Structure, Function and Regulation of the Plasma Membrane Calcium Pump in Health and Disease
Abstract
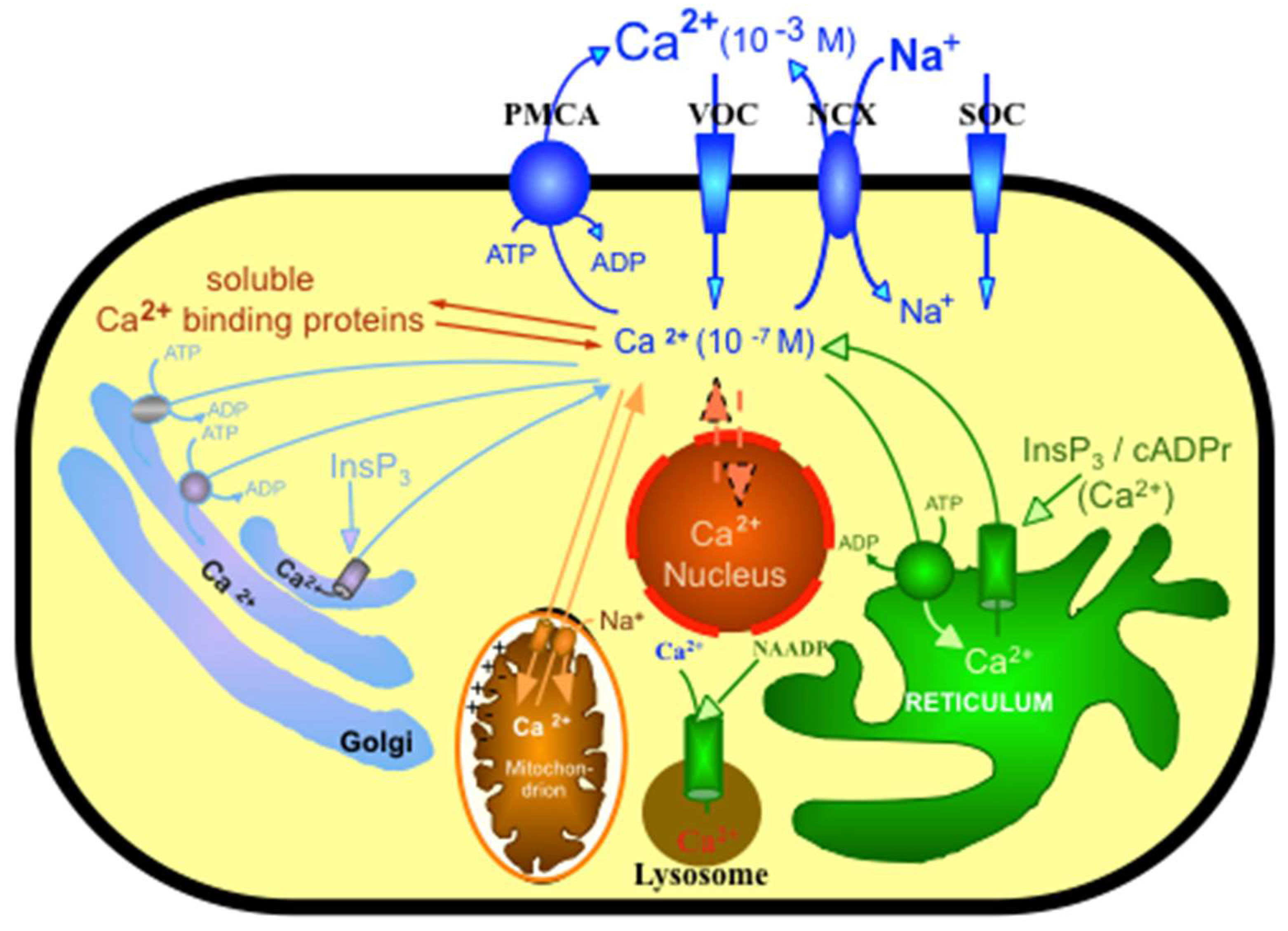
:1. Introduction
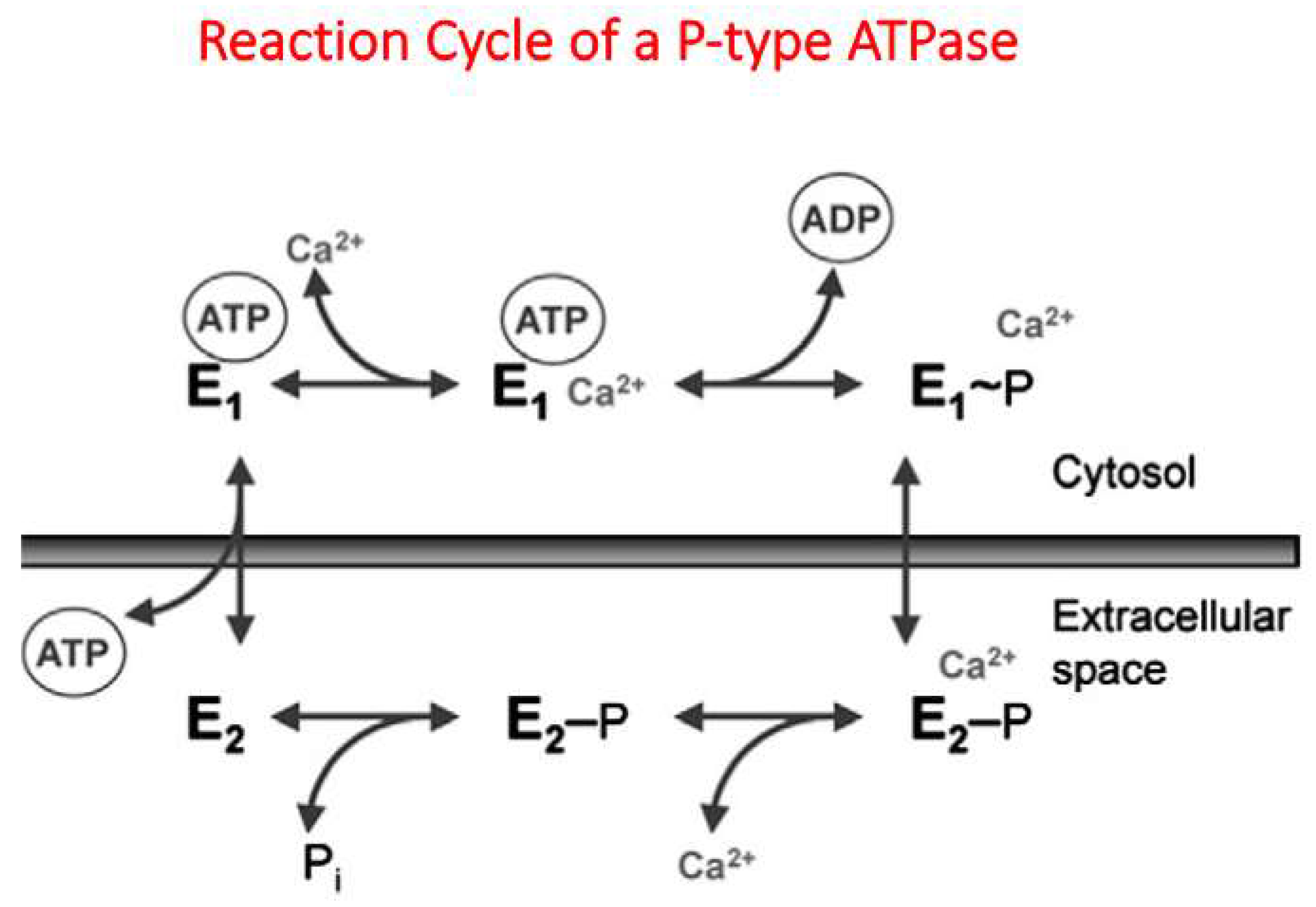
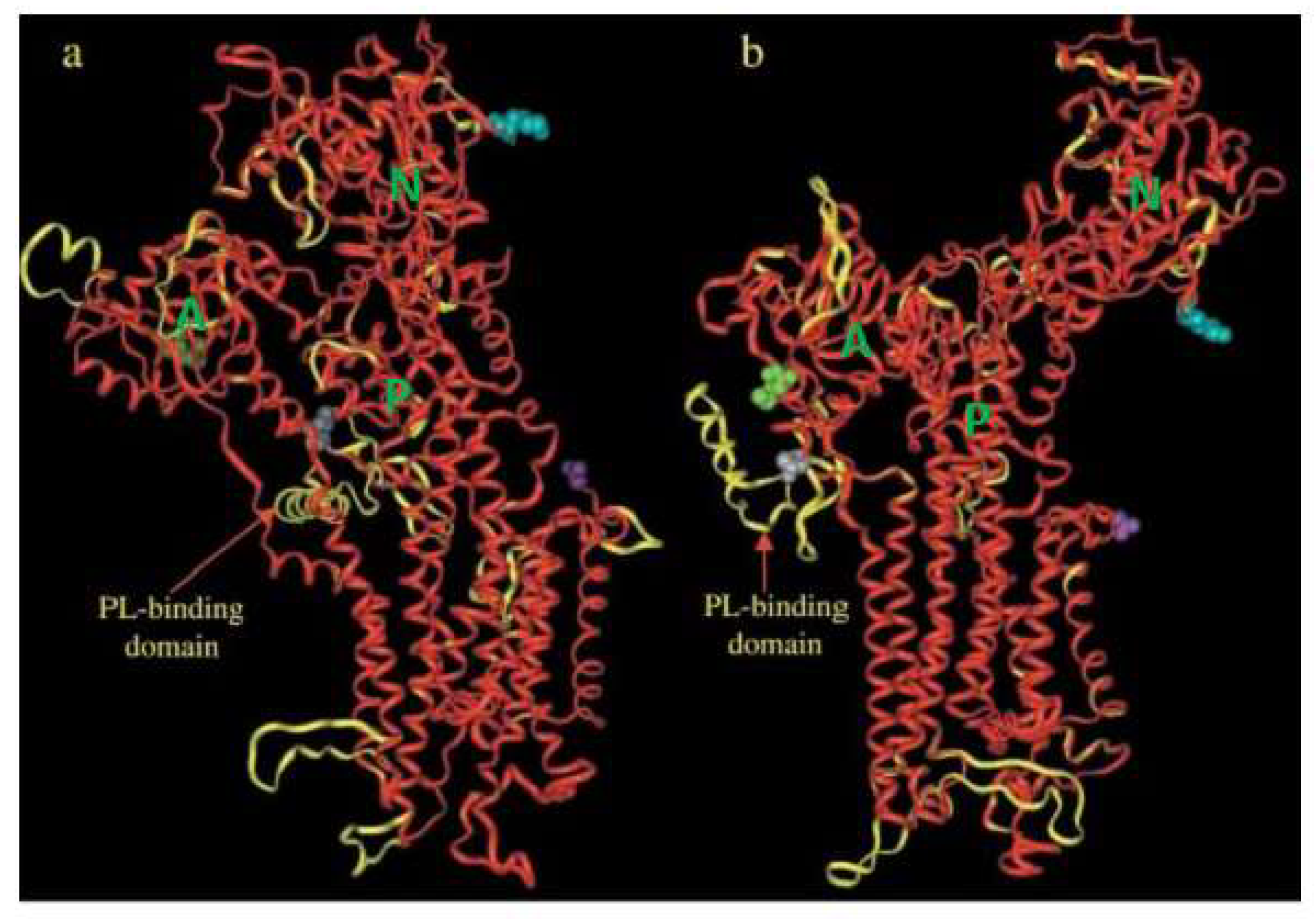
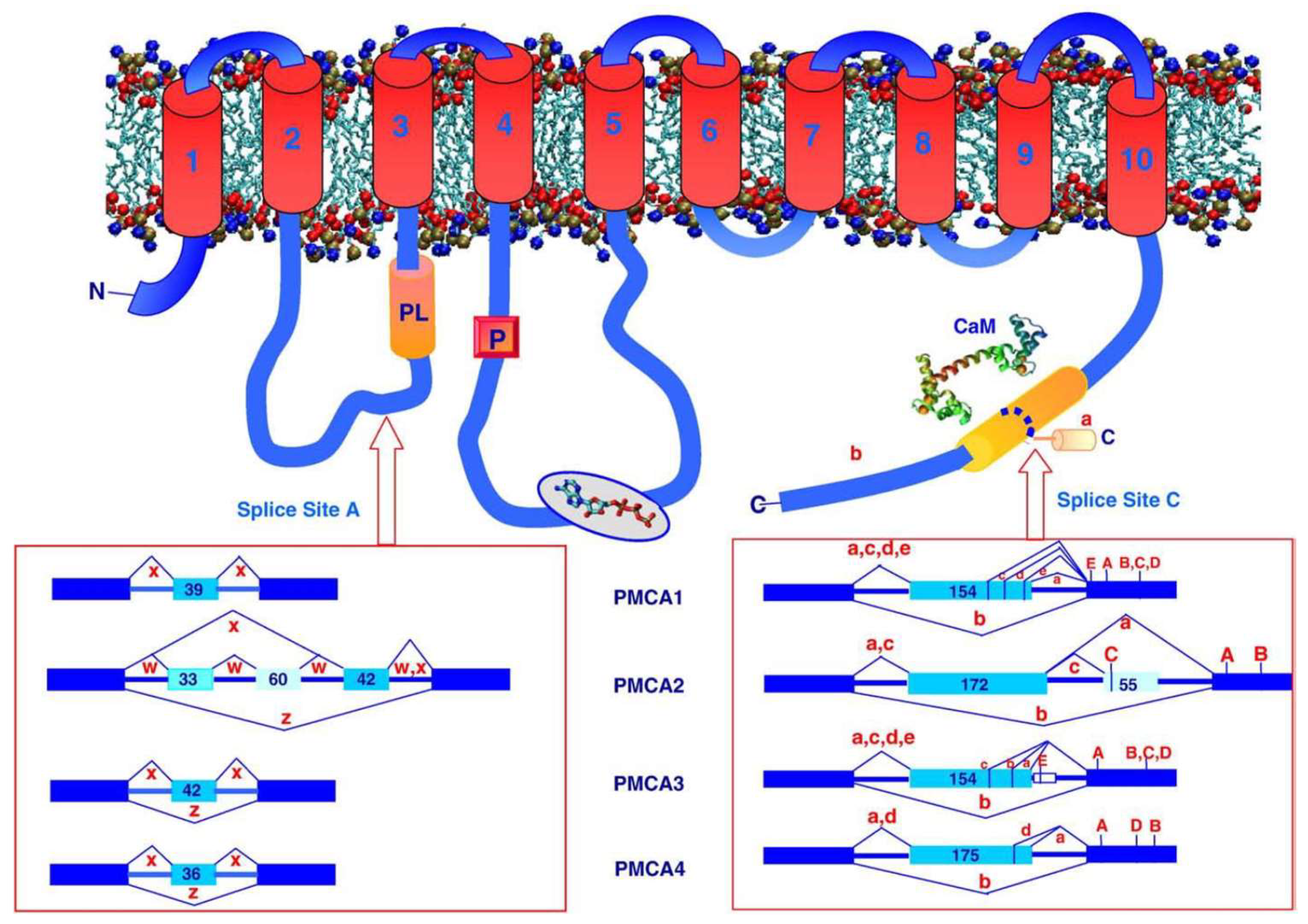
2. General Properties of PMCA
3. The Different Isoforms of PMCA
3.1. PMCA1
3.2. PMCA2
3.3. PMCA3
3.4. PMCA4
4. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.M.; Gamba, G.; Riccardi, D.; Lombardi, M.; Butters, R.; Kifor, O.; Sun, A.; Hediger, M.A.; Lytton, J.; Hebert, S.C. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 1993, 366, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.; Michalak, M. Calcium: A Matter of Life or Death; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Luan, S.; Wang, C. Calcium Signaling Mechanisms Across Kingdoms. Annu. Rev. Cell Dev. Biol. 2021, 37, 311–340. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef]
- Dunham, E.T.; Glynn, I.M. Adenosine Triphosphatase activity and the active movements of alkali metal ions. J. Physiol. 1961, 156, 274–293. [Google Scholar] [CrossRef] [Green Version]
- Schatzmann, H.J. ATP-dependent Ca++-Extrusion from human red cells. Experientia 1966, 22, 364–365. [Google Scholar] [CrossRef] [PubMed]
- Luthral, M.G.; Au, K.S.; Hanahan, D.J. Purification of an activator of human erythrocyte membrane (Ca2++Mg2+)ATPase. Biochem. Biophys. Res. Commun. 1977, 77, 678–687. [Google Scholar] [CrossRef]
- Gopinath, R.M.; Vincenzi, F.F. Phosphodiesterase protein activator mimics red blood cell cytoplasmic activator of (Ca2+-Mg2+)ATPase. Biochem. Biophys. Res. Commun. 1977, 77, 1203–1209. [Google Scholar] [CrossRef]
- Jarrett, H.W.; Penniston, J.T. Partial purification of the Ca2+-Mg2+ ATPase activator from human erythrocytes: Its similarity to the activator of 3′:5′—Cyclic nucleotide phosphodiesterase. Biochem. Biophys. Res. Commun. 1977, 77, 1210–1216. [Google Scholar] [CrossRef]
- James, P.; Maeda, M.; Fischer, R.; Verma, A.K.; Krebs, J.; Penniston, J.T.; Carafoli, E. Identification and primary structure of a calmodulin binding domain of the Ca2+ pump of human erythrocytes. J. Biol. Chem. 1988, 263, 2905–2910. [Google Scholar] [CrossRef]
- Niggli, V.; Penniston, J.T.; Carafoli, E. Purification of the (Ca2+-Mg2+)-ATPase from human erythrocyte membranes using a calmodulin affinity column. J. Biol. Chem. 1979, 254, 9955–9958. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Nissen, P. P-type ATPases. Annu. Rev. Biophys. 2011, 40, 243–266. [Google Scholar] [CrossRef]
- Pedersen, P.L.; Carafoli, E. Ion motive ATPases. I. Ubiquity, properties, and significance to cell function. Trends Biochem. Sci. 1987, 12, 146–150. [Google Scholar] [CrossRef]
- Krebs, J. The plethora of PMCA isoforms: Alternative splicing and differential expression. Biochim. Biophys. Acta Bioenerg. 2015, 1853, 2018–2024. [Google Scholar] [CrossRef] [Green Version]
- Niggli, V.; Adunyah, E.S.; Penniston, J.T.; Carafoli, E. Purified (Ca2+-Mg2+)-ATPase of the erythrocyte membrane. Reconstitution and effect of calmodulin and phospholipids. J. Biol. Chem. 1981, 256, 395–401. [Google Scholar] [CrossRef]
- Hasselbach, W. The sarcoplasmic calcium pump—A most efficient ion translocating system. Biophys. Struct. Mech. 1977, 3, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.; Vašák, M.; Scarpa, A.; Carafoli, E. Conformational differences between the E1 and E2 states of the calcium adenosine triphosphatase of the erythrocyte plasma membrane as revealed by circular dichroism and fluorescence spectroscopy. Biochemistry 1987, 26, 3921–3926. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, C.; Nakasako, M.; Nomura, H.; Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 2000, 405, 647–655. [Google Scholar] [CrossRef]
- Toyoshima, C. How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim. Biophys. Acta 2009, 1793, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Dyla, M.; Kjaergaard, M.; Poulsen, H.; Nissen, P. Structure and Mechanism of P-Type ATPase Ion Pumps. Annu. Rev. Biochem. 2020, 89, 583–603. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.; Helms, V.; Griesinger, C.; Carafoli, E. The Regulation of the Calcium Signal by Membrane Pumps. Helv. Chim. Acta 2003, 86, 3875–3888. [Google Scholar] [CrossRef]
- Gong, D.; Chi, X.; Ren, K.; Huang, G.; Zhou, G.; Yan, N.; Lei, J.; Zhou, Q. Structure of the human plasma membrane Ca2+-ATPase 1 in complex with its obligatory subunit neuroplastin. Nat. Commun. 2018, 9, 3623. [Google Scholar] [CrossRef]
- Verma, A.K.; Filoteo, A.G.; Stanford, D.R.; Wieben, E.D.; Penniston, J.T.; Strehler, E.E.; Fischer, R.; Heim, R.; Vogel, G.; Mathews, S. Complete primary structure of a human plasma membrane Ca2+ pump. J. Biol. Chem. 1988, 263, 14152–14159. [Google Scholar] [CrossRef]
- Shull, G.E.; Greeb, J. Molecular cloning of two isoforms of the plasma membrane Ca2+-transporting ATPase from rat brain. Structural and functional domains exhibit similarity to Na+, K+- and other cation transport ATPases. J. Biol. Chem. 1988, 263, 8646–8657. [Google Scholar] [CrossRef]
- Olson, S.; Wang, M.G.; Carafoli, E.; Strehler, E.E.; McBride, O. Localization of two genes encoding plasma membrane Ca2+-transporting ATPases to human chromosomes 1q25–32 and 12q21–23. Genomics 1991, 9, 629–641. [Google Scholar] [CrossRef]
- Wang, M.G.; Yi, H.; Hilfiker, H.; Carafoli, E.; Strehler, E.E.; McBride, O.W. Localization of two genes encoding plasma membrane Ca2+ ATPases isoforms 2 (ATP2B2) and 3 (ATP2B3) to human chromosomes 3p26→p25 and Xq28, respectively. Cytogenet. Genome Res. 1994, 67, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Strehler, E.E.; Zacharias, D.A. Role of Alternative Splicing in Generating Isoform Diversity Among Plasma Membrane Calcium Pumps. Physiol. Rev. 2001, 81, 21–50. [Google Scholar] [CrossRef] [Green Version]
- Ronner, P.; Gazzotti, P.; Carafoli, E. A lipid requirement for the (Ca2+ + Mg2+)-activated ATPase of erythrocyte membranes. Arch. Biochem. Biophys. 1977, 179, 578–583. [Google Scholar] [CrossRef]
- Conrard, L.; Tyteca, D. Regulation of Membrane Calcium Transport Proteins by the Surrounding Lipid Environment. Biomolecules 2019, 9, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwaritch, E.; James, P.; Vorherr, T.; Falchetto, R.; Modyanov, N.; Carafoli, E. Mapping of functional domains in the plasma membrane Ca2+ pump using trypsin proteolysis. Biochemistry 1990, 29, 8070–8076. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P.; Falchetto, R.; Vorherr, T.; Carafoli, E. Identification of two domains which mediate the binding of activating phospholipids to the plasma-membrane Ca2+ pump. Eur. J. Biochem. 1992, 204, 939–946. [Google Scholar] [CrossRef]
- Krebs, J. The Plasma Membrane Calcium Pump (PMCA): Regulation of Cytosolic Ca2+, Genetic Diversities and Its Role in Sub-plasma Membrane Microdomains. Adv. Exp. Med. Biol. 2017, 981, 3–21. [Google Scholar]
- Fujimoto, T. Calcium pump of the plasma membrane is localized in caveolae. J. Cell Biol. 1993, 120, 1147–1157. [Google Scholar] [CrossRef] [Green Version]
- Daniel, E.E.; El-Yazbi, A.; Cho, W.J. Caveolae and calcium handling, a review and a hypothesis. J. Cell. Mol. Med. 2006, 10, 529–544. [Google Scholar] [CrossRef] [Green Version]
- Pani, B.; Singh, B.B. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium 2009, 45, 625–633. [Google Scholar] [CrossRef] [Green Version]
- El-Yazbi, A.F.; Cho, W.J.; Schulz, R.; Daniel, E.E. Calcium extrusion by plasma membrane calcium pump is impaired in caveolin-1 knockout mouse small intestine. Eur. J. Pharmacol. 2008, 591, 80–87. [Google Scholar] [CrossRef]
- Nabi, I.R.; Le, P.U. Caveolae/raft-dependent endocytosis. J. Cell Biol. 2003, 161, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Miner, G.E.; Sullivan, K.D.; Zhang, C.; Rivera-Kohr, D.; Guo, A.; Hurst, L.R.; Ellis, E.C.; Starr, M.L.; Jones, B.C.; Fratti, R.A. Phosphatidylinositol 3,5-bisphosphate regulates Ca2+ transport during yeast vacuolar fusion through the Ca2+ ATPase Pmc1. Traffic 2020, 21, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E.; Zurini, M. The Ca2+-pumping ATPase of plasma membranes purification, reconstitution and properties. Biochim. Biophys. Acta Rev. Bioenerg. 1982, 683, 279–301. [Google Scholar] [CrossRef]
- Choquette, D.; Hakim, G.; Filoteo, A.G.; Plishker, G.A.; Bostwick, J.R.; Penniston, J.T. Regulation of plasma membrane Ca2+ ATPases by lipids of the phosphatidylinositol cycle. Biochem. Biophys. Res. Commun. 1984, 125, 908–915. [Google Scholar] [CrossRef]
- Kim, E.; DeMarco, S.J.; Marfatia, S.M.; Chishti, A.H.; Sheng, M.; Strehler, E.E. Plasma Membrane Ca2+ ATPase Isoform 4b Binds to Membrane-associated Guanylate Kinase (MAGUK) Proteins via Their PDZ (PSD-95/Dlg/ZO-1) Domains. J. Biol. Chem. 1998, 273, 1591–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, C.K.; Hooper, R.; Aronson, M.R.; Schultz, B.; Cangoz, T.; Nemani, N.; Zhang, Y.; Madesh, M.; Soboloff, J. The Ca2+ export pump PMCA clears near-membrane Ca2+ to facilitate store-operated Ca2+ entry and NFAT activation. Sci. Signal. 2019, 12, eaaw2627. [Google Scholar] [CrossRef]
- Krapivinsky, G.; Krapivinsky, L.; Stotz, S.C.; Manasian, Y.; Clapham, D.E. POST, partner of stromal interaction molecule 1 (STIM1), targets STIM1 to multiple transporters. Proc. Natl. Acad. Sci. USA 2011, 108, 19234–19239. [Google Scholar] [CrossRef] [Green Version]
- de Souza, L.B.; Ong, H.L.; Liu, X.; Ambudkar, I.S. PIP2 and septin control STIM1/Orai1 assembly by regulating cytoskeletal remodeling via a CDC42-WASP/WAVE-ARP2/3 protein complex. Cell Calcium 2021, 99, 102475. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.L.; Ambudkar, I.S. The Endoplasmic Reticulum–Plasma Membrane Junction: A Hub for Agonist Regulation of Ca2+ Entry. Cold Spring Harb. Perspect. Biol. 2020, 12, a035253. [Google Scholar] [CrossRef] [PubMed]
- Dalghi, M.G.; Ferreira-Gomes, M.; Rossi, J.P. Regulation of the Plasma Membrane Calcium ATPases by the actin cytoskeleton. Biochem. Biophys. Res. Commun. 2018, 506, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Subedi, K.P.; Ong, H.L.; Ambudkar, I.S. Assembly of ER-PM Junctions: A Critical Determinant in the Regulation of SOCE and TRPC1. Adv. Exp. Med. Biol. 2017, 981, 253–276. [Google Scholar]
- Kosk-Kosicka, D.; Bzdega, T. Activation of the erythrocyte Ca2+-ATPase by either self-association or interaction with calmodulin. J. Biol. Chem. 1988, 263, 18184–18189. [Google Scholar] [CrossRef]
- Smallwood, J.I.; Gügi, B.; Rasmussen, H. Regulation of erythrocyte Ca2+ pump activity by protein kinase C. J. Biol. Chem. 1988, 263, 2195–2202. [Google Scholar] [CrossRef]
- Bækgaard, L.; Luoni, L.; de Michelis, M.I.; Palmgren, M.G. The Plant Plasma Membrane Ca2+ Pump ACA8 Contains Overlapping as Well as Physically Separated Autoinhibitory and Calmodulin-binding Domains. J. Biol. Chem. 2006, 281, 1058–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geisler, M.; Axelsen, K.B.; Harper, J.F.; Palmgren, M.G. Molecular aspects of higher plant P-type Ca2+-ATPases. Biochim. Biophys. Acta Biomembr. 2000, 1465, 52–78. [Google Scholar] [CrossRef] [Green Version]
- Guerini, D.; Krebs, J.; Carafoli, E. Stimulation of the purified erythrocyte Ca2+-ATPase by tryptic fragments of calmodulin. J. Biol. Chem. 1984, 259, 15172–15177. [Google Scholar] [CrossRef]
- Maune, J.F.; Klee, C.B.; Beckingham, K. Ca2+ binding and conformational change in two series of point mutations to the individual Ca(2+)-binding sites of calmodulin. J. Biol. Chem. 1992, 267, 5286–5295. [Google Scholar] [CrossRef]
- Gao, Z.H.; Krebs, J.; VanBerkum, M.F.; Tang, W.-J.; Maune, J.F.; Means, A.R.; Stull, J.T.; Beckingham, K. Activation of four enzymes by two series of calmodulin mutants with point mutations in individual Ca2+ binding sites. J. Biol. Chem. 1993, 268, 20096–20104. [Google Scholar] [CrossRef]
- Yazawa, M.; Vorherr, T.; James, P.; Carafoli, E.; Yagi, K. Binding of calcium by calmodulin: Influence of the calmodulin binding domain of the plasma membrane calcium pump. Biochemistry 1992, 31, 3171–3176. [Google Scholar] [CrossRef]
- Soderling, T.R. Protein kinases. Regulation by autoinhibitory domains. J. Biol. Chem. 1990, 265, 1823–1826. [Google Scholar] [CrossRef]
- Kobe, B.; Kemp, B.E. Active site-directed protein regulation. Nat. Cell Biol. 1999, 402, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Falchetto, R.; Vorherr, T.; Brunner, J.; Carafoli, E. The plasma membrane Ca2+ pump contains a site that interacts with its calmodulin-binding domain. J. Biol. Chem. 1991, 266, 2930–2936. [Google Scholar] [CrossRef]
- Falchetto, R.; Vorherr, T.; Carafoli, E. The calmodulin-binding site of the plasma membrane Ca2+ pump interacts with the transduction domain of the enzyme. Protein Sci. 1992, 1, 1613–1621. [Google Scholar] [CrossRef] [Green Version]
- Schuh, K.; Uldrijan, S.; Telkamp, M.; Röthlein, N.; Neyses, L. The plasma membrane calmodulin-dependent calcium pump: A major regulator of nitric oxide synthase I. J. Cell Biol. 2001, 155, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Oceandy, D.; Cartwright, E.J.; Emerson, M.; Prehar, S.; Baudoin, F.M.; Zi, M.; Alatwi, N.; Venetucci, L.; Schuh, K.; Williams, J.C.; et al. Neuronal Nitric Oxide Synthase Signaling in the Heart Is Regulated by the Sarcolemmal Calcium Pump 4b. Circulation 2007, 115, 483–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, T.M.; Oceandy, D.; Zi, M.; Prehar, S.; Alatwi, N.; Wang, Y.; Shaheen, M.A.; Abou-Leisa, R.; Schelcher, C.; Hegab, Z.; et al. Plasma Membrane Calcium Pump (PMCA4)-Neuronal Nitric-oxide Synthase Complex Regulates Cardiac Contractility through Modulation of a Compartmentalized Cyclic Nucleotide Microdomain. J. Biol. Chem. 2011, 286, 41520–41529. [Google Scholar] [CrossRef] [Green Version]
- Calì, T.; Brini, M.; Carafoli, E. The PMCA pumps in genetically determined neuronal pathologies. Neurosci. Lett. 2018, 663, 2–11. [Google Scholar] [CrossRef]
- Zaidi, A.; Adewale, M.; McLean, L.; Ramlow, P. The plasma membrane calcium pumps—The old and the new. Neurosci. Lett. 2018, 663, 12–17. [Google Scholar] [CrossRef]
- Ikura, M.; Clore, G.M.; Gronenborn, A.M.; Zhu, G.; Klee, C.B.; Bax, A. Solution structure of a calmodulin-target peptide complex by multidimensional NMR. Science 1992, 256, 632–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meador, W.E.; Means, A.R.; Quiocho, F.A. Target Enzyme Recognition by Calmodulin: 2.4 Å Structure of a Calmodulin-Peptide Complex. Science 1992, 257, 1251–1255. [Google Scholar] [CrossRef]
- Kataoka, M.; Head, J.F.; Vorherr, T.; Krebs, J.; Carafoli, E. Small-angle X-ray scattering study of calmodulin bound to two peptides corresponding to parts of the calmodulin-binding domain of the plasma membrane Ca2+ pump. Biochemistry 1991, 30, 6247–6251. [Google Scholar] [CrossRef]
- Elshorst, B.; Hennig, M.; Försterling, H.; Diener, A.; Maurer, M.; Schulte, P.; Schwalbe, H.; Griesinger, C.; Krebs, J.; Schmid, H.; et al. NMR Solution Structure of a Complex of Calmodulin with a Binding Peptide of the Ca2+ Pump. Biochemistry 1999, 38, 12320–12332. [Google Scholar] [CrossRef] [PubMed]
- Elshorst, B. Die NMR-Lösungsstruktur des Calmodulin/C20W Komplexes. Ph.D. Thesis, Goethe University Frankfurt, Frankfurt, Germany, 2000. [Google Scholar]
- Juranic, N.; Atanasova, E.; Filoteo, A.G.; Macura, S.; Prendergast, F.G.; Penniston, J.T.; Strehler, E.E. Calmodulin wraps around its binding domain in the plasma membrane Ca2+ pump anchored by a novel 18-1 motif. J. Biol. Chem. 2010, 285, 4015–4024. [Google Scholar] [CrossRef] [Green Version]
- Tidow, H.; Poulsen, L.R.; Andreeva, A.; Knudsen, M.; Hein, K.L.; Wiuf, C.; Palmgren, M.G.; Nissen, P. A bimodular mechanism of calcium control in eukaryotes. Nature 2012, 491, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Strehler, E.E. Plasma membrane calcium ATPases: From generic Ca2+ sump pumps to versatile systems for fine-tuning cellular Ca2+. Biochem. Biophys. Res. Commun. 2015, 460, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Okunade, G.W.; Miller, M.L.; Shull, G.E. Phenotypes of SERCA and PMCA knock out mice. Biochem. Biophys. Res. Commun. 2004, 322, 1192–1203. [Google Scholar] [CrossRef]
- Okunade, G.W.; Miller, M.L.; Pyne, G.J.; Sutliff, R.L.; O’Connor, K.T.; Neumann, J.C.; Andringa, A.; Miller, D.A.; Prasad, V.; Doetschman, T.; et al. Targeted Ablation of Plasma Membrane Ca2+-ATPase (PMCA) 1 and 4 Indicates a Major Housekeeping Function for PMCA1 and a Critical Role in Hyperactivated Sperm Motility and Male Fertility for PMCA4. J. Biol. Chem. 2004, 279, 33742–33750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, P.; Neve, R.L. Expression of Plasma Membrane Calcium-Pumping ATPase mRNAs in Developing Rat Brain and Adult Brain Subregions: Evidence for Stage-Specific Expression. J. Neurochem. 1992, 59, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Filoteo, A.G.; Elwess, N.L.; Enyedi, A.; Caride, A.; Aung, H.H.; Penniston, J.T. Plasma membrane Ca2+ pump in rat brain. Patterns of alternative splices seen by isoform-specific antibodies. J. Biol. Chem. 1997, 272, 23741–23747. [Google Scholar] [CrossRef] [Green Version]
- Kip, S.N.; Gray, N.W.; Burette, A.; Canbay, A.; Weinberg, R.J.; Strehler, E.E. Changes in the expression of plasma membrane calcium extrusion systems during the maturation of hippocampal neurons. Hippocampus 2006, 16, 20–34. [Google Scholar] [CrossRef] [Green Version]
- Kenyon, K.A.; Bushong, E.A.; Mauer, A.S.; Strehler, E.E.; Weinberg, R.J.; Burette, A.C. Cellular and subcellular localization of the neuron-specific plasma membrane calcium ATPase PMCA1a in the rat brain. J. Comp. Neurol. 2010, 518, 3169–3183. [Google Scholar] [CrossRef]
- Caride, A.J.; Filoteo, A.G.; Penniston, J.T.; Strehler, E.E. The Plasma Membrane Ca2+ Pump Isoform 4a Differs from Isoform 4b in the Mechanism of Calmodulin Binding and Activation Kinetics: Implications for Ca2+ signaling. J. Biol. Chem. 2007, 282, 25640–25648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, J. Calmodulin-dependent protein kinase IV: Regulation of function and expression. Biochim. Biophys. Acta Bioenerg. 1998, 1448, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Krebs, J. The Influence of Thyroid Hormone on Ca2+ Signaling Pathways During Embryonal Development. Curr. Top. Med. Chem. 2021, 21, 1121–1128. [Google Scholar] [CrossRef]
- Korthals, M.; Tech, L.; Langnaese, K.; Gottfried, A.; Hradsky, J.; Thomas, U.; Zenclussen, A.C.; Brunner-Weinzierl, M.C.; Tedford, K.; Fischer, K.-D. Plasma membrane Ca2+ ATPase 1 (PMCA1) but not PMCA4 is critical for B-cell development and Ca2+ homeostasis in mice. Eur. J. Immunol. 2021, 51, 549–602. [Google Scholar] [CrossRef] [PubMed]
- Hegedűs, L.; Zámbó, B.; Pászty, K.; Padányi, R.; Varga, K.; Penniston, J.T.; Enyedi, Á. Molecular Diversity of Plasma Membrane Ca2+ Transporting ATPases: Their Function Under Normal and Pathological Conditions. Adv. Exp. Med. Biol. 2020, 1131, 93–129. [Google Scholar]
- Borke, J.L.; Caride, A.; Verma, A.K.; Penniston, J.T.; Kumar, R. Cellular and segmental distribution of Ca2+-pump epitopes in rat intestine. Pflug. Arch. Eur. J. Physiol. 1990, 417, 120–122. [Google Scholar] [CrossRef]
- Cai, Q.; Chandler, J.S.; Wasserman, R.H.; Kumar, R.; Penniston, J.T. Vitamin D and adaptation to dietary calcium and phosphate deficiencies increase intestinal plasma membrane calcium pump gene expression. Proc. Natl. Acad. Sci. USA 1993, 90, 1345–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, Z.C.; Craig, T.A.; Filoteo, A.G.; Westendorf, J.J.; Cartwright, E.J.; Neyses, L.; Strehler, E.E.; Kumar, R. Deletion of the intestinal plasma membrane calcium pump, isoform 1, Atp2b1, in mice is associated with decreased bone mineral density and impaired responsiveness to 1, 25-dihydroxyvitamin D3. Biochem. Biophys. Res. Commun. 2015, 467, 152–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, A.J.; Palmiter, R.D. Detecting and Avoiding Problems When Using the Cre–lox System. Trends Genet. 2018, 34, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.S.; Tempel, B.L. Atp2b2, encoding plasma membrane Ca2+-ATPase type 2, (PMCA2) exhibits tissue-specific first exon usage in hair cells, neurons, and mammary glands of mice. Neuroscience 2006, 141, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Brandt, P.; Neve, R.L.; Kammesheidt, A.; Rhoads, R.E.; Vanaman, T.C. Analysis of the tissue-specific distribution of mRNAs encoding the plasma membrane calcium-pumping ATPases and characterization of an alternately spliced form of PMCA4 at the cDNA and genomic levels. J. Biol. Chem. 1992, 267, 4376–4385. [Google Scholar] [CrossRef]
- Hilfiker, H.; Guerini, D.; Carafoli, E. Cloning and expression of isoform 2 of the human plasma membrane Ca2+ ATPase. Functional properties of the enzyme and its splicing products. J. Biol. Chem. 1994, 269, 26178–26183. [Google Scholar] [CrossRef]
- Zacharias, D.A.; Kappen, C. Developmental expression of the four plasma membrane calcium ATPase (Pmca) genes in the mouse. Biochim. Biophys. Acta Gen. Subj. 1999, 1428, 397–405. [Google Scholar] [CrossRef]
- Chicka, M.C.; Strehler, E.E. Alternative Splicing of the First Intracellular Loop of Plasma Membrane Ca2+-ATPase Isoform 2 Alters Its Membrane Targeting. J. Biol. Chem. 2003, 278, 18464–18470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enyedi, Á.; Strehler, E.E. Regulation of apical membrane enrichment and retention of plasma membrane Ca2+ ATPase splice variants by the PDZ-domain protein NHERF2. Commun. Integr. Biol. 2011, 4, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Bortolozzi, M.; Mammano, F. PMCA2 pump mutations and hereditary deafness. Neurosci. Lett. 2018, 663, 18–24. [Google Scholar] [CrossRef]
- Giacomello, M.; de Mario, A.; Lopreiato, R.; Primerano, S.; Campeol, M.; Brini, M.; Carafoli, E. Mutations in PMCA2 and hereditary deafness: A molecular analysis of the pump defect. Cell Calcium 2011, 50, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Vicario, M.; Zanni, G.; Vallese, F.; Santorelli, F.M.; Grinzato, A.; Cieri, D.; Berto, P.; Frizzarin, M.; Lopreiato, R.; Zonta, F.; et al. A V1143F mutation in the neuronal-enriched isoform 2 of the PMCA pump is linked with ataxia. Neurobiol. Dis. 2018, 115, 157–166. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Horst, R.L. Ca2+-ATPases and their expression in the mammary gland of pregnant and lactating rats. Am. J. Physiol. Cell Physiol. 1999, 276, C796–C802. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Lippolis, J.D.; Shull, G.E.; Horst, R.L. Null Mutation in the Gene Encoding Plasma Membrane Ca2+-ATPase Isoform 2 Impairs Calcium Transport into Milk. J. Biol. Chem. 2004, 279, 42369–42373. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; VanHouten, J.N.; Dann, P.; Kim, W.; Sullivan, C.; Yu, H.; Liotta, L.; Espina, V.; Stern, D.F.; Friedman, P.A.; et al. PMCA2 regulates HER2 protein kinase localization and signaling and promotes HER2-mediated breast cancer. Proc. Natl. Acad. Sci. USA 2016, 113, E282–E290. [Google Scholar] [CrossRef] [Green Version]
- Romero-Lorca, A.; Gaibar, M.; Armesilla, A.L.; Fernandez-Santander, A.; Novillo, A. Differential expression of PMCA2 mRNA isoforms in a cohort of Spanish patients with breast tumor types. Oncol. Lett. 2018, 16, 6950–6959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eakin, T.J.; Antonelli, M.C.; Malchiodi, E.L.; Baskin, D.G.; Stahl, W.L. Localization of the plasma membrane Ca2+-ATPase isoform PMCA3 in rat cerebellum, choroid plexus and hippocampus. Mol. Brain Res. 1995, 29, 71–80. [Google Scholar] [CrossRef]
- Stauffer, T.P.; Guerini, D.; Carafoli, E. Tissue Distribution of the Four Gene Products of the Plasma Membrane Ca2+ Pump: A study using specific antibodies. J. Biol. Chem. 1995, 270, 12184–12190. [Google Scholar] [CrossRef] [Green Version]
- Greeb, J.; Shull, G.E. Molecular Cloning of a Third Isoform of the Calmodulin-sensitive Plasma Membrane Ca2+-Transporting ATPase That Is Expressed Predominantly in Brain and Skeletal Muscle. J. Biol. Chem. 1989, 264, 18569–18576. [Google Scholar] [CrossRef]
- Zanni, G.; Calì, T.; Kalscheuer, V.; Ottolini, D.; Barresi, S.; Lebrun, N.; Montecchi-Palazzi, L.; Hu, H.; Chelly, J.; Bertini, E.; et al. Mutation of plasma membrane Ca2+ ATPase isoform 3 in a family with X-linked congenital cerebellar ataxia impairs Ca2+ homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, 14514–14519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calì, T.; Frizzarin, M.; Luoni, L.; Zonta, F.; Pantano, S.; Cruz, C.; Bonza, M.C.; Bertipaglia, I.; Ruzzene, M.; de Michelis, M.I.; et al. The ataxia related G1107D mutation of the plasma membrane Ca2+ ATPase isoform 3 affects its interplay with calmodulin and the autoinhibition process. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Beuschlein, F.; Boulkroun, S.; Osswald, A.; Wieland, T.; Nielsen, H.N.; Lichtenauer, U.D.; Penton, D.; Schack, V.R.; Amar, L.; Fischer, E.; et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 2013, 45, 440–444. [Google Scholar] [CrossRef]
- Williams, T.A.; Monticone, S.; Schack, V.R.; Stindl, J.; Burrello, J.; Buffolo, F.; Annaratone, L.; Castellano, I.; Beuschlein, F.; Reincke, M.; et al. Somatic ATP1A1, ATP2B3, and KCNJ5 Mutations in Aldosterone-Producing Adenomas. Hypertension 2014, 63, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Schuh, K.; Cartwright, E.J.; Jankevics, E.; Bundschu, K.; Liebermann, J.; Williams, J.C.; Armesilla, A.L.; Emerson, M.; Oceandy, D.; Knobeloch, K.-P.; et al. Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J. Biol. Chem. 2004, 279, 28220–28226. [Google Scholar] [CrossRef] [Green Version]
- Enyedi, A.; Verma, A.K.; Filoteo, A.G.; Penniston, J.T. A highly active 120-kDa truncated mutant of the plasma membrane Ca2+ pump. J. Biol. Chem. 1993, 268, 10621–10626. [Google Scholar] [CrossRef]
- Ho, P.W.-L.; Pang, S.Y.-Y.; Li, M.; Tse, Z.H.-M.; Kung, M.H.-W.; Sham, P.-C.; Ho, S.-L. PMCA4 (ATP2B4) mutation in familial spastic papraplegia causes delay in intracellular calcium extrusion. Brain Behav. 2015, 5, e00321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Ho, P.W.-L.; Pang, S.Y.-Y.; Tse, Z.H.-M.; Kung, M.H.-W.; Sham, P.-C.; Ho, S.-L. PMCA4 (ATP2B4) Mutation in Familial Spastic Paraplegia. PLoS ONE 2014, 9, e104790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naffa, R.; Padányi, R.; Ignácz, A.; Hegyi, Z.; Jezsó, B.; Tóth, S.; Varga, K.; Homolya, L.; Hegedűs, L.; Schlett, K.; et al. The Plasma Membrane Ca2+ Pump PMCA4b Regulates Melanoma Cell Migration through Remodeling of the Actin Cytoskeleton. Cancers 2021, 13, 1354. [Google Scholar] [CrossRef]
- Barak, P.; Parekh, A.B. Signaling through Ca2+ Microdomains from Store-Operated CRAC Channels. Cold Spring Harb. Perspect. Biol. 2020, 12, a035097. [Google Scholar] [CrossRef] [PubMed]
| PMCA | Location | Destination | KO | Mutation | Disease |
|---|---|---|---|---|---|
| 1 | Ubiquitous | Lethal | |||
| 2 | Neural | Postsynaptic | |||
| 2w | Apical | ||||
| 2w/a | Stereocilia | G283S | Deafness | ||
| G293S | Unbalanced | ||||
| 2w/b | Lact. gland | ||||
| 2b | Breast tumor | HER2-positive | |||
| 2 | CaM-BD | V1143F | Ataxia | ||
| 3 | Neural, skeletal mtheuscle | Presynaptic | |||
| 3 | CaM-BD | G1107D | Ataxia | ||
| 3 | Adenomas | Somatic Mutations | APA | ||
| 4 | Ubiquitous | Male infertility | |||
| 4b | R268Q | FSP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krebs, J. Structure, Function and Regulation of the Plasma Membrane Calcium Pump in Health and Disease. Int. J. Mol. Sci. 2022, 23, 1027. https://doi.org/10.3390/ijms23031027
Krebs J. Structure, Function and Regulation of the Plasma Membrane Calcium Pump in Health and Disease. International Journal of Molecular Sciences. 2022; 23(3):1027. https://doi.org/10.3390/ijms23031027
Chicago/Turabian StyleKrebs, Joachim. 2022. "Structure, Function and Regulation of the Plasma Membrane Calcium Pump in Health and Disease" International Journal of Molecular Sciences 23, no. 3: 1027. https://doi.org/10.3390/ijms23031027
APA StyleKrebs, J. (2022). Structure, Function and Regulation of the Plasma Membrane Calcium Pump in Health and Disease. International Journal of Molecular Sciences, 23(3), 1027. https://doi.org/10.3390/ijms23031027






