Alpha-Lipoic Acid Inhibits Spontaneous Diabetes and Autoimmune Recurrence in Non-Obese Diabetic Mice by Enhancing Differentiation of Regulatory T Cells and Showed Potential for Use in Cell Therapies for the Treatment of Type 1 Diabetes
Abstract
1. Introduction
2. Results
2.1. ALA Treatment Delayed the Onset of Autoimmune Diabetes
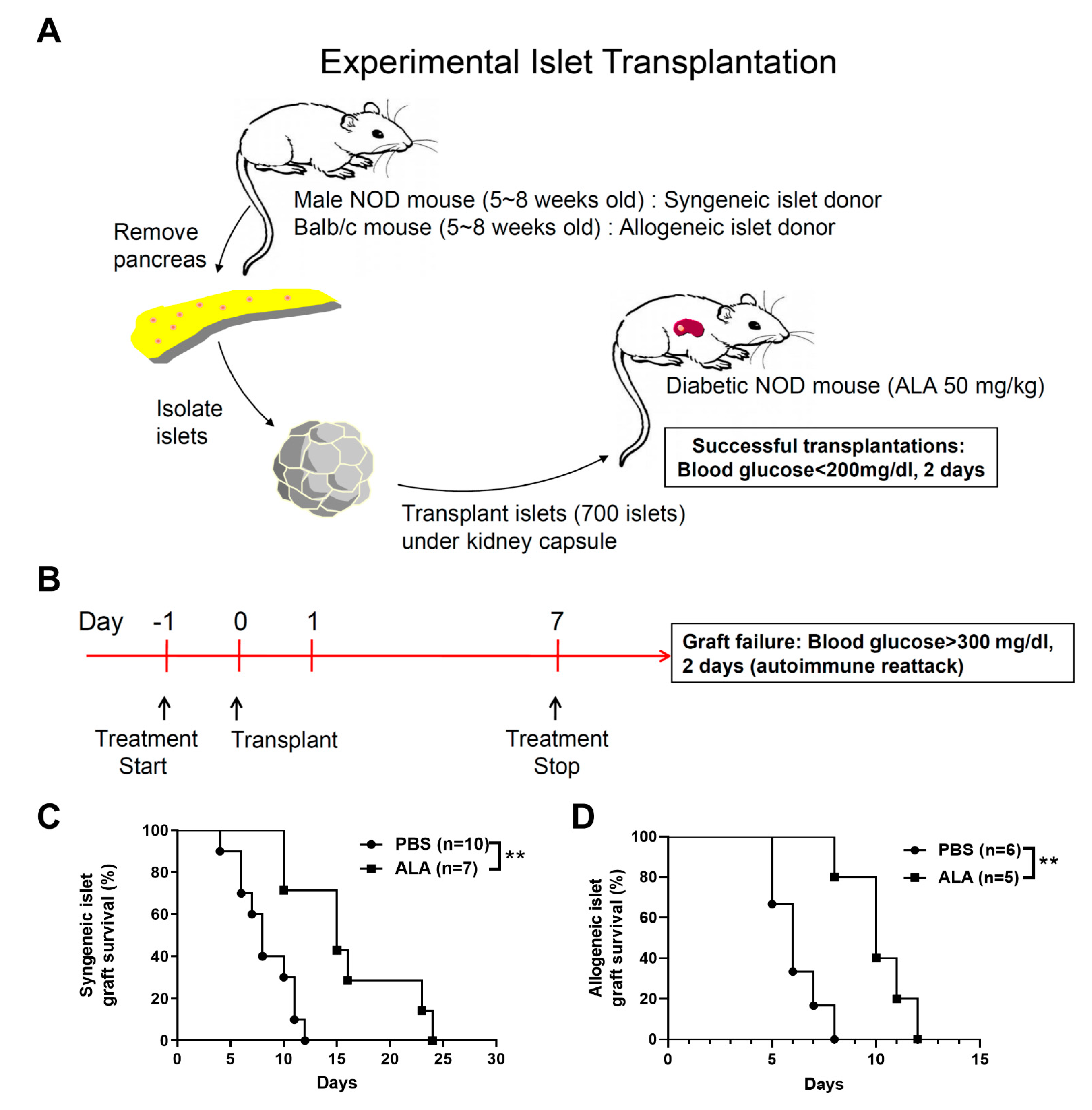
2.2. ALA Treatment Prolonged the Survival of Syngeneic and Allogeneic Islet Grafts after Islet Transplantation
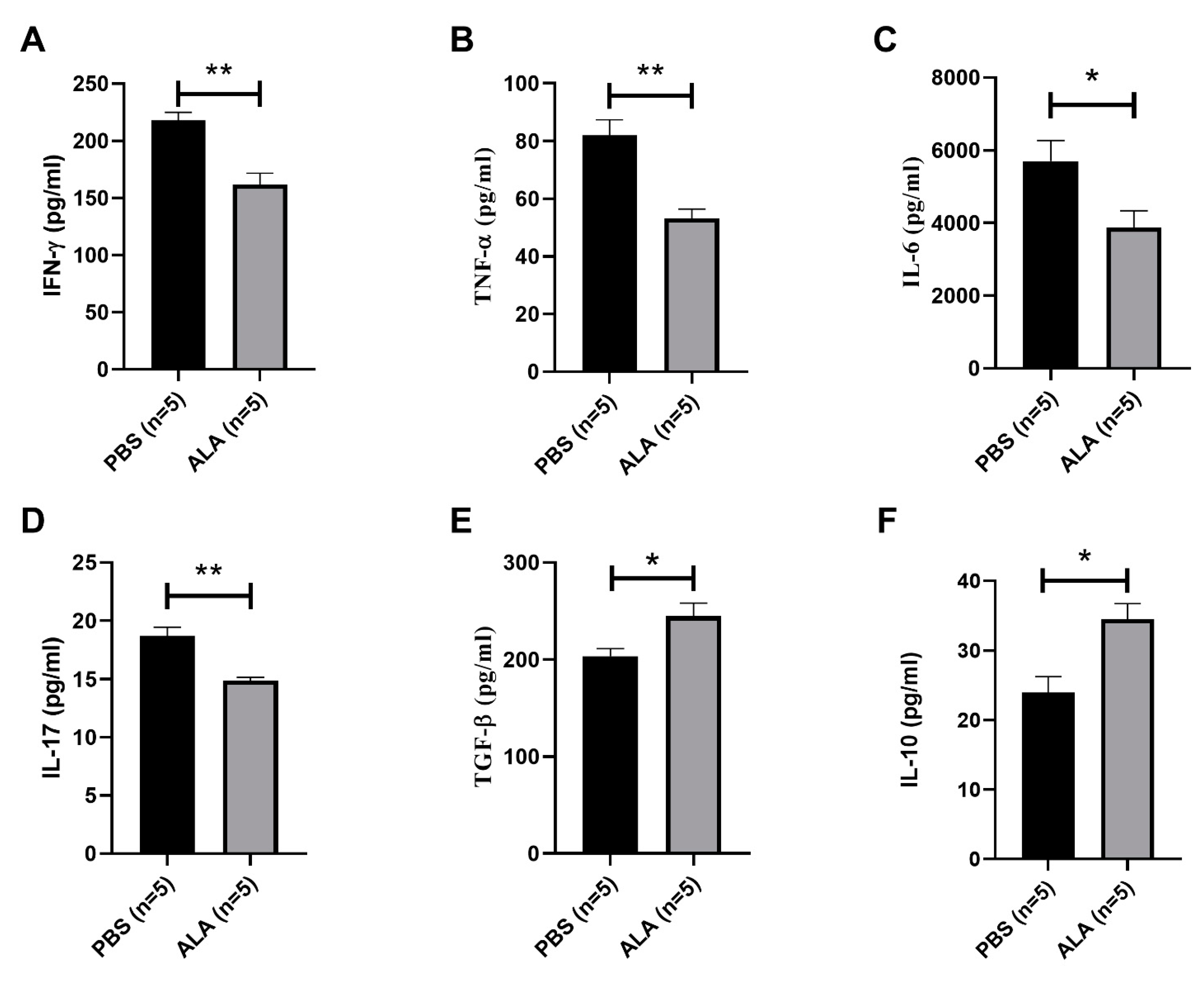
2.3. ALA Treatment Reduced the Secretion of Inflammatory Cytokines and Increased the Production of Anti-Inflammatory Cytokines
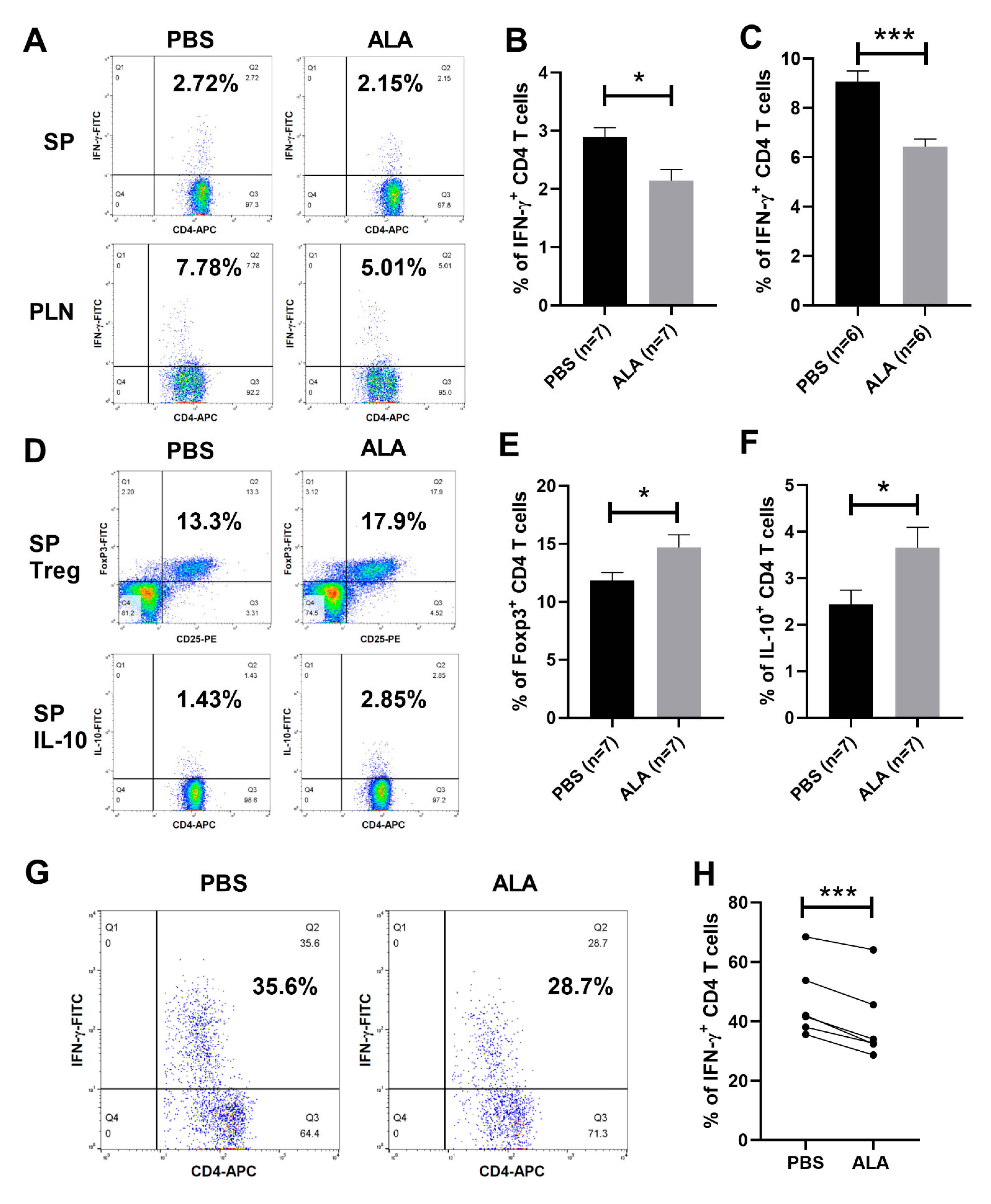
2.4. ALA Treatment Decreased the Population of Th1 Cells and Increased the Population of Tregs and IL-10-Producing CD4 T Cells
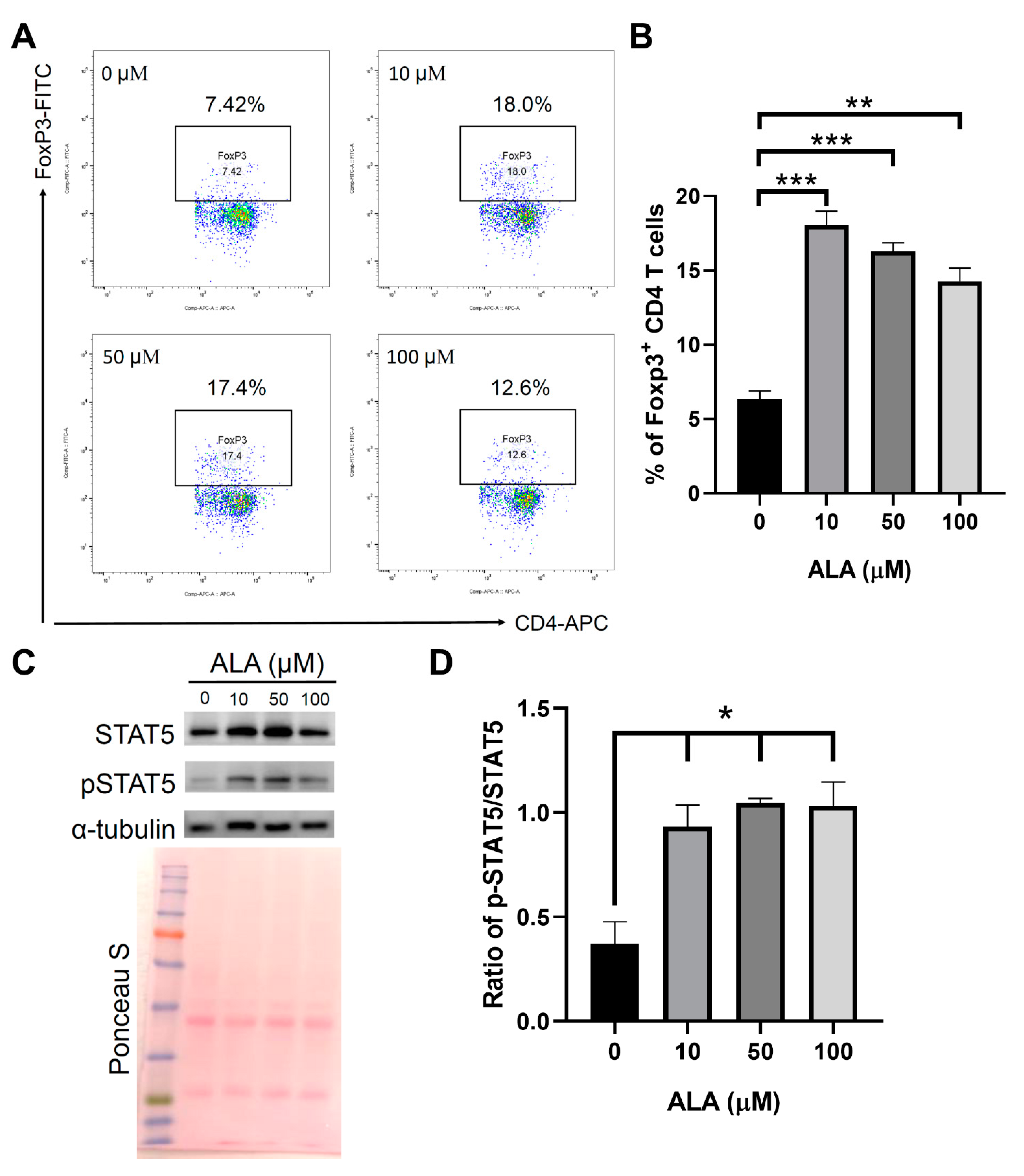
2.5. In Vitro ALA Treatment Induces the Differentiation of Tregs from the Naïve CD4 T Cells
2.6. Adoptive Transfer of ALA-Enhanced In Vitro Differentiated Tregs Exhibited a Better Protective Effect Than the Cells without ALA Treatment
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Preparation of ALA Solution and In Vivo Treatment of ALA
4.3. Islet Isolation and Transplantation
4.4. Urine Glucose and Blood Glucose Monitoring and Assessment of Insulitis
4.5. Naïve CD4 T Cell Sorting
4.6. In Vitro Treg Differentiation
4.7. Flow Cytometry
4.8. ELISA Detection of Cytokines
4.9. Adoptive Transfer of Regulatory T Cells
4.10. Protein Extraction and Western Blot
4.11. Immunofluorescence Assay
4.12. Immunohistochemical Assays
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawasaki, E.; Abiru, N.; Eguchi, K. Prevention of type 1 diabetes: From the view point of beta cell damage. Diabetes Res. Clin. Pract. 2004, 66 (Suppl. S1), S27–S32. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.; Gudbjornsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, A. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef]
- Barnett, A.H.; Eff, C.; Leslie, R.D.; Pyke, D.A. Diabetes in identical twins. A study of 200 pairs. Diabetologia 1981, 20, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Nejentsev, S.; Howson, J.M.; Walker, N.M.; Szeszko, J.; Field, S.F.; Stevens, H.E.; Reynolds, P.; Hardy, M.; King, E.; Masters, J.; et al. Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature 2007, 450, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, H.; McErlean, C.; Moraes, M.E.; Romero, M.; Marques, S.B.; Goncales, A.C.; Guariento, E.G.; Middleton, D. Identification of two novel alleles HLA-B*3569 and -B*4450 and confirmation of HLA-A*2631 in the Brazilian population. Tissue Antigens 2007, 69, 273–276. [Google Scholar] [CrossRef]
- Kaufman, D.L.; Erlander, M.G.; Clare-Salzler, M.; Atkinson, M.A.; Maclaren, N.K.; Tobin, A.J. Autoimmunity to two forms of glutamate decarboxylase in insulin-dependent diabetes mellitus. J. Clin. Investig. 1992, 89, 283–292. [Google Scholar] [CrossRef]
- Aoki, C.A.; Borchers, A.T.; Ridgway, W.M.; Keen, C.L.; Ansari, A.A.; Gershwin, M.E. NOD mice and autoimmunity. Autoimmun. Rev. 2005, 4, 373–379. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, I.A.; Corrall, R.J. Epidemiology of diabetes and its complications. N. Engl. J. Med. 1988, 318, 1619–1620. [Google Scholar] [CrossRef] [PubMed]
- Gaglia, J.L.; Shapiro, A.M.; Weir, G.C. Islet transplantation: Progress and challenge. Arch. Med. Res. 2005, 36, 273–280. [Google Scholar] [CrossRef]
- Bottino, R.; Balamurugan, A.N.; Giannoukakis, N.; Trucco, M. Islet/pancreas transplantation: Challenges for pediatrics. Pediatr. Diabetes 2002, 3, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, A.N.; Bottino, R.; Giannoukakis, N.; Smetanka, C. Prospective and challenges of islet transplantation for the therapy of autoimmune diabetes. Pancreas 2006, 32, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, T.; Bartlett, S.T.; Hadley, G.A.; Drachenberg, C.B.; Farney, A.C. Recurrent autoimmunity accelerates destruction of minor and major histoincompatible islet grafts in nonobese diabetic (NOD) mice. Am. J. Transplant. 2001, 1, 138–145. [Google Scholar] [CrossRef]
- Gysemans, C.A.; Waer, M.; Valckx, D.; Laureys, J.M.; Mihkalsky, D.; Bouillon, R.; Mathieu, C. Early graft failure of xenogeneic islets in NOD mice is accompanied by high levels of interleukin-1 and low levels of transforming growth factor-beta mRNA in the grafts. Diabetes 2000, 49, 1992–1997. [Google Scholar] [CrossRef]
- Young, H.Y.; Zucker, P.; Flavell, R.A.; Jevnikar, A.M.; Singh, B. Characterization of the role of major histocompatibility complex in type 1 diabetes recurrence after islet transplantation. Transplantation 2004, 78, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Sibley, R.K.; Sutherland, D.E.; Goetz, F.; Michael, A.F. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab. Investig. 1985, 53, 132–144. [Google Scholar]
- Tyden, G.; Reinholt, F.P.; Sundkvist, G.; Bolinder, J. Recurrence of autoimmune diabetes mellitus in recipients of cadaveric pancreatic grafts. N. Engl. J. Med. 1996, 335, 860–863. [Google Scholar] [CrossRef]
- Lin, G.J.; Sytwu, H.K.; Yu, J.C.; Chen, Y.W.; Kuo, Y.L.; Yu, C.C.; Chang, H.M.; Chan, D.C.; Huang, S.H. Dimethyl sulfoxide inhibits spontaneous diabetes and autoimmune recurrence in non-obese diabetic mice by inducing differentiation of regulatory T cells. Toxicol. Appl. Pharmacol. 2015, 282, 207–214. [Google Scholar] [CrossRef]
- Golbidi, S.; Badran, M.; Laher, I. Diabetes and alpha lipoic Acid. Front. Pharmacol. 2011, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Wollin, S.D.; Jones, P.J. Alpha-lipoic acid and cardiovascular disease. J. Nutr. 2003, 133, 3327–3330. [Google Scholar] [CrossRef]
- Jordan, S.W.; Cronan, J.E., Jr. A new metabolic link. The acyl carrier protein of lipid synthesis donates lipoic acid to the pyruvate dehydrogenase complex in Escherichia coli and mitochondria. J. Biol. Chem. 1997, 272, 17903–17906. [Google Scholar] [CrossRef]
- Machado, R.S.; Clark, D.P.; Guest, J.R. Construction and properties of pyruvate dehydrogenase complexes with up to nine lipoyl domains per lipoate acetyltransferase chain. FEMS Microbiol. Lett. 1992, 100, 243–248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sohal, R.S.; Ku, H.H.; Agarwal, S.; Forster, M.J.; Lal, H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994, 74, 121–133. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Gille, L.; Staniek, K.; Nohl, H. Dihydrolipoic acid maintains ubiquinone in the antioxidant active form by two-electron reduction of ubiquinone and one-electron reduction of ubisemiquinone. Arch. Biochem. Biophys. 1999, 363, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Gruzman, A.; Hidmi, A.; Katzhendler, J.; Haj-Yehie, A.; Sasson, S. Synthesis and characterization of new and potent alpha-lipoic acid derivatives. Bioorg. Med. Chem. 2004, 12, 1183–1190. [Google Scholar] [CrossRef]
- Biewenga, G.P.; Haenen, G.R.; Bast, A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997, 29, 315–331. [Google Scholar] [CrossRef]
- Odabasoglu, F.; Halici, Z.; Aygun, H.; Halici, M.; Atalay, F.; Cakir, A.; Cadirci, E.; Bayir, Y.; Suleyman, H. alpha-Lipoic acid has anti-inflammatory and anti-oxidative properties: An experimental study in rats with carrageenan-induced acute and cotton pellet-induced chronic inflammations. Br. J. Nutr. 2011, 105, 31–43. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wei, H.; Hagen, T.; Frei, B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 4077–4082. [Google Scholar] [CrossRef]
- Merida, S.; Sancho-Tello, M.; Muriach, M.; Miranda, M.; Navea, A.; Bosch-Morell, F. Lipoic acid lessens Th1-mediated inflammation in lipopolysaccharide-induced uveitis reducing selectively Th1 lymphocytes-related cytokines release. Free Radic. Res. 2013, 47, 593–601. [Google Scholar] [CrossRef]
- Marracci, G.H.; Jones, R.E.; McKeon, G.P.; Bourdette, D.N. Alpha lipoic acid inhibits T cell migration into the spinal cord and suppresses and treats experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2002, 131, 104–114. [Google Scholar] [CrossRef]
- Morini, M.; Roccatagliata, L.; Dell’Eva, R.; Pedemonte, E.; Furlan, R.; Minghelli, S.; Giunti, D.; Pfeffer, U.; Marchese, M.; Noonan, D.; et al. Alpha-lipoic acid is effective in prevention and treatment of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2004, 148, 146–153. [Google Scholar] [CrossRef]
- Wang, K.C.; Tsai, C.P.; Lee, C.L.; Chen, S.Y.; Lin, G.J.; Yen, M.H.; Sytwu, H.K.; Chen, S.J. alpha-Lipoic acid enhances endogenous peroxisome-proliferator-activated receptor-gamma to ameliorate experimental autoimmune encephalomyelitis in mice. Clin. Sci. 2013, 125, 329–340. [Google Scholar] [CrossRef]
- Chaudhary, P.; Marracci, G.H.; Bourdette, D.N. Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2006, 175, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Fei, M.; Xie, Q.; Zou, Y.; He, R.; Zhang, Y.; Wang, J.; Bo, L.; Li, J.; Deng, X. Alpha-lipoic acid protects mice against concanavalin A-induced hepatitis by modulating cytokine secretion and reducing reactive oxygen species generation. Int. Immunopharmacol. 2016, 35, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, K.; Gaskins, H.R.; Leiter, E.H. NIT-1, a pancreatic beta-cell line established from a transgenic NOD/Lt mouse. Diabetes 1991, 40, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P. Islet transplantation as a treatment for diabetes—A work in progress. N. Engl. J. Med. 2004, 350, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Lin, G.J.; Chu, C.H.; Yu, J.C.; Chen, T.W.; Chen, Y.W.; Chien, M.W.; Chu, C.C.; Sytwu, H.K. Triptolide ameliorates autoimmune diabetes and prolongs islet graft survival in nonobese diabetic mice. Pancreas 2013, 42, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Berkay Yilmaz, Y.; Antika, G.; Boyunegmez Tumer, T.; Fawzi Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the Use of alpha-Lipoic Acid for Therapeutic Purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef]
- Ziegler, D.; Low, P.A.; Freeman, R.; Tritschler, H.; Vinik, A.I. Predictors of improvement and progression of diabetic polyneuropathy following treatment with alpha-lipoic acid for 4 years in the NATHAN 1 trial. J. Diabetes Complicat. 2016, 30, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shi, L.J.; Li, S.G. The Immunomodulatory Effect of Alpha-Lipoic Acid in Autoimmune Diseases. Biomed. Res. Int. 2019, 2019, 8086257. [Google Scholar] [CrossRef]
- Burchill, M.A.; Yang, J.; Vogtenhuber, C.; Blazar, B.R.; Farrar, M.A. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 2007, 178, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Betts, B.C.; Veerapathran, A.; Pidala, J.; Yu, X.Z.; Anasetti, C. STAT5 polarization promotes iTregs and suppresses human T-cell alloresponses while preserving CTL capacity. J. Leukoc. Biol. 2014, 95, 205–213. [Google Scholar] [CrossRef]
- Scalapino, K.J.; Tang, Q.; Bluestone, J.A.; Bonyhadi, M.L.; Daikh, D.I. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J. Immunol. 2006, 177, 1451–1459. [Google Scholar] [CrossRef]
- Scalapino, K.J.; Daikh, D.I. Suppression of glomerulonephritis in NZB/NZW lupus prone mice by adoptive transfer of ex vivo expanded regulatory T cells. PLoS ONE 2009, 4, e6031. [Google Scholar] [CrossRef]
- Kohm, A.P.; Carpentier, P.A.; Anger, H.A.; Miller, S.D. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 2002, 169, 4712–4716. [Google Scholar] [CrossRef]
- Stephens, L.A.; Malpass, K.H.; Anderton, S.M. Curing CNS autoimmune disease with myelin-reactive Foxp3+ Treg. Eur. J. Immunol. 2009, 39, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.J.; Wu, C.H.; Yu, C.C.; Lin, J.R.; Liu, X.D.; Chen, Y.W.; Chang, H.M.; Hong, Z.J.; Cheng, C.P.; Sytwu, H.K.; et al. Adoptive transfer of DMSO-induced regulatory T cells exhibits a similar preventive effect compared to an in vivo DMSO treatment for chemical-induced experimental encapsulating peritoneal sclerosis in mice. Toxicol. Appl. Pharmacol. 2019, 378, 114641. [Google Scholar] [CrossRef] [PubMed]
- Esensten, J.H.; Muller, Y.D.; Bluestone, J.A.; Tang, Q. Regulatory T-cell therapy for autoimmune and autoinflammatory diseases: The next frontier. J. Allergy Clin. Immunol. 2018, 142, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Eggenhuizen, P.J.; Ng, B.H.; Ooi, J.D. Treg Enhancing Therapies to Treat Autoimmune Diseases. Int. J. Mol. Sci. 2020, 21, 7015. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Buckner, J.H.; Fitch, M.; Gitelman, S.E.; Gupta, S.; Hellerstein, M.K.; Herold, K.C.; Lares, A.; Lee, M.R.; Li, K.; et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 2015, 7, 315ra189. [Google Scholar] [CrossRef] [PubMed]
- Marek-Trzonkowska, N.; Mysliwiec, M.; Dobyszuk, A.; Grabowska, M.; Derkowska, I.; Juscinska, J.; Owczuk, R.; Szadkowska, A.; Witkowski, P.; Mlynarski, W.; et al. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets—Results of one year follow-up. Clin. Immunol. 2014, 153, 23–30. [Google Scholar] [CrossRef]
- Juang, J.H.; Kuo, C.H.; Hsu, B.R. Effects of multiple site implantation on islet transplantation. Transplant. Proc. 2002, 34, 2698–2699. [Google Scholar] [CrossRef]
- Verdaguer, J.; Schmidt, D.; Amrani, A.; Anderson, B.; Averill, N.; Santamaria, P. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J. Exp. Med. 1997, 186, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.R.; Huang, S.H.; Wu, C.H.; Chen, Y.W.; Hong, Z.J.; Cheng, C.P.; Sytwu, H.K.; Lin, G.J. Valproic Acid Suppresses Autoimmune Recurrence and Allograft Rejection in Islet Transplantation through Induction of the Differentiation of Regulatory T Cells and Can Be Used in Cell Therapy for Type 1 Diabetes. Pharmaceuticals 2021, 14, 475. [Google Scholar] [CrossRef] [PubMed]
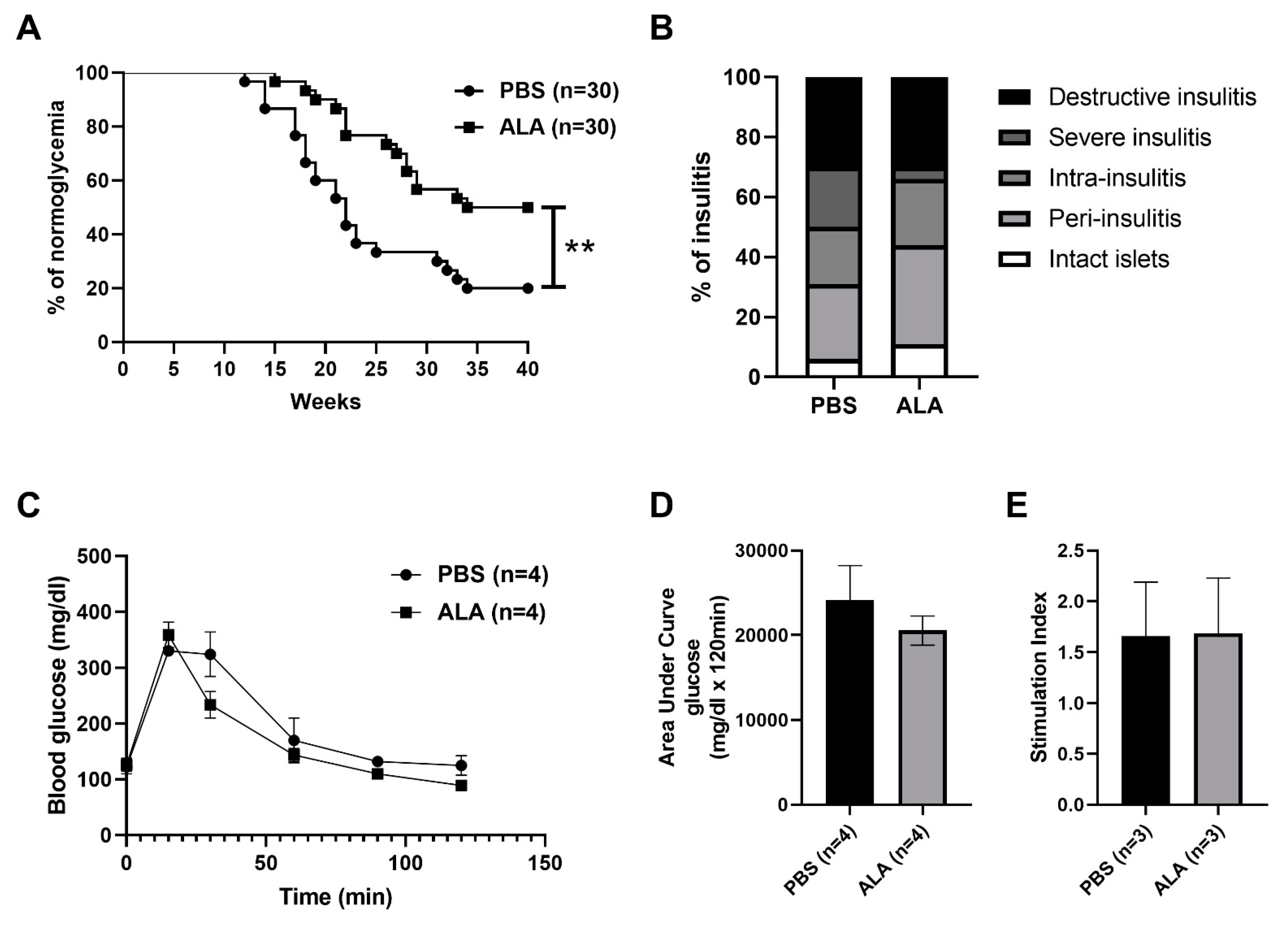
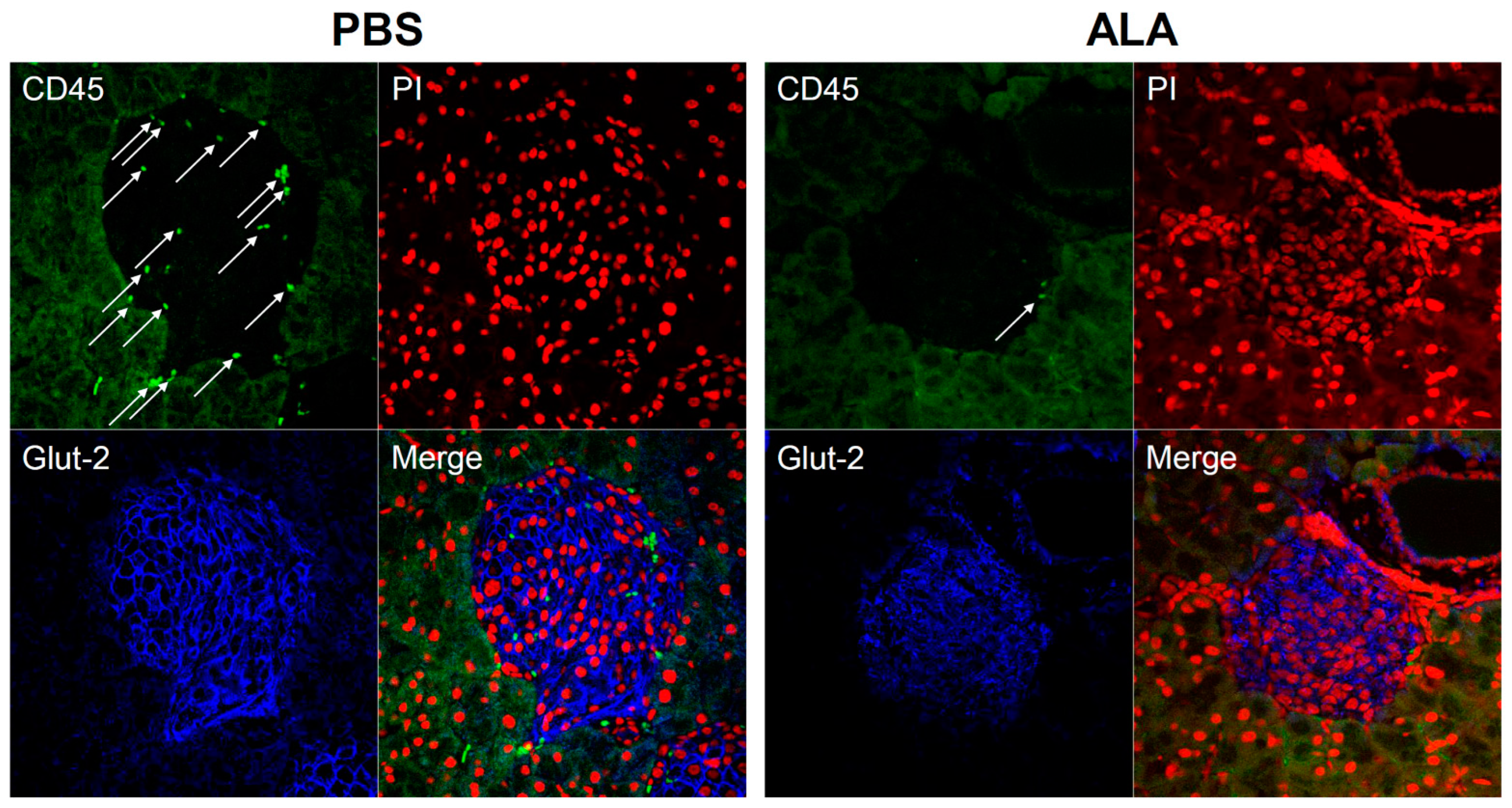

| Stage of Insulitis | % of PBS | Number | % of ALA | Number |
|---|---|---|---|---|
| Intact islet | 6% | 5 | 11% | 11 |
| Peri-insulitis | 25% | 23 | 33% | 33 |
| Intra-insulitis | 19% | 17 | 22% | 22 |
| Severe insulitis | 20% | 18 | 4% | 4 |
| Destructive insulitis | 30% | 27 | 30% | 30 |
| Group | Individual Graft Survival Time (Days) | Number | Average Survival Time |
|---|---|---|---|
| PBS | 4, 6, 6, 7, 8, 8, 10, 11, 11, 12 | 10 | 7.89 |
| ALA | 10, 10, 15, 15, 16, 23, 24 | 7 | 16.14 |
| Group | Individual Graft Survival Time (Days) | Number | Average Survival Time |
|---|---|---|---|
| PBS | 5, 5, 6, 6, 7, 8 | 6 | 6.17 |
| ALA | 8, 10, 10, 11, 12 | 5 | 10.2 |
| Group | Individual Graft Survival Time (Days) | Number | Average Survival Time |
|---|---|---|---|
| PBS | 6, 6, 7, 8, 8 | 5 | 7 |
| ALA | 7, 8, 10, 12, 14, 16, 24 | 7 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-H.; Kuo, S.-L.; Chen, S.-J.; Lin, J.-R.; Chen, Y.-W.; Hong, Z.-J.; Sytwu, H.-K.; Lin, G.-J. Alpha-Lipoic Acid Inhibits Spontaneous Diabetes and Autoimmune Recurrence in Non-Obese Diabetic Mice by Enhancing Differentiation of Regulatory T Cells and Showed Potential for Use in Cell Therapies for the Treatment of Type 1 Diabetes. Int. J. Mol. Sci. 2022, 23, 1169. https://doi.org/10.3390/ijms23031169
Huang S-H, Kuo S-L, Chen S-J, Lin J-R, Chen Y-W, Hong Z-J, Sytwu H-K, Lin G-J. Alpha-Lipoic Acid Inhibits Spontaneous Diabetes and Autoimmune Recurrence in Non-Obese Diabetic Mice by Enhancing Differentiation of Regulatory T Cells and Showed Potential for Use in Cell Therapies for the Treatment of Type 1 Diabetes. International Journal of Molecular Sciences. 2022; 23(3):1169. https://doi.org/10.3390/ijms23031169
Chicago/Turabian StyleHuang, Shing-Hwa, Shun-Li Kuo, Shyi-Jou Chen, Jeng-Rong Lin, Yuan-Wu Chen, Zhi-Jie Hong, Huey-Kang Sytwu, and Gu-Jiun Lin. 2022. "Alpha-Lipoic Acid Inhibits Spontaneous Diabetes and Autoimmune Recurrence in Non-Obese Diabetic Mice by Enhancing Differentiation of Regulatory T Cells and Showed Potential for Use in Cell Therapies for the Treatment of Type 1 Diabetes" International Journal of Molecular Sciences 23, no. 3: 1169. https://doi.org/10.3390/ijms23031169
APA StyleHuang, S.-H., Kuo, S.-L., Chen, S.-J., Lin, J.-R., Chen, Y.-W., Hong, Z.-J., Sytwu, H.-K., & Lin, G.-J. (2022). Alpha-Lipoic Acid Inhibits Spontaneous Diabetes and Autoimmune Recurrence in Non-Obese Diabetic Mice by Enhancing Differentiation of Regulatory T Cells and Showed Potential for Use in Cell Therapies for the Treatment of Type 1 Diabetes. International Journal of Molecular Sciences, 23(3), 1169. https://doi.org/10.3390/ijms23031169







