Investigating the Influence of GABA Neurons on Dopamine Neurons in the Ventral Tegmental Area Using Optogenetic Techniques
Abstract
:1. Introduction
2. Results
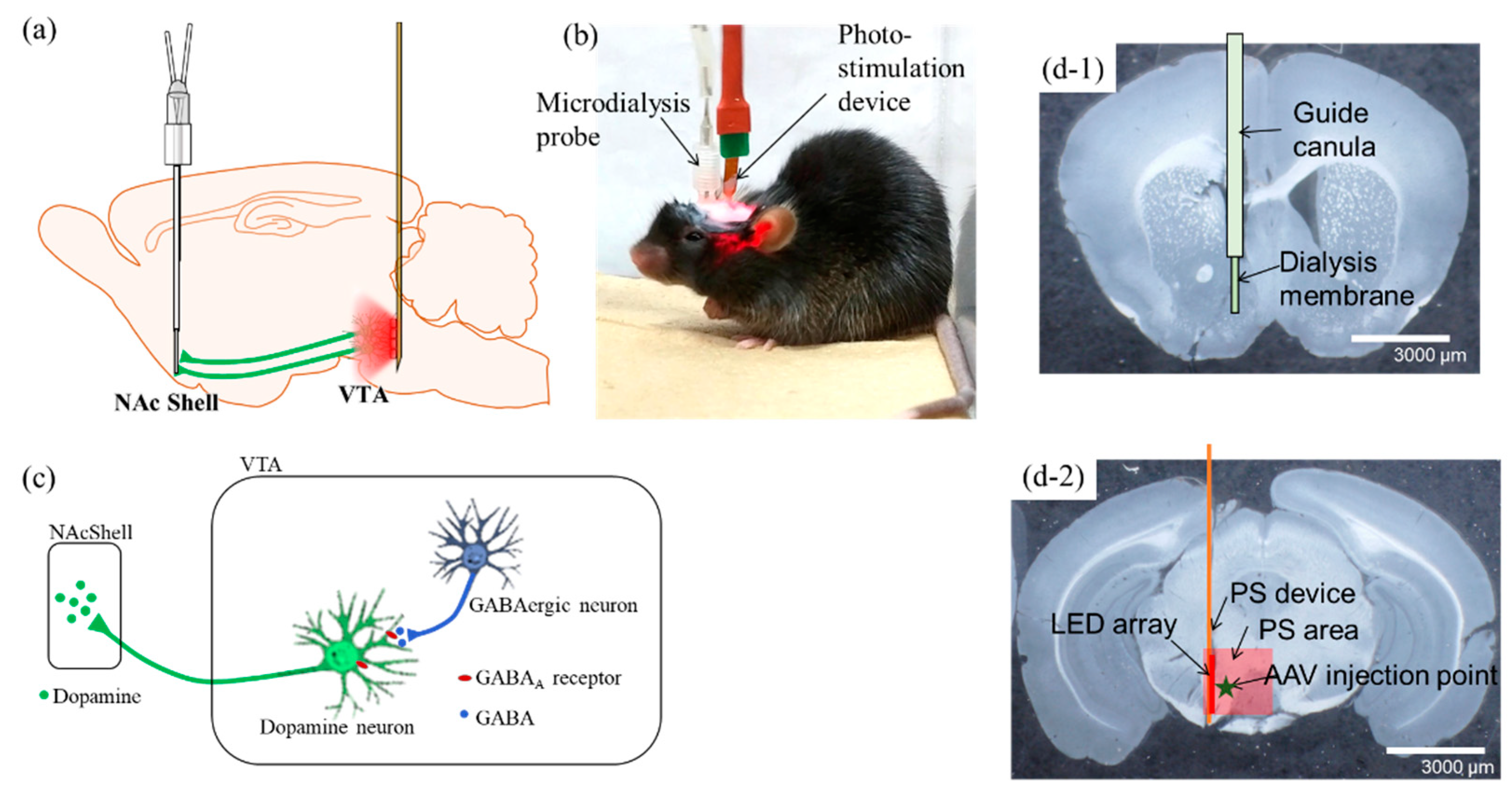
2.1. Investigation of Mesolimbic DA Neural Effects Using Optogenetics Techniques
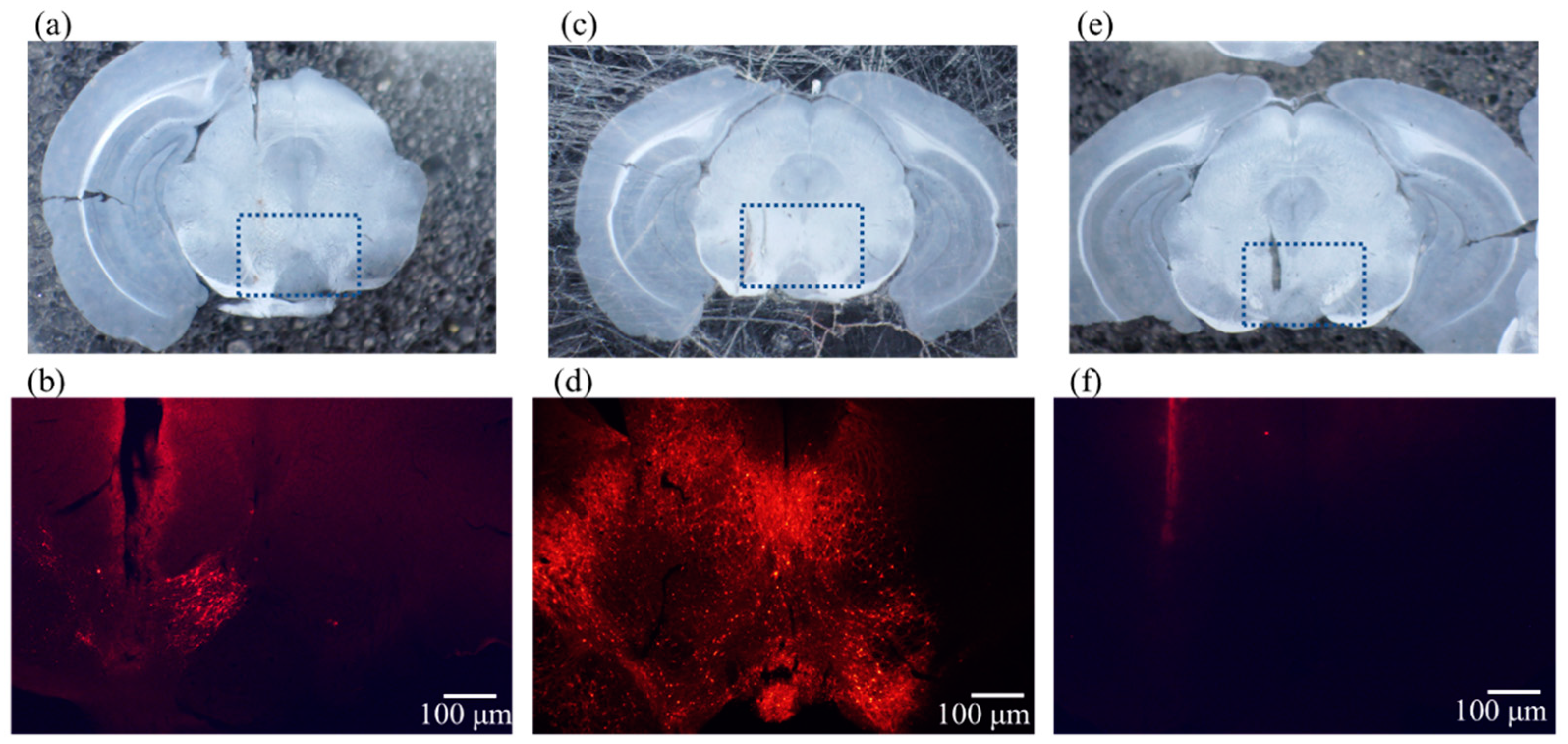
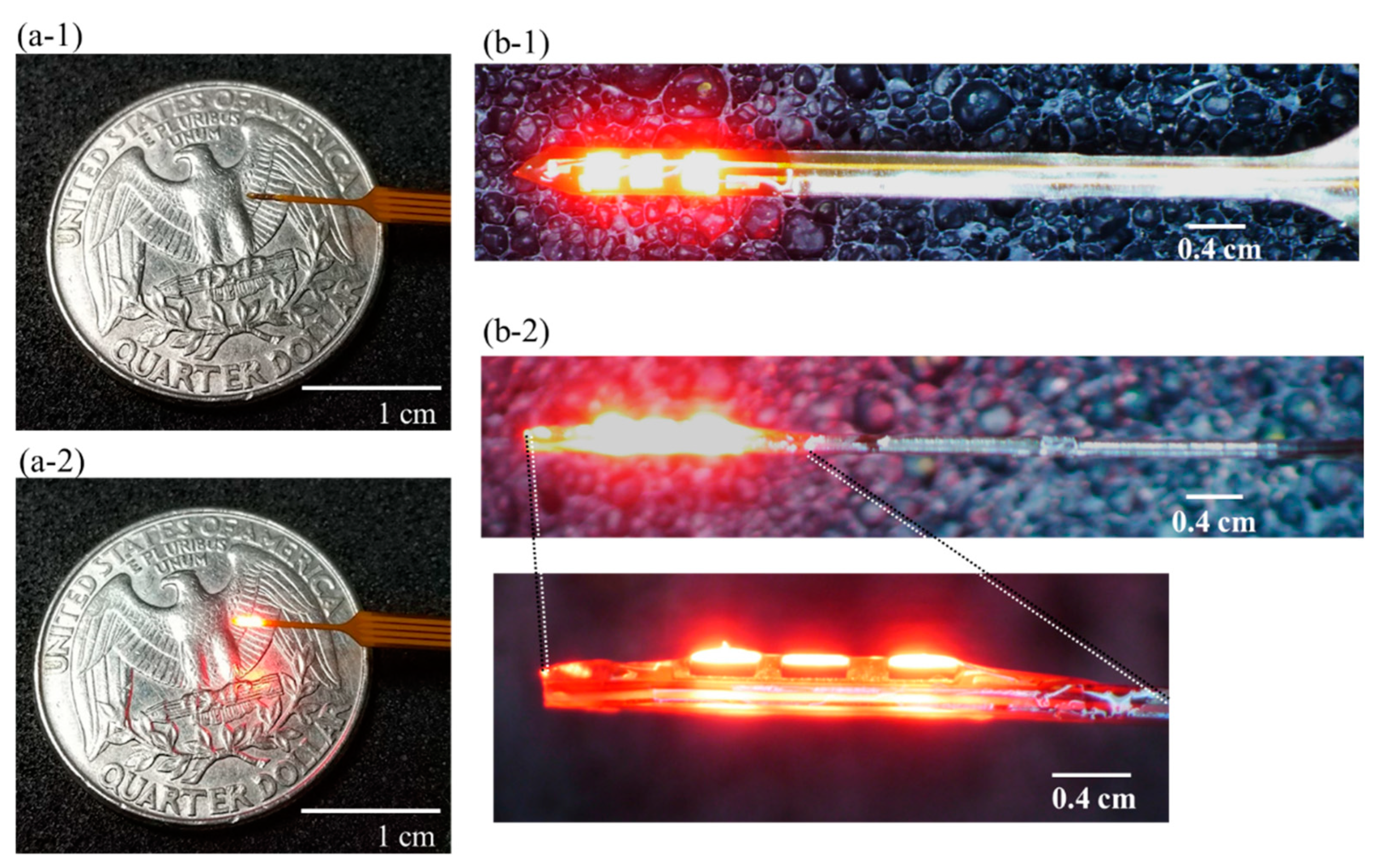
2.2. Expression Results of AAV-ChrimsonR
2.3. Optogenetics-Microdialysis Experiment
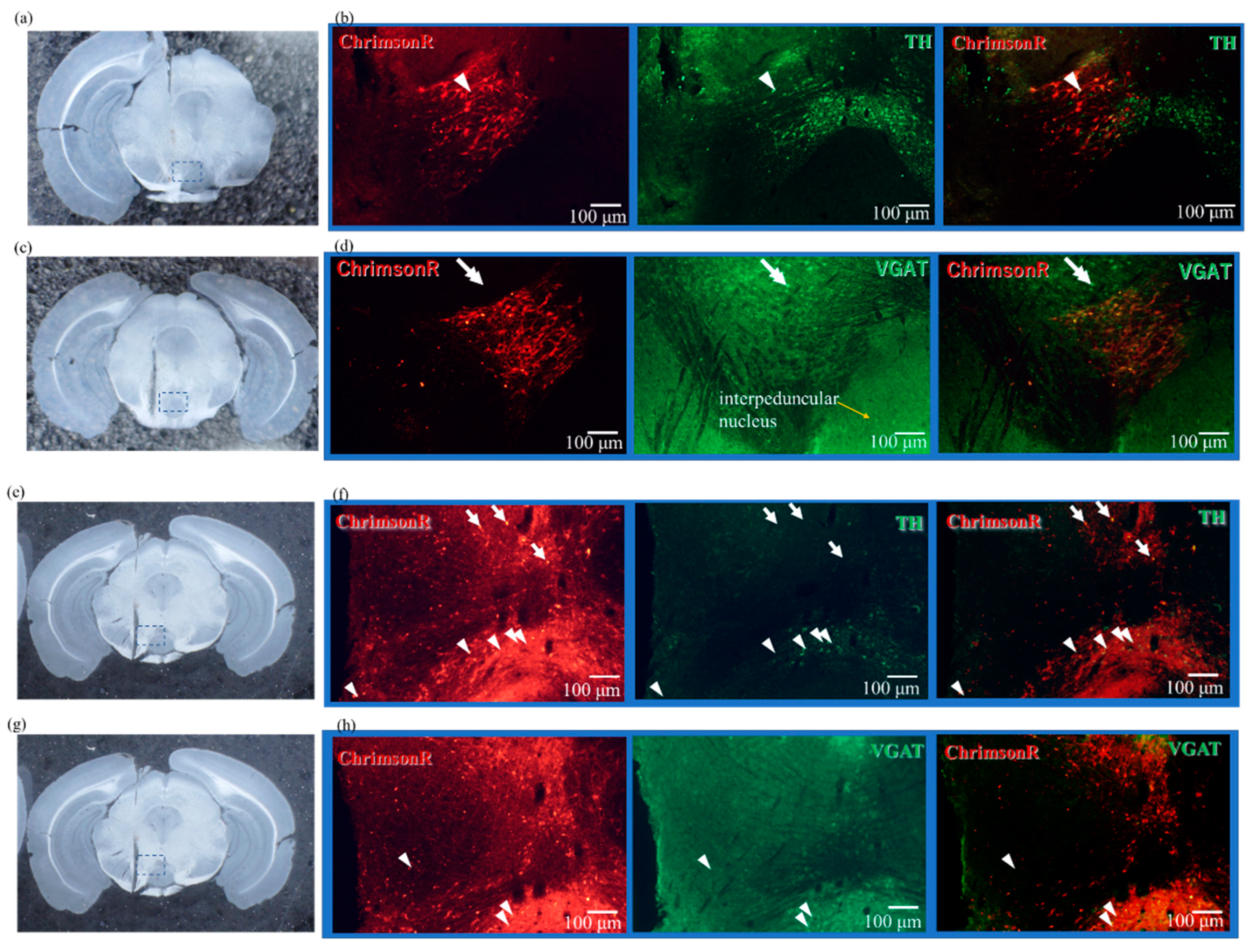
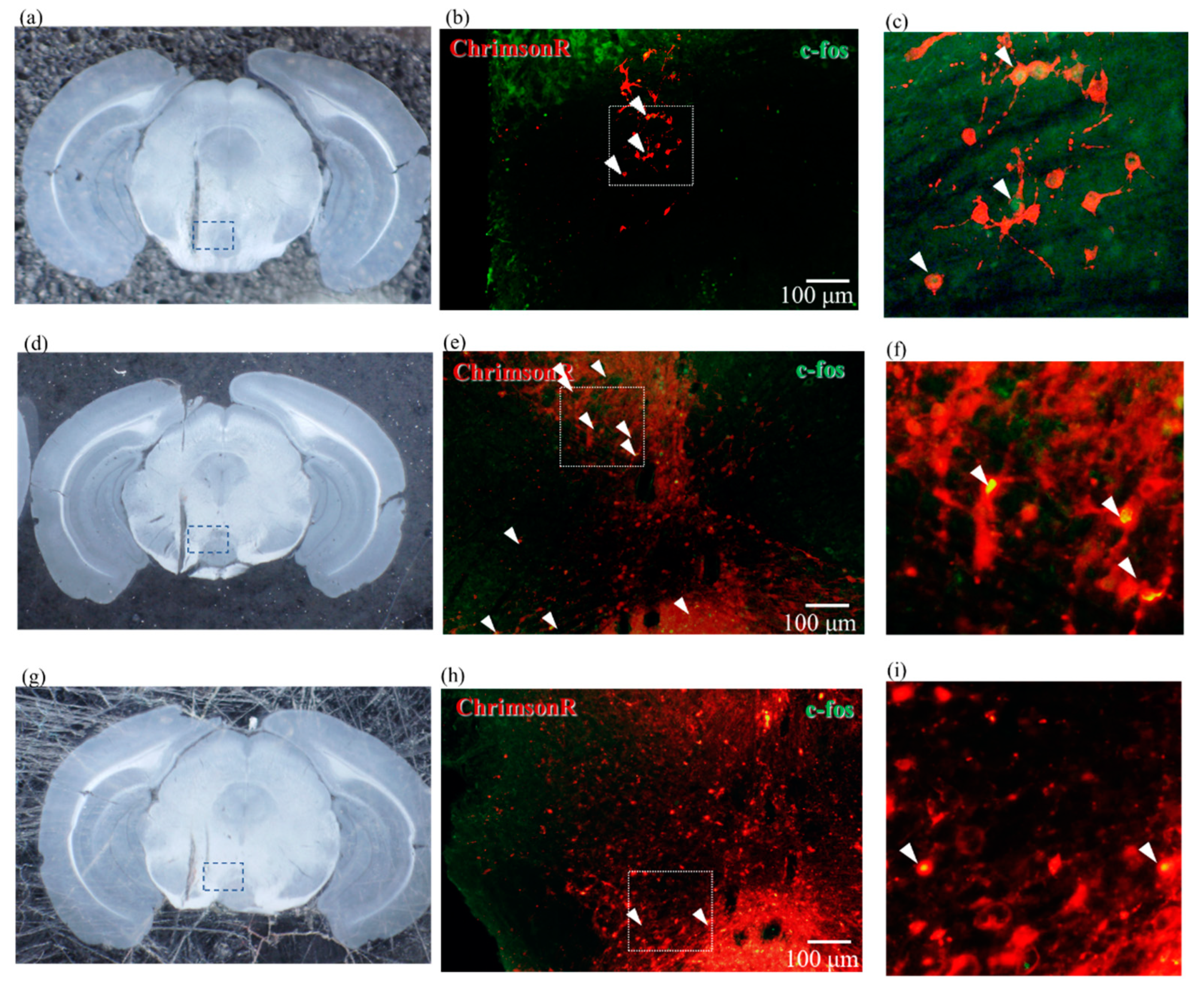
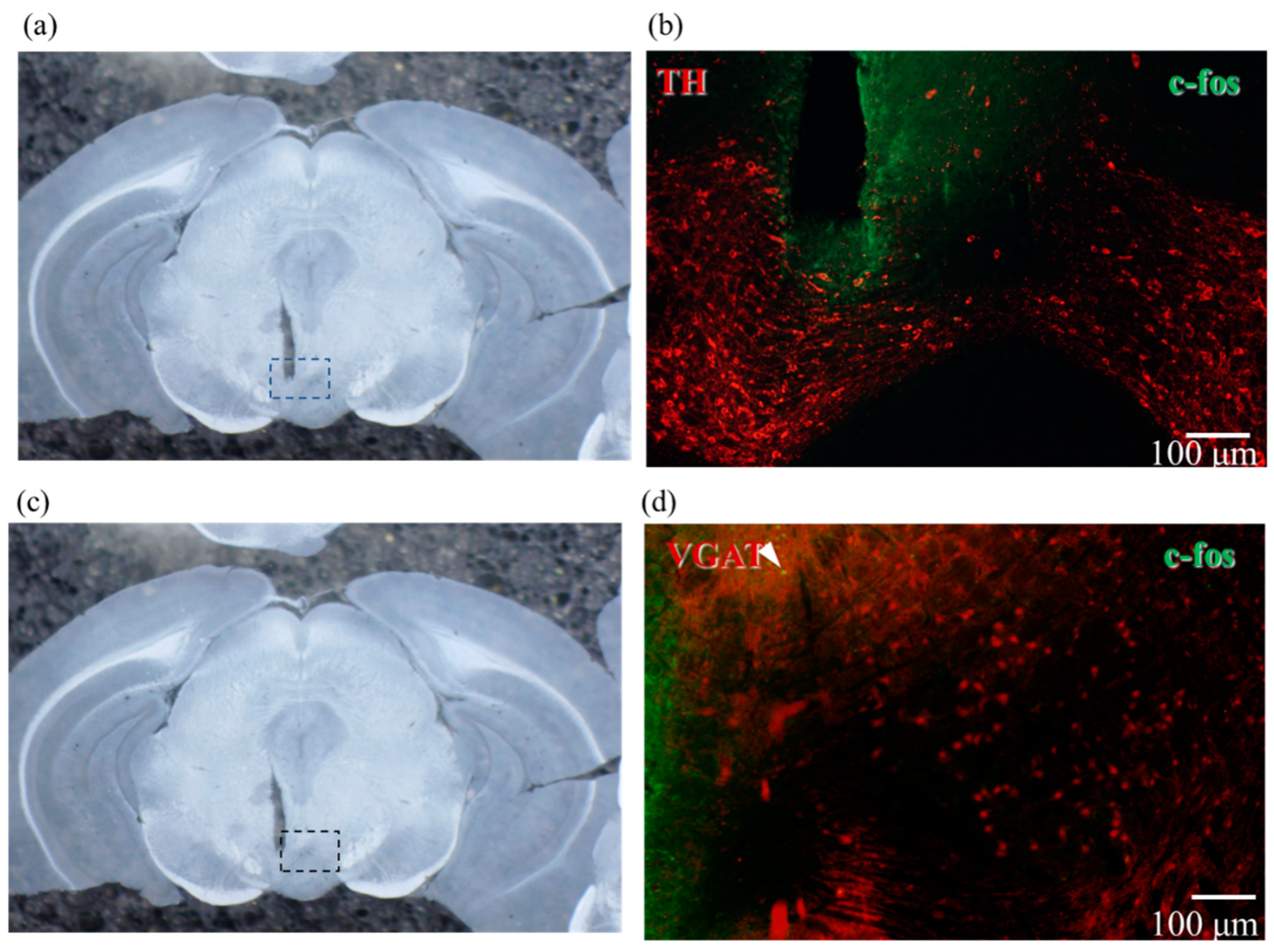
2.4. Results of Immunostaining Experiments for Expression of ChrimsonR in VTA
2.5. Confirmation of Neural Activity with C-Fos Antibody
3. Discussion
4. Materials and Methods
4.1. Ethics Statement and Animal Protocol
4.2. Photo-Stimulation (PS) Device and Fabrication
4.3. Stereotaxic Surgery
4.4. PS and Microdialysis
4.5. Experiments of PS and Bicuculline Injection
4.6. Immunostaining
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, K.A.; Gobrogge, K.L.; Wang, Z.X. The role of mesocorticolimbic dopamine in regulating interactions between drugs of abuse and social behavior. Neurosci. Biobehav. R 2011, 35, 498–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niehaus, J.L.; Cruz-Bermúdez, N.D.; Kauer, J.A. Plasticity of Addiction: A Mesolimbic Dopamine Short-Circuit? Am. J. Addict. 2009, 18, 259–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Fowler, J.S.; Wang, G.-J.; Swanson, J.M.; Telang, F. Dopamine in Drug Abuse and Addiction. Arch. Neurol. 2007, 64, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, A.; Akay, Y.M.; Zhang, D.; Akay, M. Ventral Tegmental Area Dopamine Neurons Firing Model Reveals Prenatal Nicotine Induced Alterations. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1387–1396. [Google Scholar] [CrossRef]
- Benwell, M.E.; Balfour, D.J. Regional variation in the effects of nicotine on catecholamine overflow in rat brain. Eur. J. Pharmacol. 1997, 325, 13–20. [Google Scholar] [CrossRef]
- Mereu, G.; Yoon, K.-W.P.; Boi, V.; Gessa, G.L.; Naes, L.; Westfall, T.C. Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. Eur. J. Pharmacol. 1987, 141, 395–399. [Google Scholar] [CrossRef]
- Kane, V.B.; Fu, Y.; Matta, S.G.; Sharp, B.M. Gestational Nicotine Exposure Attenuates Nicotine-Stimulated Dopamine Release in the Nucleus Accumbens Shell of Adolescent Lewis Rats. J. Pharmacol. Exp. Ther. 2004, 308, 521–528. [Google Scholar] [CrossRef]
- Roguski, E.E.; Sharp, B.M.; Chen, H.; Matta, S.G. Full-gestational exposure to nicotine and ethanol augments nicotine self-administration by altering ventral tegmental dopaminergic function due to NMDA receptors in adolescent rats. J. Neurochem. 2014, 128, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Gold, A.B.; Keller, A.B.; Perry, D.C. Prenatal exposure of rats to nicotine causes persistent alterations of nicotinic cholinergic receptors. Brain Res. 2009, 1250, 88–100. [Google Scholar] [CrossRef] [Green Version]
- Pierce, R.C.; Kumaresan, V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neurosci. Biobehav. Rev. 2006, 30, 215–238. [Google Scholar] [CrossRef]
- Feduccia, A.A.; Chatterjee, S.; Bartlett, S.E. Neuronal nicotinic acetylcholine receptors: Neuroplastic changes underlying alcohol and nicotine addictions. Front. Mol. Neurosci. 2012, 5, 83. [Google Scholar] [CrossRef] [Green Version]
- Lüscher, C.; Malenka, R.C. Drug-Evoked Synaptic Plasticity in Addiction: From Molecular Changes to Circuit Remodeling. Neuron 2011, 69, 650–663. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-L.; Qi, J.; Zhang, S.; Morales, M.I.P. Rewarding Effects of Optical Stimulation of Ventral Tegmental Area Glutamatergic Neurons. J. Neurosci. 2015, 35, 15948–15954. [Google Scholar] [CrossRef] [Green Version]
- Koyama, S.; Kawaharada, M.; Terai, H.; Ohkurano, M.; Mori, M.; Kanamaru, S.; Hirose, S. Obesity decreases excitability of putative ventral tegmental area GABAergic neurons. Physiol. Rep. 2013, 1, e00126. [Google Scholar] [CrossRef] [Green Version]
- Lowes, D.C.; Chamberlin, L.A.; Kretsge, L.N.; Holt, E.S.; Abbas, A.I.; Park, A.J.; Yusufova, L.; Bretton, Z.H.; Firdous, A.; Enikolopov, A.G.; et al. Ventral tegmental area GABA neurons mediate stress-induced blunted reward-seeking in mice. Nat. Commun. 2021, 12, 3539. [Google Scholar] [CrossRef]
- Leggio, G.M.; Di Marco, R.; Gulisano, W.; D’Ascenzo, M.; Torrisi, S.A.; Geraci, F.; Lavanco, G.; Dahl, K.; Giurdanella, G.; Castorina, A.; et al. Dopaminergic-GABAergic interplay and alcohol binge drinking. Pharmacol. Res. 2019, 141, 384–391. [Google Scholar] [CrossRef]
- Sunaga, Y.; Ohta, Y.; Murakami, T.E.; Akay, Y.M.; Ohta, J.; Akay, M. Monitoring Neuronal Dynamics in the Ventral Tegmental Area Using an Implantable Microimaging Device with Microdialysis System. IEEE Access 2021, 9, 55871–55878. [Google Scholar] [CrossRef]
- Ohta, Y.; Guinto, M.C.; Tokuda, T.; Kawahara, M.; Haruta, M.; Takehara, H.; Tashiro, H.; Sasagawa, K.; Onoe, H.; Yamaguchi, R.; et al. Micro-LED Array-Based Photo-Stimulation Devices for Optogenetics in Rat and Macaque Monkey Brains. IEEE Access 2021, 9, 127937–127949. [Google Scholar] [CrossRef]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2009, 13, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.J.; Zeng, H. Genetic Approaches to Neural Circuits in the Mouse. Annu. Rev. Neurosci. 2013, 36, 183–215. [Google Scholar] [CrossRef]
- Johnston, G.A. Advantages of an antagonist: Bicuculline and other GABA antagonists. Br. J. Pharmacol. 2013, 169, 328–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunaga, Y.; Ohta, Y.; Akay, Y.M.; Ohta, J.; Akay, M. Monitoring Neural Activities in the VTA in Response to Nicotine Intake Using a Novel Implantable Microimaging Device. IEEE Access 2020, 8, 68013–68020. [Google Scholar] [CrossRef]
- Rebusi, R.J.; Olorocisimo, J.P.; Briones, J.; Ohta, Y.; Haruta, M.; Takehara, H.; Tashiro, H.; Sasagawa, K.; Ohta, J. Simultaneous CMOS-Based Imaging of Calcium Signaling of the Central Amygdala and the Dorsal Raphe Nucleus during Nociception in Freely Moving Mice. Front. Neurosci. 2021, 15, 582. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Takagi, Y.; Kaneko, S.; Kurosawa, T. Effect of Three Types of Mixed Anesthetic Agents Alternate to Ketamine in Mice. Exp. Anim. 2011, 60, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brioni, J.D.; McGaugh, J.L. Post-training administration of GABAergic antagonists enhances retention of aversively motivated tasks. Psychopharmacology 1988, 96, 505–510. [Google Scholar] [CrossRef] [PubMed]
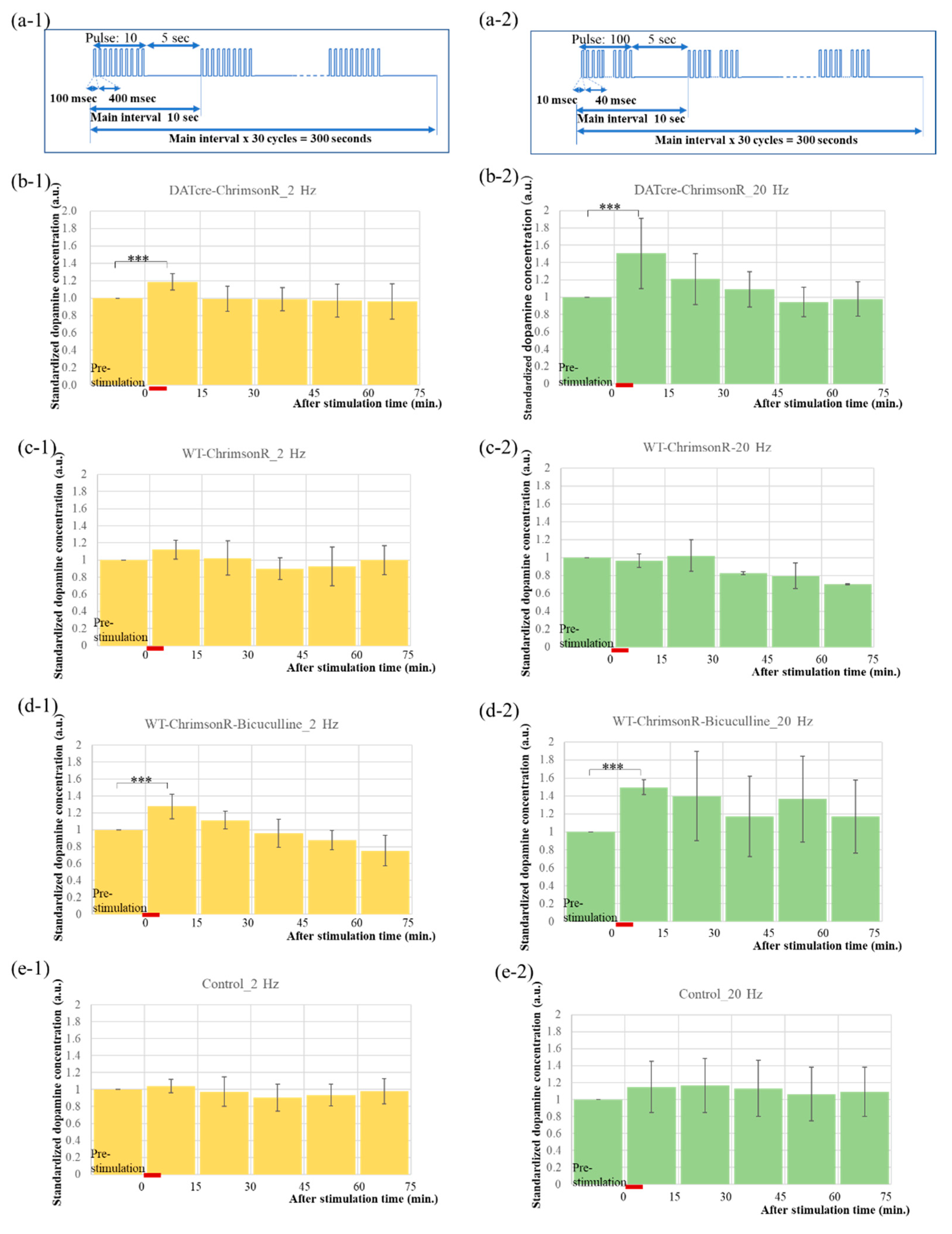
| Time (min) | DAT-ChrimsonR-2 Hz | DAT-ChrimsonR-20 Hz | WT-ChrimsonR-2 Hz | WT-ChrimsonR-20 Hz | WT-ChrimsonR-Bicuculline-2 Hz | WT-ChrimsonR-Bicuculline-20 Hz | Control-2 Hz | Control-20 Hz | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 7 | N = 6 | N = 3 | N = 3 | N = 4 | N = 3 | N = 8 | N = 6 | |||||||||
| Ave | STD | Ave | STD | Ave | STD | Ave | STD | Ave | STD | Ave | STD | Ave | STD | Ave | STD | |
| 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| 15 | 1.187 | 0.096 | 1.506 | 0.405 | 1.120 | 0.112 | 0.967 | 0.075 | 1.276 | 0.146 | 1.47 | 0.083 | 1.040 | 0.080 | 1.148 | 0.301 |
| 30 | 0.994 | 0.145 | 1.208 | 0.293 | 1.023 | 0.201 | 1.021 | 0.175 | 1.114 | 0.105 | 1.400 | 0.495 | 0.976 | 0.176 | 1.166 | 0.321 |
| 45 | 0.987 | 0.132 | 1.091 | 0.205 | 0.899 | 0.129 | 0.823 | 0.017 | 0.958 | 0.166 | 1.172 | 0.447 | 0.905 | 0.160 | 1.133 | 0.331 |
| 60 | 0.970 | 0.189 | 0.944 | 0.170 | 0.926 | 0.226 | 0.795 | 0.142 | 0.878 | 0.115 | 1.364 | 0.475 | 0.936 | 0.128 | 1.065 | 0.315 |
| 75 | 0.963 | 0.204 | 0.978 | 0.199 | 1.010 | 0.169 | 0.702 | 0.006 | 0.754 | 0.180 | 1.171 | 0.407 | 0.978 | 0.149 | 1.092 | 0.291 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohta, Y.; Murakami, T.E.; Kawahara, M.; Haruta, M.; Takehara, H.; Tashiro, H.; Sasagawa, K.; Ohta, J.; Akay, M.; Akay, Y.M. Investigating the Influence of GABA Neurons on Dopamine Neurons in the Ventral Tegmental Area Using Optogenetic Techniques. Int. J. Mol. Sci. 2022, 23, 1114. https://doi.org/10.3390/ijms23031114
Ohta Y, Murakami TE, Kawahara M, Haruta M, Takehara H, Tashiro H, Sasagawa K, Ohta J, Akay M, Akay YM. Investigating the Influence of GABA Neurons on Dopamine Neurons in the Ventral Tegmental Area Using Optogenetic Techniques. International Journal of Molecular Sciences. 2022; 23(3):1114. https://doi.org/10.3390/ijms23031114
Chicago/Turabian StyleOhta, Yasumi, Takaaki E. Murakami, Mamiko Kawahara, Makito Haruta, Hironari Takehara, Hiroyuki Tashiro, Kiyotaka Sasagawa, Jun Ohta, Metin Akay, and Yasemin M. Akay. 2022. "Investigating the Influence of GABA Neurons on Dopamine Neurons in the Ventral Tegmental Area Using Optogenetic Techniques" International Journal of Molecular Sciences 23, no. 3: 1114. https://doi.org/10.3390/ijms23031114
APA StyleOhta, Y., Murakami, T. E., Kawahara, M., Haruta, M., Takehara, H., Tashiro, H., Sasagawa, K., Ohta, J., Akay, M., & Akay, Y. M. (2022). Investigating the Influence of GABA Neurons on Dopamine Neurons in the Ventral Tegmental Area Using Optogenetic Techniques. International Journal of Molecular Sciences, 23(3), 1114. https://doi.org/10.3390/ijms23031114










