases immobilized in the liquid-crystalline cubic phase on electrodes. JBIC J. Biol. Inorg. Chem. 2006, 12, 335–344. [Google Scholar] [CrossRef]
- Angelova, A.; Ollivon, M.; Campitelli, A.; Bourgaux, C. Lipid Cubic Phases as Stable Nanochannel Network Structures for Protein Biochip Development: X-ray Diffraction Study. Langmuir 2003, 19, 6928–6935. [Google Scholar] [CrossRef]
- Murgia, S.; Biffi, S.; Mezzenga, R. Recent advances of non-lamellar lyotropic liquid crystalline nanoparticles in nanomedicine. Curr. Opin. Colloid Interface Sci. 2020, 48, 28–39. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Guidelli, R.; Becucci, L. Functional activity of peptide ion channels in tethered bilayer lipid membranes: Review. Electrochem. Sci. Adv. 2021, e2100180. [Google Scholar] [CrossRef]
- Becucci, L.; Guidelli, R. Equilibrium distribution of K+ ions in the hydrophilic spacer of tethered bilayer lipid membranes. Soft Matter 2009, 5, 2294–2301. [Google Scholar] [CrossRef]
- Becucci, L.; Moncelli, M.R.; Guidelli, R. Thallous ion movements through gramicidin channels incorporated in lipid monolayers supported by mercury. Biophys. J. 2002, 82, 852. [Google Scholar] [CrossRef]
- Su, Z.F.; Leitch, J.J.; Lipkowski, J. Electrode-supported biomimetic membranes: An electrochemical and surface science approach for characterizing biological cell membranes. Curr. Opin. Electrochem. 2018, 12, 60–72. [Google Scholar] [CrossRef]
- Su, Z.F.; Goodall, B.; Leitch, J.J.; Lipkowski, J. Ion transport mechanism in gramicidin A channels formed in floating bilayer lipid membranes supported on gold electrodes. Electrochim. Acta 2021, 375, 137892. [Google Scholar] [CrossRef]
- Guidelli, R.; Becucci, L. 4 Electrochemistry of Biomimetic Membranes. In Applications of Electrochemistry and Nanotechnology in Biology and Medicine II; Springer: Boston, MA, USA, 2012; pp. 147–266. [Google Scholar] [CrossRef]
- Brzozowska, M.; Oberts, B.P.; Blanchard, G.J.; Majewski, J.; Krysinski, P. Design and Characterization of Novel Tether Layer for Coupling of a Bilayer Lipid Membrane to the Surface of Gold. Langmuir 2009, 25, 9337–9345. [Google Scholar] [CrossRef]
- Mauzeroll, J.; Buda, M.; Bard, A.J.; Prieto, F.; Rueda, M. Detection of Tl(I) transport through a gramicidin-dioleoylphosphatidylcholine monolayer using the substrate generation-tip collection mode of scanning electrochemical microscopy. Langmuir 2002, 18, 9453–9461. [Google Scholar] [CrossRef]
- Zebrowska, A.; Krysiński, P. Incorporation of Na+, K+-ATP-ase into the Thiolipid Biomimetic Assemblies via the Fusion of Proteoliposomes. Langmuir 2004, 20, 11127–11133. [Google Scholar] [CrossRef] [PubMed]
- Naumann, R.; Baumgart, T.; Gräber, P.; Jonczyk, A.; Offenhäusser, A.; Knoll, W. Proton transport through a peptide-tethered bilayer lipid membrane by the H+-ATP synthase from chloroplasts measured by impedance spectroscopy. Biosens. Bioelectron. 2002, 17, 25–34. [Google Scholar] [CrossRef]
- Knoll, W.; Naumann, R.; Friedrich, M.; Robertson, J.W.F.; Lösche, M.; Heinrich, F.; McGillivray, D.J.; Schuster, B.; Gufler, P.C.; Pum, D.; et al. Solid supported lipid membranes: New concepts for the biomimetic functionalization of solid surfaces. Biointerphases 2008, 3, FA125. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, P.; Walter, C.; Selenschik, P.; Honigmann, A.; Wagner, R. Horizontal Bilayer for Electrical and Optical Recordings. Materials 2012, 5, 2705. [Google Scholar] [CrossRef]
- Rajapaksha, S.P.; Wang, X.; Lu, H.P. Suspended Lipid Bilayer for Optical and Electrical Measurements of Single Ion Channel Proteins. Anal. Chem. 2013, 85, 8951–8955. [Google Scholar] [CrossRef]
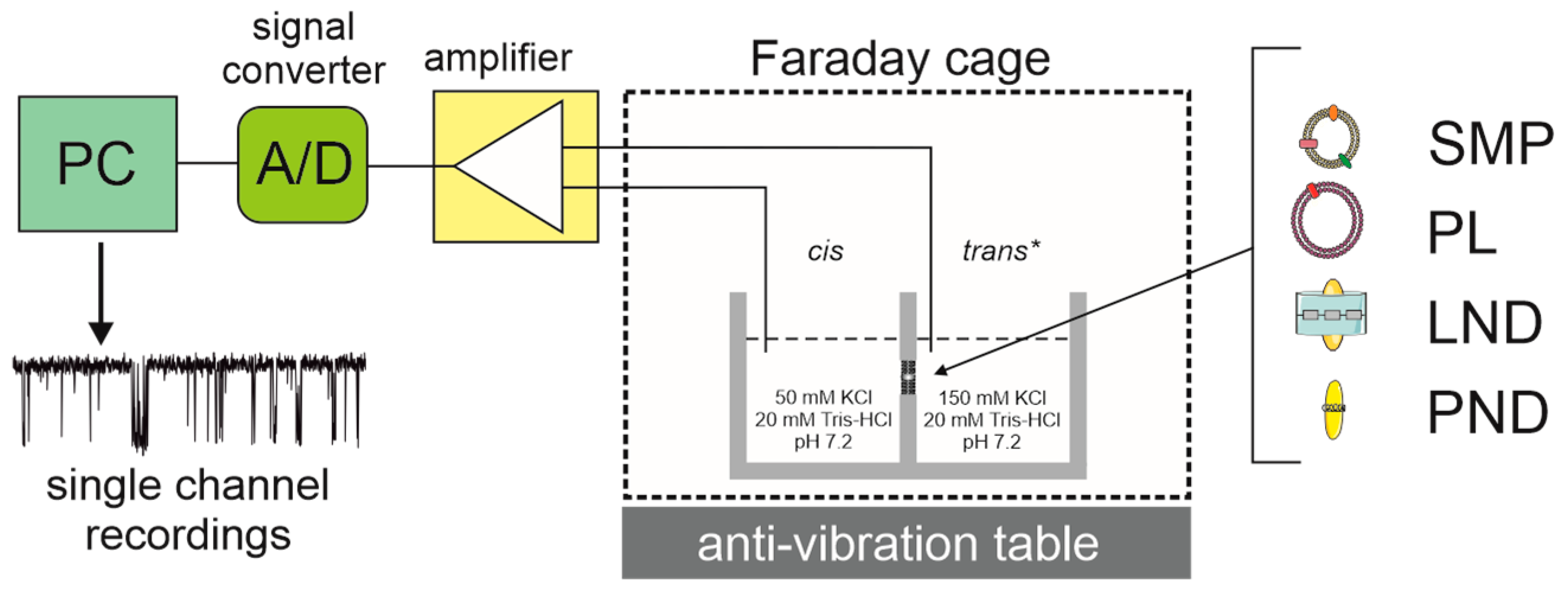
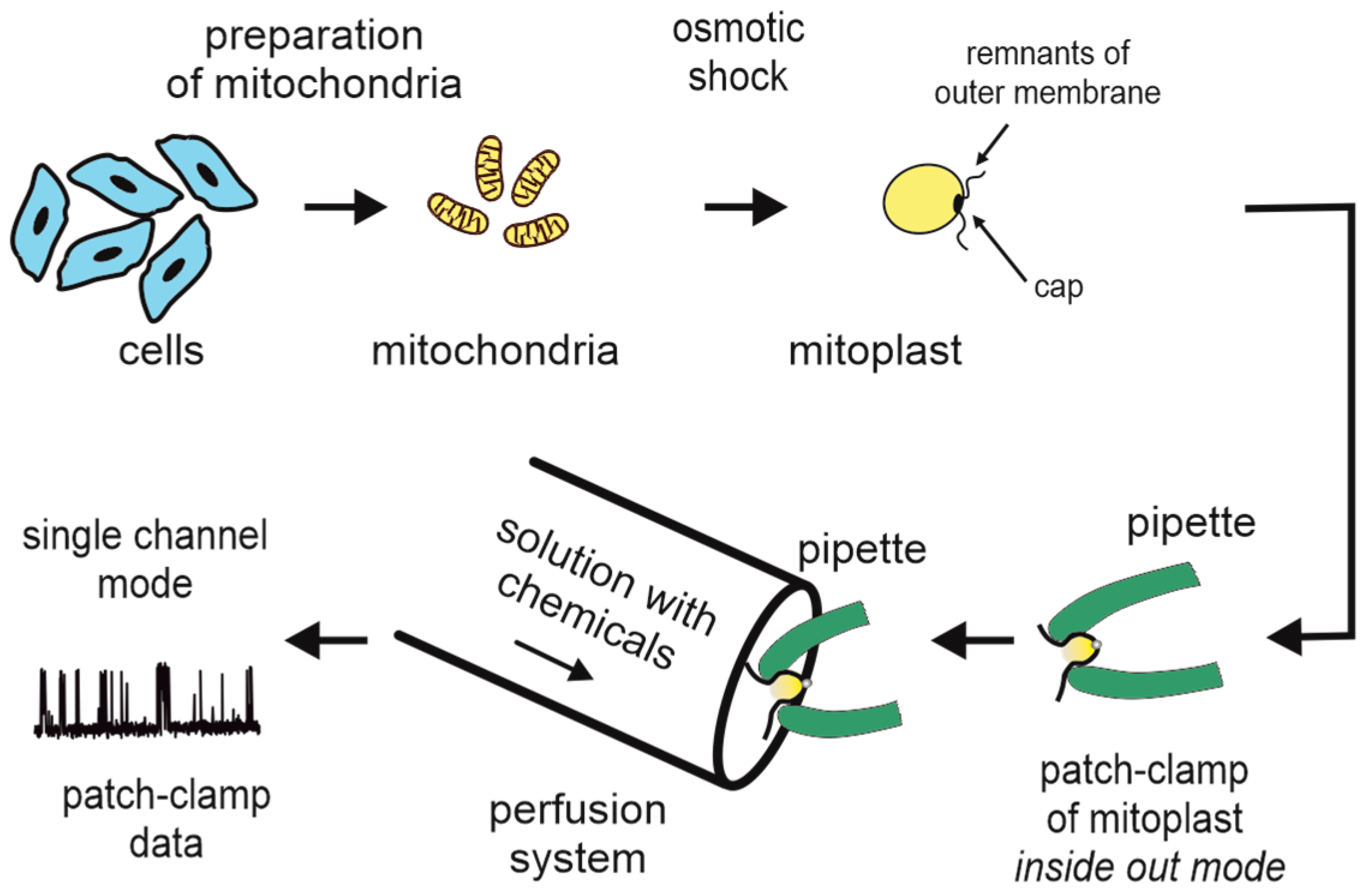
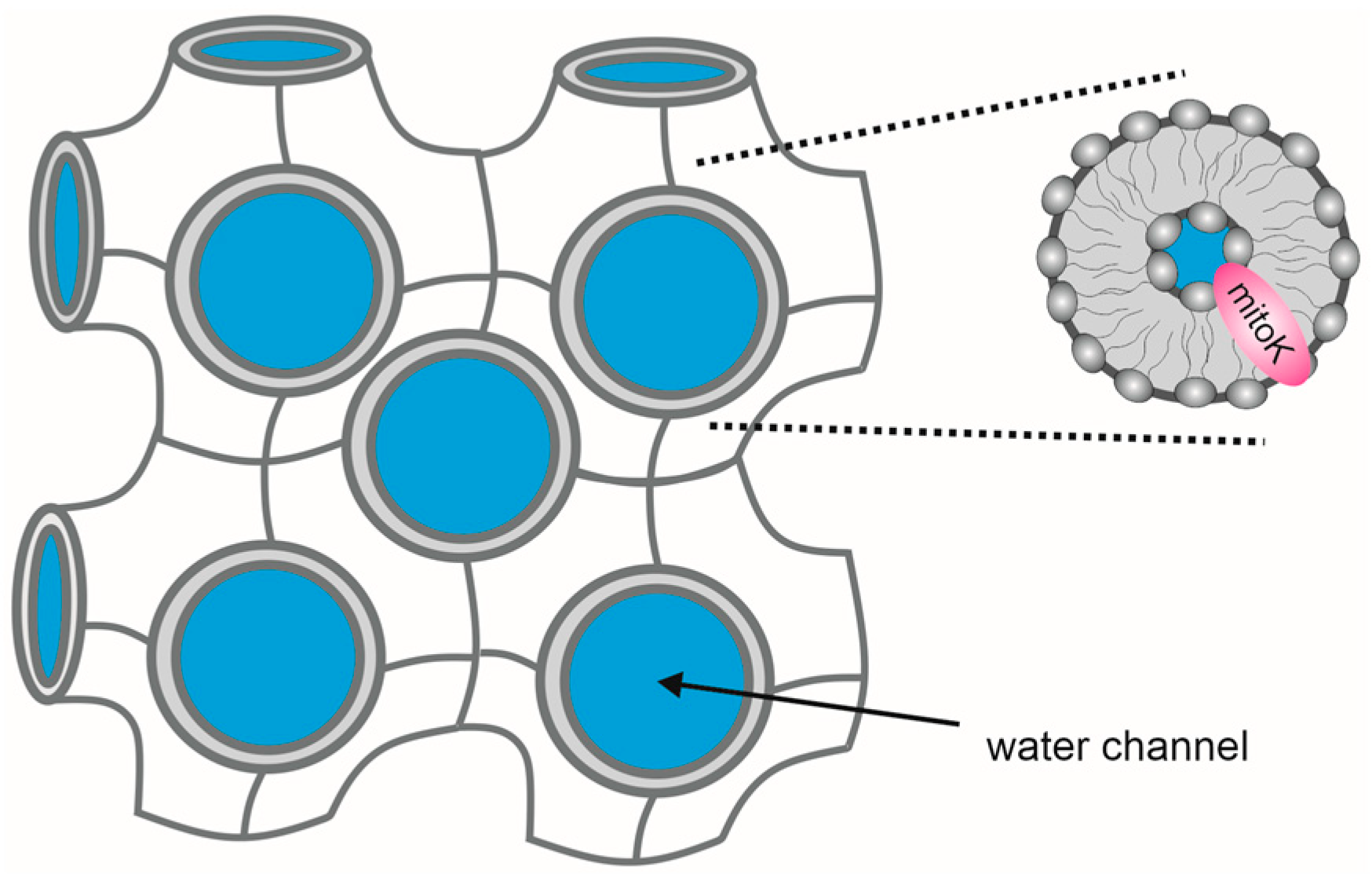

| Technique | Parameters Measured | Advantages | Limitations | References |
|---|---|---|---|---|
| Electrophysiological Techniques | ||||
| Planar lipid bilayer | Current at a controlled voltage (single-channel conductance, open probability, channel activation/inhibition) | Single-channel measurements—high sensitivity and selectivity, reconstitution into a defined lipid bilayer, easy access to both bilayer sides | Purity of channel protein preparation | [44,45,46,47,48,49,50] |
| Patch-clamp of the inner mitochondrial membrane | Current at a controlled voltage (single-channel conductance, open probability, channel activation/inhibition) | Single-channel measurements—high sensitivity and selectivity, application to native membranes | Purity of the mitochondria, nonphysiological extramembrane environment | [29,51,52,53,54,55,56,57] |
| K+ Flux Measurements by Fluorescent Probes | ||||
| Small molecule probes | K+ concentration changes | Simple application to cell, isolated mitochondria, or liposomes | Limited localization in mitochondria, poor selectivity for K+ over Na+, difficult quantification of absolute [K+], excitation with phototoxic UV-light | [58,59,60,61,62,63,64] |
| Tl+-sensitive indicators | Tl+ concentration changes | Nonfluorescent in the absence of Tl+ ions | Low solubility of thallium chloride, nonspecific subcellular localization | [65,66,67,68,69,70] |
| Genetically encoded probes | K+ concentration changes | Specific mitochondrial localization, FRET ratiometric measurements, absolute [K+] determination | Efficient transfection required, sophisticated microscope setup | [71] |
| Biochemical Techniques | ||||
| Mitochondrial swelling | Mitochondrial volume | Simple way of macroscopic ion flux measurements, can be measured in fixed cells using electron microscopy | Only isolated mitochondria, low selectivity | [62,63,72,73] |
| Respiration | Oxygen consumption | Tissue, cells, and isolated mitochondria measurements | Sensitive to nonspecific drug action on mitochondria or drug uncoupling properties | [22,23,53,72,74,75,76] |
| Mitochondrial potential | Potential changes | Cells and isolated mitochondria | Sensitive to nonspecific action on mitochondria | [22,23,53,74,75,76,77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walewska, A.; Krajewska, M.; Stefanowska, A.; Buta, A.; Bilewicz, R.; Krysiński, P.; Bednarczyk, P.; Koprowski, P.; Szewczyk, A. Methods of Measuring Mitochondrial Potassium Channels: A Critical Assessment. Int. J. Mol. Sci. 2022, 23, 1210. https://doi.org/10.3390/ijms23031210
Walewska A, Krajewska M, Stefanowska A, Buta A, Bilewicz R, Krysiński P, Bednarczyk P, Koprowski P, Szewczyk A. Methods of Measuring Mitochondrial Potassium Channels: A Critical Assessment. International Journal of Molecular Sciences. 2022; 23(3):1210. https://doi.org/10.3390/ijms23031210
Chicago/Turabian StyleWalewska, Agnieszka, Milena Krajewska, Aleksandra Stefanowska, Aleksandra Buta, Renata Bilewicz, Paweł Krysiński, Piotr Bednarczyk, Piotr Koprowski, and Adam Szewczyk. 2022. "Methods of Measuring Mitochondrial Potassium Channels: A Critical Assessment" International Journal of Molecular Sciences 23, no. 3: 1210. https://doi.org/10.3390/ijms23031210
APA StyleWalewska, A., Krajewska, M., Stefanowska, A., Buta, A., Bilewicz, R., Krysiński, P., Bednarczyk, P., Koprowski, P., & Szewczyk, A. (2022). Methods of Measuring Mitochondrial Potassium Channels: A Critical Assessment. International Journal of Molecular Sciences, 23(3), 1210. https://doi.org/10.3390/ijms23031210








