Latrotoxin-Induced Neuromuscular Junction Degeneration Reveals Urocortin 2 as a Critical Contributor to Motor Axon Terminal Regeneration
Abstract
:1. Introduction
2. Results
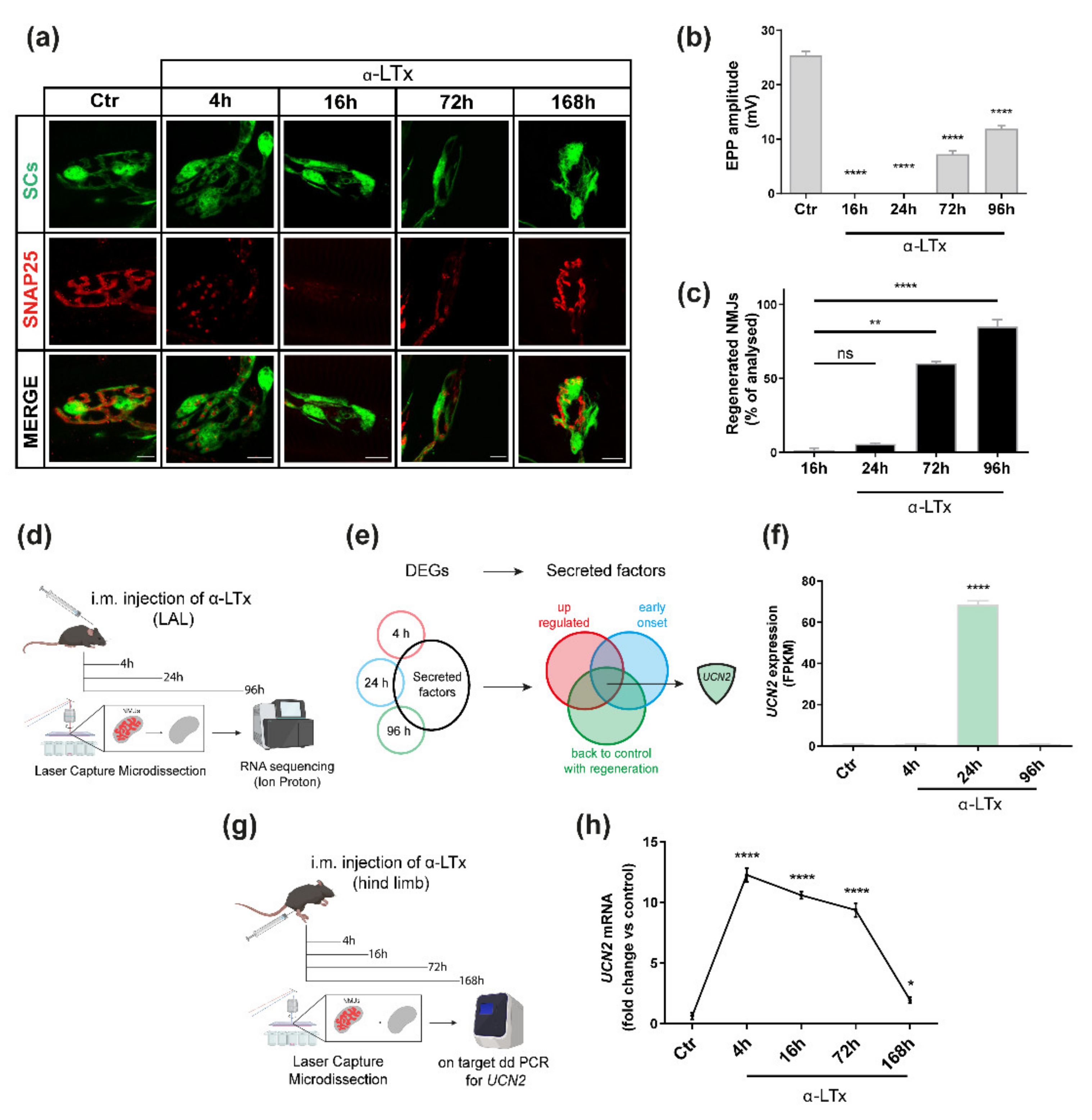
2.1. UCN2 mRNA Is Strongly Up-Regulated after NMJ Degeneration Induced by α-LTx
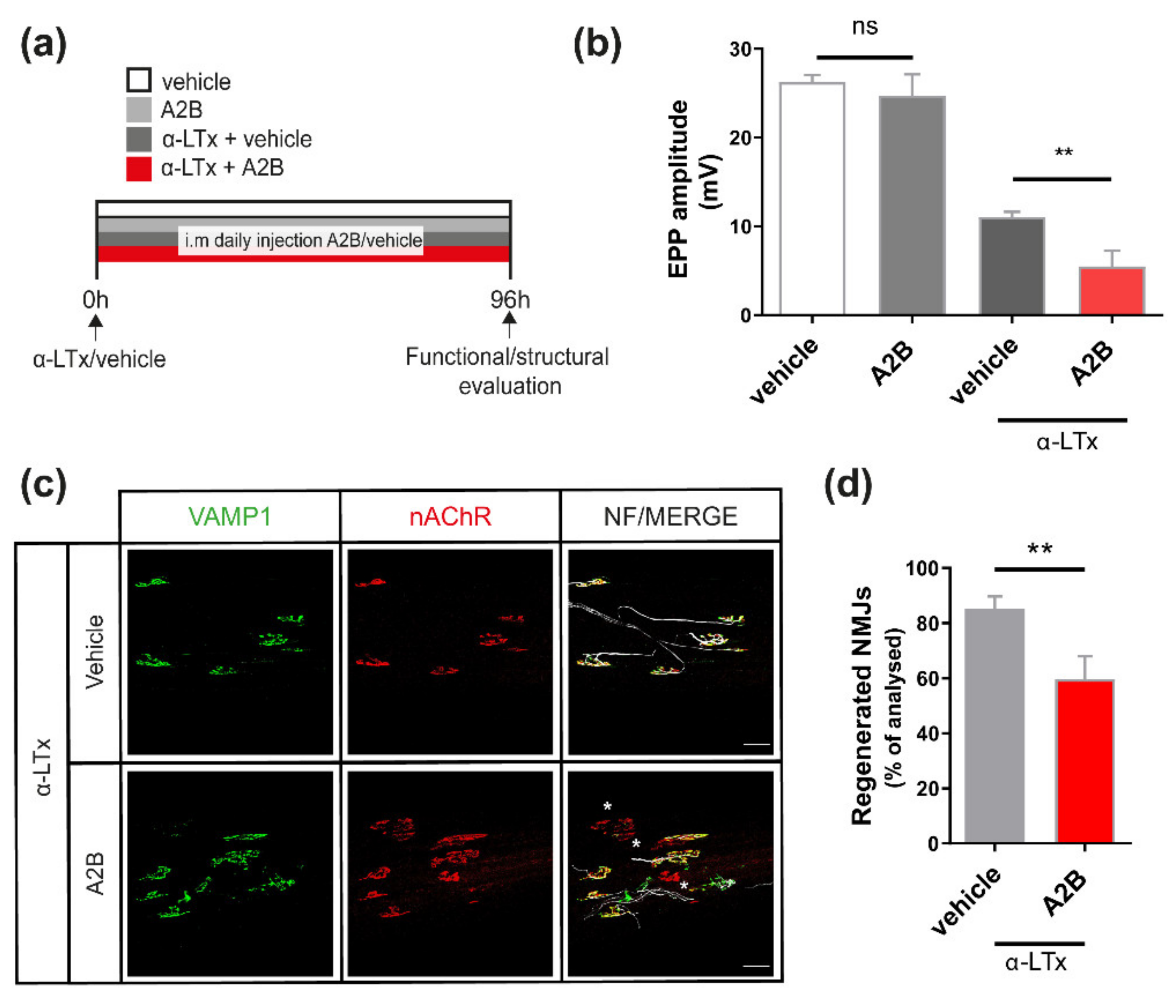
2.2. UCN2 Participates in NMJ Regeneration via CRHR2
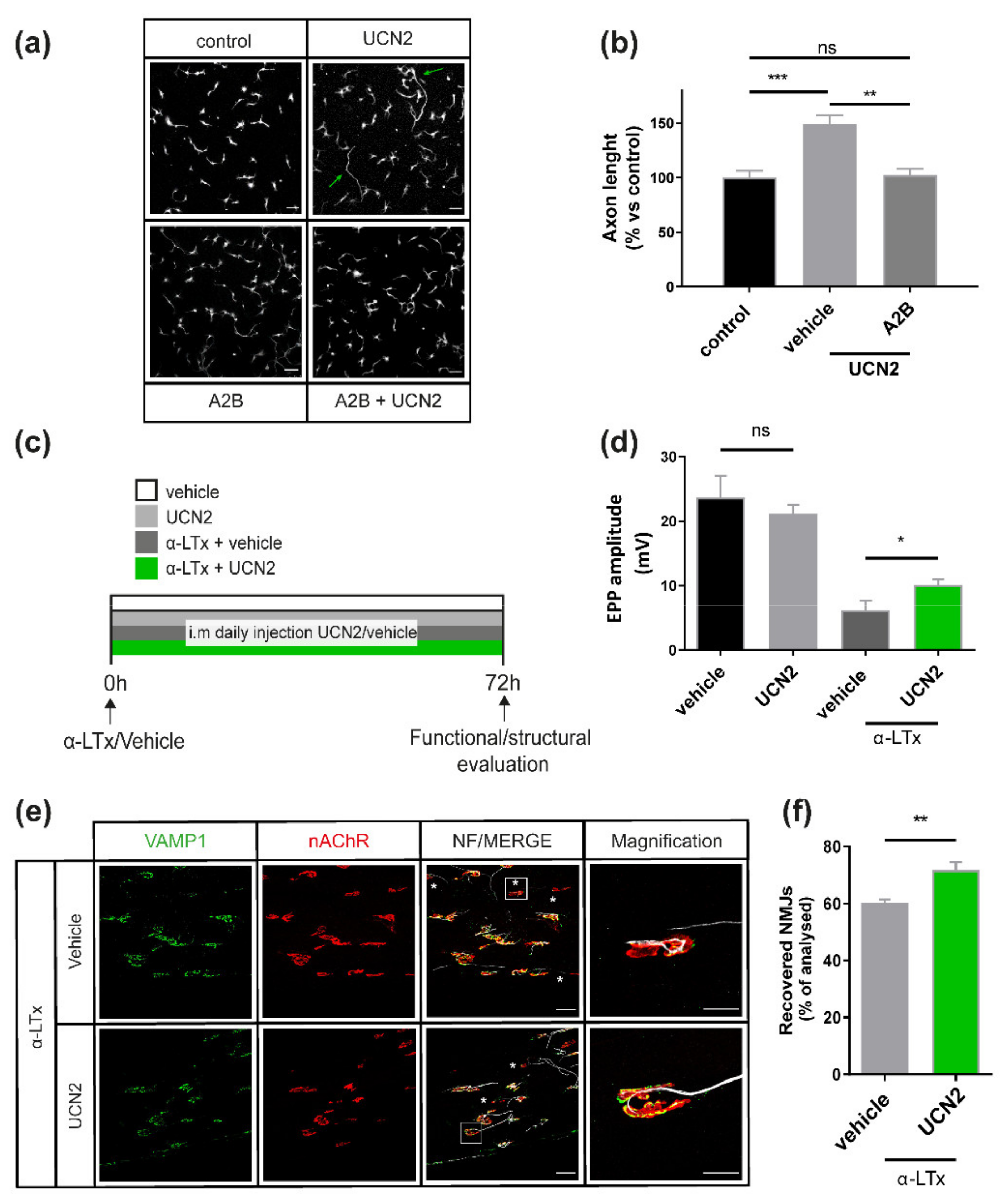
2.3. CRHR2 Expression Is Associated with Axonal Growth
2.4. UCN2 Boosts Axon Growth in Cultured SCMNs via CRHR2 and Fosters NMJ Regeneration
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Animals
4.3. Sample Preparation for NMJ Laser Microdissection
4.4. RNA Extraction and Bioinformatic Analysis
4.5. Droplet Digital PCR
4.6. Neuronal Cultures
4.7. Electrophysiological Recordings of Evoked EndPlate Potentials (EPPs)
4.8. NMJ Immunofluorescence
4.9. Microfluidic Devices
4.10. Western Blotting
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tintignac, L.A.; Brenner, H.R.; Ruegg, M.A. Mechanisms Regulating Neuromuscular Junction Development and Function and Causes of Muscle Wasting. Physiol. Rev. 2015, 95, 809–852. [Google Scholar] [CrossRef] [Green Version]
- Sanes, J.R.; Lichtman, J.W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 1999, 22, 389–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, C.B.; Feltri, M.L.; Wilson, E.R. Peripheral glia diversity. J. Anat. 2021; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.P.; Robitaille, R. Perisynaptic Schwann Cells at the Neuromuscular Synapse: Adaptable, Multitasking Glial Cells. Cold Spring Harb. Perspect. Biol. 2015, 7, a020503. [Google Scholar] [CrossRef] [PubMed]
- Ayvazyan, N.M.; O’Leary, V.B.; Dolly, J.O.; Ovsepian, S.V. Neurobiology and therapeutic utility of neurotoxins targeting postsynaptic mechanisms of neuromuscular transmission. Drug Discov. Today 2019, 24, 1968–1984. [Google Scholar] [CrossRef] [PubMed]
- Ovsepian, S.V.; O’Leary, V.B.; Ayvazyan, N.M.; Al-Sabi, A.; Ntziachristos, V.; Dolly, J.O. Neurobiology and therapeutic applications of neurotoxins targeting transmitter release. Pharm. Ther. 2019, 193, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, G.; Matteoli, M.; Montecucco, C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000, 80, 717–766. [Google Scholar] [CrossRef] [PubMed]
- Darabid, H.; Perez-Gonzalez, A.P.; Robitaille, R. Neuromuscular synaptogenesis: Coordinating partners with multiple functions. Nat. Rev. Neurosci. 2014, 15, 703–718. [Google Scholar] [CrossRef]
- Li, L.; Xiong, W.C.; Mei, L. Neuromuscular Junction Formation, Aging, and Disorders. Annu. Rev. Physiol. 2018, 80, 159–188. [Google Scholar] [CrossRef]
- Rigoni, M.; Montecucco, C. Animal models for studying motor axon terminal paralysis and recovery. J. Neurochem. 2017, 142, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Fitzsimonds, R.M.; Poo, M.M. Retrograde signaling in the development and modification of synapses. Physiol. Rev. 1998, 78, 143–170. [Google Scholar] [CrossRef]
- Rigoni, M.; Negro, S. Signals Orchestrating Peripheral Nerve Repair. Cells 2020, 9, 1768. [Google Scholar] [CrossRef]
- Duregotti, E.; Negro, S.; Scorzeto, M.; Zornetta, I.; Dickinson, B.C.; Chang, C.J.; Montecucco, C.; Rigoni, M. Mitochondrial alarmins released by degenerating motor axon terminals activate perisynaptic Schwann cells. Proc. Natl. Acad. Sci. USA 2015, 112, E497–E505. [Google Scholar] [CrossRef] [Green Version]
- Duregotti, E.; Zanetti, G.; Scorzeto, M.; Megighian, A.; Montecucco, C.; Pirazzini, M.; Rigoni, M. Snake and Spider Toxins Induce a Rapid Recovery of Function of Botulinum Neurotoxin Paralysed Neuromuscular Junction. Toxins 2015, 7, 5322–5336. [Google Scholar] [CrossRef]
- Negro, S.; Lessi, F.; Duregotti, E.; Aretini, P.; La Ferla, M.; Franceschi, S.; Menicagli, M.; Bergamin, E.; Radice, E.; Thelen, M.; et al. CXCL12alpha/SDF-1 from perisynaptic Schwann cells promotes regeneration of injured motor axon terminals. EMBO Mol. Med. 2017, 9, 1000–1010. [Google Scholar] [CrossRef]
- Mishra, R.K.; Shum, A.K.; Platanias, L.C.; Miller, R.J.; Schiltz, G.E. Discovery and characterization of novel small-molecule CXCR4 receptor agonists and antagonists. Sci. Rep. 2016, 6, 30155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stazi, M.; D’Este, G.; Mattarei, A.; Negro, S.; Lista, F.; Rigoni, M.; Megighian, A.; Montecucco, C. An agonist of the CXCR4 receptor accelerates the recovery from the peripheral neuroparalysis induced by Taipan snake envenomation. PLoS Negl. Trop. Dis 2020, 14, e0008547. [Google Scholar] [CrossRef] [PubMed]
- Negro, S.; Zanetti, G.; Mattarei, A.; Valentini, A.; Megighian, A.; Tombesi, G.; Zugno, A.; Dianin, V.; Pirazzini, M.; Fillo, S.; et al. An Agonist of the CXCR4 Receptor Strongly Promotes Regeneration of Degenerated Motor Axon Terminals. Cells 2019, 8, 1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanetti, G.; Negro, S.; Megighian, A.; Mattarei, A.; Lista, F.; Fillo, S.; Rigoni, M.; Pirazzini, M.; Montecucco, C. A CXCR4 receptor agonist strongly stimulates axonal regeneration after damage. Ann. Clin. Transl. Neurol. 2019, 6, 2395–2402. [Google Scholar] [CrossRef]
- Watanabe, O.; Meldolesi, J. The effects of alpha-latrotoxin of black widow spider venom on synaptosome ultrastructure. A morphometric analysis correlating its effects on transmitter release. J. Neurocytol. 1983, 12, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Dedic, N.; Chen, A.; Deussing, J.M. The CRF Family of Neuropeptides and their Receptors—Mediators of the Central Stress Response. Curr. Mol. Pharmacol. 2018, 11, 4–31. [Google Scholar] [CrossRef]
- Squillacioti, C.; Pelagalli, A.; Liguori, G.; Mirabella, N. Urocortins in the mammalian endocrine system. Acta Vet. Scand. 2019, 61, 46. [Google Scholar] [CrossRef] [PubMed]
- Hauger, R.L.; Grigoriadis, D.E.; Dallman, M.F.; Plotsky, P.M.; Vale, W.W.; Dautzenberg, F.M. International Union of Pharmacology. XXXVI. Current Status of the Nomenclature for Receptors for Corticotropin-Releasing Factor and Their Ligands. Pharmacol. Rev. 2003, 55, 21. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Blount, A.; Vaughan, J.; Brar, B.; Vale, W. Urocortin II gene is highly expressed in mouse skin and skeletal muscle tissues: Localization, basal expression in corticotropin-releasing factor receptor (CRFR) 1- and CRFR2-null mice, and regulation by glucocorticoids. Endocrinology 2004, 145, 2445–2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivier, J.; Gulyas, J.; Kirby, D.; Low, W.; Perrin, M.H.; Kunitake, K.; DiGruccio, M.; Vaughan, J.; Reubi, J.C.; Waser, B.; et al. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J. Med. Chem. 2002, 45, 4737–4747. [Google Scholar] [CrossRef]
- Sudhof, T.C.; Rothman, J.E. Membrane fusion: Grappling with SNARE and SM proteins. Science 2009, 323, 474–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuelsson, S.; Lange, J.S.; Hinkle, R.T.; Tarnopolsky, M.; Isfort, R.J. Corticotropin-releasing factor 2 receptor localization in skeletal muscle. J. Histochem. Cytochem. 2004, 52, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Azarnia Tehran, D.; Pirazzini, M. Preparation of Cerebellum Granule Neurons from Mouse or Rat Pups and Evaluation of Clostridial Neurotoxin Activity and Their Inhibitors by Western Blot and Immunohistochemistry. Bio-Protocol 2018, 8, e2918. [Google Scholar] [CrossRef]
- Liu, K.; Tedeschi, A.; Park, K.K.; He, Z. Neuronal Intrinsic Mechanisms of Axon Regeneration. Annu. Rev. Neurosci. 2011, 34, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Pirazzini, M.; Lista, F.; Montecucco, C. The role of the single interchains disulfide bond in tetanus and botulinum neurotoxins and the development of antitetanus and antibotulism drugs. Cell. Microbiol. 2019, 21, e13037. [Google Scholar] [CrossRef]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharm. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef]
- Rossetto, O.; Seveso, M.; Caccin, P.; Schiavo, G.; Montecucco, C. Tetanus and botulinum neurotoxins: Turning bad guys into good by research. Toxicon 2001, 39, 27–41. [Google Scholar] [CrossRef]
- Deussing, J.M.; Chen, A. The Corticotropin-Releasing Factor Family: Physiology of the Stress Response. Physiol. Rev. 2018, 98, 2225–2286. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.; Quast, K.B.; Huang, L.; Herman, A.M.; Selever, J.; Deussing, J.M.; Justice, N.J.; Arenkiel, B.R. Local CRH Signaling Promotes Synaptogenesis and Circuit Integration of Adult-Born Neurons. Dev. Cell 2014, 30, 645–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.L.; Tsai, L.Y.; Lee, E.H. Corticotropin-releasing factor produces a protein synthesis—Dependent long-lasting potentiation in dentate gyrus neurons. J. Neurophysiol. 2000, 83, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zhang, Y.-M.; Ni, X. Urocortin 2 But Not Urocortin 3 Promotes the Synaptic Formation in Hipppocampal Neurons via Induction of NGF Production by Astrocytes. Endocrinology 2016, 157, 1200–1210. [Google Scholar] [CrossRef]
- Tran, N.M.; Shekhar, K.; Whitney, I.E.; Jacobi, A.; Benhar, I.; Hong, G.; Yan, W.; Adiconis, X.; Arnold, M.E.; Lee, J.M.; et al. Single-Cell Profiles of Retinal Ganglion Cells Differing in Resilience to Injury Reveal Neuroprotective Genes. Neuron 2019, 104, 1039–1055.e12. [Google Scholar] [CrossRef]
- Inda, C.; Bonfiglio, J.J.; Dos Santos Claro, P.A.; Senin, S.A.; Armando, N.G.; Deussing, J.M.; Silberstein, S. cAMP-dependent cell differentiation triggered by activated CRHR1 in hippocampal neuronal cells. Sci. Rep. 2017, 7, 1944. [Google Scholar] [CrossRef] [Green Version]
- Swinny, J.D.; Metzger, F.; Ijkema-Paassen, J.; Gounko, N.V.; Gramsbergen, A.; Van Der Want, J.J.L. Corticotropin-releasing factor and urocortin differentially modulate rat Purkinje cell dendritic outgrowth and differentiation in vitro. Eur. J. Neurosci. 2004, 19, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Duchen, L.W.; Gomez, S.; Queiroz, L.S. The neuromuscular junction of the mouse after black widow spider venom. J. Physiol. 1981, 316, 279–291. [Google Scholar] [CrossRef]
- Orrenius, S.; Nicotera, P.; Zhivotovsky, B. Cell death mechanisms and their implications in toxicology. Toxicol. Sci. Off. J. Soc. Toxicol. 2011, 119, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Duregotti, E.; Tedesco, E.; Montecucco, C.; Rigoni, M. Calpains participate in nerve terminal degeneration induced by spider and snake presynaptic neurotoxins. Toxicon 2013, 64, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.C.; Bullens, R.W.; O’Hanlon, G.M.; Ang, C.W.; Willison, H.J.; Plomp, J.J. Detection and prevalence of alpha-latrotoxin-like effects of serum from patients with Guillain-Barre syndrome. Muscle Nerve 2002, 25, 549–558. [Google Scholar] [CrossRef]
- Rupp, A.; Morrison, I.; Barrie, J.A.; Halstead, S.K.; Townson, K.H.; Greenshields, K.N.; Willison, H.J. Motor nerve terminal destruction and regeneration following anti-ganglioside antibody and complement-mediated injury: An in and ex vivo imaging study in the mouse. Exp. Neurol. 2012, 233, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Yuki, N.; Hartung, H.P. Guillain-Barre syndrome. New Engl. J. Med. 2012, 366, 2294–2304. [Google Scholar] [CrossRef] [PubMed]
- Rodella, U.; Negro, S.; Scorzeto, M.; Bergamin, E.; Jalink, K.; Montecucco, C.; Yuki, N.; Rigoni, M. Schwann cells are activated by ATP released from neurons in an in vitro cellular model of Miller Fisher syndrome. Dis. Models Mech. 2017, 10, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Rodella, U.; Scorzeto, M.; Duregotti, E.; Negro, S.; Dickinson, B.C.; Chang, C.J.; Yuki, N.; Rigoni, M.; Montecucco, C. An animal model of Miller Fisher syndrome: Mitochondrial hydrogen peroxide is produced by the autoimmune attack of nerve terminals and activates Schwann cells. Neurobiol. Dis. 2016, 96, 95–104. [Google Scholar] [CrossRef]
- Zanetti, G.; Duregotti, E.; Locatelli, C.A.; Giampreti, A.; Lonati, D.; Rossetto, O.; Pirazzini, M. Variability in venom composition of European viper subspecies limits the cross-effectiveness of antivenoms. Sci. Rep. 2018, 8, 9818. [Google Scholar] [CrossRef]
- Rossetto, O.; Morbiato, L.; Caccin, P.; Rigoni, M.; Montecucco, C. Presynaptic enzymatic neurotoxins. J. Neurochem. 2006, 97, 1534–1545. [Google Scholar] [CrossRef]
- Rigoni, M.; Caccin, P.; Gschmeissner, S.; Koster, G.; Postle, A.D.; Rossetto, O.; Schiavo, G.; Montecucco, C. Equivalent effects of snake PLA2 neurotoxins and lysophospholipid-fatty acid mixtures. Science 2005, 310, 1678–1680. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Gorza, L.; Schiavo, G.; Schiavo, N.; Scheller, R.H.; Montecucco, C. VAMP/synaptobrevin isoforms 1 and 2 are widely and differentially expressed in nonneuronal tissues. J. Cell Biol. 1996, 132, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanetti, G.; Negro, S.; Pirazzini, M.; Caccin, P. Mouse Phrenic Nerve Hemidiaphragm Assay (MPN). Bio-Protocol 2018, 8, e2759. [Google Scholar] [CrossRef] [Green Version]
- Mallon, B.S.; Shick, H.E.; Kidd, G.J.; Macklin, W.B. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J. Neurosci. 2002, 22, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Arce, V.; Garces, A.; de Bovis, B.; Filippi, P.; Henderson, C.; Pettmann, B.; deLapeyriere, O. Cardiotrophin-1 requires LIFRbeta to promote survival of mouse motoneurons purified by a novel technique. J. Neurosci. Res. 1999, 55, 119–126. [Google Scholar] [CrossRef]
- Zanetti, G.; Negro, S.; Megighian, A.; Pirazzini, M. Electrophysiological Recordings of Evoked End-Plate Potential on Murine Neuro-muscular Synapse Preparations. Bio-Protocol 2018, 8, e2803. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, G.; Sikorra, S.; Rummel, A.; Krez, N.; Duregotti, E.; Negro, S.; Henke, T.; Rossetto, O.; Binz, T.; Pirazzini, M. Botulinum neurotoxin C mutants reveal different effects of syntaxin or SNAP-25 proteolysis on neuromuscular transmission. PLoS Pathog. 2017, 13, e1006567. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Vahidi, B.; Taylor, A.M.; Rhee, S.W.; Jeon, N.L. Microfluidic culture platform for neuroscience research. Nat. Protoc. 2006, 1, 2128–2136. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Este, G.; Stazi, M.; Negro, S.; Megighian, A.; Lista, F.; Rossetto, O.; Montecucco, C.; Rigoni, M.; Pirazzini, M. Latrotoxin-Induced Neuromuscular Junction Degeneration Reveals Urocortin 2 as a Critical Contributor to Motor Axon Terminal Regeneration. Int. J. Mol. Sci. 2022, 23, 1186. https://doi.org/10.3390/ijms23031186
D’Este G, Stazi M, Negro S, Megighian A, Lista F, Rossetto O, Montecucco C, Rigoni M, Pirazzini M. Latrotoxin-Induced Neuromuscular Junction Degeneration Reveals Urocortin 2 as a Critical Contributor to Motor Axon Terminal Regeneration. International Journal of Molecular Sciences. 2022; 23(3):1186. https://doi.org/10.3390/ijms23031186
Chicago/Turabian StyleD’Este, Giorgia, Marco Stazi, Samuele Negro, Aram Megighian, Florigio Lista, Ornella Rossetto, Cesare Montecucco, Michela Rigoni, and Marco Pirazzini. 2022. "Latrotoxin-Induced Neuromuscular Junction Degeneration Reveals Urocortin 2 as a Critical Contributor to Motor Axon Terminal Regeneration" International Journal of Molecular Sciences 23, no. 3: 1186. https://doi.org/10.3390/ijms23031186
APA StyleD’Este, G., Stazi, M., Negro, S., Megighian, A., Lista, F., Rossetto, O., Montecucco, C., Rigoni, M., & Pirazzini, M. (2022). Latrotoxin-Induced Neuromuscular Junction Degeneration Reveals Urocortin 2 as a Critical Contributor to Motor Axon Terminal Regeneration. International Journal of Molecular Sciences, 23(3), 1186. https://doi.org/10.3390/ijms23031186








