LAIR1, an ITIM-Containing Receptor Involved in Immune Disorders and in Hematological Neoplasms
Abstract
1. Introduction
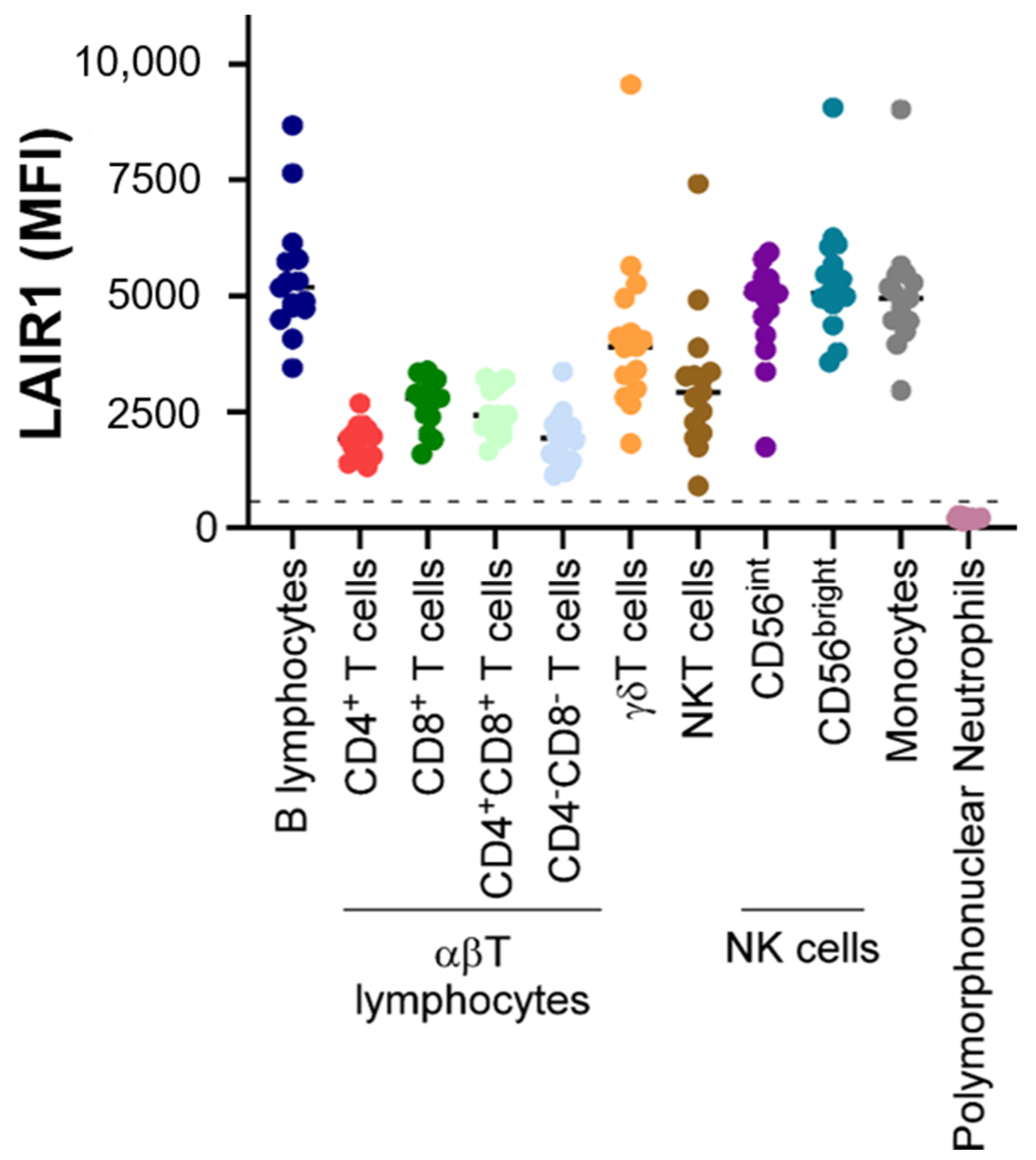
2. Expression Patterns of LAIR1
3. LAIR1 Is a Receptor Able to Bind to Collagens and Complement Component 1Q, Surfactant Protein D, and Adiponectin
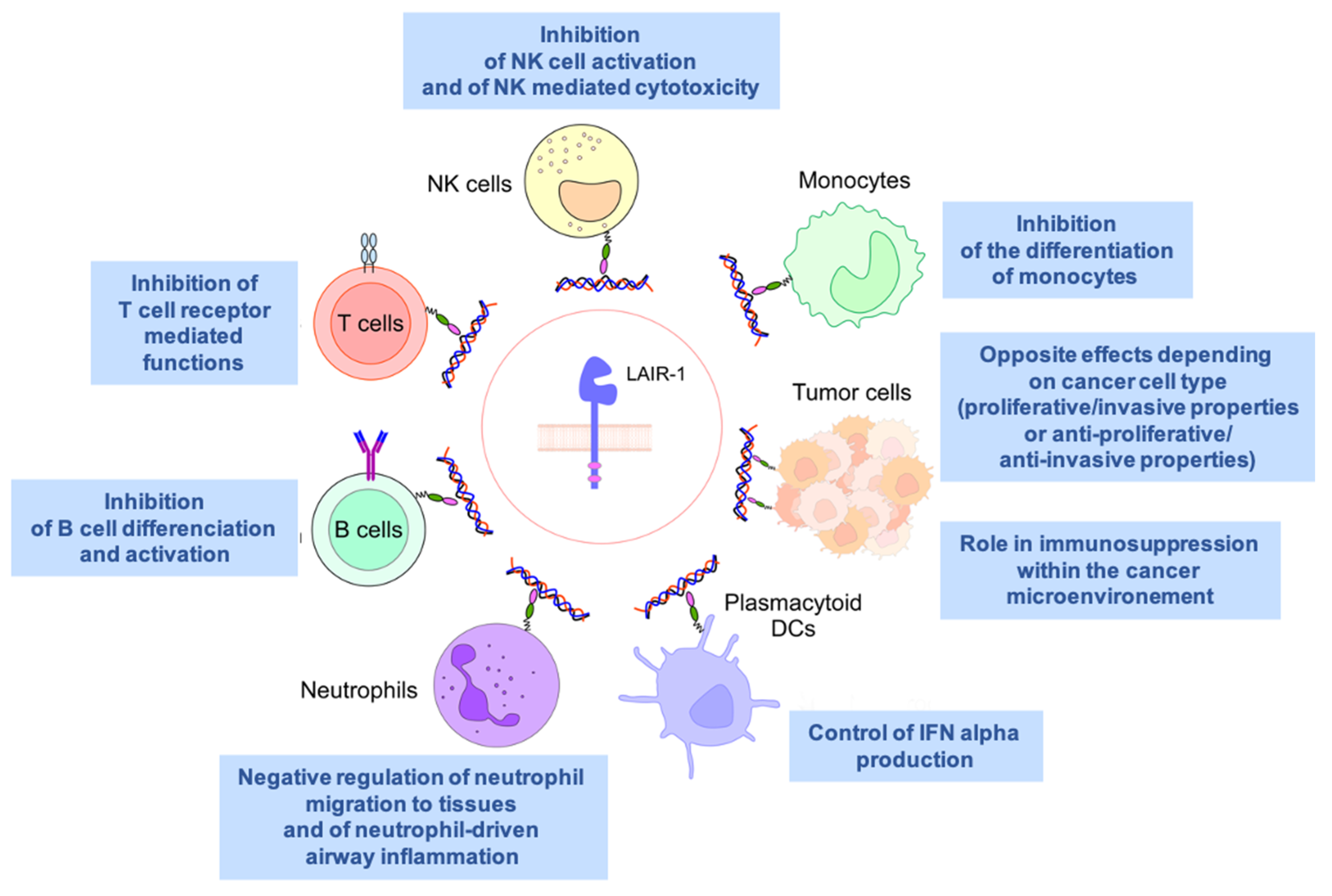
4. An Inhibitory Receptor Involved in Immunity
5. LAIR1 in Autoimmunity and Inflammatory Diseases
6. LAIR1 and Allergy
7. LAIR1 and Lupus
8. LAIR1 and Rheumatoid Arthritis
9. LAIR1 and Graft Rejection
10. LAIR1 and Liver Cirrhosis
11. A Potential Contradictory Role in Tumor Biology
| In Vitro Anti-Tumoral Properties of LAIR1 | In Vitro Pro-Tumoral Properties of LAIR1 |
|---|---|
| Ovarian cancer: Inhibition of LAIR1 expression in HO8910 cell line: ↑ proliferation, clonogenicity, and invasive properties of tumor cells [56] | Breast cancer: Knockdown of LAIR1 in SKBr3 and MDA-MB 231 cell lines: ↓ proliferation and impaired cell invasion properties [58] |
| Cervical cancer: Overexpression of LAIR1 in ME-180 cell line: inhibition of proliferation and reversion of the anti-apoptosis tendency [57] | Renal cell carcinoma: Knockdown of LAIR1 in Caki-1 and Caki-2 cell lines: ↓ Akt phosphorylation [61] |
| Acute Myeloid Leukemia (AML): Knockdown of LAIR1 in AML cell lines: ↓ cell growth and ↑ apoptosis; failure to engraft in a murine model [22] | |
| B Acute Lymphoblastic Leukemia (B ALL): ITIM cytoplasmic motifs of LAIR1 described as critical for the survival of B ALL cells [65] |
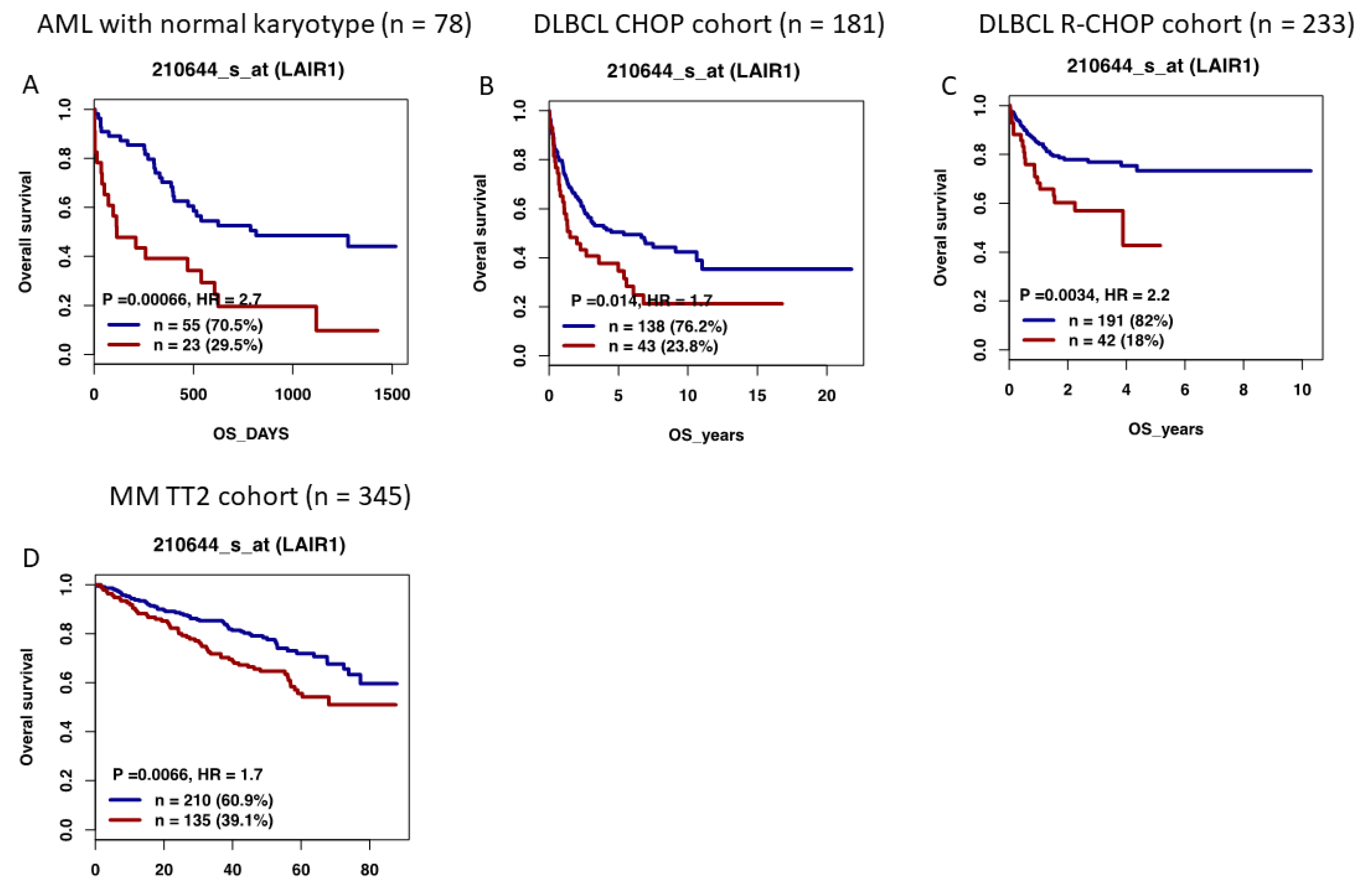
12. High LAIR1 Expression Is Associated with a Poor Outcome in Several Hematological Malignancies
13. LAIR1 as a Future Therapeutic Target
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyaard, L.; Adema, G.J.; Chang, C.; Woollatt, E.; Sutherland, G.R.; Lanier, L.L.; Phillips, J.H. LAIR1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 1997, 7, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Billadeau, D.D.; Leibson, P.J. ITAMs versus ITIMs: Striking a balance during cell regulation. J. Clin. Investig. 2002, 109, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, A.; Ruiter Td, T.; Clevers, H.; Meyaard, L. Differential contribution of the immunoreceptor tyrosine-based inhibitory motifs of human leukocyte-associated Ig-like receptor-1 to inhibitory function and phosphatase recruitment. Int. Immunol. 2003, 15, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Ravetch, J.V.; Lanier, L.L. Immune inhibitory receptors. Science 2000, 290, 84–89. [Google Scholar] [CrossRef]
- Lebbink, R.J.; van den Berg, M.C.; de Ruiter, T.; Raynal, N.; van Roon, J.A.; Lenting, P.J.; Jin, B.; Meyaard, L. The soluble leukocyte-associated Ig-like receptor (LAIR)-2 antagonizes the collagen/LAIR1 inhibitory immune interaction. J. Immunol. 2008, 180, 1662–1669. [Google Scholar] [CrossRef]
- Meyaard, L. The inhibitory collagen receptor LAIR1 (CD305). J. Leukoc. Biol. 2008, 83, 799–803. [Google Scholar] [CrossRef]
- Meyaard, L. LAIR1, a widely distributed human ITIM-bearing receptor on hematopoietic cells. Curr. Top. Microbiol. Immunol. 1999, 244, 151–157. [Google Scholar] [CrossRef]
- Florian, S.; Sonneck, K.; Czerny, M.; Hennersdorf, F.; Hauswirth, A.W.; Buhring, H.J.; Valent, P. Detection of novel leukocyte differentiation antigens on basophils and mast cells by HLDA8 antibodies. Allergy 2006, 61, 1054–1062. [Google Scholar] [CrossRef]
- Verbrugge, A.; de Ruiter, T.; Geest, C.; Coffer, P.J.; Meyaard, L. Differential expression of leukocyte-associated Ig-like receptor-1 during neutrophil differentiation and activation. J. Leukoc. Biol. 2006, 79, 828–836. [Google Scholar] [CrossRef]
- Bonaccorsi, I.; Cantoni, C.; Carrega, P.; Oliveri, D.; Lui, G.; Conte, R.; Navarra, M.; Cavaliere, R.; Traggiai, E.; Gattorno, M.; et al. The immune inhibitory receptor LAIR1 is highly expressed by plasmacytoid dendritic cells and acts complementary with NKp44 to control IFNalpha production. PLoS ONE 2010, 5, e15080. [Google Scholar] [CrossRef]
- Rodriguez-Bayona, B.; Ramos-Amaya, A.; Brieva, J.A. Differential expression of SLAMS and other modulatory molecules by human plasma cells during normal maturation. Immunol. Lett. 2011, 134, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Maasho, K.; Masilamani, M.; Valas, R.; Basu, S.; Coligan, J.E.; Borrego, F. The inhibitory leukocyte-associated Ig-like receptor-1 (LAIR1) is expressed at high levels by human naive T cells and inhibits TCR mediated activation. Mol. Immunol. 2005, 42, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- van der Vuurst de Vries, A.R.; Clevers, H.; Logtenberg, T.; Meyaard, L. Leukocyte-associated immunoglobulin-like receptor-1 (LAIR1) is differentially expressed during human B cell differentiation and inhibits B cell receptor-mediated signaling. Eur. J. Immunol. 1999, 29, 3160–3167. [Google Scholar] [CrossRef]
- Devin, J.; Kassambara, A.; Bruyer, A.; Moreaux, J.; Bret, C. Phenotypic Characterization of Diffuse Large B-Cell Lymphoma Cells and Prognostic Impact. J. Clin. Med. 2019, 8, 1074. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Y.; Ma, Q.; Wang, N.; Guo, W.; Jin, B.; Fang, L.; Chen, L. LAIR1 activation inhibits inflammatory macrophage phenotype in vitro. Cell. Immunol. 2018, 331, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Tomasello, E.; Ferrero, E.; Zocchi, M.R.; Moretta, L. p40/LAIR1 regulates the differentiation of peripheral blood precursors to dendritic cells induced by granulocyte-monocyte colony-stimulating factor. Eur. J. Immunol. 1998, 28, 2086–2091. [Google Scholar] [CrossRef]
- Ouyang, W.; Ma, D.; Lin, D.; Sun, Y.; Liu, X.; Li, Q.; Jia, W.; Cao, Y.; Zhu, Y.; Jin, B. 9.1C3 is identical to LAIR1, which is expressed on hematopoietic progenitors. Biochem. Biophys. Res. Commun. 2003, 310, 1236–1240. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, X.; Zhao, H.; Fu, Q.; Cao, Y.; Wang, Y.; Feng, X.; Fu, A. Leukocyte-associated immunoglobulin-like receptor-1 is expressed on human megakaryocytes and negatively regulates the maturation of primary megakaryocytic progenitors and cell line. Biochem. Biophys. Res. Commun. 2011, 405, 128–133. [Google Scholar] [CrossRef]
- Smith, C.W.; Thomas, S.G.; Raslan, Z.; Patel, P.; Byrne, M.; Lordkipanidze, M.; Bem, D.; Meyaard, L.; Senis, Y.A.; Watson, S.P.; et al. Mice Lacking the Inhibitory Collagen Receptor LAIR1 Exhibit a Mild Thrombocytosis and Hyperactive Platelets. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 823–835. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Huang, Y.; Zhang, C.; Boquan, J.; Ran, Z. Expression of leukocyte-associated immunoglobulin-like receptor-1 (LAIR1) on osteoclasts and its potential role in rheumatoid arthritis. Clinics 2013, 68, 475–481. [Google Scholar] [CrossRef]
- Lebbink, R.J.; de Ruiter, T.; Adelmeijer, J.; Brenkman, A.B.; van Helvoort, J.M.; Koch, M.; Farndale, R.W.; Lisman, T.; Sonnenberg, A.; Lenting, P.J.; et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR1. J. Exp. Med. 2006, 203, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Lu, Z.; Cui, C.; Deng, M.; Fan, Y.; Dong, B.; Han, X.; Xie, F.; Tyner, J.W.; Coligan, J.E.; et al. The ITIM-containing receptor LAIR1 is essential for acute myeloid leukaemia development. Nat. Cell Biol. 2015, 17, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Santiago-Schwarz, F.; Al-Abed, Y.; Diamond, B. C1q limits dendritic cell differentiation and activation by engaging LAIR1. Proc. Natl. Acad. Sci. USA 2012, 109, E3160–E3167. [Google Scholar] [CrossRef] [PubMed]
- Olde Nordkamp, M.J.; van Eijk, M.; Urbanus, R.T.; Bont, L.; Haagsman, H.P.; Meyaard, L. Leukocyte-associated Ig-like receptor-1 is a novel inhibitory receptor for surfactant protein D. J. Leukoc. Biol. 2014, 96, 105–111. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhuang, R.; Ma, Y.; Zhang, C.; Tang, K.; Yi, H.; Jin, B. Adiponectin’s globular domain inhibits T cell activation by interacting with LAIR1. Biochem. Biophys. Res. Commun. 2021, 573, 117–124. [Google Scholar] [CrossRef]
- Meyaard, L.; Hurenkamp, J.; Clevers, H.; Lanier, L.L.; Phillips, J.H. Leukocyte-associated Ig-like receptor-1 functions as an inhibitory receptor on cytotoxic T cells. J. Immunol. 1999, 162, 5800–5804. [Google Scholar] [PubMed]
- Saverino, D.; Fabbi, M.; Merlo, A.; Ravera, G.; Grossi, C.E.; Ciccone, E. Surface density expression of the leukocyte-associated Ig-like receptor-1 is directly related to inhibition of human T-cell functions. Hum. Immunol. 2002, 63, 534–546. [Google Scholar] [CrossRef]
- Jansen, C.A.; Cruijsen, C.W.; de Ruiter, T.; Nanlohy, N.; Willems, N.; Janssens-Korpela, P.L.; Meyaard, L. Regulated expression of the inhibitory receptor LAIR1 on human peripheral T cells during T cell activation and differentiation. Eur. J. Immunol. 2007, 37, 914–924. [Google Scholar] [CrossRef]
- Tang, X.; Tian, L.; Esteso, G.; Choi, S.C.; Barrow, A.D.; Colonna, M.; Borrego, F.; Coligan, J.E. Leukocyte-associated Ig-like receptor-1-deficient mice have an altered immune cell phenotype. J. Immunol. 2012, 188, 548–558. [Google Scholar] [CrossRef]
- Merlo, A.; Tenca, C.; Fais, F.; Battini, L.; Ciccone, E.; Grossi, C.E.; Saverino, D. Inhibitory receptors CD85j, LAIR1, and CD152 down-regulate immunoglobulin and cytokine production by human B lymphocytes. Clin. Diagn. Lab. Immunol. 2005, 12, 705–712. [Google Scholar] [CrossRef]
- Rygiel, T.P.; Stolte, E.H.; de Ruiter, T.; van de Weijer, M.L.; Meyaard, L. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR1. Mol. Immunol. 2011, 49, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, T.; Garcia, S.; Pascoal Ramos, M.I.; Giovannone, B.; Radstake, T.; Marut, W.; Meyaard, L. Leukocyte Associated Immunoglobulin Like Receptor 1 Regulation and Function on Monocytes and Dendritic Cells During Inflammation. Front. Immunol. 2020, 11, 1793. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Esparza, M.; Ruiz-Alcaraz, A.J.; Carmona-Martinez, V.; Fernandez-Fernandez, M.D.; Anton, G.; Munoz-Tornero, M.; Lencina, M.; Pagan, I.; de la Pena, J.; Garcia-Penarrubia, P. Expression of LAIR1 (CD305) on Human Blood Monocytes as a Marker of Hepatic Cirrhosis Progression. J. Immunol. Res. 2019, 2019, 2974753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lv, K.; Zhang, C.M.; Jin, B.Q.; Zhuang, R.; Ding, Y. The role of LAIR1 (CD305) in T cells and monocytes/macrophages in patients with rheumatoid arthritis. Cell. Immunol. 2014, 287, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Geerdink, R.J.; Hennus, M.P.; Westerlaken, G.H.A.; Abrahams, A.C.; Albers, K.I.; Walk, J.; Wesselink, E.; Janssen, R.; Bont, L.; Meyaard, L. LAIR1 limits neutrophil extracellular trap formation in viral bronchiolitis. J. Allergy Clin. Immunol. 2018, 141, 811–814. [Google Scholar] [CrossRef]
- Verbrugge, A.; Rijkers, E.S.; de Ruiter, T.; Meyaard, L. Leukocyte-associated Ig-like receptor-1 has SH2 domain-containing phosphatase-independent function and recruits C-terminal Src kinase. Eur. J. Immunol. 2006, 36, 190–198. [Google Scholar] [CrossRef]
- Kumawat, K.; Geerdink, R.J.; Hennus, M.P.; Roda, M.A.; van Ark, I.; Leusink-Muis, T.; Folkerts, G.; van Oort-Jansen, A.; Mazharian, A.; Watson, S.P.; et al. LAIR1 Limits Neutrophilic Airway Inflammation. Front. Immunol. 2019, 10, 842. [Google Scholar] [CrossRef]
- Omiya, R.; Tsushima, F.; Narazaki, H.; Sakoda, Y.; Kuramasu, A.; Kim, Y.; Xu, H.; Tamura, H.; Zhu, G.; Chen, L.; et al. Leucocyte-associated immunoglobulin-like receptor-1 is an inhibitory regulator of contact hypersensitivity. Immunology 2009, 128, 543–555. [Google Scholar] [CrossRef]
- Helou, D.G.; Shafiei-Jahani, P.; Hurrell, B.P.; Painter, J.D.; Quach, C.; Howard, E.; Akbari, O. LAIR1 acts as an immune checkpoint on activated ILC2s and regulates the induction of airway hyperreactivity. J. Allergy Clin. Immunol. 2021, 149, 223–236.e6. [Google Scholar] [CrossRef]
- Walport, M.J. Complement and systemic lupus erythematosus. Arthritis Res. 2002, 4 (Suppl. 3), S279–S293. [Google Scholar] [CrossRef]
- Hosszu, K.K.; Santiago-Schwarz, F.; Peerschke, E.I.; Ghebrehiwet, B. Evidence that a C1q/C1qR system regulates monocyte-derived dendritic cell differentiation at the interface of innate and acquired immunity. Innate Immun. 2010, 16, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Diamond, B.; Volpe, B.T.; Aranow, C.B.; Mackay, M.C.; Santiago-Schwarz, F. Evidence for C1q-mediated crosslinking of CD33/LAIR1 inhibitory immunoreceptors and biological control of CD33/LAIR1 expression. Sci. Rep. 2017, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Leffler, J.; Bengtsson, A.A.; Blom, A.M. The complement system in systemic lupus erythematosus: An update. Ann. Rheum. Dis. 2014, 73, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Colombo, B.M.; Canevali, P.; Magnani, O.; Rossi, E.; Puppo, F.; Zocchi, M.R.; Poggi, A. Defective expression and function of the leukocyte associated Ig-like receptor 1 in B lymphocytes from systemic lupus erythematosus patients. PLoS ONE 2012, 7, e31903. [Google Scholar] [CrossRef]
- Chang, M.H.; Nigrovic, P.A. Antibody-dependent and -independent mechanisms of inflammatory arthritis. JCI Insight 2019, 4, e125278. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Dong, H.; Yi, X.; Zhang, J.; Liu, X.; Zhuang, R.; Ding, Y. LAIR1 shedding from human fibroblast-like synoviocytes in rheumatoid arthritis following TNF-alpha stimulation. Clin. Exp. Immunol. 2018, 192, 193–205. [Google Scholar] [CrossRef]
- Kim, S.; Easterling, E.R.; Price, L.C.; Smith, S.L.; Coligan, J.E.; Park, J.E.; Brand, D.D.; Rosloniec, E.F.; Stuart, J.M.; Kang, A.H.; et al. The Role of Leukocyte-Associated Ig-like Receptor-1 in Suppressing Collagen-Induced Arthritis. J. Immunol. 2017, 199, 2692–2700. [Google Scholar] [CrossRef]
- Park, J.E.; Brand, D.D.; Rosloniec, E.F.; Yi, A.K.; Stuart, J.M.; Kang, A.H.; Myers, L.K. Leukocyte-associated immunoglobulin-like receptor 1 inhibits T-cell signaling by decreasing protein phosphorylation in the T-cell signaling pathway. J. Biol. Chem. 2020, 295, 2239–2247. [Google Scholar] [CrossRef]
- Myers, L.K.; Winstead, M.; Kee, J.D.; Park, J.J.; Zhang, S.; Li, W.; Yi, A.K.; Stuart, J.M.; Rosloniec, E.F.; Brand, D.D.; et al. 1,25-Dihydroxyvitamin D3 and 20-Hydroxyvitamin D3 Upregulate LAIR1 and Attenuate Collagen Induced Arthritis. Int. J. Mol. Sci. 2021, 22, 13342. [Google Scholar] [CrossRef]
- Agashe, V.V.; Jankowska-Gan, E.; Keller, M.; Sullivan, J.A.; Haynes, L.D.; Kernien, J.F.; Torrealba, J.R.; Roenneburg, D.; Dart, M.; Colonna, M.; et al. Leukocyte-Associated Ig-like Receptor 1 Inhibits Th1 Responses but Is Required for Natural and Induced Monocyte-Dependent Th17 Responses. J. Immunol. 2018, 201, 772–781. [Google Scholar] [CrossRef]
- Ouyang, W.; Xue, J.; Liu, J.; Jia, W.; Li, Z.; Xie, X.; Liu, X.; Jian, J.; Li, Q.; Zhu, Y.; et al. Establishment of an ELISA system for determining soluble LAIR1 levels in sera of patients with HFRS and kidney transplant. J. Immunol. Methods 2004, 292, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Duprez, A.; Guerret-Stocker, S.; Plenat, F.; Vignaud, J.M.; Hartmann, D.; Grimaud, J.A. [Histo-immunolocalization of human and murine type I and III collagens in human cancers grafted to congenital athymic mice]. C. R. Acad. Sci. III 1987, 304, 155–160. [Google Scholar] [PubMed]
- Gu, Y.; Bi, Y.; Wei, H.; Li, J.; Huang, Z.; Liao, C.; Liao, W.; Huang, Y. Expression and clinical significance of inhibitory receptor Leukocyte-associated immunoglobulin-like receptor-1 on peripheral blood T cells of chronic hepatitis B patients: A cross-sectional study. Medicine 2021, 100, e26667. [Google Scholar] [CrossRef] [PubMed]
- Vijver, S.V.; Singh, A.; Mommers-Elshof, E.; Meeldijk, J.; Copeland, R.; Boon, L.; Langermann, S.; Flies, D.; Meyaard, L.; Ramos, M.I.P. Collagen Fragments Produced in Cancer Mediate T Cell Suppression Through Leukocyte-Associated Immunoglobulin-Like Receptor 1. Front. Immunol. 2021, 12, 733561. [Google Scholar] [CrossRef]
- Peng, D.H.; Rodriguez, B.L.; Diao, L.; Chen, L.; Wang, J.; Byers, L.A.; Wei, Y.; Chapman, H.A.; Yamauchi, M.; Behrens, C.; et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8(+) T cell exhaustion. Nat. Commun. 2020, 11, 4520. [Google Scholar] [CrossRef]
- Cao, Q.; Fu, A.; Yang, S.; He, X.; Wang, Y.; Zhang, X.; Zhou, J.; Luan, X.; Yu, W.; Xue, J. Leukocyte-associated immunoglobulin-like receptor-1 expressed in epithelial ovarian cancer cells and involved in cell proliferation and invasion. Biochem. Biophys. Res. Commun. 2015, 458, 399–404. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Miao, F.; Cao, Y.; Xue, J.; Cao, Q.; Zhang, X. Clinical significance of leukocyte-associated immunoglobulin-like receptor-1 expression in human cervical cancer. Exp. Ther. Med. 2016, 12, 3699–3705. [Google Scholar] [CrossRef]
- Joseph, C.; Alsaleem, M.A.; Toss, M.S.; Kariri, Y.A.; Althobiti, M.; Alsaeed, S.; Aljohani, A.I.; Narasimha, P.L.; Mongan, N.P.; Green, A.R.; et al. The ITIM-Containing Receptor: Leukocyte-Associated Immunoglobulin-Like Receptor-1 (LAIR1) Modulates Immune Response and Confers Poor Prognosis in Invasive Breast Carcinoma. Cancers 2020, 13, 80. [Google Scholar] [CrossRef]
- Yang, L.L.; Zhang, M.J.; Wu, L.; Mao, L.; Chen, L.; Yu, G.T.; Deng, W.W.; Zhang, W.F.; Liu, B.; Sun, W.K.; et al. LAIR1 overexpression and correlation with advanced pathological grade and immune suppressive status in oral squamous cell carcinoma. Head Neck 2019, 41, 1080–1086. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, L.; Zhou, J.; Liu, L.; Fu, Q.; Fu, A.; Feng, X.; Xin, R.; Liu, H.; Gao, Y.; et al. Clinicopathologic significance of LAIR1 expression in hepatocellular carcinoma. Curr. Probl. Cancer 2019, 43, 18–26. [Google Scholar] [CrossRef]
- Jingushi, K.; Uemura, M.; Nakano, K.; Hayashi, Y.; Wang, C.; Ishizuya, Y.; Yamamoto, Y.; Hayashi, T.; Kinouchi, T.; Matsuzaki, K.; et al. Leukocyteassociated immunoglobulinlike receptor 1 promotes tumorigenesis in RCC. Oncol. Rep. 2019, 41, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Cheng, S.; Mu, Y.; Liu, Y.; Yi, X.; Jiang, D.; Ding, Y.; Zhuang, R. LAIR1 overexpression inhibits epithelial-mesenchymal transition in osteosarcoma via GLUT1-related energy metabolism. World J. Surg. Oncol. 2020, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Pellegatta, F.; Leone, B.E.; Moretta, L.; Zocchi, M.R. Engagement of the leukocyte-associated Ig-likereceptor-1 induces programmed cell death and prevents NF-kappaB nuclear translocation in human myeloid leukemias. Eur. J. Immunol. 2000, 30, 2751–2758. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, M.R.; Pellegatta, F.; Pierri, I.; Gobbi, M.; Poggi, A. Leukocyte-associated Ig-like receptor-1 prevents granulocyte-monocyte colony stimulating factor-dependent proliferation and Akt1/PKB alpha activation in primary acute myeloid leukemia cells. Eur. J. Immunol. 2001, 31, 3667–3675. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shojaee, S.; Buchner, M.; Geng, H.; Woong Lee, J.; Klemm, L.; Titz, B.; Graeber, T.G.; Park, E.; Xim Tan, Y.; et al. Signaling threshold and negative B cell selection in acute lymphoblastic leukemia. Nature 2015, 521, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Catellani, S.; Bruzzone, A.; Caligaris-Cappio, F.; Gobbi, M.; Zocchi, M.R. Lack of the leukocyte-associated Ig-like receptor-1 expression in high-risk chronic lymphocytic leukaemia results in the absence of a negative signal regulating kinase activation and cell division. Leukemia 2008, 22, 980–988. [Google Scholar] [CrossRef]
- Rawstron, A.C.; Shingles, J.; de Tute, R.; Bennett, F.; Jack, A.S.; Hillmen, P. Chronic lymphocytic leukaemia (CLL) and CLL-type monoclonal B-cell lymphocytosis (MBL) show differential expression of molecules involved in lymphoid tissue homing. Cytom. B Clin. Cytom. 2010, 78 (Suppl. 1), S42–S46. [Google Scholar] [CrossRef]
- Perbellini, O.; Falisi, E.; Giaretta, I.; Boscaro, E.; Novella, E.; Facco, M.; Fortuna, S.; Finotto, S.; Amati, E.; Maniscalco, F.; et al. Clinical significance of LAIR1 (CD305) as assessed by flow cytometry in a prospective series of patients with chronic lymphocytic leukemia. Haematologica 2014, 99, 881–887. [Google Scholar] [CrossRef]
- van Dongen, J.J.; Lhermitte, L.; Bottcher, S.; Almeida, J.; van der Velden, V.H.; Flores-Montero, J.; Rawstron, A.; Asnafi, V.; Lecrevisse, Q.; Lucio, P.; et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia 2012, 26, 1908–1975. [Google Scholar] [CrossRef]
- Kassambara, A.; Reme, T.; Jourdan, M.; Fest, T.; Hose, D.; Tarte, K.; Klein, B. GenomicScape: An easy-to-use web tool for gene expression data analysis. Application to investigate the molecular events in the differentiation of B cells into plasma cells. PLoS Comput. Biol. 2015, 11, e1004077. [Google Scholar] [CrossRef]
- Metzeler, K.H.; Hummel, M.; Bloomfield, C.D.; Spiekermann, K.; Braess, J.; Sauerland, M.C.; Heinecke, A.; Radmacher, M.; Marcucci, G.; Whitman, S.P.; et al. An 86-probe-set gene-expression signature predicts survival in cytogenetically normal acute myeloid leukemia. Blood 2008, 112, 4193–4201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Lin, C.C.; Hsu, C.L.; Hung, S.Y.; Yao, C.Y.; Lee, S.H.; Tsai, C.H.; Hou, H.A.; Chou, W.C.; Tien, H.F. Distinct clinical and biological characteristics of acute myeloid leukemia with higher expression of long noncoding RNA KIAA0125. Ann. Hematol. 2021, 100, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Leich, E.; Salaverria, I.; Bea, S.; Zettl, A.; Wright, G.; Moreno, V.; Gascoyne, R.D.; Chan, W.C.; Braziel, R.M.; Rimsza, L.M.; et al. Follicular lymphomas with and without translocation t(14;18) differ in gene expression profiles and genetic alterations. Blood 2009, 114, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Blenk, S.; Engelmann, J.C.; Pinkert, S.; Weniger, M.; Schultz, J.; Rosenwald, A.; Muller-Hermelink, H.K.; Muller, T.; Dandekar, T. Explorative data analysis of MCL reveals gene expression networks implicated in survival and prognosis supported by explorative CGH analysis. BMC Cancer 2008, 8, 106. [Google Scholar] [CrossRef]
- Cardesa-Salzmann, T.M.; Colomo, L.; Gutierrez, G.; Chan, W.C.; Weisenburger, D.; Climent, F.; Gonzalez-Barca, E.; Mercadal, S.; Arenillas, L.; Serrano, S.; et al. High microvessel density determines a poor outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus chemotherapy. Haematologica 2011, 96, 996–1001. [Google Scholar] [CrossRef]
- Lenz, G.; Wright, G.; Dave, S.S.; Xiao, W.; Powell, J.; Zhao, H.; Xu, W.; Tan, B.; Goldschmidt, N.; Iqbal, J.; et al. Stromal gene signatures in large-B-cell lymphomas. N. Engl. J. Med. 2008, 359, 2313–2323. [Google Scholar] [CrossRef]
- Barlogie, B.; Tricot, G.; Rasmussen, E.; Anaissie, E.; van Rhee, F.; Zangari, M.; Fassas, A.; Hollmig, K.; Pineda-Roman, M.; Shaughnessy, J.; et al. Total therapy 2 without thalidomide in comparison with total therapy 1: Role of intensified induction and posttransplantation consolidation therapies. Blood 2006, 107, 2633–2638. [Google Scholar] [CrossRef]
- Herviou, L.; Kassambara, A.; Boireau, S.; Robert, N.; Requirand, G.; Muller-Tidow, C.; Vincent, L.; Seckinger, A.; Goldschmidt, H.; Cartron, G.; et al. PRC2 targeting is a therapeutic strategy for EZ score defined high-risk multiple myeloma patients and overcome resistance to IMiDs. Clin. Epigenet. 2018, 10, 121. [Google Scholar] [CrossRef]
- Ogluszka, M.; Orzechowska, M.; Jedroszka, D.; Witas, P.; Bednarek, A.K. Evaluate Cutpoints: Adaptable continuous data distribution system for determining survival in Kaplan-Meier estimator. Comput. Methods Programs Biomed. 2019, 177, 133–139. [Google Scholar] [CrossRef]
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef]
- Giussani, M.; Triulzi, T.; Sozzi, G.; Tagliabue, E. Tumor Extracellular Matrix Remodeling: New Perspectives as a Circulating Tool in the Diagnosis and Prognosis of Solid Tumors. Cells 2019, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.I.P.; Tian, L.; de Ruiter, E.J.; Song, C.; Paucarmayta, A.; Singh, A.; Elshof, E.; Vijver, S.V.; Shaik, J.; Bosiacki, J.; et al. Cancer immunotherapy by NC410, a LAIR-2 Fc protein blocking human LAIR-collagen interaction. eLife 2021, 10, e62927. [Google Scholar] [CrossRef] [PubMed]

| Breast cancer | Association of high expression of LAIR1 with higher histological grade and poor outcome, hormone receptor negativity, extracellular matrix remodeling events [58] |
| Oral squamous cell carcinoma | Association of high expression of LAIR1 with advanced pathological grades and immune-suppressive profiles [59] |
| Hepatocellular carcinoma | Association of high expression of LAIR1 with poor cancer differentiation and with worse overall survival [60] |
| Renal cell carcinoma | Association of high expression of LAIR1 with poor progression-free survival [61] |
| Osteosarcoma | Association of high expression of LAIR1 with advanced stage [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Laethem, F.; Donaty, L.; Tchernonog, E.; Lacheretz-Szablewski, V.; Russello, J.; Buthiau, D.; Almeras, M.; Moreaux, J.; Bret, C. LAIR1, an ITIM-Containing Receptor Involved in Immune Disorders and in Hematological Neoplasms. Int. J. Mol. Sci. 2022, 23, 16136. https://doi.org/10.3390/ijms232416136
Van Laethem F, Donaty L, Tchernonog E, Lacheretz-Szablewski V, Russello J, Buthiau D, Almeras M, Moreaux J, Bret C. LAIR1, an ITIM-Containing Receptor Involved in Immune Disorders and in Hematological Neoplasms. International Journal of Molecular Sciences. 2022; 23(24):16136. https://doi.org/10.3390/ijms232416136
Chicago/Turabian StyleVan Laethem, François, Lucie Donaty, Emmanuelle Tchernonog, Vanessa Lacheretz-Szablewski, Jennifer Russello, Delphine Buthiau, Marion Almeras, Jérôme Moreaux, and Caroline Bret. 2022. "LAIR1, an ITIM-Containing Receptor Involved in Immune Disorders and in Hematological Neoplasms" International Journal of Molecular Sciences 23, no. 24: 16136. https://doi.org/10.3390/ijms232416136
APA StyleVan Laethem, F., Donaty, L., Tchernonog, E., Lacheretz-Szablewski, V., Russello, J., Buthiau, D., Almeras, M., Moreaux, J., & Bret, C. (2022). LAIR1, an ITIM-Containing Receptor Involved in Immune Disorders and in Hematological Neoplasms. International Journal of Molecular Sciences, 23(24), 16136. https://doi.org/10.3390/ijms232416136





