Rab21 Protein Is Degraded by Both the Ubiquitin-Proteasome Pathway and the Autophagy-Lysosome Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture, Plasmid, and Transfection
2.2. Immunofluorescence
2.3. Western Blotting
2.4. Statistical Analysis
3. Results
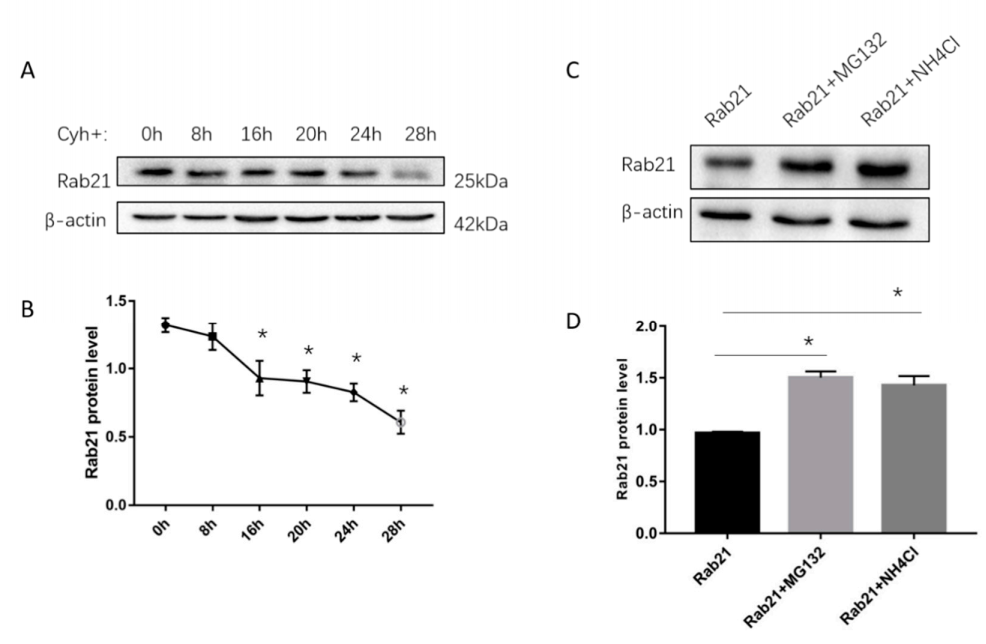
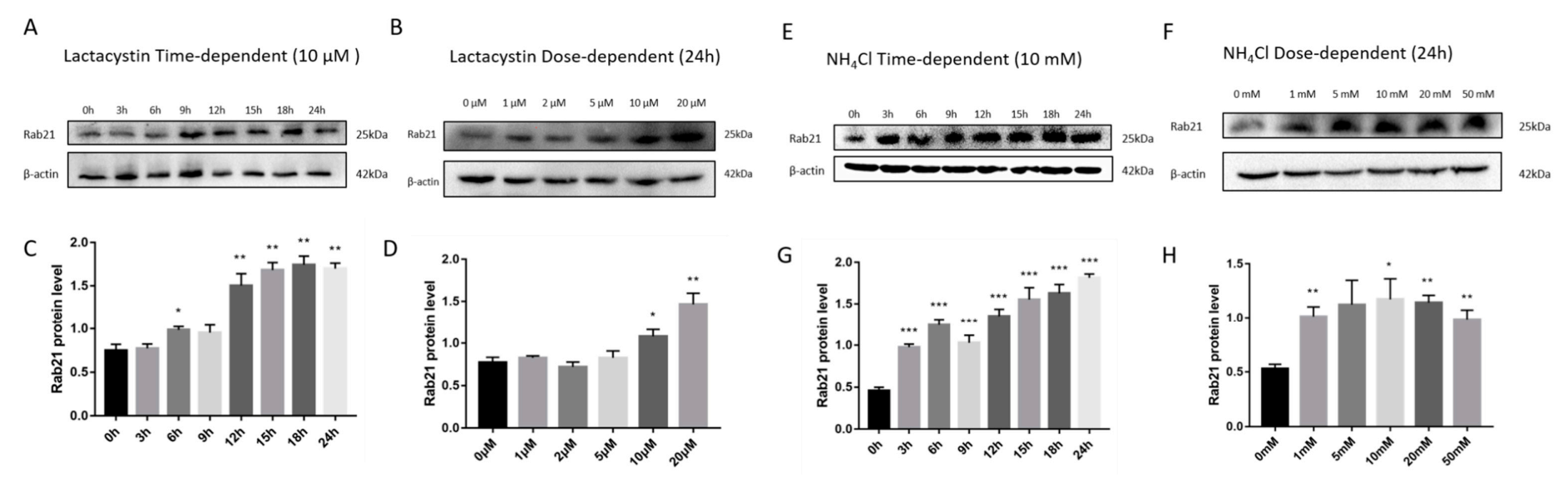
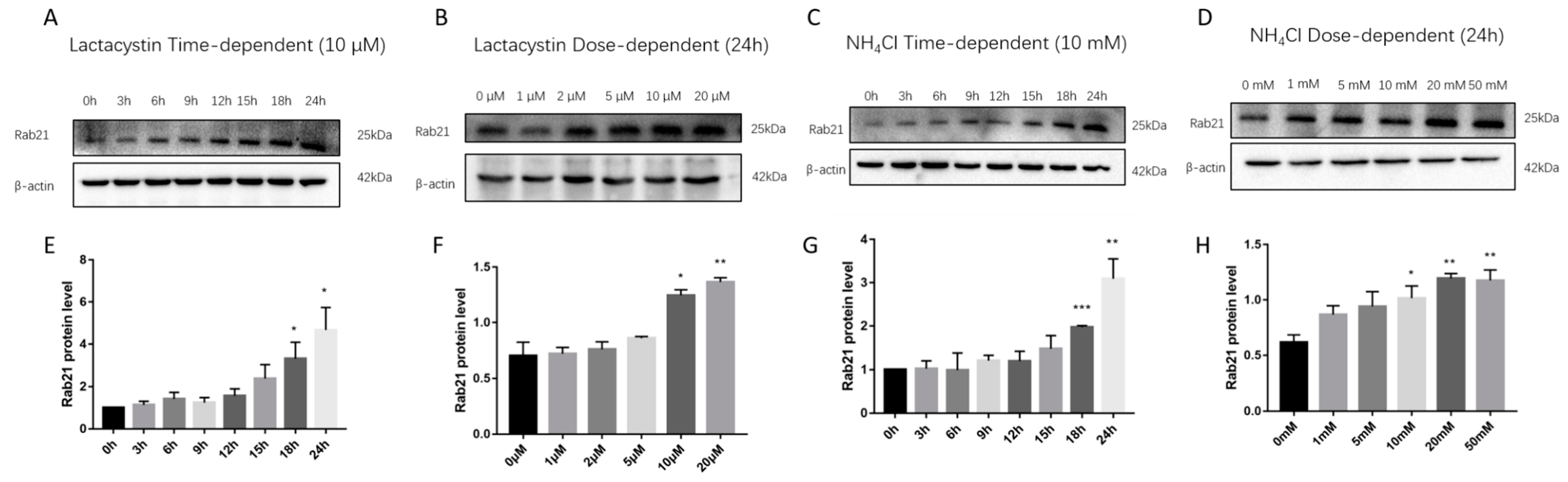
3.1. Rab21 Is Degraded by Proteasome- and Lysosome-Dependent Proteolysis
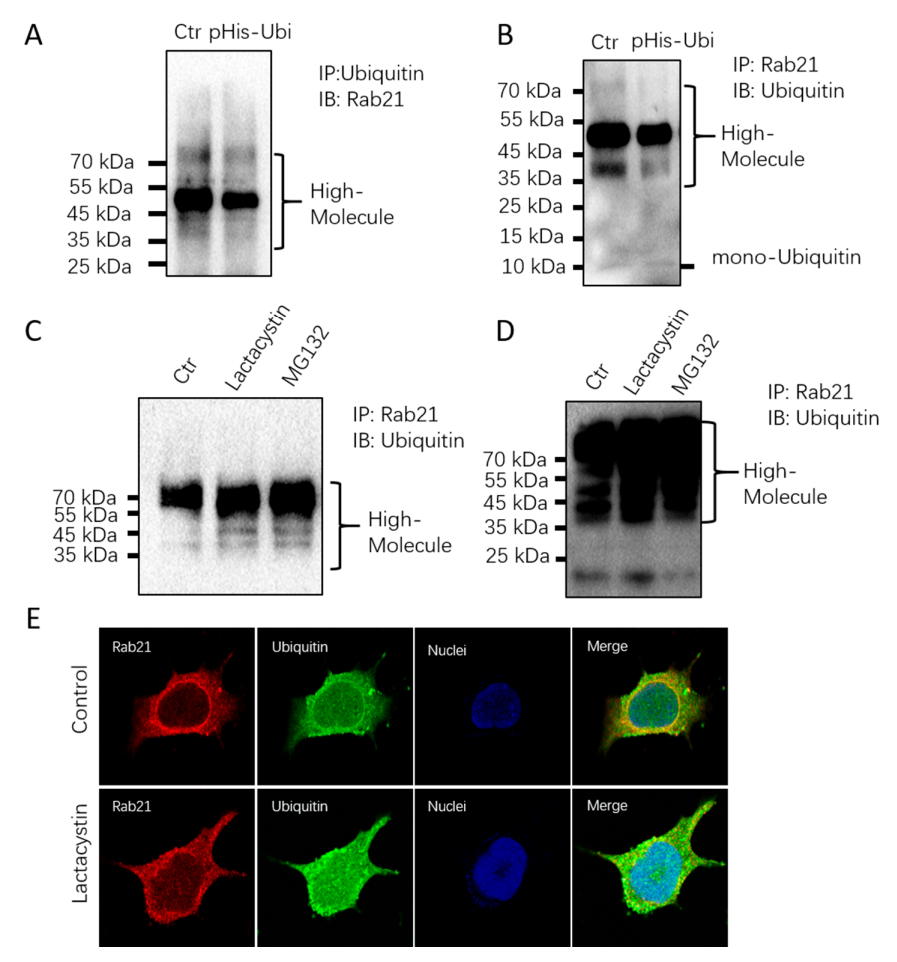
3.2. Rab21 Is Ubiquitinated
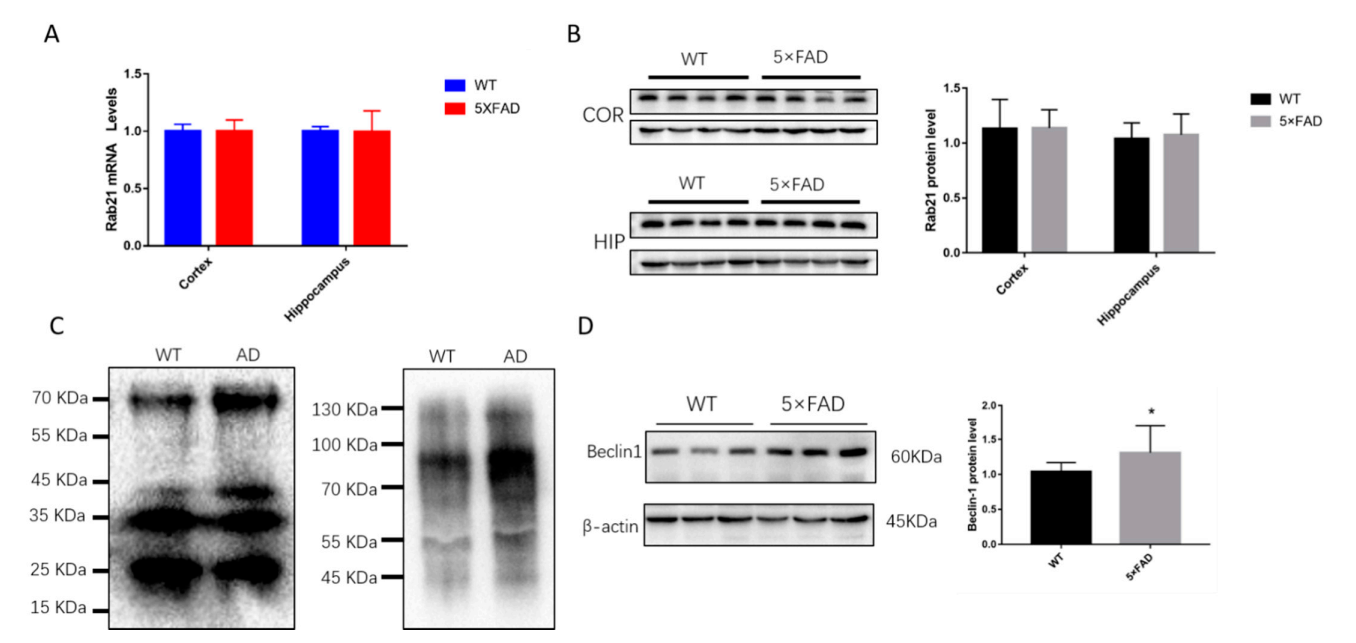
3.3. The Ubiquitination of Rab21 Is Increased in Alzheimer’s Disease (AD) Model Mice
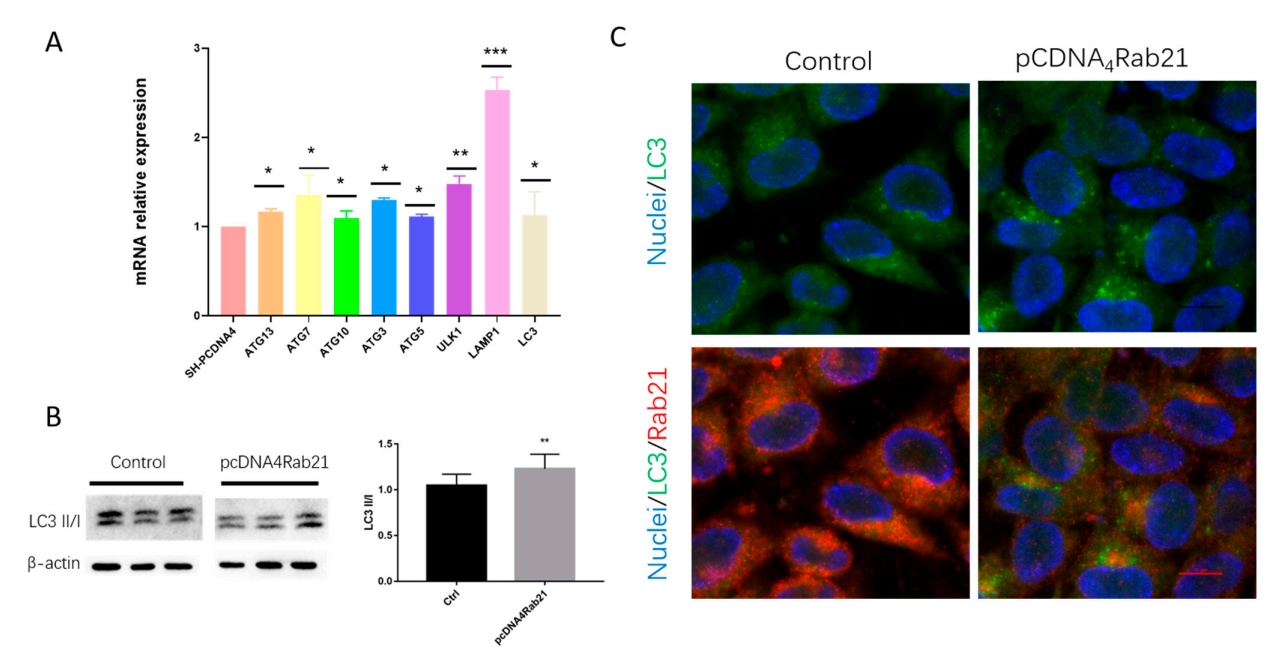
3.4. Rab21 Upregulate Autophagy-Related Genes
4. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Olmo, T.; Lacarrière-Keïta, C.; Normandin, C.; Jean, D.; Boisvert, F.-M.; Jean, S. RAB21 interacts with TMED10 and modulates its localization and abundance. Biol. Open 2019, 8, bio045336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicinanza, M.; D’angelo, G.; Di Campli, A.; De Matteis, M.A. Function and dysfunction of the PI system in membrane trafficking. EMBO J. 2008, 27, 2457–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pylypenko, O.; Hammich, H.; Yu, I.-M.; Houdusse, A. Rab GTPases and their interacting protein partners: Structural insights into Rab functional diversity. Small GTPases 2018, 9, 22–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutagalung, A.H.; Novick, P.J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 2011, 91, 119–149. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Zou, S.; Chen, Y.; Lipatova, Z.; Sun, D.; Zhu, X.; Li, R.; Wu, Z.; You, W.; Cong, X.; et al. A Rab5 GTPase module is important for autophagosome closure. PLoS Genet. 2017, 13, e1007020. [Google Scholar] [CrossRef] [Green Version]
- Hyttinen, J.M.; Niittykoski, M.; Salminen, A.; Kaarniranta, K. Maturation of autophagosomes and endosomes: A key role for Rab7. Biochim. Biophys. Acta 2013, 1833, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Jiang, X.; Tian, R.; Zhao, P.; Li, L.; Wang, X.; Chen, S.; Zhu, Y.; Mei, M.; Bao, S.; et al. RAB2 regulates the formation of autophagosome and autolysosome in mammalian cells. Autophagy 2019, 15, 1774–1786. [Google Scholar] [CrossRef] [Green Version]
- Daro, E.; Van Der Sluijs, P.; Galli, T.; Mellman, I. Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc. Natl. Acad. Sci. USA 1996, 93, 9559–9564. [Google Scholar] [CrossRef] [Green Version]
- Kouranti, I.; Sachse, M.; Arouche, N.; Goud, B.; Echard, A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr. Biol. 2006, 16, 1719–1725. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, O.; Reinsch, S.; Urbe, S.; Zerial, M.; Parton, R. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996, 135, 913–924. [Google Scholar] [CrossRef]
- Szodorai, A.; Kuan, Y.H.; Hunzelmann, S.; Engel, U.; Sakane, A.; Sasaki, T.; Kins, S.; Takai, Y.; Kirsch, J.; Müller, U.; et al. APP anterograde transport requires Rab3A GTPase activity for assembly of the transport vesicle. J. Neurosci. 2009, 29, 14534–14544. [Google Scholar] [CrossRef] [Green Version]
- Dulubova, I.; Lou, X.; Lu, J.; Huryeva, I.; Alam, A.; Schneggenburger, R.; Rizo, J.; Südhof, T.C. A Munc13/RIM/Rab3 tripartite complex: From priming to plasticity? EMBO J. 2005, 24, 2839–2850. [Google Scholar] [CrossRef]
- Deinhardt, K.; Salinas, S.; Verastegui, C.; Watson, R.; Worth, D.; Hanrahan, S.; Bucci, C.; Schiavo, G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron 2006, 52, 293–305. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.; Tran, I.C.; Backos, D.S.; Esteban, J.A. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron 2005, 45, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Simpson, J.; Griffiths, G.; Wessling-Resnick, M.; Fransen, J.; Bennett, H.; Jones, A.T. A role for the small GTPase Rab21 in the early endocytic pathway. J. Cell Sci. 2004, 117 Pt 26, 6297–6311. [Google Scholar] [CrossRef] [Green Version]
- Pellinen, T.; Arjonen, A.; Vuoriluoto, K.; Kallio, K.; Fransen, J.A.; Ivaska, J. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J. Cell Biol. 2006, 173, 767–780. [Google Scholar] [CrossRef]
- Del Olmo, T.; Lauzier, A.; Normandin, C.; Larcher, R.; Lecours, M.; Jean, D.; Lessard, L.; Steinberg, F.; Boisvert, F.; Jean, S. APEX2-mediated RAB proximity labeling identifies a role for RAB21 in clathrin-independent cargo sorting. EMBO Rep. 2019, 20, e47192. [Google Scholar] [CrossRef]
- Wang, N.; Meng, W.; Jia, R.; Xiang, S. Rab GTPase 21 mediates caerulin-induced TRAF3-MKK3-p38 activation and acute pancreatitis response. Biochem. Biophys. Res. Commun. 2019, 518, 50–58. [Google Scholar] [CrossRef]
- Ge, J.; Chen, Q.; Liu, B.; Wang, L.; Zhang, S.; Ji, B. Knockdown of Rab21 inhibits proliferation and induces apoptosis in human glioma cells. Cell. Mol. Biol. Lett. 2017, 22, 30. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Xie, Y.; Chen, Y.; Yang, Q.; Quan, Z.; Dai, R.; Qing, H. Rab21, a Novel PS1 Interactor, Regulates gamma-Secretase Activity via PS1 Subcellular Distribution. Mol. Neurobiol. 2018, 55, 3841–3855. [Google Scholar]
- Qing, H.; Zhou, W.; Christensen, M.A.; Sun, X.; Tong, Y.; Song, W. Degradation of BACE by the ubiquitin-proteasome pathway. FASEB J. 2004, 18, 1571–1573. [Google Scholar] [CrossRef] [PubMed]
- Tooze, S.A.; Abada, A.; Elazar, Z. Endocytosis and autophagy: Exploitation or cooperation? Cold Spring Harb. Perspect. Biol. 2014, 6, a018358. [Google Scholar] [CrossRef]
- Udayar, V.; Buggia-Prévot, V.; Guerreiro, R.L.; Siegel, G.; Rambabu, N.; Soohoo, A.L.; Ponnusamy, M.; Siegenthaler, B.; Rajendran, L.; Thinakaran, G.; et al. A paired RNAi and RabGAP overexpression screen identifies Rab11 as a regulator of beta-amyloid production. Cell Rep. 2013, 5, 1536–1551. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, N.V.; Desjardins, A.; Leclerc, N. Tau secretion is correlated to an increase of Golgi dynamics. PLoS ONE 2017, 12, e0178288. [Google Scholar] [CrossRef]
- Rodriguez, L.; Mohamed, N.-V.; Desjardins, A.; Lippe, R.; A Fon, E.; Leclerc, N. Rab7A regulates tau secretion. J. Neurochem. 2017, 141, 592–605. [Google Scholar] [CrossRef]
- Ihara, Y.; Morishima-Kawashima, M.; Nixon, R. The ubiquitin-proteasome system and the autophagic-lysosomal system in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006361. [Google Scholar] [CrossRef] [Green Version]
- Ihara, Y.; Abraham, C.; Selkoe, D.J. Antibodies to paired helical filaments in Alzheimer’s disease do not recognize normal brain proteins. Nature 1983, 304, 727–730. [Google Scholar] [CrossRef]
- Parihar, N.; Bhatt, L.K. Deubiquitylating enzymes: Potential target in autoimmune diseases. Inflammopharmacology 2021, 29, 1683–1699. [Google Scholar] [CrossRef]
- Trelford, C.B.; Di Guglielmo, G.M. Molecular mechanisms of mammalian autophagy. Biochem. J. 2021, 478, 3395–3421. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, K.; Zhao, H. Redefining the Scope of Targeted Protein Degradation: Translational Opportunities in Hijacking the Autophagy-Lysosome Pathway. Biochemistry 2021. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.H.; Jun, C.B.; Ro, S.-H.; Kim, Y.-M.; Otto, N.M.; Cao, J.; Kundu, M.; Kim, D.-H. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell 2009, 20, 1992–2003. [Google Scholar] [CrossRef] [Green Version]
- Menon, M.B.; Dhamija, S. Beclin 1 Phosphorylation—At the Center of Autophagy Regulation. Front. Cell Dev. Biol. 2018, 6, 137. [Google Scholar] [CrossRef] [Green Version]
- Devereaux, K.; Dall’Armi, C.; Alcazar-Roman, A.R.; Ogasawara, Y.; Zhou, X.; Wang, F.; Yamamoto, A.; De Camilli, P.; Di Paolo, G. Regulation of mammalian autophagy by class II and III PI 3-kinases through PI3P synthesis. PLoS ONE 2013, 8, e76405. [Google Scholar] [CrossRef] [Green Version]
- Kraft, L.J.; Manral, P.; Dowler, J.; Kenworthy, A.K. Nuclear LC3 Associates with Slowly Diffusing Complexes that Survey the Nucleolus. Traffic 2016, 17, 369–399. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Dang, Y.; Su, W.; Liu, C.; Ma, H.; Shan, Y.; Pei, Y.; Wan, B.; Guo, J.; Yu, L. Molecular cloning and characterization of rat LC3A and LC3B—Two novel markers of autophagosome. Biochem. Biophys. Res. Commun. 2006, 339, 437–442. [Google Scholar] [CrossRef]
- Kimura, S.; Noda, T.; Yoshimori, T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 2007, 3, 452–460. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Wu, A.; Li, H.; Zhang, J.; Ni, J.; Quan, Z.; Qing, H. Rab21 Protein Is Degraded by Both the Ubiquitin-Proteasome Pathway and the Autophagy-Lysosome Pathway. Int. J. Mol. Sci. 2022, 23, 1131. https://doi.org/10.3390/ijms23031131
Liu P, Wu A, Li H, Zhang J, Ni J, Quan Z, Qing H. Rab21 Protein Is Degraded by Both the Ubiquitin-Proteasome Pathway and the Autophagy-Lysosome Pathway. International Journal of Molecular Sciences. 2022; 23(3):1131. https://doi.org/10.3390/ijms23031131
Chicago/Turabian StyleLiu, Pinduo, Anping Wu, Hui Li, Jun Zhang, Junjun Ni, Zhenzhen Quan, and Hong Qing. 2022. "Rab21 Protein Is Degraded by Both the Ubiquitin-Proteasome Pathway and the Autophagy-Lysosome Pathway" International Journal of Molecular Sciences 23, no. 3: 1131. https://doi.org/10.3390/ijms23031131
APA StyleLiu, P., Wu, A., Li, H., Zhang, J., Ni, J., Quan, Z., & Qing, H. (2022). Rab21 Protein Is Degraded by Both the Ubiquitin-Proteasome Pathway and the Autophagy-Lysosome Pathway. International Journal of Molecular Sciences, 23(3), 1131. https://doi.org/10.3390/ijms23031131




