The Role of Vitamin A in Retinal Diseases
Abstract
1. Introduction
1.1. Food Sources of Vitamin A
1.2. Hypervitaminosis A
1.3. Hypovitaminosis A
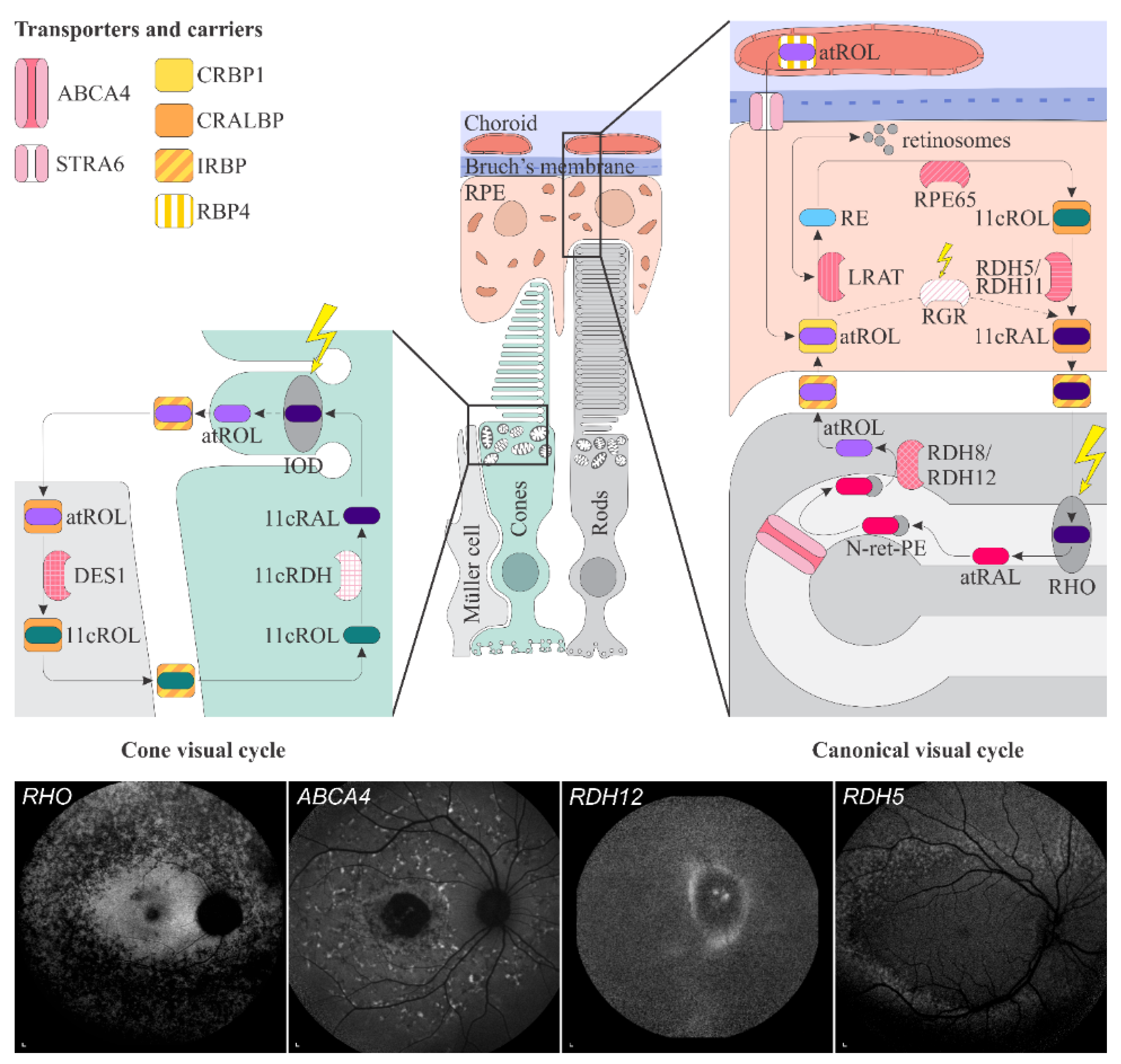
1.4. Vitamin A Pathways in the Retina
1.4.1. Transport from Blood Circulation to the Photoreceptor Outer Segments
1.4.2. Phototransduction
1.4.3. The Visual Cycle
1.4.4. Accumulation and Clearance of Toxic Derivatives of Vitamin A
2. Retinal Diseases Directly or Indirectly Associated with Vitamin A and Its Pathways
2.1. Retinal Signs of Hypovitaminosis A
2.2. Retinal Diseases Associated with Pathogenic Variants in Genes Encoding Proteins Involved with Visual Cycle or Phototransduction
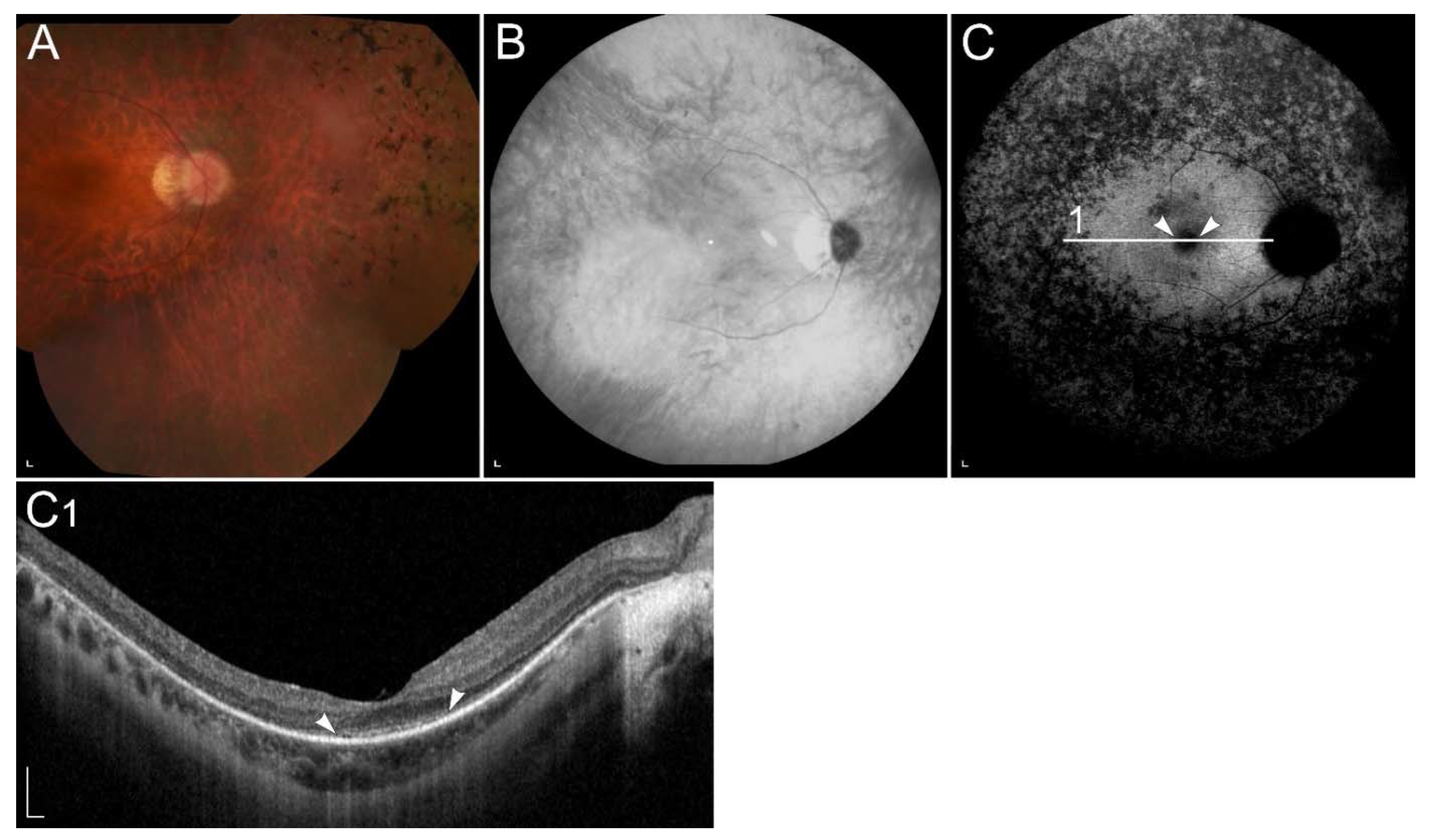
2.2.1. ABCA4-Retinopathy
2.2.2. Retinopathy Due to Pathogenic Variants in RPE65
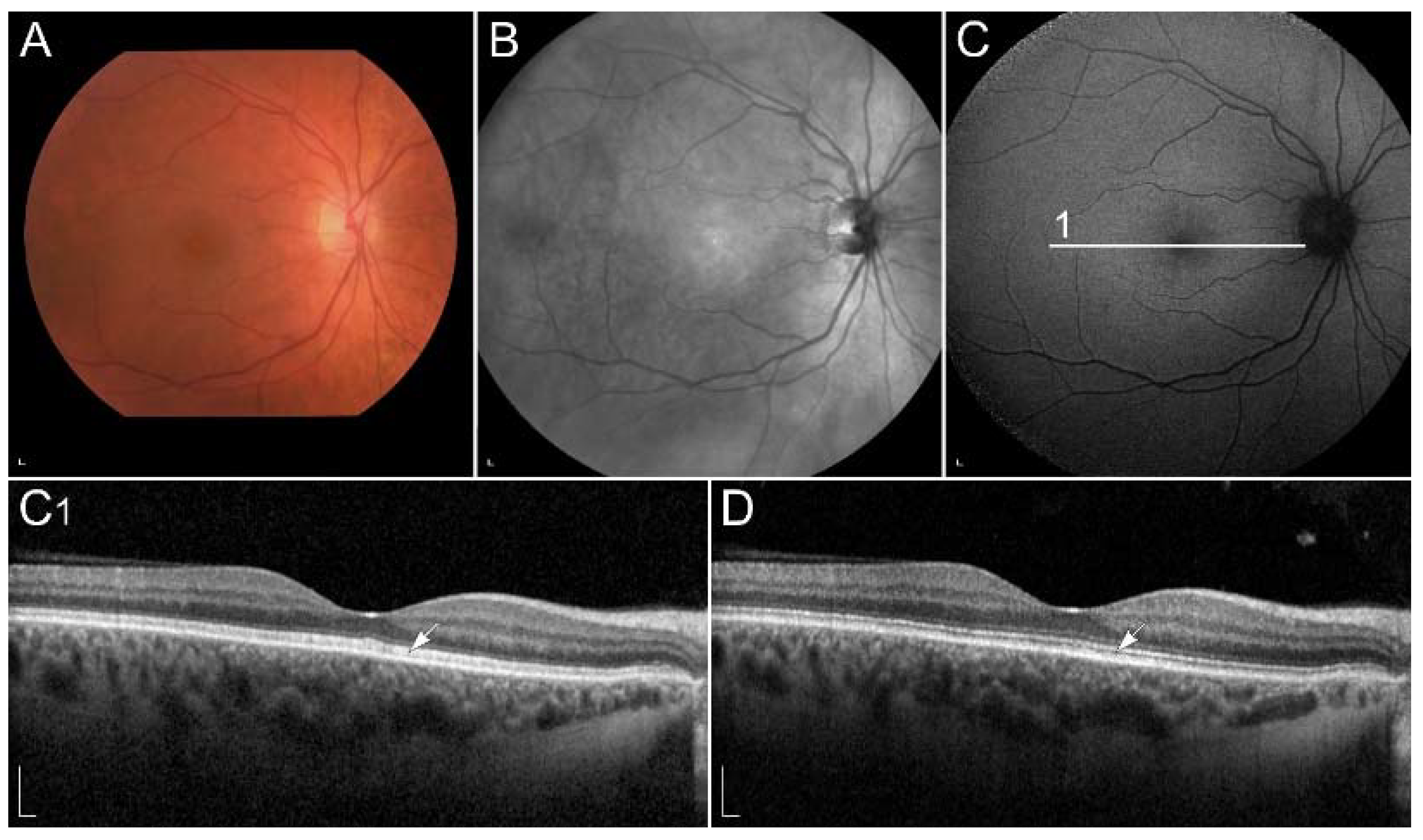
2.2.3. Retinitis Pigmentosa Due to Pathogenic Variants in RHO
2.2.4. Retinopathy Due to Pathogenic Variants in RDH5 or RDH11
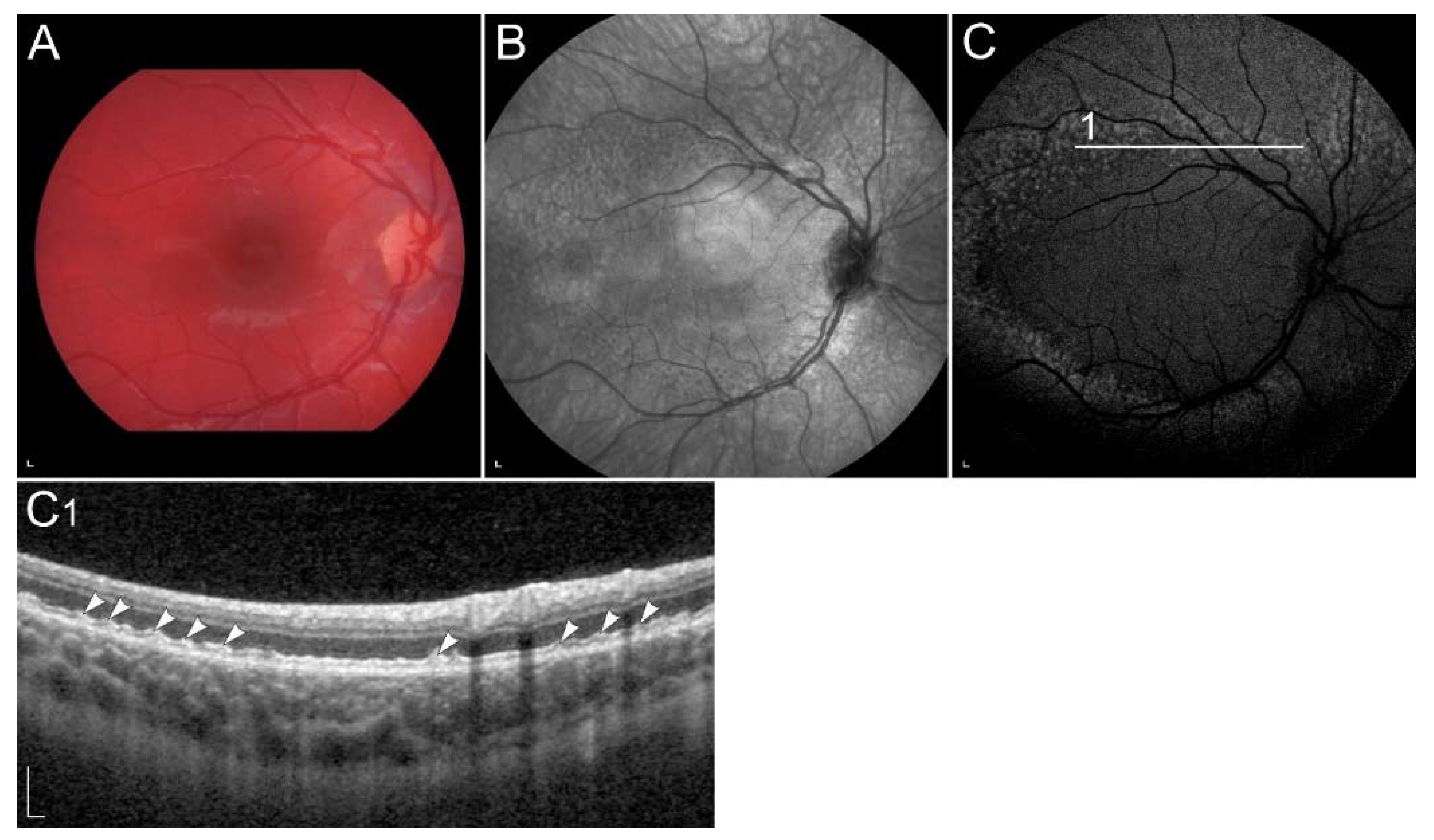
2.2.5. Retinopathy Due to Pathogenic Variants in RDH8 or RDH12
2.2.6. Retinopathy Due to Pathogenic Variants RLBP1
2.2.7. Retinopathy Due to Pathogenic Variants in RBP3
2.2.8. Retinopathy Due to Pathogenic Variants in RBP4
2.2.9. Retinopathy Due to Pathogenic Variants RGR
2.2.10. Retinopathy Due to Pathogenic Variants in LRAT
2.2.11. Retinopathy Due to Pathogenic Variants STRA6
2.3. Retinal Diseases Involving Local Vitamin A Deficiency
2.3.1. Sorsby Fundus Dystrophy TIMP3
2.3.2. Age-Related Macular Degeneration
3. Summary of the Relevant Clinical Trials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef] [PubMed]
- Blaner, W.S.; Li, Y.; Brun, P.J.; Yuen, J.J.; Lee, S.A.; Clugston, R.D. Vitamin A Absorption, Storage and Mobilization. Subcell Biochem. 2016, 81, 95–125. [Google Scholar] [CrossRef]
- O’Byrne, S.M.; Wongsiriroj, N.; Libien, J.; Vogel, S.; Goldberg, I.J.; Baehr, W.; Palczewski, K.; Blaner, W.S. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J. Biol. Chem. 2005, 280, 35647–35657. [Google Scholar] [CrossRef]
- Orban, T.; Palczewska, G.; Palczewski, K. Retinyl ester storage particles (retinosomes) from the retinal pigmented epithelium resemble lipid droplets in other tissues. J. Biol. Chem. 2011, 286, 17248–17258. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.K.; Vogel, S. Vitamin A metabolism and adipose tissue biology. Nutrients 2011, 3, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Chytil, F. The lungs and vitamin A. Am. J. Physiol. 1992, 262, L517–L527. [Google Scholar] [CrossRef] [PubMed]
- Redmond, T.M. RPE65 takes on another role in the vertebrate retina. Proc. Natl. Acad. Sci. USA 2017, 114, 10818–10820. [Google Scholar] [CrossRef]
- Toti, E.; Chen, C.Y.O.; Palmery, M.; Villaño Valencia, D.; Peluso, I. Non-Provitamin A and Provitamin A Carotenoids as Immunomodulators: Recommended Dietary Allowance, Therapeutic Index, or Personalized Nutrition? Oxid. Med. Cell. Longev. 2018, 2018, 4637861. [Google Scholar] [CrossRef]
- Álvarez, R.; Vaz, B.; Gronemeyer, H.; de Lera, Á.R. Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chem. Rev. 2014, 114, 1–125. [Google Scholar] [CrossRef] [PubMed]
- Marzęda, P.; Łuszczki, J.J. Role of vitamin A in health and illness. J. Pre-Clin. Clin. Res. 2019, 13, 137–142. [Google Scholar] [CrossRef]
- Gannon, B.M.; Jones, C.; Mehta, S. Vitamin A Requirements in Pregnancy and Lactation. Curr. Dev. Nutr. 2020, 4, nzaa142. [Google Scholar] [CrossRef]
- Russell, R.M. The vitamin A spectrum: From deficiency to toxicity. Am. J. Clin. Nutr. 2000, 71, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Blomhoff, R. Vitamin A and Carotenoid Toxicity. Food Nutr. Bull. 2001, 22, 320–334. [Google Scholar] [CrossRef]
- Layton, A. The use of isotretinoin in acne. Dermato-Endocrinology 2009, 1, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Bergler-Czop, B.; Bilewicz-Stebel, M.; Stańkowska, A.; Bilewicz-Wyrozumska, T. Side effects of retinoid therapy on the quality of vision. Acta Pharm. 2016, 66, 471–478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gollapalli, D.R.; Rando, R.R. The specific binding of retinoic acid to RPE65 and approaches to the treatment of macular degeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 10030–10035. [Google Scholar] [CrossRef]
- Gamble, M.V.; Mata, N.L.; Tsin, A.T.; Mertz, J.R.; Blaner, W.S. Substrate specificities and 13-cis-retinoic acid inhibition of human, mouse and bovine cis-retinol dehydrogenases. Biochim. Biophys. Acta 2000, 1476, 3–8. [Google Scholar] [CrossRef]
- Radu, R.A.; Mata, N.L.; Nusinowitz, S.; Liu, X.; Sieving, P.A.; Travis, G.H. Treatment with isotretinoin inhibits lipofuscin accumulation in a mouse model of recessive Stargardt’s macular degeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 4742–4747. [Google Scholar] [CrossRef] [PubMed]
- Edigin, E.; Asemota, I.R.; Olisa, E.; Nwaichi, C. Carotenemia: A Case Report. Cureus 2019, 11, e5218. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.A.; Wesołowska, A.; Pawlus, B.; Hamułka, J. Health Effects of Carotenoids during Pregnancy and Lactation. Nutrients 2017, 9, 838. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L., Jr.; Valanis, B.; Williams, J.H., Jr.; et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J. Natl. Cancer Inst. 1996, 88, 1550–1559. [Google Scholar] [CrossRef]
- Albanes, D.; Heinonen, O.P.; Huttunen, J.K.; Taylor, P.R.; Virtamo, J.; Edwards, B.K.; Haapakoski, J.; Rautalahti, M.; Hartman, A.M.; Palmgren, J. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am. J. Clin. Nutr. 1995, 62, 1427S–1430S. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; Sangiovanni, J.P.; Danis, R.P.; Ferris, F.L., 3rd; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; Bressler, S.B.; et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, V.H.; Kowe, P.A.; Kulkarni, M.M.; Singh, R.P. Co-existent Erythromelanosis Follicularis Faciei et Colli and Erythroses Pigmentosa Mediofacialis in a Patient of Generalized Keratosis Pilaris—A Rare Report in a Young Female. Indian Dermatol. Online J. 2020, 11, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C. The eye signs of vitamin A deficiency. Community Eye Health 2013, 26, 66–67. [Google Scholar] [PubMed]
- Choi, E.H.; Daruwalla, A.; Suh, S.; Leinonen, H.; Palczewski, K. Retinoids in the visual cycle: Role of the retinal G protein-coupled receptor. J. Lipid Res. 2021, 62, 100040. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta 2012, 1821, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.H.; Kopec, R.E. Chapter 50—Digestion and Intestinal A. In Physiology of the Gastrointestinal Tract, 6th ed.; Said, H.M., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1133–1151. [Google Scholar] [CrossRef]
- Perusek, L.; Maeda, T. Vitamin A derivatives as treatment options for retinal degenerative diseases. Nutrients 2013, 5, 2646–2666. [Google Scholar] [CrossRef]
- Penniston, K.L.; Tanumihardjo, S.A. The acute and chronic toxic effects of vitamin A. Am. J. Clin. Nutr. 2006, 83, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Kawaguchi, R.; Kassai, M.; Sun, H. Retina, retinol, retinal and the natural history of vitamin A as a light sensor. Nutrients 2012, 4, 2069–2096. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms of Transport and Delivery of Vitamin A and Carotenoids to the Retinal Pigment Epithelium. Mol. Nutr. Food Res. 2019, 63, e1801046. [Google Scholar] [CrossRef]
- Greaves, R.F.; Woollard, G.A.; Hoad, K.E.; Walmsley, T.A.; Johnson, L.A.; Briscoe, S.; Koetsier, S.; Harrower, T.; Gill, J.P. Laboratory medicine best practice guideline: Vitamins a, e and the carotenoids in blood. Clin. Biochem. Rev. 2014, 35, 81–113. [Google Scholar]
- Seeliger, M.W.; Biesalski, H.K.; Wissinger, B.; Gollnick, H.; Gielen, S.; Frank, J.; Beck, S.; Zrenner, E. Phenotype in retinol deficiency due to a hereditary defect in retinol binding protein synthesis. Investig. Ophthalmol. Vis. Sci. 1999, 40, 3–11. [Google Scholar]
- van Bennekum, A.M.; Wei, S.; Gamble, M.V.; Vogel, S.; Piantedosi, R.; Gottesman, M.; Episkopou, V.; Blaner, W.S. Biochemical basis for depressed serum retinol levels in transthyretin-deficient mice. J. Biol. Chem. 2001, 276, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Raz, A.; Goodman, D.S. Retinol-binding protein: The transport protein for vitamin A in human plasma. J. Clin. Investig. 1968, 47, 2025–2044. [Google Scholar] [CrossRef]
- Berry, D.C.; Croniger, C.M.; Ghyselinck, N.B.; Noy, N. Transthyretin blocks retinol uptake and cell signaling by the holo-retinol-binding protein receptor STRA6. Mol. Cell. Biol. 2012, 32, 3851–3859. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, R.; Yu, J.; Wiita, P.; Honda, J.; Sun, H. An essential ligand-binding domain in the membrane receptor for retinol-binding protein revealed by large-scale mutagenesis and a human polymorphism. J. Biol. Chem. 2008, 283, 15160–15168. [Google Scholar] [CrossRef]
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef]
- Kelly, M.; Widjaja-Adhi, M.A.K.; Palczewski, G.; von Lintig, J. Transport of vitamin A across blood-tissue barriers is facilitated by STRA6. FASEB J. 2016, 30, 2985–2995. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, R.; Zhong, M.; Kassai, M.; Ter-Stepanian, M.; Sun, H. STRA6-catalyzed vitamin A influx, efflux, and exchange. J. Membr. Biol. 2012, 245, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.; Sivaprasadarao, A.; DeSousa, M.M.; Findlay, J.B. The transfer of retinol from serum retinol-binding protein to cellular retinol-binding protein is mediated by a membrane receptor. J. Biol. Chem. 1998, 273, 3336–3342. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, R.; Zhong, M.; Kassai, M.; Ter-Stepanian, M.; Sun, H. Vitamin A Transport Mechanism of the Multitransmembrane Cell-Surface Receptor STRA6. Membranes 2015, 5, 425–453. [Google Scholar] [CrossRef] [PubMed]
- Saari, J.C.; Nawrot, M.; Garwin, G.G.; Kennedy, M.J.; Hurley, J.B.; Ghyselinck, N.B.; Chambon, P. Analysis of the visual cycle in cellular retinol-binding protein type I (CRBPI) knockout mice. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1730–1735. [Google Scholar]
- Sears, A.E.; Palczewski, K. Lecithin:Retinol Acyltransferase: A Key Enzyme Involved in the Retinoid (visual) Cycle. Biochemistry 2016, 55, 3082–3091. [Google Scholar] [CrossRef]
- Kiser, P.D.; Golczak, M.; Maeda, A.; Palczewski, K. Key enzymes of the retinoid (visual) cycle in vertebrate retina. Biochim. Biophys. Acta 2012, 1821, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Saari, J.C. Vitamin A metabolism in rod and cone visual cycles. Annu. Rev. Nutr. 2012, 32, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Stecher, H.; Gelb, M.H.; Saari, J.C.; Palczewski, K. Preferential release of 11-cis-retinol from retinal pigment epithelial cells in the presence of cellular retinaldehyde-binding protein. J. Biol. Chem. 1999, 274, 8577–8585. [Google Scholar] [CrossRef]
- Winston, A.; Rando, R.R. Regulation of isomerohydrolase activity in the visual cycle. Biochemistry 1998, 37, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhang, T.; Madigan, M.C.; Fernando, N.; Aggio-Bruce, R.; Zhou, F.; Pierce, M.; Chen, Y.; Huang, L.; Natoli, R.; et al. Interphotoreceptor Retinoid-Binding Protein (IRBP) in Retinal Health and Disease. Front. Cell. Neurosci. 2020, 14, 577935. [Google Scholar] [CrossRef] [PubMed]
- Saari, J.C.; Nawrot, M.; Stenkamp, R.E.; Teller, D.C.; Garwin, G.G. Release of 11-cis-retinal from cellular retinaldehyde-binding protein by acidic lipids. Mol. Vis. 2009, 15, 844–854. [Google Scholar] [PubMed]
- Wang, J.-S.; Kefalov, V.J. The cone-specific visual cycle. Prog. Retin. Eye Res. 2011, 30, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Molday, R.S.; Moritz, O.L. Photoreceptors at a glance. J. Cell Sci. 2015, 128, 4039–4045. [Google Scholar] [CrossRef] [PubMed]
- Kevany, B.M.; Palczewski, K. Phagocytosis of retinal rod and cone photoreceptors. Physiology 2010, 25, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiao, X.; Li, S.; Jia, X.; Guo, X.; Zhang, Q. RGR variants in different forms of retinal diseases: The undetermined role of truncation mutations. Mol. Med. Rep. 2016, 14, 4811–4815. [Google Scholar] [CrossRef] [PubMed]
- Morimura, H.; Saindelle-Ribeaudeau, F.; Berson, E.L.; Dryja, T.P. Mutations in RGR, encoding a light-sensitive opsin homologue, in patients with retinitis pigmentosa. Nat. Genet. 1999, 23, 393–394. [Google Scholar] [CrossRef]
- Wenzel, A.; Oberhauser, V.; Pugh, E.N., Jr.; Lamb, T.D.; Grimm, C.; Samardzija, M.; Fahl, E.; Seeliger, M.W.; Remé, C.E.; von Lintig, J. The retinal G protein-coupled receptor (RGR) enhances isomerohydrolase activity independent of light. J. Biol. Chem. 2005, 280, 29874–29884. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Van Hooser, J.P.; Driessen, C.A.; Filipek, S.; Janssen, J.J.; Palczewski, K. Evaluation of the role of the retinal G protein-coupled receptor (RGR) in the vertebrate retina in vivo. J. Neurochem. 2003, 85, 944–956. [Google Scholar] [CrossRef]
- Kefalov, V.J. Rod and cone visual pigments and phototransduction through pharmacological, genetic, and physiological approaches. J. Biol. Chem. 2012, 287, 1635–1641. [Google Scholar] [CrossRef]
- Sahu, B.; Maeda, A. Retinol Dehydrogenases Regulate Vitamin A Metabolism for Visual Function. Nutrients 2016, 8, 746. [Google Scholar] [CrossRef]
- Wang, J.S.; Kefalov, V.J. An alternative pathway mediates the mouse and human cone visual cycle. Curr. Biol. 2009, 19, 1665–1669. [Google Scholar] [CrossRef]
- Mata, N.L.; Radu, R.A.; Clemmons, R.C.; Travis, G.H. Isomerization and oxidation of vitamin a in cone-dominant retinas: A novel pathway for visual-pigment regeneration in daylight. Neuron 2002, 36, 69–80. [Google Scholar] [CrossRef]
- Kaylor, J.J.; Yuan, Q.; Cook, J.; Sarfare, S.; Makshanoff, J.; Miu, A.; Kim, A.; Kim, P.; Habib, S.; Roybal, C.N.; et al. Identification of DES1 as a vitamin A isomerase in Müller glial cells of the retina. Nat. Chem. Biol. 2013, 9, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kiser, P.D.; Kolesnikov, A.V.; Kiser, J.Z.; Dong, Z.; Chaurasia, B.; Wang, L.; Summers, S.A.; Hoang, T.; Blackshaw, S.; Peachey, N.S.; et al. Conditional deletion of Des1 in the mouse retina does not impair the visual cycle in cones. FASEB J. 2019, 33, 5782–5792. [Google Scholar] [CrossRef]
- Sato, S.; Frederiksen, R.; Cornwall, M.C.; Kefalov, V.J. The retina visual cycle is driven by cis retinol oxidation in the outer segments of cones. Vis. Neurosci. 2017, 34, E004. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.O.; Crouch, R.K. The interphotoreceptor retinoid binding (IRBP) is essential for normal retinoid processing in cone photoreceptors. Adv. Exp. Med. Biol. 2010, 664, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Morshedian, A.; Kaylor, J.J.; Ng, S.Y.; Tsan, A.; Frederiksen, R.; Xu, T.; Yuan, L.; Sampath, A.P.; Radu, R.A.; Fain, G.L.; et al. Light-Driven Regeneration of Cone Visual Pigments through a Mechanism Involving RGR Opsin in Müller Glial Cells. Neuron 2019, 102, 1172–1183. [Google Scholar] [CrossRef]
- Young, R.W. The renewal of rod and cone outer segments in the rhesus monkey. J. Cell Biol. 1971, 49, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Molday, R.S.; Zhong, M.; Quazi, F. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim. Biophys. Acta 2009, 1791, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Lenis, T.L.; Hu, J.; Ng, S.Y.; Jiang, Z.; Sarfare, S.; Lloyd, M.B.; Esposito, N.J.; Samuel, W.; Jaworski, C.; Bok, D.; et al. Expression of ABCA4 in the retinal pigment epithelium and its implications for Stargardt macular degeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E11120–E11127. [Google Scholar] [CrossRef]
- Molday, R.S. Insights into the Molecular Properties of ABCA4 and Its Role in the Visual Cycle and Stargardt Disease. Prog. Mol. Biol. Transl. Sci. 2015, 134, 415–431. [Google Scholar] [CrossRef]
- Kim, H.J.; Montenegro, D.; Zhao, J.; Sparrow, J.R. Bisretinoids of the Retina: Photo-Oxidation, Iron-Catalyzed Oxidation, and Disease Consequences. Antioxidants 2021, 10, 1382. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Wu, Y.; Kim, C.Y.; Zhou, J. Phospholipid meets all-trans-retinal: The making of RPE bisretinoids. J. Lipid Res. 2010, 51, 247–261. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Gregory-Roberts, E.; Yamamoto, K.; Blonska, A.; Ghosh, S.K.; Ueda, K.; Zhou, J. The bisretinoids of retinal pigment epithelium. Prog. Retin. Eye Res. 2012, 31, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.; Klufas, M.A.; Sarraf, D. Clinical applications of fundus autofluorescence in retinal disease. Int. J. Retin. Vitr. 2016, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Sodi, A.; Franco, F.; Murro, V.; Biagini, D.; Miele, A.; Abbruzzese, G.; Mucciolo, D.P.; Virgili, G.; Menchini, U.; et al. Dietary profile of patients with Stargardt’s disease and Retinitis Pigmentosa: Is there a role for a nutritional approach? BMC Ophthalmol. 2016, 16, 13. [Google Scholar] [CrossRef][Green Version]
- Anand-Apte, B.; Chao, J.R.; Singh, R.; Stöhr, H. Sorsby fundus dystrophy: Insights from the past and looking to the future. J. Neurosci. Res. 2019, 97, 88–97. [Google Scholar] [CrossRef]
- Brito-García, N.; Del Pino-Sedeño, T.; Trujillo-Martín, M.M.; Coco, R.M.; Rodríguez de la Rúa, E.; Del Cura-González, I.; Serrano-Aguilar, P. Effectiveness and safety of nutritional supplements in the treatment of hereditary retinal dystrophies: A systematic review. Eye 2017, 31, 273–285. [Google Scholar] [CrossRef]
- Miyazono, S.; Shimauchi-Matsukawa, Y.; Tachibanaki, S.; Kawamura, S. Highly efficient retinal metabolism in cones. Proc. Natl. Acad. Sci. USA 2008, 105, 16051–16056. [Google Scholar] [CrossRef]
- Saker, S.; Morales, M.; Jhittay, H.; Wen, Y.; Amoaku, W. Electrophysiological and microperimetry changes in vitamin A deficiency retinopathy. Doc. Ophthalmol. 2015, 130, 231–240. [Google Scholar] [CrossRef] [PubMed]
- McBain, V.A.; Egan, C.A.; Pieris, S.J.; Supramaniam, G.; Webster, A.R.; Bird, A.C.; Holder, G.E. Functional observations in vitamin A deficiency: Diagnosis and time course of recovery. Eye 2007, 21, 367–376. [Google Scholar] [CrossRef]
- Kakiuchi, D.; Uehara, T.; Shiotani, M.; Nakano-Ito, K.; Suganuma, A.; Aoki, T.; Tsukidate, K.; Sawada, K. Oscillatory potentials in electroretinogram as an early marker of visual abnormalities in vitamin A deficiency. Mol. Med. Rep. 2015, 11, 995–1003. [Google Scholar] [CrossRef]
- Jevnikar, K.; Šuštar, M.; Kozjek, N.R.; Štrucl, A.M.; Markelj, Š.; Hawlina, M.; Fakin, A. Disruption of the outer segments of the photoreceptors on OCT as a feature of vitamin A deficiency. Retin. Cases Brief Rep. 2020. [Google Scholar] [CrossRef] [PubMed]
- Aleman, T.S.; Garrity, S.T.; Brucker, A.J. Retinal structure in vitamin A deficiency as explored with multimodal imaging. Doc. Ophthalmol. 2013, 127, 239–243. [Google Scholar] [CrossRef]
- Apushkin, M.A.; Fishman, G.A. Improvement in visual function and fundus findings for a patient with vitamin A-deficient retinopathy. Retina 2005, 25, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Lima de Carvalho, J.R., Jr.; Tsang, S.H.; Sparrow, J.R. Vitamin a Deficiency Monitored by Quantitative Short Wavelength Fundus Autofluorescence in a Case of Bariatric Surgery. Retin. Cases Brief Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Lima de Carvalho, J.R.; Ryu, J.; Tsang, S.H.; Sparrow, J.R. Short-Wavelength and Near-Infrared Autofluorescence in Patients with Deficiencies of the Visual Cycle and Phototransduction. Sci. Rep. 2020, 10, 8998. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, A.; Trief, D.; Wang, N.-K.; Chang, S.; Tsang, S.H. Vitamin A deficiency in New York City. Lancet 2010, 376, 267. [Google Scholar] [CrossRef][Green Version]
- Wang, N.K.; Chuang, L.H.; Lai, C.C.; Chou, C.L.; Chu, H.Y.; Yeung, L.; Chen, Y.P.; Chen, K.J.; Wu, W.C.; Chen, T.L.; et al. Multimodal fundus imaging in fundus albipunctatus with RDH5 mutation: A newly identified compound heterozygous mutation and review of the literature. Doc. Ophthalmol. 2012, 125, 51–62. [Google Scholar] [CrossRef]
- Owsley, C.; McGwin, G.; Jackson, G.R.; Heimburger, D.C.; Piyathilake, C.J.; Klein, R.; White, M.F.; Kallies, K. Effect of short-term, high-dose retinol on dark adaptation in aging and early age-related maculopathy. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Cremers, F.P.M.; Lee, W.; Collin, R.W.J.; Allikmets, R. Clinical spectrum, genetic complexity and therapeutic approaches for retinal disease caused by ABCA4 mutations. Prog. Retin. Eye Res. 2020, 79, 100861. [Google Scholar] [CrossRef]
- Tsybovsky, Y.; Molday, R.S.; Palczewski, K. The ATP-binding cassette transporter ABCA4: Structural and functional properties and role in retinal disease. Adv. Exp. Med. Biol. 2010, 703, 105–125. [Google Scholar] [CrossRef]
- Quazi, F.; Molday, R.S. ATP-binding cassette transporter ABCA4 and chemical isomerization protect photoreceptor cells from the toxic accumulation of excess 11-cis-retinal. Proc. Natl. Acad. Sci. USA 2014, 111, 5024–5029. [Google Scholar] [CrossRef] [PubMed]
- Conley, S.M.; Cai, X.; Makkia, R.; Wu, Y.; Sparrow, J.R.; Naash, M.I. Increased cone sensitivity to ABCA4 deficiency provides insight into macular vision loss in Stargardt’s dystrophy. Biochim. Biophys. Acta 2012, 1822, 1169–1179. [Google Scholar] [CrossRef]
- Fakin, A.; Robson, A.G.; Fujinami, K.; Moore, A.T.; Michaelides, M.; Holder, G.E.; Webster, A.R. Phenotype and Progression of Retinal Degeneration Associated with Nullizigosity of ABCA4. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4668–4678. [Google Scholar] [CrossRef]
- Tanna, P.; Strauss, R.W.; Fujinami, K.; Michaelides, M. Stargardt disease: Clinical features, molecular genetics, animal models and therapeutic options. Br. J. Ophthalmol. 2017, 101, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Federspiel, C.A.; Bertelsen, M.; Kessel, L. Vitamin A in Stargardt disease-an evidence-based update. Ophthalmic Genet. 2018, 39, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Radu, R.A.; Yuan, Q.; Hu, J.; Peng, J.H.; Lloyd, M.; Nusinowitz, S.; Bok, D.; Travis, G.H. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following Vitamin A supplementation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3821–3829. [Google Scholar] [CrossRef]
- Piotter, E.; McClements, M.E.; MacLaren, R.E. Therapy Approaches for Stargardt Disease. Biomolecules 2021, 11, 1179. [Google Scholar] [CrossRef]
- Hussain, R.M.; Ciulla, T.A.; Berrocal, A.M.; Gregori, N.Z.; Flynn, H.W.; Lam, B.L. Stargardt macular dystrophy and evolving therapies. Expert Opin. Biol. Ther. 2018, 18, 1049–1059. [Google Scholar] [CrossRef]
- Marmor, M.F.; Jain, A.; Moshfeghi, D. Total rod ERG suppression with high dose compassionate Fenretinide usage. Doc. Ophthalmol. 2008, 117, 257–261. [Google Scholar] [CrossRef]
- Zhang, J.; Kiser, P.D.; Badiee, M.; Palczewska, G.; Dong, Z.; Golczak, M.; Tochtrop, G.P.; Palczewski, K. Molecular pharmacodynamics of emixustat in protection against retinal degeneration. J. Clin. Investig. 2015, 125, 2781–2794. [Google Scholar] [CrossRef]
- Dobri, N.; Qin, Q.; Kong, J.; Yamamoto, K.; Liu, Z.; Moiseyev, G.; Ma, J.X.; Allikmets, R.; Sparrow, J.R.; Petrukhin, K. A1120, a nonretinoid RBP4 antagonist, inhibits formation of cytotoxic bisretinoids in the animal model of enhanced retinal lipofuscinogenesis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Mata, N.L.; Lichter, J.B.; Vogel, R.; Han, Y.; Bui, T.V.; Singerman, L.J. Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina 2013, 33, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Charbel Issa, P.; Barnard, A.R.; Herrmann, P.; Washington, I.; MacLaren, R.E. Rescue of the Stargardt phenotype in Abca4 knockout mice through inhibition of vitamin A dimerization. Proc. Natl. Acad. Sci. USA 2015, 112, 8415–8420. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, Y.; Ma, L.; Washington, I. Deuterium enrichment of vitamin A at the C20 position slows the formation of detrimental vitamin A dimers in wild-type rodents. J. Biol. Chem. 2011, 286, 7958–7965. [Google Scholar] [CrossRef]
- Ma, L.; Kaufman, Y.; Zhang, J.; Washington, I. C20-D3-vitamin A slows lipofuscin accumulation and electrophysiological retinal degeneration in a mouse model of Stargardt disease. J. Biol. Chem. 2011, 286, 7966–7974. [Google Scholar] [CrossRef]
- Cai, X.; Conley, S.M.; Naash, M.I. RPE65: Role in the visual cycle, human retinal disease, and gene therapy. Ophthalmic Genet. 2009, 30, 57–62. [Google Scholar] [CrossRef]
- Cideciyan, A.V. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog. Retin. Eye Res. 2010, 29, 398–427. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Moiseyev, G.; Nikolaeva, O.; Ma, J.-x. Identification of the key residues determining the product specificity of isomerohydrolase. Biochemistry 2012, 51, 4217–4225. [Google Scholar] [CrossRef]
- Li, S.; Xiao, X.; Yi, Z.; Sun, W.; Wang, P.; Zhang, Q. RPE65 mutation frequency and phenotypic variation according to exome sequencing in a tertiary centre for genetic eye diseases in China. Acta Ophthalmol. 2020, 98, e181–e190. [Google Scholar] [CrossRef] [PubMed]
- Aoun, M.; Passerini, I.; Chiurazzi, P.; Karali, M.; De Rienzo, I.; Sartor, G.; Murro, V.; Filimonova, N.; Seri, M.; Banfi, S. Inherited Retinal Diseases Due to RPE65 Variants: From Genetic Diagnostic Management to Therapy. Int. J. Mol. Sci. 2021, 22, 7207. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, N.; Moore, A.T.; Weleber, R.G.; Michaelides, M. Leber congenital amaurosis/early-onset severe retinal dystrophy: Clinical features, molecular genetics and therapeutic interventions. Br. J. Ophthalmol. 2017, 101, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.G.; Aleman, T.S.; Cideciyan, A.V.; Roman, A.J.; Sumaroka, A.; Windsor, E.A.; Schwartz, S.B.; Heon, E.; Stone, E.M. Defining the residual vision in leber congenital amaurosis caused by RPE65 mutations. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, B.; Wabbels, B.; Wegscheider, E.; Hamel, C.P.; Drexler, W.; Preising, M.N. Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology 2004, 111, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, N.; Georgiou, M.; Bainbridge, J.W.B.; Bertelsen, M.; Larsen, M.; Blanco-Kelly, F.; Ayuso, C.; Tran, H.V.; Munier, F.L.; Kalitzeos, A.; et al. Retinal Structure in RPE65-Associated Retinal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 47. [Google Scholar] [CrossRef]
- Katz, M.L.; Redmond, T.M. Effect of Rpe65 knockout on accumulation of lipofuscin fluorophores in the retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3023–3030. [Google Scholar]
- Van Hooser, J.P.; Aleman, T.S.; He, Y.-G.; Cideciyan, A.V.; Kuksa, V.; Pittler, S.J.; Stone, E.M.; Jacobson, S.G.; Palczewski, K. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc. Natl. Acad. Sci. USA 2000, 97, 8623. [Google Scholar] [CrossRef]
- Maeda, T.; Dong, Z.; Jin, H.; Sawada, O.; Gao, S.; Utkhede, D.; Monk, W.; Palczewska, G.; Palczewski, K. QLT091001, a 9-cis-retinal analog, is well-tolerated by retinas of mice with impaired visual cycles. Investig. Ophthalmol. Vis. Sci. 2013, 54, 455–466. [Google Scholar] [CrossRef]
- Scholl, H.P.N.; Moore, A.T.; Koenekoop, R.K.; Wen, Y.; Fishman, G.A.; van den Born, L.I.; Bittner, A.; Bowles, K.; Fletcher, E.C.; Collison, F.T.; et al. Safety and Proof-of-Concept Study of Oral QLT091001 in Retinitis Pigmentosa Due to Inherited Deficiencies of Retinal Pigment Epithelial 65 Protein (RPE65) or Lecithin:Retinol Acyltransferase (LRAT). PLoS ONE 2015, 10, e0143846. [Google Scholar] [CrossRef]
- Koenekoop, R.K.; Sui, R.; Sallum, J.; van den Born, L.I.; Ajlan, R.; Khan, A.; den Hollander, A.I.; Cremers, F.P.; Mendola, J.D.; Bittner, A.K.; et al. Oral 9-cis retinoid for childhood blindness due to Leber congenital amaurosis caused by RPE65 or LRAT mutations: An open-label phase 1b trial. Lancet 2014, 384, 1513–1520. [Google Scholar] [CrossRef]
- Wang, X.; Yu, C.; Tzekov, R.T.; Zhu, Y.; Li, W. The effect of human gene therapy for RPE65-associated Leber’s congenital amaurosis on visual function: A systematic review and meta-analysis. Orphanet J. Rare Dis. 2020, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Miraldi Utz, V.; Coussa, R.G.; Antaki, F.; Traboulsi, E.I. Gene therapy for RPE65-related retinal disease. Ophthalmic Genet. 2018, 39, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Daiger, S.P.; Sullivan, L.S.; Bowne, S.J. Genes and mutations causing retinitis pigmentosa. Clin. Genet. 2013, 84, 132–141. [Google Scholar] [CrossRef]
- Beryozkin, A.; Levy, G.; Blumenfeld, A.; Meyer, S.; Namburi, P.; Morad, Y.; Gradstein, L.; Swaroop, A.; Banin, E.; Sharon, D. Genetic Analysis of the Rhodopsin Gene Identifies a Mosaic Dominant Retinitis Pigmentosa Mutation in a Healthy Individual. Investig. Ophthalmol. Vis. Sci. 2016, 57, 940–947. [Google Scholar] [CrossRef]
- Xiao, T.; Xu, K.; Zhang, X.; Xie, Y.; Li, Y. Sector Retinitis Pigmentosa caused by mutations of the RHO gene. Eye 2019, 33, 592–599. [Google Scholar] [CrossRef]
- Dryja, T.P.; Mukai, S.; Petersen, R.; Rapaport, J.M.; Walton, D.; Yandell, D.W. Parental origin of mutations of the retinoblastoma gene. Nature 1989, 339, 556–558. [Google Scholar] [CrossRef]
- Berson, E.L.; Rosner, B.; Sandberg, M.A.; Hayes, K.C.; Nicholson, B.W.; Weigel-DiFranco, C.; Willett, W. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch. Ophthalmol. 1993, 111, 761–772. [Google Scholar] [CrossRef]
- Berson, E.L.; Rosner, B.; Sandberg, M.A.; Weigel-DiFranco, C.; Willett, W.C. ω-3 intake and visual acuity in patients with retinitis pigmentosa receiving vitamin A. Arch. Ophthalmol. 2012, 130, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Berson, E.L.; Rosner, B.; Sandberg, M.A.; Weigel-DiFranco, C.; Brockhurst, R.J.; Hayes, K.C.; Johnson, E.J.; Anderson, E.J.; Johnson, C.A.; Gaudio, A.R.; et al. Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin A. Arch. Ophthalmol. 2010, 128, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, H.; Melia, M.; Dagnelie, G. Lutein supplementation in retinitis pigmentosa: PC-based vision assessment in a randomized double-masked placebo-controlled clinical trial [NCT00029289]. BMC Ophthalmol. 2006, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.O.; Crouch, R.K. Retinol dehydrogenases (RDHs) in the visual cycle. Exp. Eye Res. 2010, 91, 788–792. [Google Scholar] [CrossRef]
- Simon, A.; Lagercrantz, J.; Bajalica-Lagercrantz, S.; Eriksson, U. Primary structure of human 11-cis retinol dehydrogenase and organization and chromosomal localization of the corresponding gene. Genomics 1996, 36, 424–430. [Google Scholar] [CrossRef]
- Simon, A.; Hellman, U.; Wernstedt, C.; Eriksson, U. The retinal pigment epithelial-specific 11-cis retinol dehydrogenase belongs to the family of short chain alcohol dehydrogenases. J. Biol. Chem. 1995, 270, 1107–1112. [Google Scholar] [CrossRef]
- Krill, A.E.; Klien, B.A. Flecked Retina Syndrome. Arch. Ophthalmol. 1965, 74, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Simon, A.; Eriksson, U.; Harris, E.; Berson, E.L.; Dryja, T.P. Mutations in the gene encoding 11-cis retinol dehydrogenase cause delayed dark adaptation and fundus albipunctatus. Nat. Genet. 1999, 22, 188–191. [Google Scholar] [CrossRef]
- Sergouniotis, P.I.; Sohn, E.H.; Li, Z.; McBain, V.A.; Wright, G.A.; Moore, A.T.; Robson, A.G.; Holder, G.E.; Webster, A.R. Phenotypic variability in RDH5 retinopathy (Fundus Albipunctatus). Ophthalmology 2011, 118, 1661–1670. [Google Scholar] [CrossRef]
- Katagiri, S.; Hayashi, T.; Nakamura, M.; Mizobuchi, K.; Gekka, T.; Komori, S.; Ueno, S.; Terasaki, H.; Sakuramoto, H.; Kuniyoshi, K.; et al. RDH5-Related Fundus Albipunctatus in a Large Japanese Cohort. Investig. Ophthalmol. Vis. Sci. 2020, 61, 53. [Google Scholar] [CrossRef]
- Schatz, P.; Preising, M.; Lorenz, B.; Sander, B.; Larsen, M.; Eckstein, C.; Rosenberg, T. Lack of autofluorescence in fundus albipunctatus associated with mutations in RDH5. Retina 2010, 30, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Skorczyk-Werner, A.; Pawłowski, P.; Michalczuk, M.; Warowicka, A.; Wawrocka, A.; Wicher, K.; Bakunowicz-Łazarczyk, A.; Krawczyński, M.R. Fundus albipunctatus: Review of the literature and report of a novel RDH5 gene mutation affecting the invariant tyrosine (p.Tyr175Phe). J. Appl. Genet. 2015, 56, 317–327. [Google Scholar] [CrossRef]
- Driessen, C.A.; Winkens, H.J.; Hoffmann, K.; Kuhlmann, L.D.; Janssen, B.P.; Van Vugt, A.H.; Van Hooser, J.P.; Wieringa, B.E.; Deutman, A.F.; Palczewski, K.; et al. Disruption of the 11-cis-retinol dehydrogenase gene leads to accumulation of cis-retinols and cis-retinyl esters. Mol. Cell Biol. 2000, 20, 4275–4287. [Google Scholar] [CrossRef]
- Niwa, Y.; Kondo, M.; Ueno, S.; Nakamura, M.; Terasaki, H.; Miyake, Y. Cone and Rod Dysfunction in Fundus Albipunctatus with RDH5 Mutation: An Electrophysiological Study. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Rotenstreich, Y.; Harats, D.; Shaish, A.; Pras, E.; Belkin, M. Treatment of a retinal dystrophy, fundus albipunctatus, with oral 9-cis-β-carotene. Br. J. Ophthalmol. 2010, 94, 616–621. [Google Scholar] [CrossRef]
- Haeseleer, F.; Jang, G.-F.; Imanishi, Y.; Driessen, C.A.G.G.; Matsumura, M.; Nelson, P.S.; Palczewski, K. Dual-substrate specificity short chain retinol dehydrogenases from the vertebrate retina. J. Biol. Chem. 2002, 277, 45537–45546. [Google Scholar] [CrossRef]
- Kim, T.S.; Maeda, A.; Maeda, T.; Heinlein, C.; Kedishvili, N.; Palczewski, K.; Nelson, P.S. Delayed dark adaptation in 11-cis-retinol dehydrogenase-deficient mice: A role of RDH11 in visual processes in vivo. J. Biol. Chem. 2005, 280, 8694–8704. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.A.; Lee, W.; Cai, C.; Gambin, T.; Nõupuu, K.; Sujirakul, T.; Ayuso, C.; Jhangiani, S.; Muzny, D.; Boerwinkle, E.; et al. New syndrome with retinitis pigmentosa is caused by nonsense mutations in retinol dehydrogenase RDH11. Hum. Mol. Genet. 2014, 23, 5774–5780. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Lee, W.; Sengillo, J.D.; Allikmets, R.; Garg, K.; Tsang, S.H. Peripapillary sparing in RDH12-associated Leber congenital amaurosis. Ophthalmic Genet. 2017, 38, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Daruwalla, A.; Choi, E.H.; Palczewski, K.; Kiser, P.D. Structural biology of 11-cis-retinaldehyde production in the classical visual cycle. Biochem. J. 2018, 475, 3171–3188. [Google Scholar] [CrossRef]
- Ba-Abbad, R.; Arno, G.; Robson, A.G.; Bouras, K.; Georgiou, M.; Wright, G.; Webster, A.R.; Michaelides, M. Macula-predominant retinopathy associated with biallelic variants in RDH12. Ophthalmic Genet. 2020, 41, 612–615. [Google Scholar] [CrossRef]
- Mackay, D.S.; Dev Borman, A.; Moradi, P.; Henderson, R.H.; Li, Z.; Wright, G.A.; Waseem, N.; Gandra, M.; Thompson, D.A.; Bhattacharya, S.S.; et al. RDH12 retinopathy: Novel mutations and phenotypic description. Mol. Vis. 2011, 17, 2706–2716. [Google Scholar] [PubMed]
- Fahim, A.T.; Bouzia, Z.; Branham, K.H.; Kumaran, N.; Vargas, M.E.; Feathers, K.L.; Perera, N.D.; Young, K.; Khan, N.W.; Heckenlively, J.R.; et al. Detailed clinical characterisation, unique features and natural history of autosomal recessive RDH12 associated retinal degeneration. Br. J. Ophthalmol. 2019, 103, 1789. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.A.; Place, E.M.; Ferenchak, K.; Zampaglione, E.; Wagner, N.E.; Chao, K.R.; DiTroia, S.P.; Navarro-Gomez, D.; Mukai, S.; Huckfeldt, R.M.; et al. Expanding the phenotypic spectrum in RDH12-associated retinal disease. Cold Spring Harb. Mol. Case Stud. 2020, 6, a004754. [Google Scholar] [CrossRef] [PubMed]
- Bunt-Milam, A.H.; Saari, J.C. Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. J. Cell Biol. 1983, 97, 703–712. [Google Scholar] [CrossRef]
- Bernal, S.; Calaf, M.; Adan, A.; Solans, T.; Valverde, D.; Ayuso, C.; Baiget, M. Evaluation of RLBP1 in 50 autosomal recessive retinitis pigmentosa and 4 retinitis punctata albescens Spanish families. Ophthalmic Genet. 2001, 22, 19–25. [Google Scholar] [CrossRef]
- Kolesnikov, A.V.; Kiser, P.D.; Palczewski, K.; Kefalov, V.J. Function of mammalian M-cones depends on the level of CRALBP in Müller cells. J. Gen. Physiol. 2021, 153, e202012675. [Google Scholar] [CrossRef]
- Xue, Y.; Shen, S.Q.; Jui, J.; Rupp, A.C.; Byrne, L.C.; Hattar, S.; Flannery, J.G.; Corbo, J.C.; Kefalov, V.J. CRALBP supports the mammalian retinal visual cycle and cone vision. J. Clin. Investig. 2015, 125, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Lima de Carvalho, J.R., Jr.; Kim, H.J.; Ueda, K.; Zhao, J.; Owji, A.P.; Yang, T.; Tsang, S.H.; Sparrow, J.R. Effects of deficiency in the RLBP1-encoded visual cycle protein CRALBP on visual dysfunction in humans and mice. J. Biol. Chem. 2020, 295, 6767–6780. [Google Scholar] [CrossRef] [PubMed]
- Torres-Costa, S.; Ferreira, C.S.; Grangeia, A.; Santos-Silva, R.; Brandão, E.; Estrela-Silva, S.; Falcão-Reis, F. A novel homozygous frameshift variant in the cellular retinaldehyde-binding protein 1 (RLBP1) gene causes retinitis punctata albescens. Eur. J. Ophthalmol. 2021, 31, NP74–NP80. [Google Scholar] [CrossRef]
- Scimone, C.; Donato, L.; Esposito, T.; Rinaldi, C.; D’Angelo, R.; Sidoti, A. A novel RLBP1 gene geographical area-related mutation present in a young patient with retinitis punctata albescens. Hum. Genom. 2017, 11, 18. [Google Scholar] [CrossRef]
- Dessalces, E.; Bocquet, B.; Bourien, J.; Zanlonghi, X.; Verdet, R.; Meunier, I.; Hamel, C.P. Early-Onset Foveal Involvement in Retinitis Punctata Albescens With Mutations in RLBP1. JAMA Ophthalmol. 2013, 131, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, B.; El Alami Trebki, H.; Roux, A.F.; Labesse, G.; Brabet, P.; Arndt, C.; Zanlonghi, X.; Defoort-Dhellemmes, S.; Hamroun, D.; Boulicot-Séguin, C.; et al. Retinitis Punctata Albescens and RLBP1-Allied Phenotypes: Phenotype–Genotype Correlation and Natural History in the Aim of Gene Therapy. Ophthalmol. Sci. 2021, 1, 100052. [Google Scholar] [CrossRef]
- Farrar, G.J.; Carrigan, M.; Dockery, A.; Millington-Ward, S.; Palfi, A.; Chadderton, N.; Humphries, M.; Kiang, A.S.; Kenna, P.F.; Humphries, P. Toward an elucidation of the molecular genetics of inherited retinal degenerations. Hum. Mol. Genet. 2017, 26, R2–R11. [Google Scholar] [CrossRef] [PubMed]
- Kiser, P.D.; Palczewski, K. Retinoids and Retinal Diseases. Annu. Rev. Vis. Sci. 2016, 2, 197–234. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, Z.; Hu, J.; Gordon, W.C.; Bazan, N.G.; Haas, A.L.; Bok, D.; Jin, M. Secretory Defect and Cytotoxicity: The Potential Disease Mechanisms for the Retinitis Pigmentosa (RP)-Associated Interphotoreceptor Retinoid-Binding Protein (IRBP). J. Biol. Chem. 2013, 288, 11395–11406. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, A.I.; McGee, T.L.; Ziviello, C.; Banfi, S.; Dryja, T.P.; Gonzalez-Fernandez, F.; Ghosh, D.; Berson, E.L. A homozygous missense mutation in the IRBP gene (RBP3) associated with autosomal recessive retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1864–1872. [Google Scholar] [CrossRef]
- Arno, G.; Hull, S.; Robson, A.G.; Holder, G.E.; Cheetham, M.E.; Webster, A.R.; Plagnol, V.; Moore, A.T. Lack of Interphotoreceptor Retinoid Binding Protein Caused by Homozygous Mutation of RBP3 Is Associated with High Myopia and Retinal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2358–2365. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Frank, J.; Beck, S.C.; Heinrich, F.; Illek, B.; Reifen, R.; Gollnick, H.; Seeliger, M.W.; Wissinger, B.; Zrenner, E. Biochemical but not clinical vitamin A deficiency results from mutations in the gene for retinol binding protein. Am. J. Clin. Nutr. 1999, 69, 931–936. [Google Scholar] [CrossRef]
- Cukras, C.; Gaasterland, T.; Lee, P.; Gudiseva, H.V.; Chavali, V.R.; Pullakhandam, R.; Maranhao, B.; Edsall, L.; Soares, S.; Reddy, G.B.; et al. Exome analysis identified a novel mutation in the RBP4 gene in a consanguineous pedigree with retinal dystrophy and developmental abnormalities. PLoS ONE 2012, 7, e50205. [Google Scholar] [CrossRef]
- Khan, K.N.; Carss, K.; Raymond, F.L.; Islam, F.; Nihr BioResource-Rare Diseases, C.; Moore, A.T.; Michaelides, M.; Arno, G. Vitamin A deficiency due to bi-allelic mutation of RBP4: There’s more to it than meets the eye. Ophthalmic Genet. 2017, 38, 465–466. [Google Scholar] [CrossRef]
- Chou, C.M.; Nelson, C.; Tarlé, S.A.; Pribila, J.T.; Bardakjian, T.; Woods, S.; Schneider, A.; Glaser, T. Biochemical Basis for Dominant Inheritance, Variable Penetrance, and Maternal Effects in RBP4 Congenital Eye Disease. Cell 2015, 161, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Cehajic-Kapetanovic, J.; Jasani, K.M.; Shanks, M.; Clouston, P.; MacLaren, R.E. A novel homozygous c.67C>T variant in retinol binding protein 4 (RBP4) associated with retinitis pigmentosa and childhood acne vulgaris. Ophthalmic Genet. 2020, 41, 288–292. [Google Scholar] [CrossRef]
- Chen, X.N.; Korenberg, J.R.; Jiang, M.; Shen, D.; Fong, H.K. Localization of the human RGR opsin gene to chromosome 10q23. Hum. Genet. 1996, 97, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Pandey, S.; Fong, H.K. An opsin homologue in the retina and pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3669–3678. [Google Scholar]
- Pandey, S.; Blanks, J.C.; Spee, C.; Jiang, M.; Fong, H.K. Cytoplasmic retinal localization of an evolutionary homolog of the visual pigments. Exp. Eye Res. 1994, 58, 605–613. [Google Scholar] [CrossRef]
- Ba-Abbad, R.; Leys, M.; Wang, X.; Chakarova, C.; Waseem, N.; Carss, K.J.; Raymond, F.L.; Bujakowska, K.M.; Pierce, E.A.; Mahroo, O.A.; et al. Clinical Features of a Retinopathy Associated With a Dominant Allele of the RGR Gene. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4812–4820. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Yang, C.-M.; Yang, C.-H.; Hou, Y.-C.; Chen, T.-C. Leber’s Congenital Amaurosis: Current Concepts of Genotype-Phenotype Correlations. Genes 2021, 12, 1261. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, L.; Jiao, X.; Riazuddin, S.; Riazuddin, S.A.; Fielding Hetmancik, J. A novel LRAT mutation affecting splicing in a family with early onset retinitis pigmentosa. Hum. Genom. 2018, 12, 35. [Google Scholar] [CrossRef]
- Dev Borman, A.; Ocaka, L.A.; Mackay, D.S.; Ripamonti, C.; Henderson, R.H.; Moradi, P.; Hall, G.; Black, G.C.; Robson, A.G.; Holder, G.E.; et al. Early onset retinal dystrophy due to mutations in LRAT: Molecular analysis and detailed phenotypic study. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3927–3938. [Google Scholar] [CrossRef]
- Saini, A.; Almasarweh, S.; Acosta, S.; Jayakar, P.; Janvier, M.; Wong, T.C.; Salyakina, D.; Sasaki, J. Syndromic Microphthalmia 9: Role of rapid genome sequencing and novel mutations in STRA6 gene. Prog. Pediatric Cardiol. 2021, 101443. [Google Scholar] [CrossRef]
- Ruiz, A.; Mark, M.; Jacobs, H.; Klopfenstein, M.; Hu, J.; Lloyd, M.; Habib, S.; Tosha, C.; Radu, R.A.; Ghyselinck, N.B.; et al. Retinoid Content, Visual Responses, and Ocular Morphology Are Compromised in the Retinas of Mice Lacking the Retinol-Binding Protein Receptor, STRA6. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3027–3039. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, N.; Golzio, C.; Odent, S.; Lequeux, L.; Vigouroux, A.; Martinovic-Bouriel, J.; Tiziano, F.D.; Masini, L.; Piro, F.; Maragliano, G.; et al. Phenotypic spectrum of STRA6 mutations: From Matthew-Wood syndrome to non-lethal anophthalmia. Hum. Mutat. 2009, 30, E673–E681. [Google Scholar] [CrossRef] [PubMed]
- Pasutto, F.; Flinter, F.; Rauch, A.; Reis, A. Novel STRA6 null mutations in the original family described with Matthew-Wood syndrome. Am. J. Med. Genet. A 2018, 176, 134–138. [Google Scholar] [CrossRef]
- Pasutto, F.; Sticht, H.; Hammersen, G.; Gillessen-Kaesbach, G.; Fitzpatrick, D.R.; Nürnberg, G.; Brasch, F.; Schirmer-Zimmermann, H.; Tolmie, J.L.; Chitayat, D.; et al. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am. J. Hum. Genet. 2007, 80, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Golzio, C.; Martinovic-Bouriel, J.; Thomas, S.; Mougou-Zrelli, S.; Grattagliano-Bessieres, B.; Bonniere, M.; Delahaye, S.; Munnich, A.; Encha-Razavi, F.; Lyonnet, S.; et al. Matthew-Wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6. Am. J. Hum. Genet. 2007, 80, 1179–1187. [Google Scholar] [CrossRef]
- Christensen, D.R.G.; Brown, F.E.; Cree, A.J.; Ratnayaka, J.A.; Lotery, A.J. Sorsby fundus dystrophy—A review of pathology and disease mechanisms. Exp. Eye Res. 2017, 165, 35–46. [Google Scholar] [CrossRef]
- Weber, B.H.; Vogt, G.; Pruett, R.C.; Stöhr, H.; Felbor, U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby’s fundus dystrophy. Nat. Genet. 1994, 8, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Gliem, M.; Müller, P.L.; Mangold, E.; Holz, F.G.; Bolz, H.J.; Stöhr, H.; Weber, B.H.; Charbel Issa, P. Sorsby Fundus Dystrophy: Novel Mutations, Novel Phenotypic Characteristics, and Treatment Outcomes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2664–2676. [Google Scholar] [CrossRef] [PubMed]
- Sorsby, A.; Mason, M.E. A fundus dystrophy with unusual features. Br. J. Ophthalmol. 1949, 33, 67–97. [Google Scholar] [CrossRef]
- Baston, A.; Gerhardt, C.; Zandi, S.; Garweg, J.G. Visual Outcome after Intravitreal Anti-VEGF Therapy for Macular Neovascularisation Secondary to Sorsby’s Fundus Dystrophy: A Systematic Review. J. Clin. Med. 2021, 10, 2433. [Google Scholar] [CrossRef]
- Naessens, S.; De Zaeytijd, J.; Syx, D.; Vandenbroucke, R.E.; Smeets, F.; Van Cauwenbergh, C.; Leroy, B.P.; Peelman, F.; Coppieters, F. The N-terminal p.(Ser38Cys) TIMP3 mutation underlying Sorsby fundus dystrophy is a founder mutation disrupting an intramolecular disulfide bond. Hum. Mutat. 2019, 40, 539–551. [Google Scholar] [CrossRef]
- Gourier, H.C.Y.; Chong, N.V. Can Novel Treatment of Age-Related Macular Degeneration Be Developed by Better Understanding of Sorsby’s Fundus Dystrophy. J. Clin. Med. 2015, 4, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.A.; Zhu, M.; Billson, F. The interaction of indocyanine green with human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Armento, A.; Ueffing, M.; Clark, S.J. The complement system in age-related macular degeneration. Cell. Mol. Life Sci. 2021, 78, 4487–4505. [Google Scholar] [CrossRef]
- Lee, Y.; Hussain, A.A.; Seok, J.H.; Kim, S.H.; Marshall, J. Modulating the Transport Characteristics of Bruch’s Membrane With Steroidal Glycosides and its Relevance to Age-Related Macular Degeneration (AMD). Investig. Ophthalmol. Vis. Sci. 2015, 56, 8403–8418. [Google Scholar] [CrossRef] [PubMed]
- Chong, N.H.; Keonin, J.; Luthert, P.J.; Frennesson, C.I.; Weingeist, D.M.; Wolf, R.L.; Mullins, R.F.; Hageman, G.S. Decreased thickness and integrity of the macular elastic layer of Bruch’s membrane correspond to the distribution of lesions associated with age-related macular degeneration. Am. J. Pathol. 2005, 166, 241–251. [Google Scholar] [CrossRef]
- Spraul, C.W.; Grossniklaus, H.E. Characteristics of Drusen and Bruch’s membrane in postmortem eyes with age-related macular degeneration. Arch. Ophthalmol. 1997, 115, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Fraser, R.G.; Tan, R.; Caruso, E.; Lek, J.J.; Guymer, R.H.; Luu, C.D. Longitudinal Changes in Retinotopic Rod Function in Intermediate Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD19–AMD24. [Google Scholar] [CrossRef]
- Nebbioso, M.; Barbato, A.; Pescosolido, N. Scotopic microperimetry in the early diagnosis of age-related macular degeneration: Preliminary study. BioMed Res. Int. 2014, 2014, 671529. [Google Scholar] [CrossRef]
- Rabiolo, A.; Sacconi, R.; Cicinelli, M.V.; Querques, L.; Bandello, F.; Querques, G. Spotlight on reticular pseudodrusen. Clin. Ophthalmol. 2017, 11, 1707–1718. [Google Scholar] [CrossRef] [PubMed]

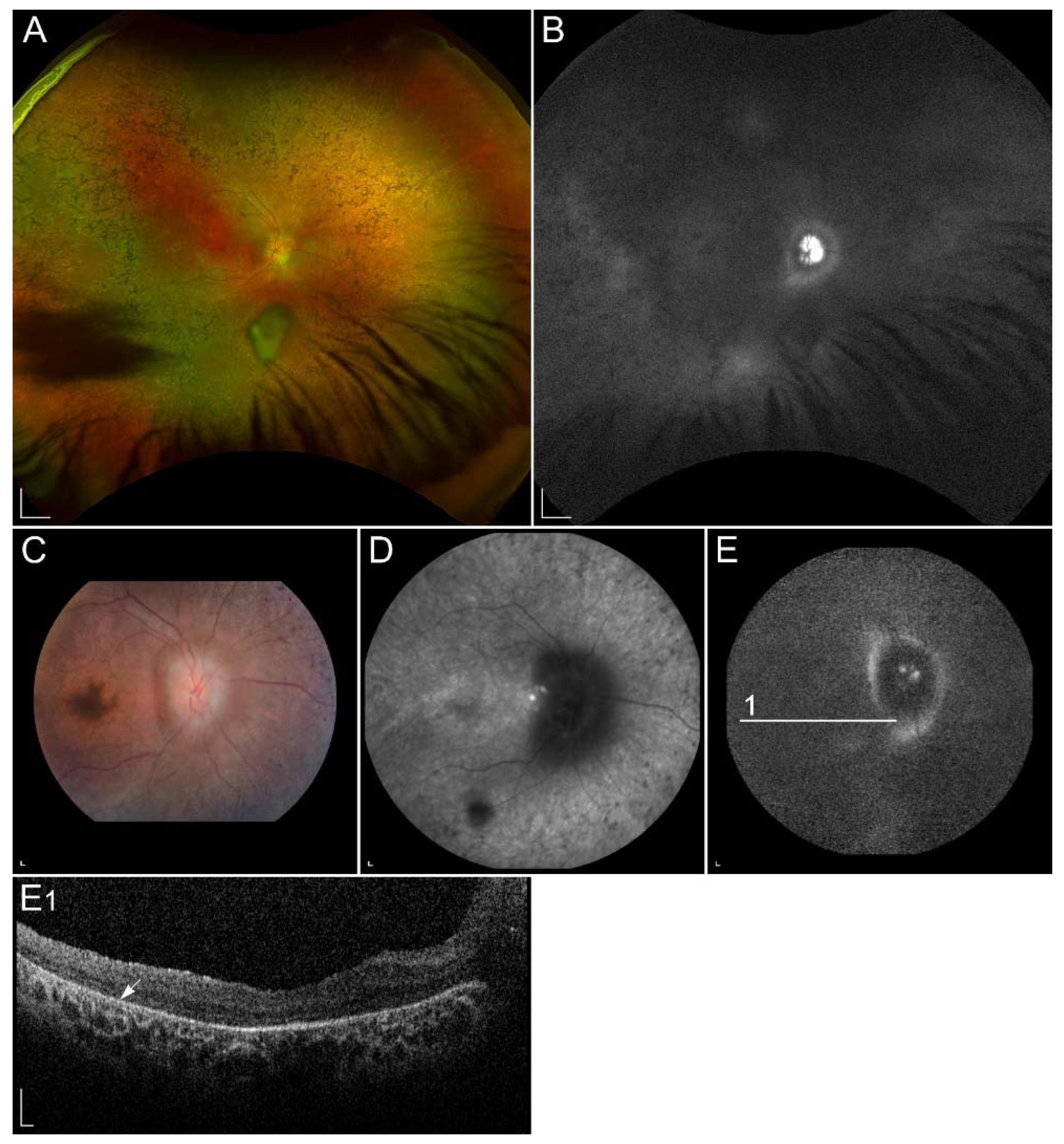

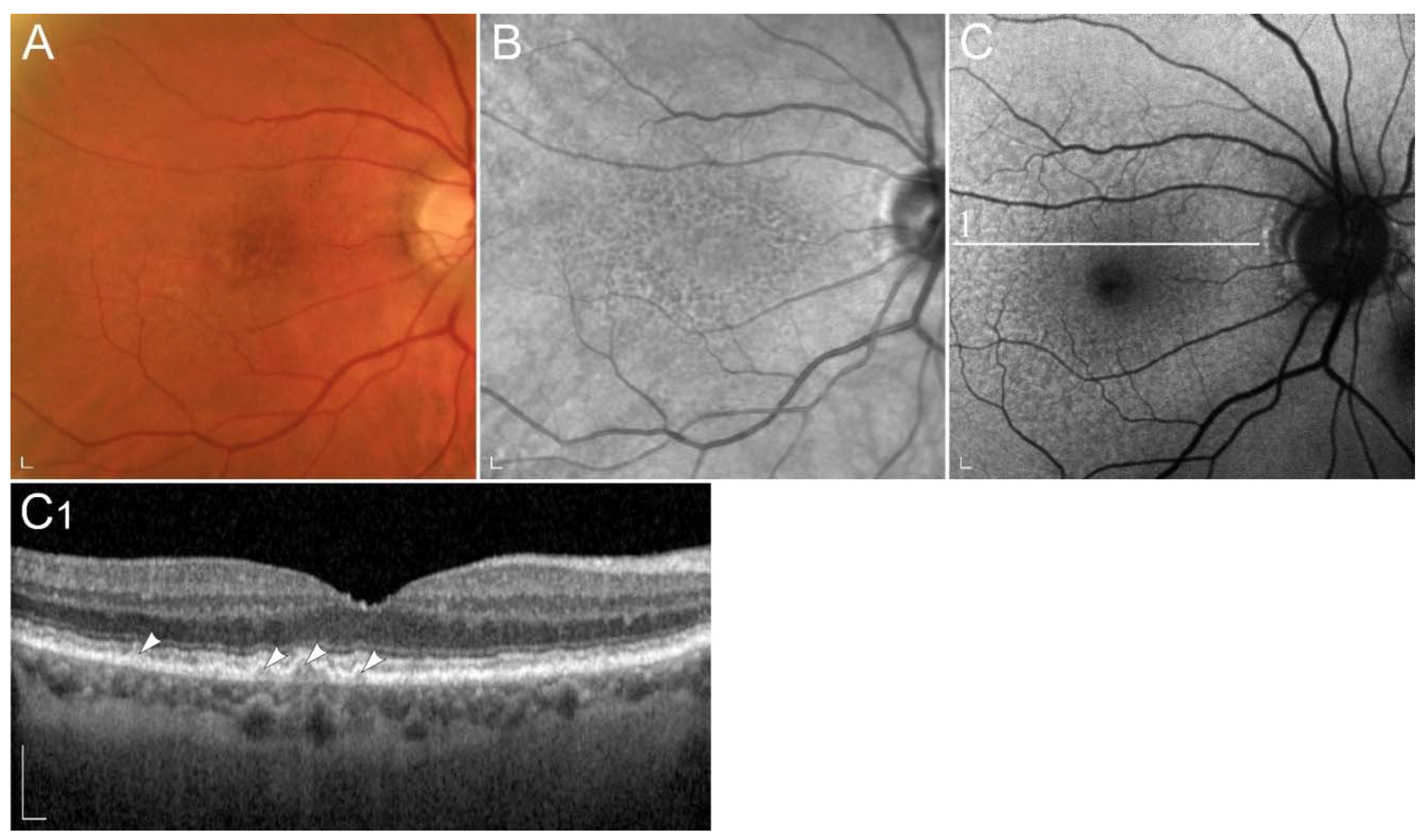
| Gene (Protein) | Location | Role | Associated Disease | Proposed Pathogenic Mechanism |
|---|---|---|---|---|
| ABCA4 (ABCA4) | 1p22.1 | Transport of retinoids to the cytoplasmic membrane of rods and cones. | STGD1 | Accumulation of toxic retinoids in the RPE, direct cone toxicity. |
| LRAT (LRAT) | 4q32.1 | Conversion of all-trans-retinol to all-trans-retinyl esters | LCA, RP | 11-cis-retinol deficiency, reduced lipofuscin accumulation in the RPE. |
| RBP1 (CRBP1) | 3q23 | Transport of all-trans-retinol in the retina. | No human retinal diseases have been associated with pathogenic variants in RBP1 gene. | / |
| RBP3 (IRBP) | 10q11.22 | Transport of retinoids between photoreceptors, RPE and Müller cells. | RP, unusual retinal dystrophy | Disabled protection and solubilization of visual cycle retinoids. |
| RBP4 (RBP4) | 10q23.33 | Transport of all-trans-retinol in the crculation and its delivery to the STRA6. | Night blindness, microphthalmia, anophthalmia, coloboma, retinal dystrophy, acne vulgaris | Reduced plasma concentrations of RBP4 and all-trans-retinol. |
| RDH5 (RDH5) | 12q13-q14 | Conversion of 11-cis-retinol to 11-cis-retinal. | Fundus albipunctatus | Slowed production of 11-cis-retinal, reduced lipofuscin accumulation in the RPE. |
| RDH8 (RDH8) | 19p13.2 | Conversion of all-trans-retinal to all-trans-retinol. | Pathogenic variants are not linked to any retinal disease | / |
| RDH11 (RDH11) | 14q24.1 | Oxidation and reduction of cis-retinoids and trans-retinoids. | Syndromic RP | Slowed production of 11-cis-retinal, reduced lipofuscin accumulation in the RPE. |
| RDH12 (RDH12) | 14q24.1 | Conversion of all-trans-retinal to all-trans-retinol. | LCA | Decreased synthesis of 11-cis-retinal, reduced lipofuscin accumulation in the RPE. |
| RGR (RGR) | 10q23.1 | Contributes to the regeneration of 11-cis-retinal. | Association with specific ocular diseases has been rarely reported | Decreased synthesis of 11-cis-retinal. |
| RHO (RHO) | 3q22.1 | G protein-coupled photosensitive receptor in rods | Congenital stationary night blindness, RP | Dysfunction in phototransduction. |
| RLBP1 (CRALBP) | 15q26 | Transports of 11-cis-retinoids in RPE and Müller cells. | RP, fundus albipunctatus, Bothnia dystrophy, Newfoundland rod-cone dystrophy and retinitis punctata albescens. | Regeneration of 11-cis-retinal is prevented, reduced lipofuscin accumulation in the RPE. |
| RPE65 (RPE65) | 1p31.3 | Conversion of all-trans-retinal to 11-cis-retinal. | LCA and EOSRD (LCA2), RP, fundus albipunctatus, cone-rod dystrophy | 11-cis-retinol deficiency, leading to visual cycle interruption, reduced lipofuscin accumulation in the RPE, retinyl esters accumulation in the RPE. |
| STRA6 (STRA6) | 15q24.1 | Release of retinol from RBP4 and its translocation across the RPE | Matthew-Wood syndrome | Severe reduction of vitamin A transport into RPE cells. |
| TIMP3 (TIMP3) | 22q12.3 | Regulation of extracellular matrix turnover, inflammation, pro-apoptotic and anti-angiogenic activities. | SFD | Impaired transfer of vitamin A through the thickened Bruch’s membrane. |
| NCT Number | Disease | Drug | Sponsor | Number of Subjects | Phase of the Study | Mechanism |
|---|---|---|---|---|---|---|
| NCT03772665 | STGD1 | Emixustat (inhibitor of RPE65) | Kubota Vision Inc. | 194 | 3 | Slower regeneration of 11-cis-retinal. |
| NCT03364153 | STGD1 | Zimura (anti-C5 aptamer) | IVERIC bio, Inc. | 120 | 2 | Prevention of the destructive effects of the activated complement cascade. |
| NCT02402660 and NCT04239625 | STGD1 | ALK-001 (C20-deuterated vitamin A) | Alkeus Pharmaceuticals, Inc. | 140 | 2 | Impaired dimerization of vitamin A and therefore reduced production of A2E. |
| NCT03374657 | RP | CPK850 (RLBP1 promoter) | Novartis Pharmaceuticals | Recruiting | 1 and 2 | Gene therapy. |
| NCT03478865 and NCT03478878 | AMD | Vitamin A palmitate | National Eye Institute (NEI) | Recruiting | 1 | Vitamin A supplementation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajovic, J.; Meglič, A.; Glavač, D.; Markelj, Š.; Hawlina, M.; Fakin, A. The Role of Vitamin A in Retinal Diseases. Int. J. Mol. Sci. 2022, 23, 1014. https://doi.org/10.3390/ijms23031014
Sajovic J, Meglič A, Glavač D, Markelj Š, Hawlina M, Fakin A. The Role of Vitamin A in Retinal Diseases. International Journal of Molecular Sciences. 2022; 23(3):1014. https://doi.org/10.3390/ijms23031014
Chicago/Turabian StyleSajovic, Jana, Andrej Meglič, Damjan Glavač, Špela Markelj, Marko Hawlina, and Ana Fakin. 2022. "The Role of Vitamin A in Retinal Diseases" International Journal of Molecular Sciences 23, no. 3: 1014. https://doi.org/10.3390/ijms23031014
APA StyleSajovic, J., Meglič, A., Glavač, D., Markelj, Š., Hawlina, M., & Fakin, A. (2022). The Role of Vitamin A in Retinal Diseases. International Journal of Molecular Sciences, 23(3), 1014. https://doi.org/10.3390/ijms23031014








