Paclitaxel-Induced Epidermal Alterations: An In Vitro Preclinical Assessment in Primary Keratinocytes and in a 3D Epidermis Model
Abstract
1. Introduction
2. Results
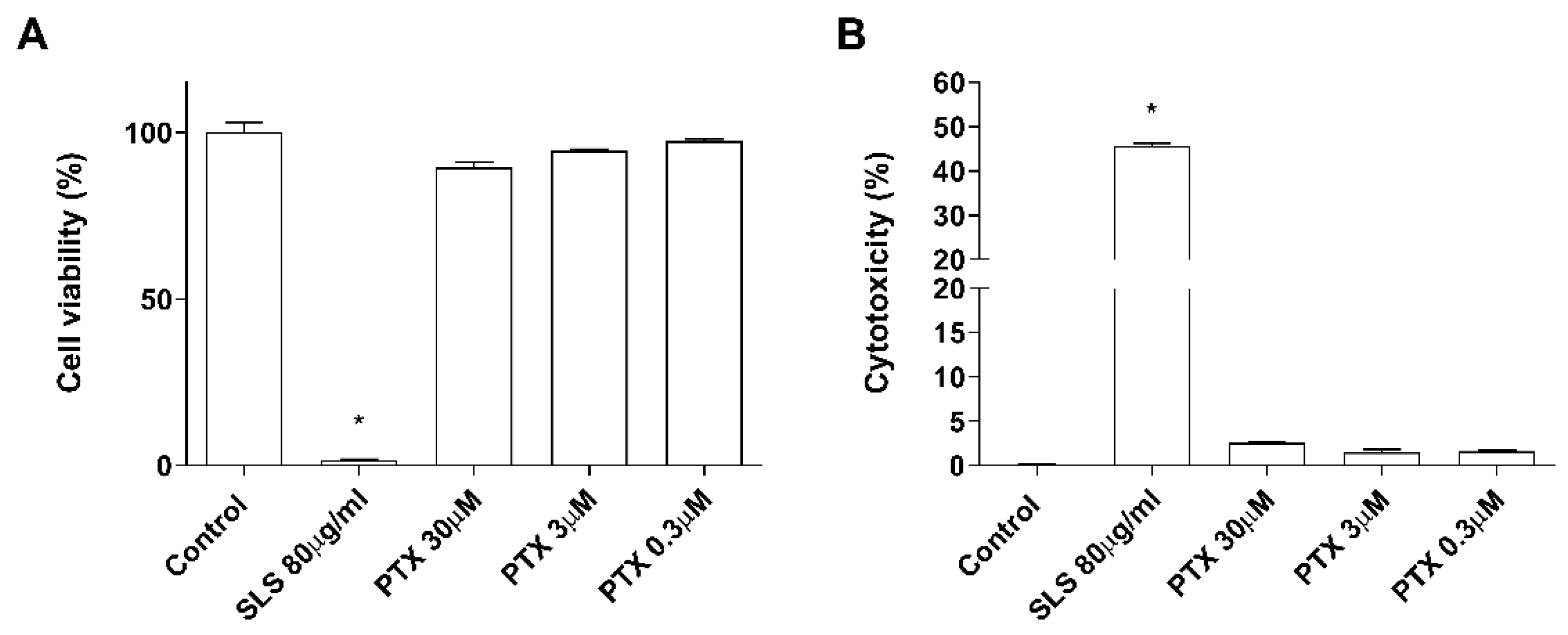
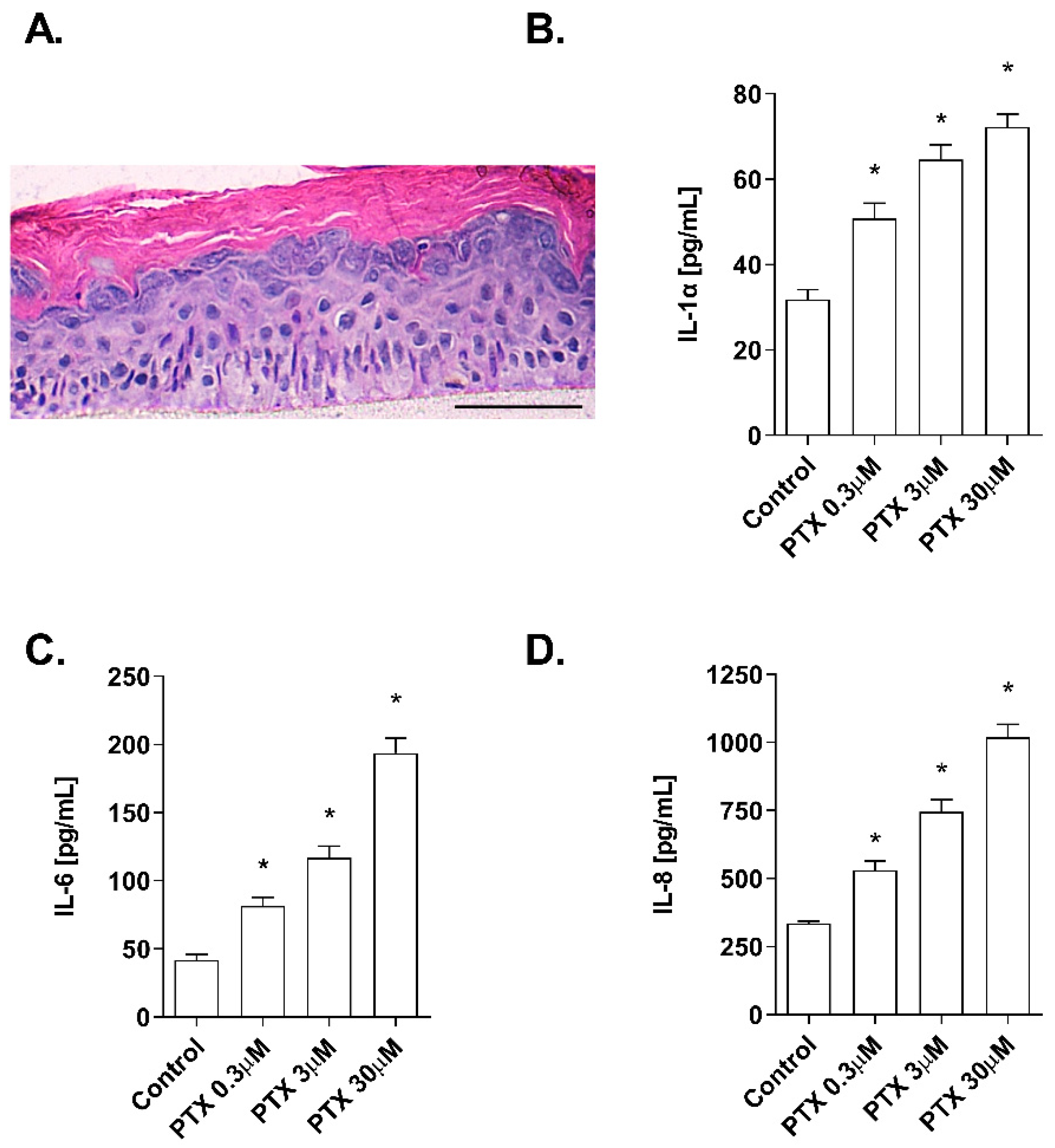
2.1. Non-Cytotoxic Doses of Paclitaxel Induce Inflammation in a 3D Epidermis Model
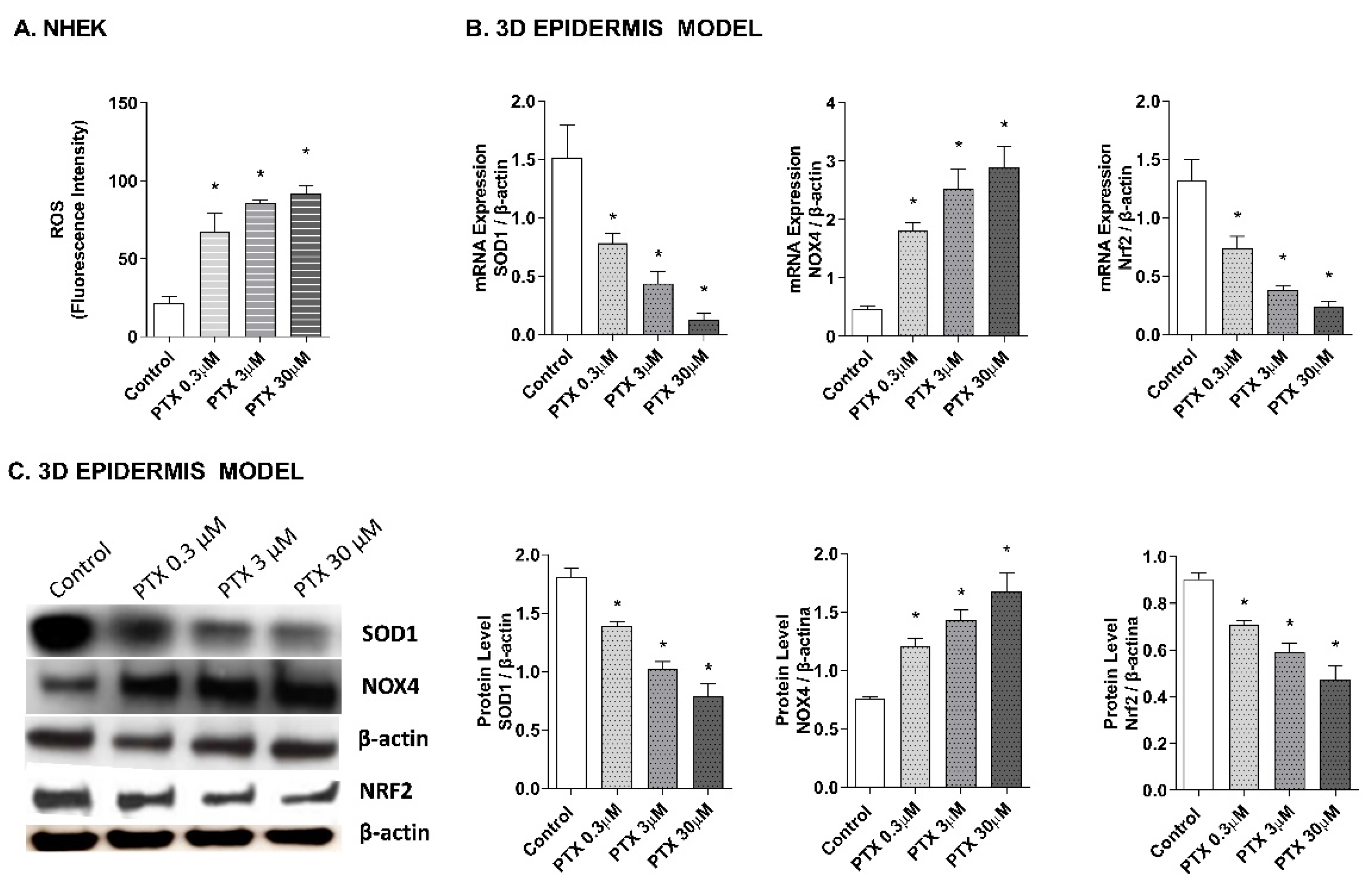
2.2. Paclitaxel-Induced Oxidative Stress Response
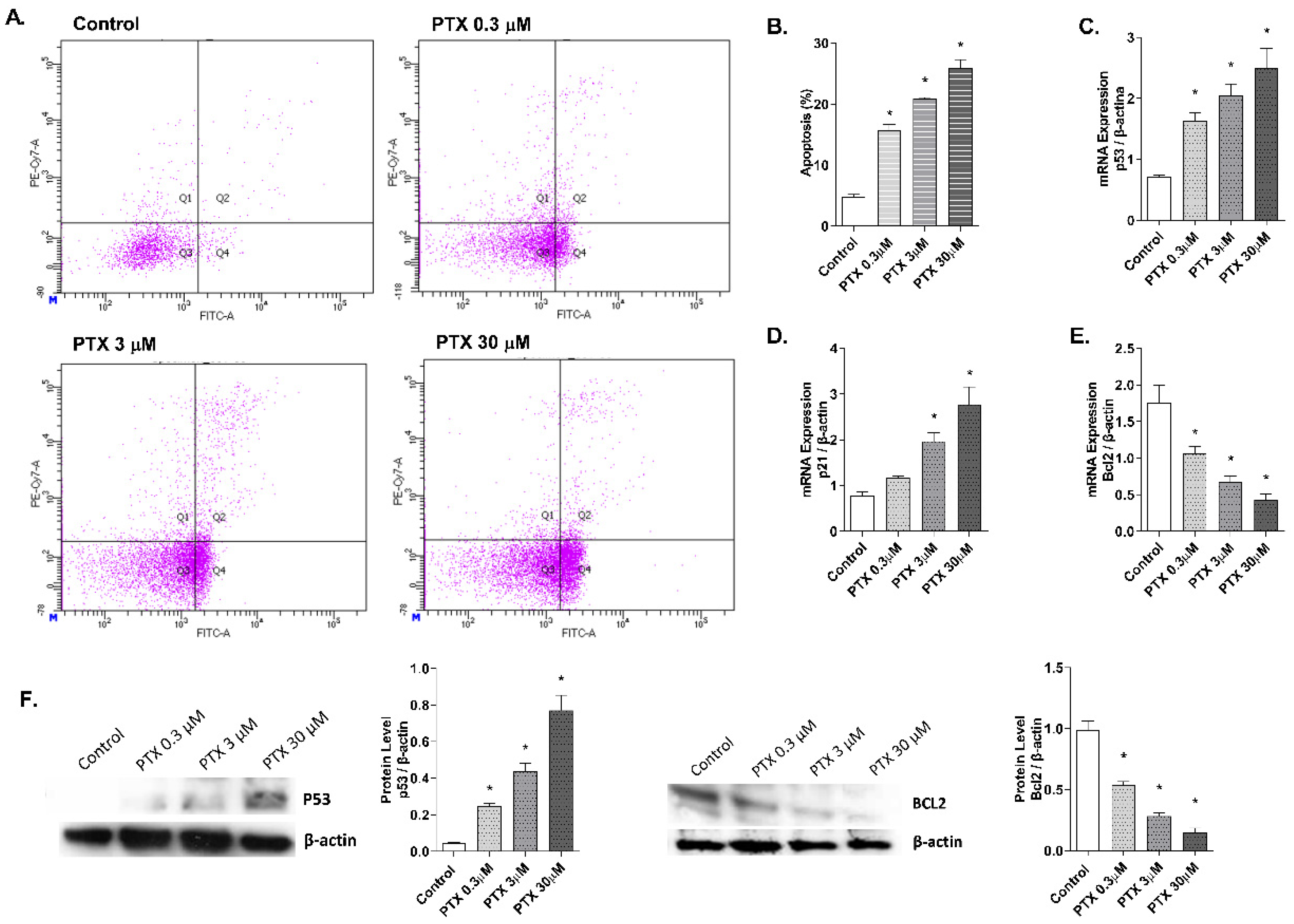
2.3. Paclitaxel-Induced Apoptosis
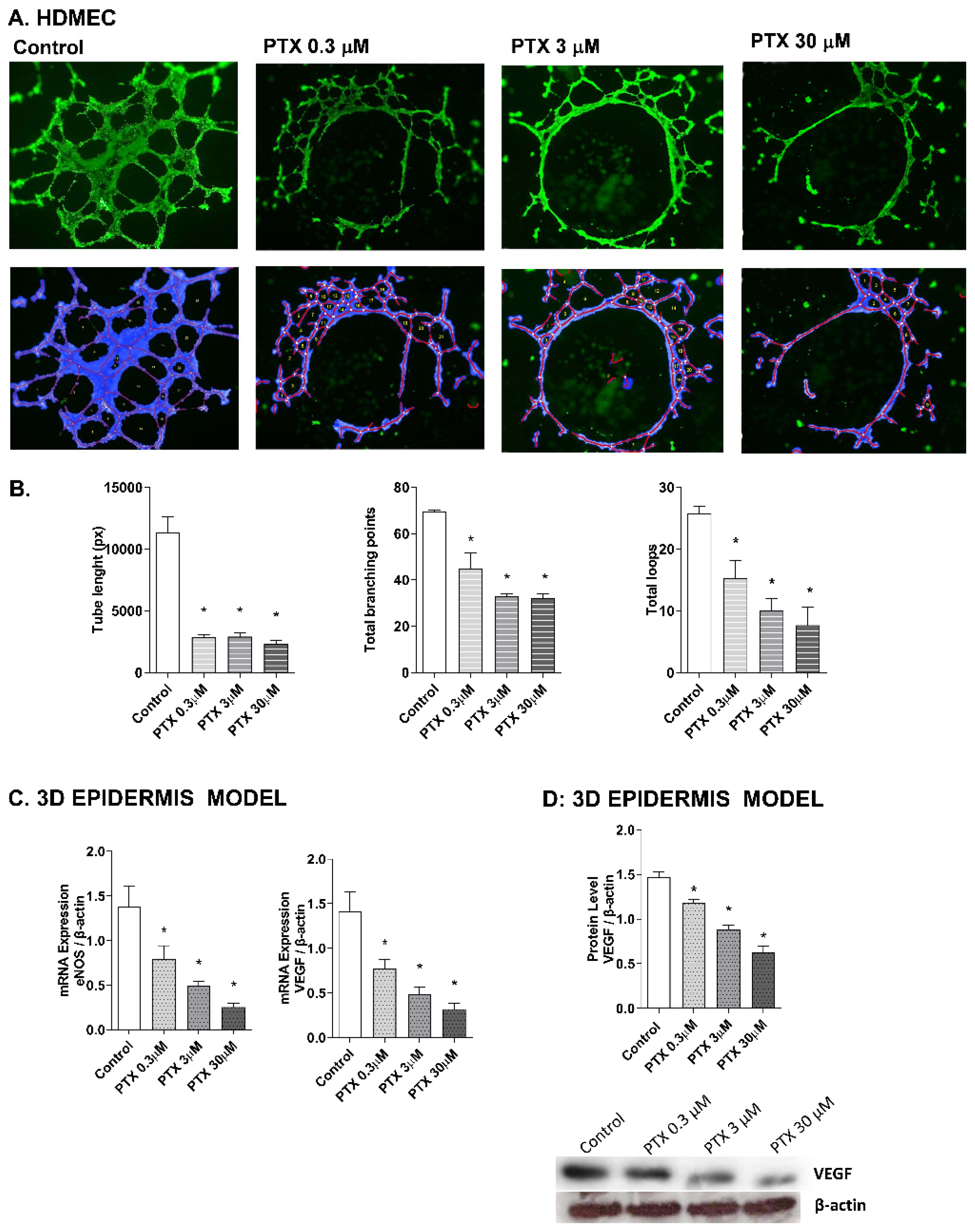
2.4. Paclitaxel-Targeted Angiogenesis
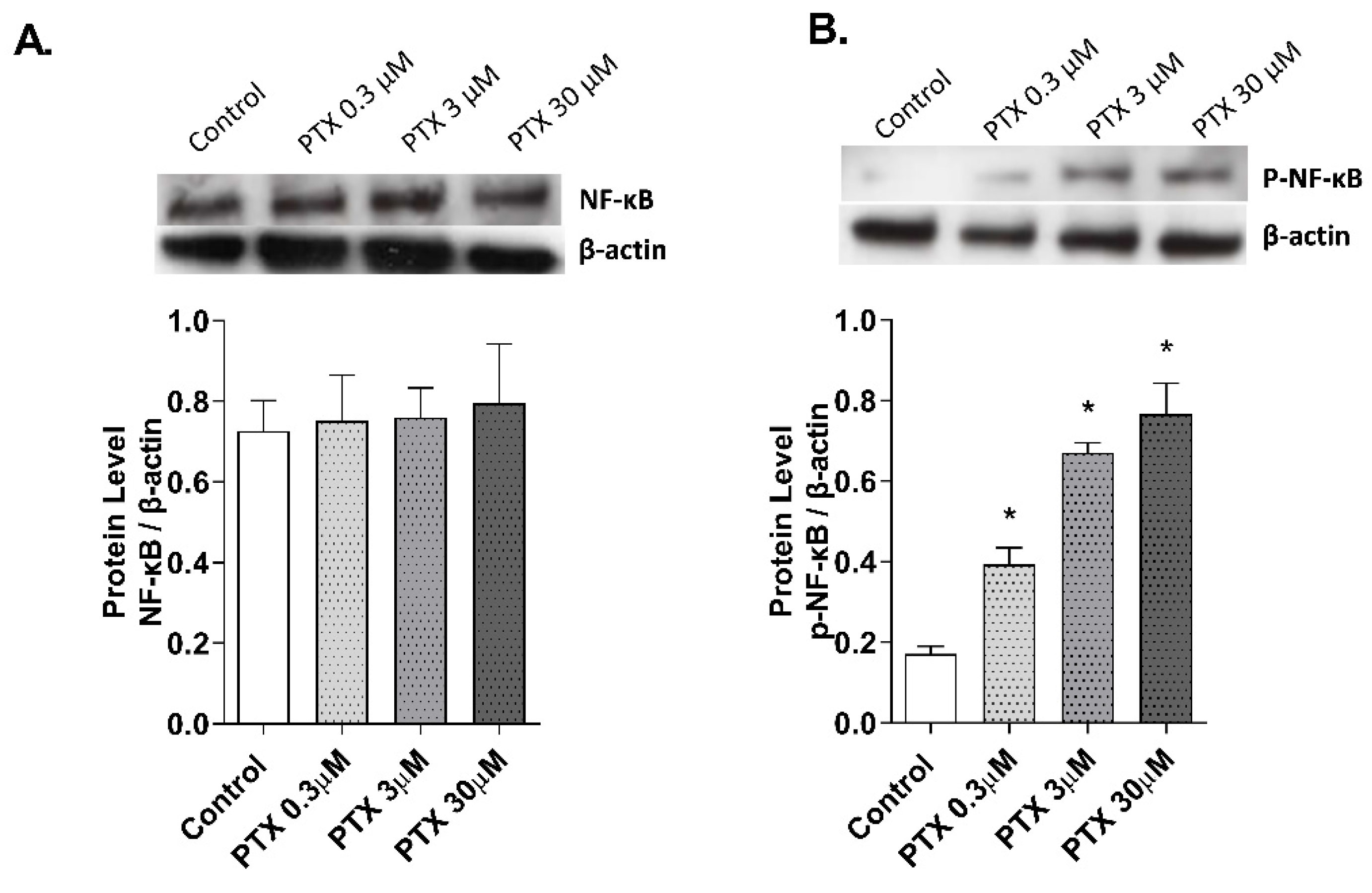
2.5. NF-κB Transcription Factor Activation by Paclitaxel
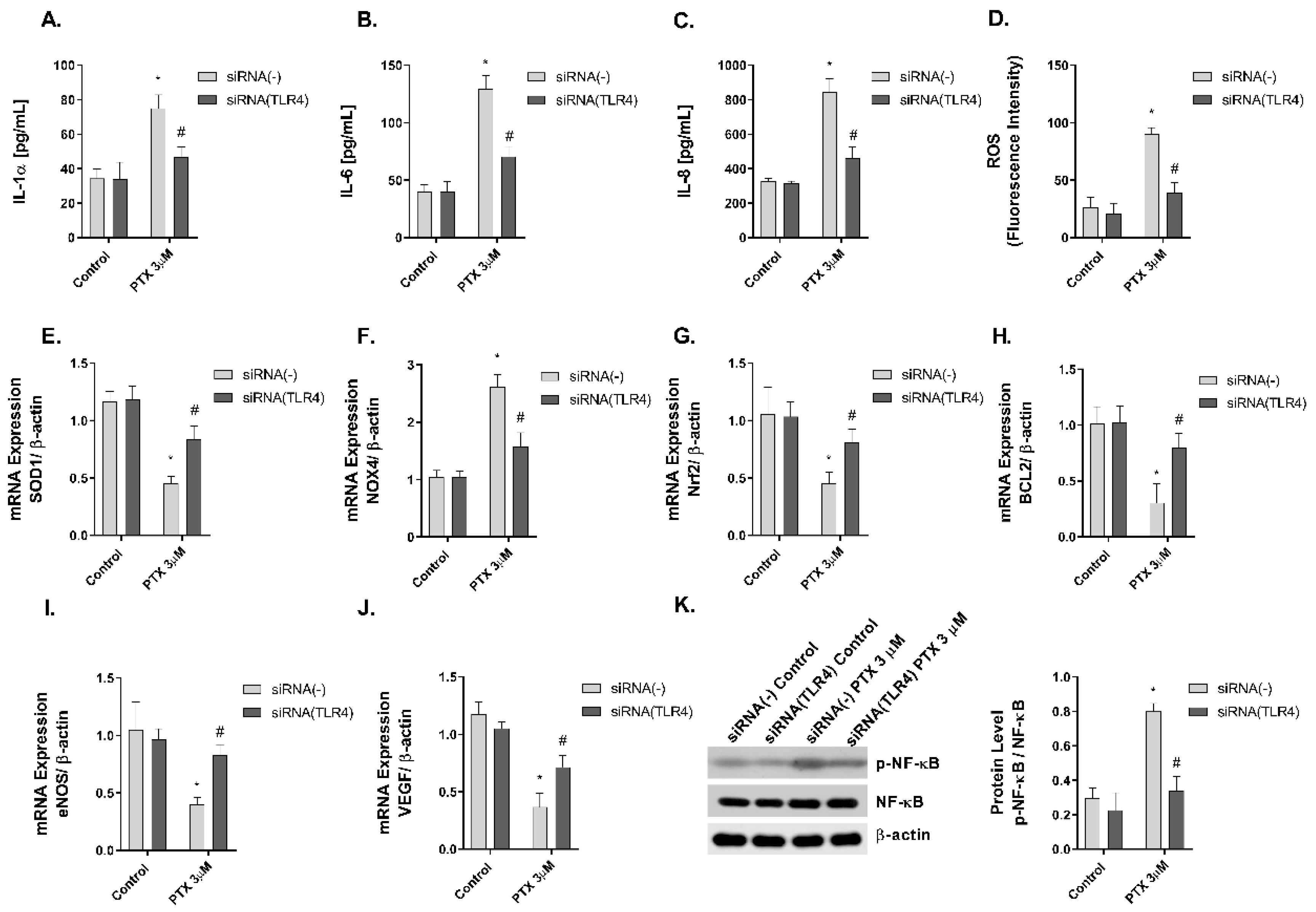
2.6. TLR4 Mediates Paclitaxel Effects on Human Keratinocytes
3. Discussion
4. Materials and Methods
4.1. Cell Culture and 3D Epidermis Model Reconstruction
4.2. Cell Viability and Cell Death Assay
4.3. Cytokine Determination by ELISA
4.4. DCF Fluorescence Measurement of Reactive Oxygen Species
4.5. Real-Time RT-PCR and siRNA Experiments
4.6. Western Blotting Analysis
4.7. Apoptosis
4.8. Angiogenesis
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alkahtani, H.M.; AlQuadeib, B.T. Chapter three—Paclitaxel. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: London, UK, 2019; pp. 205–238. [Google Scholar]
- Zeng, Q.; Yang, F.; Li, C.; Xu, L.; He, X.; Mai, F.; Zeng, C.; Zhang, C.; Zha, Q.; Ouyang, D. Paclitaxel Enhances the Innate Immunity by Promoting NLRP3 Inflammasome Activation in Macrophages. Front. Immunol. 2019, 10, 72. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; McNally, O.M.; Quinn, M.; Plebanski, M. Paclitaxel and Its Evolving Role in the Management of Ovarian Cancer. BioMed Res. Int. 2015, 2015, 413076. [Google Scholar] [CrossRef] [PubMed]
- Byrd-Leifer, C.A.; Block, E.F.; Takeda, K.; Akira, S.; Ding, A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur. J. Immunol. 2001, 31, 2448–2457. [Google Scholar] [CrossRef]
- Fan, W. Possible mechanisms of paclitaxel-induced apoptosis. Biochem. Pharmacol. 1999, 57, 1215–1221. [Google Scholar]
- Wang, T.-H.; Chan, Y.-H.; Chen, C.-W.; Kung, W.-H.; Lee, Y.-S.; Wang, S.-T.; Chang, T.-C.; Wang, H.-S. Paclitaxel (Taxol) upregulates expression of functional interleukin-6 in human ovarian cancer cells through multiple signaling pathways. Oncogene 2006, 25, 4857–4866. [Google Scholar] [CrossRef][Green Version]
- Pfannenstiel, L.W.; Lam, S.S.K.; Emens, L.A.; Jaffee, E.M.; Armstrong, T.D. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell. Immunol. 2010, 263, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhao, B.; Chang, H.; Xiao, M.; Wu, Y.; Liu, Y. Paclitaxel suppresses proliferation and induces apoptosis through regulation of ROS and the AKT/MAPK signaling pathway in canine mammary gland tumor cells. Mol. Med. Rep. 2018, 17, 8289–8299. [Google Scholar] [CrossRef]
- Wang, T.H.; Wang, H.S.; Soong, Y.K. Paclitaxel-induced cell death: Where the cell cycle and apoptosis come together. Cancer 2000, 88, 2619–2628. [Google Scholar] [CrossRef]
- Lee, L.F.; Li, G.; Templeton, D.J.; Ting, J.P. Paclitaxel (Taxol)-induced gene expression and cell death are both mediated by the activation of c-Jun NH2-terminal kinase (JNK/SAPK). J. Biol. Chem. 1998, 273, 28253–28260. [Google Scholar] [CrossRef]
- Kate, S.; Gulia, S.; Gupta, S. Severe skin toxicity due to weekly paclitaxel administration. Ind. J. Med. Paediatr. Oncol. 2015, 36, 62. [Google Scholar] [CrossRef]
- Su, M.-H.; Chen, G.-Y.; Lin, J.-H.; Lee, H.H.; Chung, K.-C.; Wang, P.-H. Paclitaxel-related dermatological problems: Not only alopecia occurs. Taiwan. J. Obstet. Gynecol. 2019, 58, 877–879. [Google Scholar] [CrossRef]
- Weinberg, J.M.; Egan, C.L.; Tangoren, I.A.; Li, L.J.; Laughinghouse, K.A.; Guzzo, C.A. Generalized pustular dermatosis following paclitaxel therapy. Int. J. Dermatol. 1997, 36, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Masson Regnault, M.; Gadaud, N.; Boulinguez, S.; Tournier, E.; Lamant, L.; Gladieff, L.; Roche, H.; Guenounou, S.; Recher, C.; Sibaud, V. Chemotherapy-Related Reticulate Hyperpigmentation: A Case Series and Review of the Literature. Dermatology 2015, 231, 312–318. [Google Scholar] [CrossRef]
- Spicknall, K.E.; Mutasim, D.F. Localized toxic erythema of chemotherapy during treatment with paclitaxel. Int. J. Dermatol. 2014, 53, e3–e5. [Google Scholar] [CrossRef] [PubMed]
- Childress, J.; Lokich, J. Cutaneous hand and foot toxicity associated with cancer chemotherapy. Am. J. Clin. Oncol. 2003, 26, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V.; Lebœuf, N.R.; Roche, H.; Belum, V.R.; Gladieff, L.; Deslandres, M.; Montastruc, M.; Eche, A.; Vigarios, E.; Dalenc, F.; et al. Dermatological adverse events with taxane chemotherapy. Eur. J. Dermatol. 2016, 26, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Cheng, M.; Lee, N.-R.; Chang, W.-H.; Lee, Y.-L.; Wang, P.-H. Paclitaxel-related nail toxicity. Taiwan. J. Obstet. Gynecol. 2019, 58, 709–711. [Google Scholar] [CrossRef]
- Mitchell, E.; Mellor, C.E.L.; Purba, T.S. XMU-MP-1 induces growth arrest in a model human mini-organ and antagonises cell cycle-dependent paclitaxel cytotoxicity. Cell Div. 2020, 15, 11. [Google Scholar] [CrossRef]
- Purba, T.S.; Ng’andu, K.; Brunken, L.; Smart, E.; Mitchell, E.; Hassan, N.; O’Brien, A.; Mellor, C.; Jackson, J.; Shahmalak, A.; et al. CDK4/6 inhibition mitigates stem cell damage in a novel model for taxane-induced alopecia. EMBO Mol. Med. 2019, 11, e11031. [Google Scholar] [CrossRef]
- Hokeness, K.; Qiu, L.H.; Vezeridis, M.; Yan, B.F.; Mehta, S.; Wan, Y.S. IFN-gamma enhances paclitaxel-induced apoptosis that is modulated by activation of caspases 8 and 3 with a concomitant down regulation of the AKT survival pathway in cultured human keratinocytes. Oncol. Rep. 2005, 13, 965–969. [Google Scholar] [PubMed]
- Salas-Martínez, A.-M.; Caballero, C.I.; González-Vela, M.C.; Mira, M.-C.; Laso-Dosal, F.; Val-Bernal, J.-F. Paclitaxel-induced cutaneous change mimicking malignancy in a previous cutaneous eruption. Rev. Esp. Patol. 2018, 51, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Torres, L.; Llamas-Velasco, M.; Machan, S.; Haro, R.; de Asis, S.; Carmo, M.; Loredo, A.; del Puerto, C.; Fried, I.; Kempf, W.; et al. Taxanes-induced cutaneous eruption: Another histopathologic mimicker of malignancy. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Lisse, T.S.; Middleton, L.J.; Pellegrini, A.D.; Martin, P.B.; Spaulding, E.L.; Lopes, O.; Brochu, E.A.; Carter, E.V.; Waldron, A.; Rieger, S. Paclitaxel-induced epithelial damage and ectopic MMP-13 expression promotes neurotoxicity in zebrafish. Proc. Natl. Acad. Sci. USA 2016, 113, E2189–E2198. [Google Scholar] [CrossRef]
- Stark, H.J.; Baur, M.; Breitkreutz, D.; Mirancea, N.; Fusenig, N.E. Organotypic keratinocyte cocultures in defined medium with regular epidermal morphogenesis and differentiation. J. Invest. Dermatol. 1999, 112, 681–691. [Google Scholar] [CrossRef]
- Do Nascimento Pedrosa, T.; Catarino, C.M.; Pennacchi, P.C.; de Assis, S.R.; Gimenes, F.; Consolaro, M.E.L.; de Moraes Barros, B.; Maria-Engler, S.S. A new reconstructed human epidermis for in vitro skin irritation testing. Toxicol. In Vitro 2017, 42, 31–37. [Google Scholar] [CrossRef]
- Arnette, C.; Koetsier, J.L.; Hoover, P.; Getsios, S.; Green, K.J. In Vitro Model of the Epidermis: Connecting Protein Function to 3D Structure. Methods Enzymol. 2016, 569, 287–308. [Google Scholar] [PubMed]
- Pruniéras, M.; Régnier, M.; Woodley, D. Methods for Cultivation of Keratinocytes with an Air-Liquid Interface. J. Invest. Dermatol. 1983, 81, S28–S33. [Google Scholar] [CrossRef]
- Lamb, R.; Ambler, C.A. Keratinocytes propagated in serum-free, feeder-free culture conditions fail to form stratified epidermis in a reconstituted skin model. PLoS ONE 2013, 8, e52494. [Google Scholar]
- Bisson, F.; Rochefort, E.; Lavoie, A.; Larouche, D.; Zaniolo, K.; Simard-Bisson, C.; Damour, O.; Auger, F.A.; Guérin, S.L.; Germain, L. Irradiated human dermal fibroblasts are as efficient as mouse fibroblasts as a feeder layer to improve human epidermal cell culture lifespan. Int. J. Mol. Sci. 2013, 14, 4684–4704. [Google Scholar] [CrossRef]
- De Weger, V.A.; Beijnen, J.H.; Schellens, J.H.M. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—A review. Anticancer Drugs 2014, 25, 488–494. [Google Scholar] [CrossRef]
- Perkins, M.A.; Osterhues, M.A.; Farage, M.A.; Robinson, M.K. A noninvasive method to assess skin irritation and compromised skin conditions using simple tape adsorption of molecular markers of inflammation. Skin Res. Technol. 2001, 7, 227–237. [Google Scholar] [CrossRef]
- Jensen, L.E. Targeting the IL-1 family members in skin inflammation. Curr. Opin. Investig. Drugs 2010, 11, 1211–1220. [Google Scholar] [PubMed]
- Gröne, A. Keratinocytes and cytokines. Vet. Immunol. Immunopathol. 2002, 88, 1–12. [Google Scholar] [CrossRef]
- Collins, T.S.; Lee, L.F.; Ting, J.P. Paclitaxel up-regulates interleukin-8 synthesis in human lung carcinoma through an NF-kappaB- and AP-1-dependent mechanism. Cancer Immunol. Immunother. 2000, 49, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Hu, Y.; Lu, W.; Pelicano, H.; Huang, P. Novel action of paclitaxel against cancer cells: Bystander effect mediated by reactive oxygen species. Cancer Res. 2007, 67, 3512–3517. [Google Scholar] [CrossRef]
- Kim, H.S.; Oh, J.M.; Jin, D.H.; Yang, K.-H.; Moon, E.-Y. Paclitaxel induces vascular endothelial growth factor expression through reactive oxygen species production. Pharmacology 2008, 81, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Belotti, D.; Vergani, V.; Drudis, T.; Borsotti, P.; Pitelli, M.R.; Viale, G.; Giavazzi, R.; Taraboletti, G. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin. Cancer Res. 1996, 2, 1843–1849. [Google Scholar]
- Ai, B.; Bie, Z.; Zhang, S.; Li, A. Paclitaxel targets VEGF-mediated angiogenesis in ovarian cancer treatment. Am. J. Cancer Res. 2016, 6, 1624–1635. [Google Scholar]
- Mikuła-Pietrasik, J.; Witucka, A.; Pakuła, M.; Uruski, P.; Begier-Krasińska, B.; Niklas, A.; Tykarski, A.; Książek, K. Comprehensive review on how platinum- and taxane-based chemotherapy of ovarian cancer affects biology of normal cells. Cell. Mol. Life Sci. 2019, 76, 681–697. [Google Scholar] [CrossRef]
- Wang, A.C.; Su, Q.B.; Wu, F.X.; Zhang, X.L.; Liu, P.S. Role of TLR4 for paclitaxel chemotherapy in human epithelial ovarian cancer cells. Eur. J. Clin. Investig. 2009, 39, 157–164. [Google Scholar] [CrossRef]
- Verweij, J.; Clavel, M.; Chevalier, B. Paclitaxel (Taxol) and docetaxel (Taxotere): Not simply two of a kind. Ann. Oncol. 1994, 5, 495–505. [Google Scholar] [CrossRef]
- Coquette, A.; Berna, N.; Vandenbosch, A.; Rosdy, M.; De Wever, B.; Poumay, Y. Analysis of interleukin-1α (IL-1α) and interleukin-8 (IL-8) expression and release in in vitro reconstructed human epidermis for the prediction of in vivo skin irritation and/or sensitization. Toxicol. In Vitro 2003, 17, 311–321. [Google Scholar] [CrossRef]
- Nickoloff, B.J.; Naidu, Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J. Am. Acad. Dermatol. 1994, 30, 535–546. [Google Scholar] [CrossRef]
- Pusztai, L.; Mendoza, T.R.; Reuben, J.M.; Martinez, M.M.; Willey, J.S.; Lara, J.; Syed, A.; Fritsche, H.A.; Bruera, E.; Booser, D.; et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine 2004, 25, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.F.; Schuerer-Maly, C.C.; Lofquist, A.K.; van Haaften-Day, C.; Ting, J.P.; White, C.M.; Martin, B.K.; Haskill, J.S. Taxol-dependent transcriptional activation of IL-8 expression in a subset of human ovarian cancer. Cancer Res. 1996, 56, 1303–1308. [Google Scholar]
- Lieu, C.H.; Chang, Y.N.; Lai, Y.K. Dual cytotoxic mechanisms of submicromolar taxol on human leukemia HL-60 cells. Biochem. Pharmacol. 1997, 53, 1587–1596. [Google Scholar] [CrossRef]
- Zhang, C.C.; Yang, J.M.; White, E.; Murphy, M.; Levine, A.; Hait, W.N. The role of MAP4 expression in the sensitivity to paclitaxel and resistance to vinca alkaloids in p53 mutant cells. Oncogene 1998, 16, 1617–1624. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef]
- Hayashi, S.; Yamamoto, A.; You, F.; Yamashita, K.; Ikegame, Y.; Tawada, M.; Yoshimori, T.; Shimizu, S.; Nakashima, S. The Stent-Eluting Drugs Sirolimus and Paclitaxel Suppress Healing of the Endothelium by Induction of Autophagy. Am. J. Pathol. 2009, 175, 2226–2234. [Google Scholar] [CrossRef][Green Version]
- Hadzic, T.; Aykin-Burns, N.; Zhu, Y.; Coleman, M.C.; Leick, K.; Jacobson, G.M.; Spitz, D.R. Paclitaxel combined with inhibitors of glucose and hydroperoxide metabolism enhances breast cancer cell killing via H2O2-mediated oxidative stress. Free Radic. Biol. Med. 2010, 48, 1024–1033. [Google Scholar] [CrossRef]
- Alexandre, J.; Batteux, F.; Nicco, C.; Chéreau, C.; Laurent, A.; Guillevin, L.; Weill, B.; Goldwasser, F. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int. J. Cancer 2006, 119, 41–48. [Google Scholar] [CrossRef]
- Peiris-Pagès, M.; Sotgia, F.; Lisanti, M.P. Chemotherapy induces the cancer-associated fibroblast phenotype, activating paracrine Hedgehog-GLI signalling in breast cancer cells. Oncotarget 2015, 6, 10728–10745. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Shibuya, S.; Ozawa, Y.; Nojiri, H.; Izuo, N.; Yokote, K.; Shimizu, T. Superoxide dismutase 1 loss disturbs intracellular redox signaling, resulting in global age-related pathological changes. Biomed. Res. Int. 2014, 2014, 140165. [Google Scholar] [CrossRef] [PubMed]
- Peiris-Pagès, M.; Smith, D.L.; Győrffy, B.; Sotgia, F.; Lisanti, M.P. Proteomic identification of prognostic tumour biomarkers, using chemotherapy-induced cancer-associated fibroblasts. Aging 2015, 7, 816–838. [Google Scholar] [CrossRef] [PubMed]
- Merchan, J.R.; Jayaram, D.R.; Supko, J.G.; He, X.; Bubley, G.J.; Sukhatme, V.P. Increased endothelial uptake of paclitaxel as a potential mechanism for its antiangiogenic effects: Potentiation by Cox-2 inhibition. Int. J. Cancer 2005, 113, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Jiang, W.G.; Ahmed, A.; Boulton, M. Vascular endothelial growth factor-induced endothelial cell proliferation is regulated by interaction between VEGFR-2, SH-PTP1 and eNOS. Microvasc. Res. 2006, 71, 20–31. [Google Scholar] [CrossRef]
- Man, M.-Q.; Wakefield, J.S.; Mauro, T.M.; Elias, P.M. Role of nitric oxide in regulating epidermal permeability barrier function. Exp. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Graves, D.T. Keratinocyte Function in Normal and Diabetic Wounds and Modulation by FOXO1. J. Diabetes Res. 2020, 2020, 3714704. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Eun, H.C. Angiogenesis in skin aging and photoaging. J. Dermatol. 2007, 34, 593–600. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef]
- Bracci, L.; Schiavoni, G.; Sistigu, A.; Belardelli, F. Immune-based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014, 21, 15–25. [Google Scholar] [CrossRef]
- Iotzova-Weiss, G.; Freiberger, S.N.; Johansen, P.; Kamarachev, J.; Guenova, E.; Dziunycz, P.J.; Roux, G.A.; Neu, J.; Hofbauer, G.F.L. TLR4 as a negative regulator of keratinocyte proliferation. PLoS ONE 2017, 12, e0185668. [Google Scholar] [CrossRef]
- Mikami, E.; Kudo, M.; Ohashi, R.; Kawahara, K.; Kawamoto, Y.; Teduka, K.; Fujii, T.; Kitamura, T.; Kure, S.; Ishino, K.; et al. Toll-like receptor 4 plays a tumor-suppressive role in cutaneous squamous cell carcinoma. Int. J. Oncol. 2019, 54, 2179–2188. [Google Scholar] [CrossRef]
- Mak, V.H.; Cumpstone, M.B.; Kennedy, A.H.; Harmon, C.S.; Guy, R.H.; Potts, R.O. Barrier function of human keratinocyte cultures grown at the air-liquid interface. J. Investig. Dermatol. 1991, 96, 323–327. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero, P.; Milara, J.; Pérez-Leal, M.; Estornut, C.; Roger, I.; Pérez-Fidalgo, A.; Sanz, C.; Cortijo, J. Paclitaxel-Induced Epidermal Alterations: An In Vitro Preclinical Assessment in Primary Keratinocytes and in a 3D Epidermis Model. Int. J. Mol. Sci. 2022, 23, 1142. https://doi.org/10.3390/ijms23031142
Montero P, Milara J, Pérez-Leal M, Estornut C, Roger I, Pérez-Fidalgo A, Sanz C, Cortijo J. Paclitaxel-Induced Epidermal Alterations: An In Vitro Preclinical Assessment in Primary Keratinocytes and in a 3D Epidermis Model. International Journal of Molecular Sciences. 2022; 23(3):1142. https://doi.org/10.3390/ijms23031142
Chicago/Turabian StyleMontero, Paula, Javier Milara, Martín Pérez-Leal, Cristina Estornut, Inés Roger, Alejandro Pérez-Fidalgo, Celia Sanz, and Julio Cortijo. 2022. "Paclitaxel-Induced Epidermal Alterations: An In Vitro Preclinical Assessment in Primary Keratinocytes and in a 3D Epidermis Model" International Journal of Molecular Sciences 23, no. 3: 1142. https://doi.org/10.3390/ijms23031142
APA StyleMontero, P., Milara, J., Pérez-Leal, M., Estornut, C., Roger, I., Pérez-Fidalgo, A., Sanz, C., & Cortijo, J. (2022). Paclitaxel-Induced Epidermal Alterations: An In Vitro Preclinical Assessment in Primary Keratinocytes and in a 3D Epidermis Model. International Journal of Molecular Sciences, 23(3), 1142. https://doi.org/10.3390/ijms23031142





