Current and New Challenges in the Management of Pancreatic Neuroendocrine Tumors: The Role of miRNA-Based Approaches as New Reliable Biomarkers
Abstract
:1. Introduction
2. Current Diagnostic and Therapeutic Strategies in PanNETs
2.1. Diagnostic
2.2. Treatment
2.2.1. Surgery
2.2.2. Somatostatin Analogs
2.2.3. Other Systemic Therapies
2.2.4. Peptide Receptor Radionuclide Therapy
3. Microbiome and Microbiota in PanNETs
4. Circulating Tumor Cells in PanNETs
5. Genetic Insights in PanNETs
6. MiRNA-Based Epigenetic Challenges in PanNETs
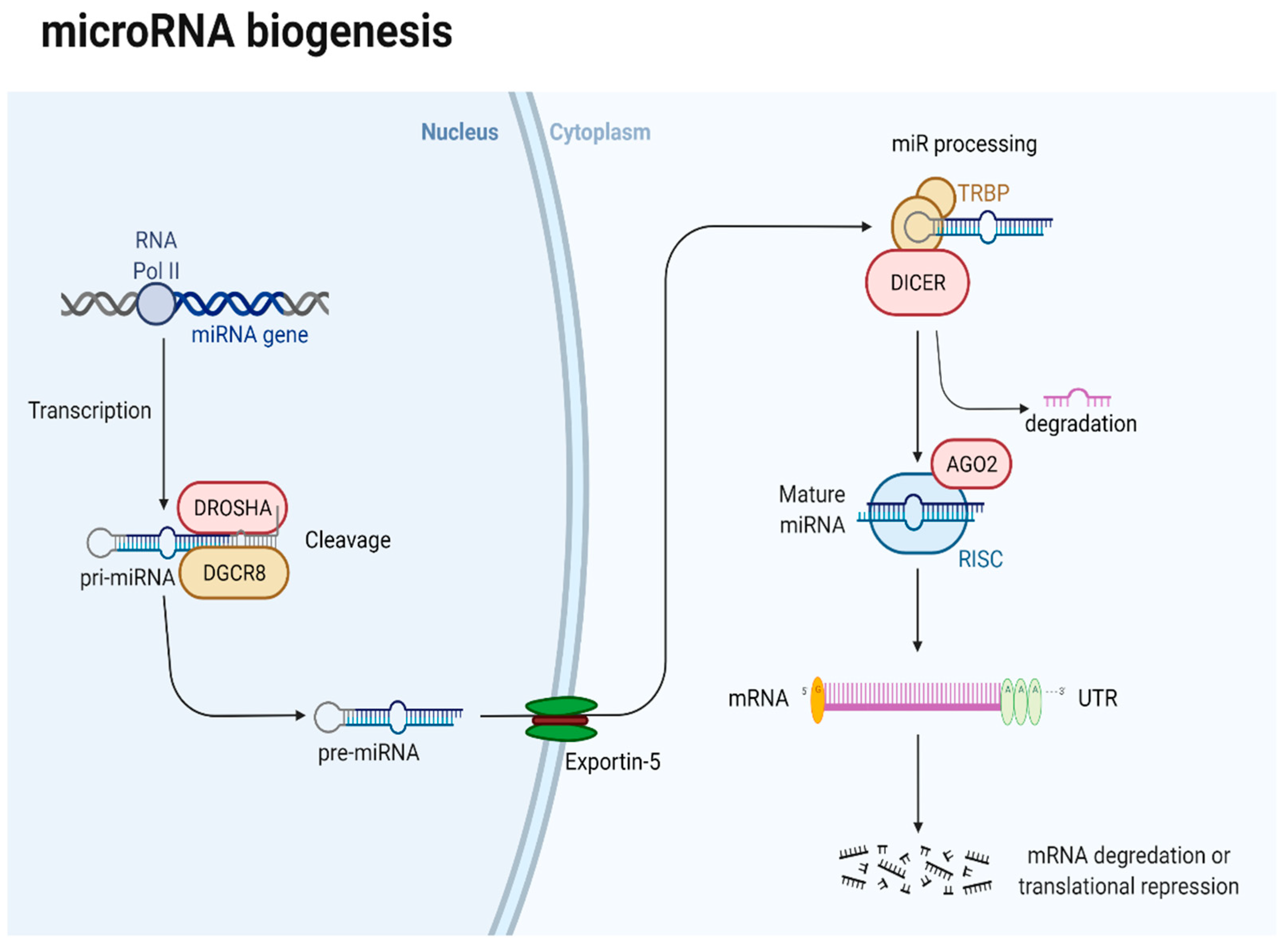
6.1. Brief Overview of miRNA in PanNETs
6.2. Clinical Implications of miRNAs in PanNETs
6.2.1. The Prognostic Role of miRNAs in PanNETs
6.2.2. The Diagnostic Role of miRNAs in PanNETs
6.2.3. The Therapeutic Role of miRNAs in PanNETs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, M.R.; Harris, C.; Baeg, K.J.; Aronson, A.; Wisnivesky, J.P.; Kim, M.K. Incidence Trends of Gastroenteropancreatic Neuroendocrine Tumors in the United States. Clin. Gastroenterol. Hepatol. 2019, 17, 2212–2217.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, J.; Kim, K.W.; Kim, H.J.; Kim, D.W.; Kim, K.P.; Hong, S.-M.; Ryu, J.-S.; Tirumani, S.H.; Krajewski, K.; Ramaiya, N. What Is New in the 2017 World Health Organization Classification and 8th American Joint Committee on Cancer Staging System for Pancreatic Neuroendocrine Neoplasms? Korean J. Radiol. 2019, 20, 5. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Rabe, K.G.; Rubin, J.; Petersen, G.M. Pancreatic neuroendocrine tumors (PNETs): Incidence, prognosis and recent trend toward improved survival. Ann. Oncol. 2008, 19, 1727–1733. [Google Scholar] [CrossRef]
- Lesén, E.; Granfeldt, D.; Houchard, A.; Berthon, A.; Dinet, J.; Gabriel, S.; Björstad, Å.; Björholt, I.; Elf, A.K.; Johanson, V. Cost-of-illness of metastatic gastroenteropancreatic neuroendocrine tumours in Sweden—A population-based register-linkage study. Eur. J. Cancer Care 2019, 28, e12983. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kowdley, K.V. MicroRNAs in Common Human Diseases. Genomics. Proteom. Bioinform. 2012, 10, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.W.; Kim, M.K.; Kim, H.G. Diagnosis of Pancreatic Neuroendocrine Tumors. Clin. Endosc. 2017, 50, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Reubi, J.C. Peptide receptor expression in GEP-NET. Virchows Arch. 2007, 451, 47–50. [Google Scholar] [CrossRef]
- Ito, T.; Jensen, R.T. Molecular imaging in neuroendocrine tumors. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 71, 199–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.; Ito, T.; Jensen, R.T. Imaging of pancreatic neuroendocrine tumors: Recent advances, current status, and controversies. Expert Rev. Anticancer Ther. 2018, 18, 837–860. [Google Scholar] [CrossRef] [PubMed]
- Campana, D.; Ambrosini, V.; Pezzilli, R.; Fanti, S.; Labate, A.M.M.; Santini, D.; Ceccarelli, C.; Nori, F.; Franchi, R.; Corinaldesi, R.; et al. Standardized Uptake Values of 68Ga-DOTANOC PET: A Promising Prognostic Tool in Neuroendocrine Tumors. J. Nucl. Med. 2010, 51, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Ambrosini, V.; Campana, D.; Polverari, G.; Peterle, C.; Diodato, S.; Ricci, C.; Allegri, V.; Casadei, R.; Tomassetti, P.; Fanti, S. Prognostic Value of 68Ga-DOTANOC PET/CT SUVmax in Patients with Neuroendocrine Tumors of the Pancreas. J. Nucl. Med. 2015, 56, 1843–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panagiotidis, E.; Alshammari, A.; Michopoulou, S.; Skoura, E.; Naik, K.; Maragkoudakis, E.; Mohmaduvesh, M.; Al-Harbi, M.; Belda, M.; Caplin, M.E.; et al. Comparison of the Impact of 68 Ga-DOTATATE and 18 F-FDG PET/CT on Clinical Management in Patients with Neuroendocrine Tumors. J. Nucl. Med. 2017, 58, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, K.; Czernin, J.; Wolin, E.M.; Gupta, P.; Barrio, M.; Gutierrez, A.; Schiepers, C.; Mosessian, S.; Phelps, M.E.; Allen-Auerbach, M.S. Impact of 68Ga-DOTATATE PET/CT on the Management of Neuroendocrine Tumors: The Referring Physician’s Perspective. J. Nucl. Med. 2015, 56, 70–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calais, J.; Czernin, J.; Eiber, M.; Fendler, W.P.; Gartmann, J.; Heaney, A.P.; Hendifar, A.E.; Pisegna, J.R.; Hecht, J.R.; Wolin, E.M.; et al. Most of the Intended Management Changes After 68 Ga-DOTATATE PET/CT Are Implemented. J. Nucl. Med. 2017, 58, 1793–1796. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yang, Y.; Li, Z.; Cheng, C.; Yang, T.; Wang, C.; Liu, L.; Liu, S. Diagnostic Value of Circulating Chromogranin A for Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0124884. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Zhang, C.; Tang, M.; Xu, X.; Liu, L.; Ji, Y.; Pan, B.; Lou, W. The value of serum chromogranin A as a predictor of tumor burden, therapeutic response, and nomogram-based survival in well-moderate nonfunctional pancreatic neuroendocrine tumors with liver metastases. Eur. J. Gastroenterol. Hepatol. 2015, 27, 527–535. [Google Scholar] [CrossRef]
- Ma, Z.-Y.; Gong, Y.-F.; Zhuang, H.-K.; Zhou, Z.-X.; Huang, S.-Z.; Zou, Y.-P.; Huang, B.-W.; Sun, Z.-H.; Zhang, C.-Z.; Tang, Y.-Q.; et al. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J. Gastroenterol. 2020, 26, 2305–2322. [Google Scholar] [CrossRef]
- Yao, J.C.; Pavel, M.; Phan, A.T.; Kulke, M.H.; Hoosen, S.; St. Peter, J.; Cherfi, A.; Öberg, K.E. Chromogranin A and Neuron-Specific Enolase as Prognostic Markers in Patients with Advanced pNET Treated with Everolimus. J. Clin. Endocrinol. Metab. 2011, 96, 3741–3749. [Google Scholar] [CrossRef] [Green Version]
- Bocchini, M.; Nicolini, F.; Severi, S.; Bongiovanni, A.; Ibrahim, T.; Simonetti, G.; Grassi, I.; Mazza, M. Biomarkers for Pancreatic Neuroendocrine Neoplasms (PanNENs) Management—An Updated Review. Front. Oncol. 2020, 10, 831. [Google Scholar] [CrossRef]
- Vinik, A.; Perry, R.R.; Casellini, C.; Hughes, M.S.; Feliberti, E. Pathophysiology and Treatment of Pancreatic Neuroendocrine Tumors (PNETs): New Developments; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Scott, A.T.; Breheny, P.J.; Keck, K.J.; Bellizzi, A.M.; Dillon, J.S.; O’Dorisio, T.M.; Howe, J.R. Effective cytoreduction can be achieved in patients with numerous neuroendocrine tumor liver metastases (NETLMs). Surgery 2019, 165, 166–175. [Google Scholar] [CrossRef]
- Rindi, G.; Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Klöppel, G.; et al. Consensus Guidelines Update for the Management of Functional p-NETs (F-p-NETs) and Non-Functional p-NETs (NF-p-NETs). Neuroendocrinology 2016, 103, 153. [Google Scholar] [CrossRef] [Green Version]
- Partelli, S.; Mazza, M.; Andreasi, V.; Muffatti, F.; Crippa, S.; Tamburrino, D.; Falconi, M. Management of small asymptomatic nonfunctioning pancreatic neuroendocrine tumors: Limitations to apply guidelines into real life. Surgery 2019, 166, 157–163. [Google Scholar] [CrossRef]
- Sadot, E.; Reidy-Lagunes, D.L.; Tang, L.H.; Do, R.K.G.; Gonen, M.; D’Angelica, M.I.; DeMatteo, R.P.; Kingham, T.P.; Koerkamp, B.G.; Untch, B.R.; et al. Observation versus Resection for Small Asymptomatic Pancreatic Neuroendocrine Tumors: A Matched Case–Control Study. Ann. Surg. Oncol. 2016, 23, 1361–1370. [Google Scholar] [CrossRef] [Green Version]
- Perri, G.; Prakash, L.R.; Katz, M.H.G. Pancreatic neuroendocrine tumors. Curr. Opin. Gastroenterol. 2019, 35, 468–477. [Google Scholar] [CrossRef]
- Jilesen, A.P.J.; van Eijck, C.H.J.; Busch, O.R.C.; van Gulik, T.M.; Gouma, D.J.; van Dijkum, E.J.M.N. Postoperative Outcomes of Enucleation and Standard Resections in Patients with a Pancreatic Neuroendocrine Tumor. World J. Surg. 2016, 40, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Heckler, M.; Mihaljevic, A.L.; Probst, P.; Klaiber, U.; Heger, U.; Schimmack, S.; Büchler, M.W.; Hackert, T. Systematic Review and Metaanalysis of Lymph Node Metastases of Resected Pancreatic Neuroendocrine Tumors. Ann. Surg. Oncol. 2020, 28, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Nakashima, A.; Kondo, N.; Sakabe, R.; Ohge, H.; Sueda, T. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann. Surg. Oncol. 2011, 18, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Jutric, Z.; Grendar, J.; Hoen, H.M.; Cho, S.W.; Cassera, M.A.; Newell, P.H.; Hammill, C.W.; Hansen, P.D.; Wolf, R.F. Regional Metastatic Behavior of Nonfunctional Pancreatic Neuroendocrine Tumors. Pancreas 2017, 46, 898–903. [Google Scholar] [CrossRef]
- Fairweather, M.; Swanson, R.; Wang, J.; Brais, L.K.; Dutton, T.; Kulke, M.H.; Clancy, T.E. Management of Neuroendocrine Tumor Liver Metastases: Long-Term Outcomes and Prognostic Factors from a Large Prospective Database. Ann. Surg. Oncol. 2017, 24, 2319–2325. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, J.; Xiu, D.; Tao, M.; Ma, Z.; Jiang, B.; Li, Z.; Li, L.; Wang, L.; Wang, H.; et al. Meta-analysis of Liver Resection Versus Nonsurgical Treatments for Pancreatic Neuroendocrine Tumors with Liver Metastases. Ann. Surg. Oncol. 2016, 23, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; O’’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.-F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Zheng, M.; Li, Y.; Li, T.; Zhang, L.; Zhou, L. Resection of the primary tumor improves survival in patients with gastro-entero-pancreatic neuroendocrine neoplasms with liver metastases: A SEER-based analysis. Cancer Med. 2019, 8, 5128–5136. [Google Scholar] [CrossRef] [Green Version]
- Cloyd, J.M.; Wiseman, J.T.; Pawlik, T.M. Surgical management of pancreatic neuroendocrine liver metastases. J. Gastrointest. Oncol. 2020, 11, 590–600. [Google Scholar] [CrossRef]
- Gomes-Porras, M.; Cárdenas-Salas, J.; Álvarez-Escolá, C. Somatostatin Analogs in Clinical Practice: A Review. Int. J. Mol. Sci. 2020, 21, 1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vezzosi, D.; Bennet, A.; Rochaix, P.; Courbon, F.; Selves, J.; Pradere, B.; Buscail, L.; Susini, C.; Caron, P. Octreotide in insulinoma patients: Efficacy on hypoglycemia, relationships with Octreoscan scintigraphy and immunostaining with anti-sst2A and anti-sst5 antibodies. Eur. J. Endocrinol. 2005, 152, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Kulke, M.H.; Hörsch, D.; Caplin, M.E.; Anthony, L.B.; Bergsland, E.; Öberg, K.; Welin, S.; Warner, R.R.P.; Lombard-Bohas, C.; Kunz, P.L.; et al. Telotristat Ethyl, a Tryptophan Hydroxylase Inhibitor for the Treatment of Carcinoid Syndrome. J. Clin. Oncol. 2017, 35, 14–23. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Rinke, A.; Müller, H.-H.; Schade-Brittinger, C.; Klose, K.-J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.-F.; Bläker, M.; et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients With Metastatic Neuroendocrine Midgut Tumors: A Report From the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, M.; Lombard-Bohas, C.; Cadiot, G.; Matysiak-Budnik, T.; Rebours, V.; Vullierme, M.-P.; Couvelard, A.; Hentic, O.; Ruszniewski, P. Ki67 proliferation index, hepatic tumor load, and pretreatment tumor growth predict the antitumoral efficacy of lanreotide in patients with malignant digestive neuroendocrine tumors. Eur. J. Gastroenterol. Hepatol. 2013, 25, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Bayonas, A.; Jiménez-Fonseca, P.; Lamarca, Á.; Barriuso, J.; Castaño, Á.; Benavent, M.; Alonso, V.; del Carmen Riesco-Martínez, M.; Alonso-Gordoa, T.; Custodio, A.; et al. Prediction of Progression-Free Survival in Patients with Advanced, Well-Differentiated, Neuroendocrine Tumors Being Treated with a Somatostatin Analog: The GETNE-TRASGU Study. J. Clin. Oncol. 2019, 37, 2571–2580. [Google Scholar] [CrossRef]
- Yao, J.C.; Pavel, M.; Lombard-Bohas, C.; Van Cutsem, E.; Voi, M.; Brandt, U.; He, W.; Chen, D.; Capdevila, J.; de Vries, E.G.E.; et al. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers from the Randomized, Phase III RADIANT-3 Study. J. Clin. Oncol. 2016, 34, 3906–3913. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Fazio, N.; Cella, C.A.; Del Re, M.; Laffi, A.; Rubino, M.; Zagami, P.; Spada, F. Pharmacodynamics, clinical findings and approval status of current and emerging tyrosine-kinase inhibitors for pancreatic neuroendocrine tumors. Expert Opin. Drug Metab. Toxicol. 2019, 15, 993–1004. [Google Scholar] [CrossRef]
- Clewemar Antonodimitrakis, P.; Sundin, A.; Wassberg, C.; Granberg, D.; Skogseid, B.; Eriksson, B. Streptozocin and 5-Fluorouracil for the Treatment of Pancreatic Neuroendocrine Tumors: Efficacy, Prognostic Factors and Toxicity. Neuroendocrinology 2016, 103, 345–353. [Google Scholar] [CrossRef]
- Dilz, L.-M.; Denecke, T.; Steffen, I.G.; Prasad, V.; von Weikersthal, L.F.; Pape, U.-F.; Wiedenmann, B.; Pavel, M. Streptozocin/5-fluorouracil chemotherapy is associated with durable response in patients with advanced pancreatic neuroendocrine tumours. Eur. J. Cancer 2015, 51, 1253–1262. [Google Scholar] [CrossRef]
- Akirov, A.; Larouche, V.; Alshehri, S.; Asa, S.L.; Ezzat, S. Treatment Options for Pancreatic Neuroendocrine Tumors. Cancers 2019, 11, 828. [Google Scholar] [CrossRef] [Green Version]
- Kunz, P.L.; Catalano, P.J.; Nimeiri, H.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Yao, J.C.; Kulke, M.H.; Hendifar, A.E.; Shanks, J.C.; et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211). J. Clin. Oncol. 2018, 36, 4004. [Google Scholar] [CrossRef]
- van der Zwan, W.A.; Bodei, L.; Mueller-Brand, J.; de Herder, W.W.; Kvols, L.K.; Kwekkeboom, D.J. GEP–NETs UPDATE: Radionuclide therapy in neuroendocrine tumors. Eur. J. Endocrinol. 2015, 172, R1–R8. [Google Scholar] [CrossRef] [Green Version]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177 Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-Related Quality of Life in Patients with Progressive Midgut Neuroendocrine Tumors Treated With 177 Lu-Dotatate in the Phase III NETTER-1 Trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef]
- Starr, J.S.; Sonbol, M.B.; Hobday, T.J.; Sharma, A.; Kendi, A.T.; Halfdanarson, T.R. Peptide Receptor Radionuclide Therapy for the Treatment of Pancreatic Neuroendocrine Tumors: Recent Insights. Onco Targets Ther. 2020, 13, 3545–3555. [Google Scholar] [CrossRef]
- Zandee, W.T.; Brabander, T.; Blažević, A.; Kam, B.L.R.; Teunissen, J.J.M.; Feelders, R.A.; Hofland, J.; de Herder, W.W. Symptomatic and Radiological Response to 177Lu-DOTATATE for the Treatment of Functioning Pancreatic Neuroendocrine Tumors. J. Clin. Endocrinol. Metab. 2019, 104, 1336–1344. [Google Scholar] [CrossRef]
- Börnigen, D.; Morgan, X.C.; Franzosa, E.A.; Ren, B.; Xavier, R.J.; Garrett, W.S.; Huttenhower, C. Functional profiling of the gut microbiome in disease-associated inflammation. Genome Med. 2013, 5, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.R.; Bleich, R.M.; Arthur, J.C. Microbiota Effects on Carcinogenesis: Initiation, Promotion, and Progression. Annu. Rev. Med. 2021, 72, 243–261. [Google Scholar] [CrossRef]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.M.; Zhang, Z.Y.; Zhang, C.H.; Wu, J.; Wang, Y.X.; Zhang, G.X. Intestinal Microbial Community Differs between Acute Pancreatitis Patients and Healthy Volunteers. Biomed. Environ. Sci. 2018, 31, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Capurso, G.; Signoretti, M.; Archibugi, L.; Stigliano, S.; Delle Fave, G. Systematic review and meta-analysis: Small intestinal bacterial overgrowth in chronic pancreatitis. United Eur. Gastroenterol. J. 2016, 4, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Knip, M.; Honkanen, J. Modulation of Type 1 Diabetes Risk by the Intestinal Microbiome. Curr. Diab. Rep. 2017, 17, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Fuhler, G.M.; BN, N.; Jose, T.; Bruno, M.J.; Peppelenbosch, M.P.; Konstantinov, S.R. Pancreatic cyst fluid harbors a unique microbiome. Microbiome 2017, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.H.; Satagopan, J.; Xu, Y.; Ling, L.; Leong, S.; Orlow, I.; Saldia, A.; Li, P.; Nunes, P.; Madonia, V.; et al. The oral microbiota in patients with pancreatic cancer, patients with IPMNs, and controls: A pilot study. Cancer Causes Control 2017, 28, 959–969. [Google Scholar] [CrossRef]
- Gesualdo, M.; Rizzi, F.; Bonetto, S.; Rizza, S.; Cravero, F.; Saracco, G.M.; De Angelis, C.G. Pancreatic Diseases and Microbiota: A Literature Review and Future Perspectives. J. Clin. Med. 2020, 9, 3535. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, G.; You, L.; Yang, J.; Feng, M.; Qiu, J.; Zhao, F.; Liu, Y.; Cao, Z.; Zheng, L.; et al. Role of the microbiome in occurrence, development and treatment of pancreatic cancer. Mol. Cancer 2019, 18, 173. [Google Scholar] [CrossRef] [Green Version]
- Zambirinis, C.P.; Ochi, A.; Barilla, R.; Greco, S.; Deutsch, M.; Miller, G. Induction of TRIF- or MYD88-dependent pathways perturbs cell cycle regulation in pancreatic cancer. Cell Cycle 2013, 12, 1153–1154. [Google Scholar] [CrossRef] [Green Version]
- Silke, J.; O’Reilly, L.A. NF-κB and Pancreatic Cancer; Chapter and Verse. Cancers 2021, 13, 4510. [Google Scholar] [CrossRef]
- Vitale, G.; Dicitore, A.; Barrea, L.; Sbardella, E.; Razzore, P.; Campione, S.; Faggiano, A.; Colao, A.; Albertelli, M.; Altieri, B.; et al. From microbiota toward gastro-enteropancreatic neuroendocrine neoplasms: Are we on the highway to hell? Rev. Endocr. Metab. Disord. 2021, 22, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Clift, A.K.; Kidd, M.; Bodei, L.; Toumpanakis, C.; Baum, R.P.; Oberg, K.; Modlin, I.M.; Frilling, A. Neuroendocrine Neoplasms of the Small Bowel and Pancreas. Neuroendocrinology 2020, 110, 444–476. [Google Scholar] [CrossRef] [PubMed]
- Sammallahti, H.; Kokkola, A.; Rezasoltani, S.; Ghanbari, R.; Asadzadeh Aghdaei, H.; Knuutila, S.; Puolakkainen, P.; Sarhadi, V.K. Microbiota Alterations and Their Association with Oncogenomic Changes in Pancreatic Cancer Patients. Int. J. Mol. Sci. 2021, 22, 12978. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-X.; Bai, F. Single-cell analyses of circulating tumor cells. Cancer Biol. Med. 2015, 12, 184–192. [Google Scholar] [CrossRef]
- Cabel, L.; Proudhon, C.; Gortais, H.; Loirat, D.; Coussy, F.; Pierga, J.Y.; Bidard, F.C. Circulating tumor cells: Clinical validity and utility. Int. J. Clin. Oncol. 2017, 22, 421–430. [Google Scholar] [CrossRef]
- Khan, M.S.; Tsigani, T.; Rashid, M.; Rabouhans, J.S.; Yu, D.; Luong, T.V.; Caplin, M.; Meyer, T. Circulating Tumor Cells and EpCAM Expression in Neuroendocrine Tumors. Clin. Cancer Res. 2011, 17, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Kirkwood, A.; Tsigani, T.; Garcia-Hernandez, J.; Hartley, J.A.; Caplin, M.E.; Meyer, T. Circulating tumor cells as prognostic markers in neuroendocrine tumors. J. Clin. Oncol. 2013, 31, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Kirkwood, A.A.; Tsigani, T.; Lowe, H.; Goldstein, R.; Hartley, J.A.; Caplin, M.E.; Meyer, T. Early Changes in Circulating Tumor Cells Are Associated with Response and Survival Following Treatment of Metastatic Neuroendocrine Neoplasms. Clin. Cancer Res. 2016, 22, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Zatelli, M.C.; Grossrubatscher, E.M.; Guadagno, E.; Sciammarella, C.; Faggiano, A.; Colao, A. Circulating tumor cells and miRNAs as prognostic markers in neuroendocrine neoplasms. Endocr. Relat. Cancer 2017, 24, R223–R237. [Google Scholar] [CrossRef]
- Rizzo, F.M.; Vesely, C.; Childs, A.; Marafioti, T.; Khan, M.S.; Mandair, D.; Cives, M.; Ensell, L.; Lowe, H.; Akarca, A.U.; et al. Circulating tumour cells and their association with bone metastases in patients with neuroendocrine tumours. Br. J. Cancer 2019, 120, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Capurso, G.; Festa, S.; Valente, R.; Piciucchi, M.; Panzuto, F.; Jensen, R.T.; Delle Fave, G. Molecular pathology and genetics of pancreatic endocrine tumours. J. Mol. Endocrinol. 2012, 49, R37–R50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakker, R.V. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4). Mol. Cell. Endocrinol. 2014, 386, 2–15. [Google Scholar] [CrossRef]
- Scarpa, A.; Chang, D.K.; Nones, K.; Corbo, V.; Patch, A.M.; Bailey, P.; Lawlor, R.T.; Johns, A.L.; Miller, D.K.; Mafficini, A.; et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 2017, 543, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, R.; Jiang, X.; Lu, J.; Jiang, J.; Zhang, C.; Li, X.; Ning, G. Nuclear-Cytoplasmic Shuttling of Menin Regulates Nuclear Translocation of β-Catenin. Mol. Cell. Biol. 2009, 29, 5477–5487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurung, B.; Feng, Z.; Iwamoto, D.V.; Thiel, A.; Jin, G.; Fan, C.-M.; Ng, J.M.Y.; Curran, T.; Hua, X. Menin Epigenetically Represses Hedgehog Signaling in MEN1 Tumor Syndrome. Cancer Res. 2013, 73, 2650–2658. [Google Scholar] [CrossRef] [Green Version]
- Heppner, C.; Bilimoria, K.Y.; Agarwal, S.K.; Kester, M.; Whitty, L.J.; Guru, S.C.; Chandrasekharappa, S.C.; Collins, F.S.; Spiegel, A.M.; Marx, S.J.; et al. The tumor suppressor protein menin interacts with NF-κB proteins and inhibits NF-κB-mediated transactivation. Oncogene 2001, 20, 4917–4925. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, C.E.; Scheel, D.W.; McGlynn, K.; Kim, H.; Miyatsuka, T.; Wang, J.; Nguyen, V.; Zhao, S.; Mavropoulos, A.; Abraham, A.G.; et al. Menin determines K-RAS proliferative outputs in endocrine cells. J. Clin. Investig. 2014, 124, 4093–4101. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ozawa, A.; Zaman, S.; Prasad, N.B.; Chandrasekharappa, S.C.; Agarwal, S.K.; Marx, S.J. The Tumor Suppressor Protein Menin Inhibits AKT Activation by Regulating Its Cellular Localization. Cancer Res. 2011, 71, 371–382. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, A.M.; Schmid, S.; Rudolph, T.; Anlauf, M.; Prinz, C.; Klöppel, G.; Moch, H.; Heitz, P.U.; Komminoth, P.; Perren, A. VHL inactivation is an important pathway for the development of malignant sporadic pancreatic endocrine tumors. Endocr. Relat. Cancer 2009, 16, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Mafficini, A.; Scarpa, A. Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr. Rev. 2019, 40, 506–536. [Google Scholar] [CrossRef]
- Brems, H.; Beert, E.; de Ravel, T.; Legius, E. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol. 2009, 10, 508–515. [Google Scholar] [CrossRef]
- Minnetti, M.; Grossman, A. Somatic and germline mutations in NETs: Implications for their diagnosis and management. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 115–127. [Google Scholar] [CrossRef]
- Jiao, Y.; Shi, C.; Edil, B.H.; de Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Schulick, R.D.; Tang, L.H.; Wolfgang, C.L.; Choti, M.A.; et al. DAXX/ATRX, MEN1, and mTOR Pathway Genes Are Frequently Altered in Pancreatic Neuroendocrine Tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef] [Green Version]
- Heaphy, C.M.; de Wilde, R.F.; Jiao, Y.; Klein, A.P.; Edil, B.H.; Shi, C.; Bettegowda, C.; Rodriguez, F.J.; Eberhart, C.G.; Hebbar, S.; et al. Altered Telomeres in Tumors with ATRX and DAXX Mutations. Science 2011, 333, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Wilde, R.F.; Heaphy, C.M.; Maitra, A.; Meeker, A.K.; Edil, B.H.; Wolfgang, C.L.; Ellison, T.A.; Schulick, R.D.; Molenaar, I.Q.; Valk, G.D.; et al. Loss of ATRX or DAXX expression and concomitant acquisition of the alternative lengthening of telomeres phenotype are late events in a small subset of MEN-1 syndrome pancreatic neuroendocrine tumors. Mod. Pathol. 2012, 25, 1033–1039. [Google Scholar] [CrossRef]
- Missiaglia, E.; Dalai, I.; Barbi, S.; Beghelli, S.; Falconi, M.; della Peruta, M.; Piemonti, L.; Capurso, G.; Di Florio, A.; delle Fave, G.; et al. Pancreatic Endocrine Tumors: Expression Profiling Evidences a Role for AKT-mTOR Pathway. J. Clin. Oncol. 2010, 28, 245–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donninger, H.; Vos, M.D.; Clark, G.J. The RASSF1A tumor suppressor. J. Cell Sci. 2007, 120, 3163–3172. [Google Scholar] [CrossRef] [Green Version]
- House, M.G.; Herman, J.G.; Guo, M.Z.; Hooker, C.M.; Schulick, R.D.; Lillemoe, K.D.; Cameron, J.L.; Hruban, R.H.; Maitra, A.; Yeo, C.J.; et al. Aberrant Hypermethylation Tumor Suppressor Genes in Pancreatic Endocrine Neoplasms. Ann. Surg. 2003, 238, 423–432. [Google Scholar] [CrossRef]
- Serrano, J.; Goebel, S.U.; Peghini, P.L.; Lubensky, I.A.; Gibril, F.; Jensen, R.T. Alterations in the p16INK4a/CDKN2A tumor suppressor gene in gastrinomas. J. Clin. Endocrinol. Metab. 2000, 85, 4146–4156. [Google Scholar] [CrossRef]
- Bartsch, D.K.; Kersting, M.; Wild, A.; Ramaswamy, A.; Gerdes, B.; Schuermann, M.; Simon, B.; Rothmund, M. Low frequency of p16(INK4a) alterations in insulinomas. Digestion 2000, 62, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.; Ramaswamy, A.; Langer, P.; Celik, I.; Fendrich, V.; Chaloupka, B.; Simon, B.; Bartsch, D.K. Frequent methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene in pancreatic endocrine tumors. J. Clin. Endocrinol. Metab. 2003, 88, 1367–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cives, M.; Simone, V.; Rizzo, F.M.; Silvestris, F. NETs: Organ-related epigenetic derangements and potential clinical applications. Oncotarget 2016, 7, 57414–57429. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef]
- Balacescu, O.; Sur, D.; Cainap, C.; Visan, S.; Cruceriu, D.; Manzat-Saplacan, R.; Muresan, M.-S.; Balacescu, L.; Lisencu, C.; Irimie, A. The Impact of miRNA in Colorectal Cancer Progression and Its Liver Metastases. Int. J. Mol. Sci. 2018, 19, 3711. [Google Scholar] [CrossRef] [Green Version]
- miRbase. Available online: http://mirbase.org/ (accessed on 10 February 2021).
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates that Thousands of Human Genes are MicroRNA Targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; How Huang, K.; Jen Lee, M.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Di Leva, G.; Garofalo, M.; Croce, C.M. MicroRNAs in Cancer. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 287–314. [Google Scholar] [CrossRef] [Green Version]
- Krichevsky, A.M.; Gabriely, G. miR-21: A small multi-faceted RNA. J. Cell. Mol. Med. 2008, 13, 39–53. [Google Scholar] [CrossRef]
- Pfeffer, S.R.; Yang, C.H.; Pfeffer, L.M. The Role of miR-21 in Cancer. Drug Dev. Res. 2015, 76, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Grolmusz, V.K.; Kövesdi, A.; Borks, K.; Igaz, P.; Patócs, A. Prognostic relevance of proliferation-related miRNAs in pancreatic neuroendocrine neoplasms. Eur. J. Endocrinol. 2018, 179, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, N.; Knief, J.; Kacprowski, T.; Lazar-Karsten, P.; Keck, T.; Billmann, F.; Schmid, S.; Luley, K.; Lehnert, H.; Brabant, G.; et al. MicroRNA analysis of gastroenteropancreatic neuroendocrine tumors and metastases. Oncotarget 2018, 9, 28379–28390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roldo, C.; Missiaglia, E.; Hagan, J.P.; Falconi, M.; Capelli, P.; Bersani, S.; Calin, G.A.; Volinia, S.; Liu, C.-G.; Scarpa, A.; et al. MicroRNA Expression Abnormalities in Pancreatic Endocrine and Acinar Tumors Are Associated with Distinctive Pathologic Features and Clinical Behavior. J. Clin. Oncol. 2006, 24, 4677–4684. [Google Scholar] [CrossRef]
- Mao, L.; Liu, S.; Hu, L.; Jia, L.; Wang, H.; Guo, M.; Chen, C.; Liu, Y.; Xu, L. miR-30 Family: A Promising Regulator in Development and Disease. Biomed Res. Int. 2018, 2018, 9623412. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.H.; Zhang, H.D.; Tang, J.H. MiR-30a: A Novel Biomarker and Potential Therapeutic Target for Cancer. J. Oncol. 2018, 2018, 5167829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Jeong, D.E.; Heo, S.; Ji, E.; Rho, J.G.; Jung, M.; Ahn, S.; Kim, Y.-J.; Kim, Y.-S.; Nam, S.W.; et al. Reduced expression of the RNA-binding protein HuD in pancreatic neuroendocrine tumors correlates with low p27 Kip1 levels and poor prognosis. J. Pathol. 2018, 246, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Ma, J.; Zhou, W.; Zhou, X.; Cao, B.; Fan, D.; Hong, L. Biological implications and clinical value of mir-210 in gastrointestinal cancer. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Thorns, C.; Schurmann, C.; Gebauer, N.; Wallaschofski, H.; Kümpers, C.; Bernard, V.; Feller, A.C.; Keck, T.; Habermann, J.K.; Begum, N.; et al. Global microRNA profiling of pancreatic neuroendocrine neoplasias. Anticancer Res. 2014, 34, 2249–2254. [Google Scholar]
- Cao, D.; Di, M.; Liang, J.; Shi, S.; Tan, Q.; Wang, Z. MicroRNA-183 in Cancer Progression. J. Cancer 2020, 11, 1315–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-Y.; Feng, H.-M. MEG3 Suppresses Human Pancreatic Neuroendocrine Tumor Cells Growth and Metastasis by Down-Regulation of Mir-183. Cell. Physiol. Biochem. 2017, 44, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Michael, I.P.; Saghafinia, S.; Hanahan, D. A set of microRNAs coordinately controls tumorigenesis, invasion, and metastasis. Proc. Natl. Acad. Sci. USA 2019, 116, 24184–24195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.-Q.; Chen, Q.-C.; Qiu, Z.-T.; Tan, W.-L.; Mo, C.-Q.; Gao, S.-W. Integrative microRNA-mRNA and protein-protein interaction analysis in pancreatic neuroendocrine tumors. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2842–2852. [Google Scholar] [PubMed]
- Klieser, E.; Urbas, R.; Swierczynski, S.; Stättner, S.; Primavesi, F.; Jäger, T.; Mayr, C.; Kiesslich, T.; Di Fazio, P.; Helm, K.; et al. HDAC-Linked “Proliferative” miRNA Expression Pattern in Pancreatic Neuroendocrine Tumors. Int. J. Mol. Sci. 2018, 19, 2781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalcanti, E.; Galleggiante, V.; Coletta, S.; Stasi, E.; Chieppa, M.; Armentano, R.; Serino, G. Altered miRNAs Expression Correlates with Gastroenteropancreatic Neuroendocrine Tumors Grades. Front. Oncol. 2020, 10, 1187. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, H.; Kim, H.W.; Lee, J.-C.; Paik, K.-H.; Kang, J.; Kim, J.; Yoon, Y.-S.; Han, H.-S.; Sohn, I.; et al. High Expression of MicroRNA-196a Indicates Poor Prognosis in Resected Pancreatic Neuroendocrine Tumor. Medicine 2015, 94, e2224. [Google Scholar] [CrossRef]
- Gill, P.; Kim, E.; Chua, T.C.; Clifton-Bligh, R.J.; Nahm, C.B.; Mittal, A.; Gill, A.J.; Samra, J.S. MiRNA-3653 Is a Potential Tissue Biomarker for Increased Metastatic Risk in Pancreatic Neuroendocrine Tumours. Endocr. Pathol. 2019, 30, 128–133. [Google Scholar] [CrossRef]
- Vicentini, C.; Calore, F.; Nigita, G.; Fadda, P.; Simbolo, M.; Sperandio, N.; Luchini, C.; Lawlor, R.T.; Croce, C.M.; Corbo, V.; et al. Exosomal miRNA signatures of pancreatic lesions. BMC Gastroenterol. 2020, 20, 137. [Google Scholar] [CrossRef]
- Matthaei, H.; Wylie, D.; Lloyd, M.B.; Dal Molin, M.; Kemppainen, J.; Mayo, S.C.; Wolfgang, C.L.; Schulick, R.D.; Langfield, L.; Andruss, B.F.; et al. miRNA Biomarkers in Cyst Fluid Augment the Diagnosis and Management of Pancreatic Cysts. Clin. Cancer Res. 2012, 18, 4713–4724. [Google Scholar] [CrossRef] [Green Version]
- Kövesdi, A.; Kurucz, P.A.; Nyírő, G.; Darvasi, O.; Patócs, A.; Butz, H. Circulating miRNA Increases the Diagnostic Accuracy of Chromogranin A in Metastatic Pancreatic Neuroendocrine Tumors. Cancers 2020, 12, 2488. [Google Scholar] [CrossRef]
- Panarelli, N.; Tyryshkin, K.; Wong, J.J.M.; Majewski, A.; Yang, X.; Scognamiglio, T.; Kim, M.K.; Bogardus, K.; Tuschl, T.; Chen, Y.-T.; et al. Evaluating gastroenteropancreatic neuroendocrine tumors through microRNA sequencing. Endocr. Relat. Cancer 2019, 26, 47–57. [Google Scholar] [CrossRef]
- Dettori, D.; Orso, F.; Penna, E.; Baruffaldi, D.; Brundu, S.; Maione, F.; Turco, E.; Giraudo, E.; Taverna, D. Therapeutic Silencing of miR-214 Inhibits Tumor Progression in Multiple Mouse Models. Mol. Ther. 2018, 26, 2008–2018. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Choi, S.; Zhang, T.; Chen, Z.; Chi, Y.; Huang, S.; Xiang, J.Z.; Du, Y.-C.N. miR-431 Promotes Metastasis of Pancreatic Neuroendocrine Tumors by Targeting DAB2 Interacting Protein, a Ras GTPase Activating Protein Tumor Suppressor. Am. J. Pathol. 2020, 190, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Na, H.; Hua, X.; Wei, Y.; Ye, T.; Zhang, Y.; Jian, G.; Zeng, W.; Yan, L.; Tang, Q. A retrospective study of NENs and miR-224 promotes apoptosis of BON-1 cells by targeting PCSK9 inhibition. Oncotarget 2017, 8, 6929–6939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamana, H.; Kato, K.; Kobara, H.; Fujihara, S.; Fujita, K.; Namima, D.; Fujita, N.; Kobayashi, K.; Kamada, H.; Morishita, A.; et al. Metformin Inhibits Proliferation and Tumor Growth of QGP-1 Pancreatic Neuroendocrine Tumor Cells by Inducing Cell Cycle Arrest and Apoptosis. Anticancer Res. 2020, 40, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Hosseinahli, N.; Aghapour, M.; Duijf, P.H.G.; Baradaran, B. Treating cancer with microRNA replacement therapy: A literature review. J. Cell. Physiol. 2018, 233, 5574–5588. [Google Scholar] [CrossRef] [PubMed]

| miRNA | Expression | Function | Clinical Implication | Ref. No. |
|---|---|---|---|---|
| miR-96-5p | ⬆ | Oncogenic, FoxO1a inhibition | High tumor grade | [127] |
| miR-130b-3p | ⬆ | N/A | ||
| miR-194-5p | ⬇ | N/A | ||
| miR-132-3p | ⬆ | Tumor-suppressing and tumor-promoting function | Low tumor grade Vascular invasion Somatostatin expression | [126] |
| miR-34a-5p | ⬆ | N/A | Somatostatin, gastrin, and serotonin expression | |
| miR-145-5p | ⬆ | N/A | High tumor grade Lymphatic invasion Serotonin expression | |
| miR-449a | ⬆ | Oncogenic via histone deacetylases 3/4 | High tumor grade, mitotic and proliferative activity, lymph-node invasion | |
| miR-183-5p | ⬆ | Tumor suppressor | High tumor grade Tumor size Somatostatin-receptor expression | |
| miR-196a | ⬆ | N/A | Advanced tumor Lymph-node invasion High mitotic and proliferative activity Recurrence | [128] |
| miR-660 miR-30a-5p miR-339-3p miR-345 | ⬇ | N/A | Metastatic disease | [115] |
| miR-210 | ⬆ | Oncogenic | Metastatic disease | [115,121] |
| miR-642 | ⬆ | Oncogenic | Proliferative activity | [121] |
| miR-3653 | ⬆ | Oncogenic associated with ATRX mutations | Metastatic disease | [129] |
| miR-21 | ⬆ | Oncogenic | Tumor grade Metastatic disease Proliferative activity | [115] [114] [116] |
| miRNA | Expression | Sample | Sample Size | Diagnostic Implication | Ref. No. |
|---|---|---|---|---|---|
| miR-193b | ⬆ | Serum, FFPE | 37 | Healthy vs PanNETs | [121] |
| miR-103 miR-107 | ⬆ | FFPE | 96 | Healthy vs. tumor | [116] |
| miR-155 | ⬇ | ||||
| miR-99a, 99b, 100, 125a, 125b-1, 125b-2, 129-2, 130a, 132, 342 | ⬆ | PanNETs vs. pancreatic adenocarcinoma/normal pancreas | |||
| miR-451a, 372-3p, 106a-5p, 17-5p, 25-3p, 644a | ⬆ | Serum, FFPE | 140 | PanNETs vs. chronic pancreatitis | [130] |
| miR-22-3p, 1246, 4454, 7975, 320e | ⬇ | ||||
| miR-451a, 26b-5p, 25-3p, 16-5p | ⬆ | PanNETs vs. pancreatic adenocarcinoma | |||
| miR-1322, 1285-5p, 320e | ⬇ | ||||
| miR-24, 30a-3p, 18a, 92a, 342-3p, 99b, 106b, 142-3p, 532-3p | N/A | FFPE | 120 | High-grade IPMNs, cystic PanNETs, and SPN vs. low-grade IPMN, SCA | [110] |
| miR-615 and -92b miR-429 and -487b | N/A | FFPE | 81 | PanNETs vs. ileal, appendicular, rectal neuroendocrine tumors | [133] |
| miRNA | Expression | Model | Treatment | Outcomes | Ref. No. |
|---|---|---|---|---|---|
| miR-214 | ⬆ | Mouse | miR-214 inhibition | -Reduced tumor volume -Decreased volume of peripancreatic lymphatic metastases -Reduced tumor vascularization | [134] |
| miR-431 | ⬆ | Cell lines | miR-431-targeted locked nucleic acids | -Reduced invasiveness | [135] |
| miR-224 | ⬇ | Cell lines | miR-224 agomir | -Promotes apoptosis -Inhibits proliferation, invasion | [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havasi, A.; Sur, D.; Cainap, S.S.; Lungulescu, C.-V.; Gavrilas, L.-I.; Cainap, C.; Vlad, C.; Balacescu, O. Current and New Challenges in the Management of Pancreatic Neuroendocrine Tumors: The Role of miRNA-Based Approaches as New Reliable Biomarkers. Int. J. Mol. Sci. 2022, 23, 1109. https://doi.org/10.3390/ijms23031109
Havasi A, Sur D, Cainap SS, Lungulescu C-V, Gavrilas L-I, Cainap C, Vlad C, Balacescu O. Current and New Challenges in the Management of Pancreatic Neuroendocrine Tumors: The Role of miRNA-Based Approaches as New Reliable Biomarkers. International Journal of Molecular Sciences. 2022; 23(3):1109. https://doi.org/10.3390/ijms23031109
Chicago/Turabian StyleHavasi, Andrei, Daniel Sur, Simona Sorana Cainap, Cristian-Virgil Lungulescu, Laura-Ioana Gavrilas, Calin Cainap, Catalin Vlad, and Ovidiu Balacescu. 2022. "Current and New Challenges in the Management of Pancreatic Neuroendocrine Tumors: The Role of miRNA-Based Approaches as New Reliable Biomarkers" International Journal of Molecular Sciences 23, no. 3: 1109. https://doi.org/10.3390/ijms23031109
APA StyleHavasi, A., Sur, D., Cainap, S. S., Lungulescu, C.-V., Gavrilas, L.-I., Cainap, C., Vlad, C., & Balacescu, O. (2022). Current and New Challenges in the Management of Pancreatic Neuroendocrine Tumors: The Role of miRNA-Based Approaches as New Reliable Biomarkers. International Journal of Molecular Sciences, 23(3), 1109. https://doi.org/10.3390/ijms23031109








