A General Signal Pathway to Regulate Multiple Detoxification Genes Drives the Evolution of Helicoverpa armigera Adaptation to Xenobiotics
Abstract
1. Introduction
2. Results
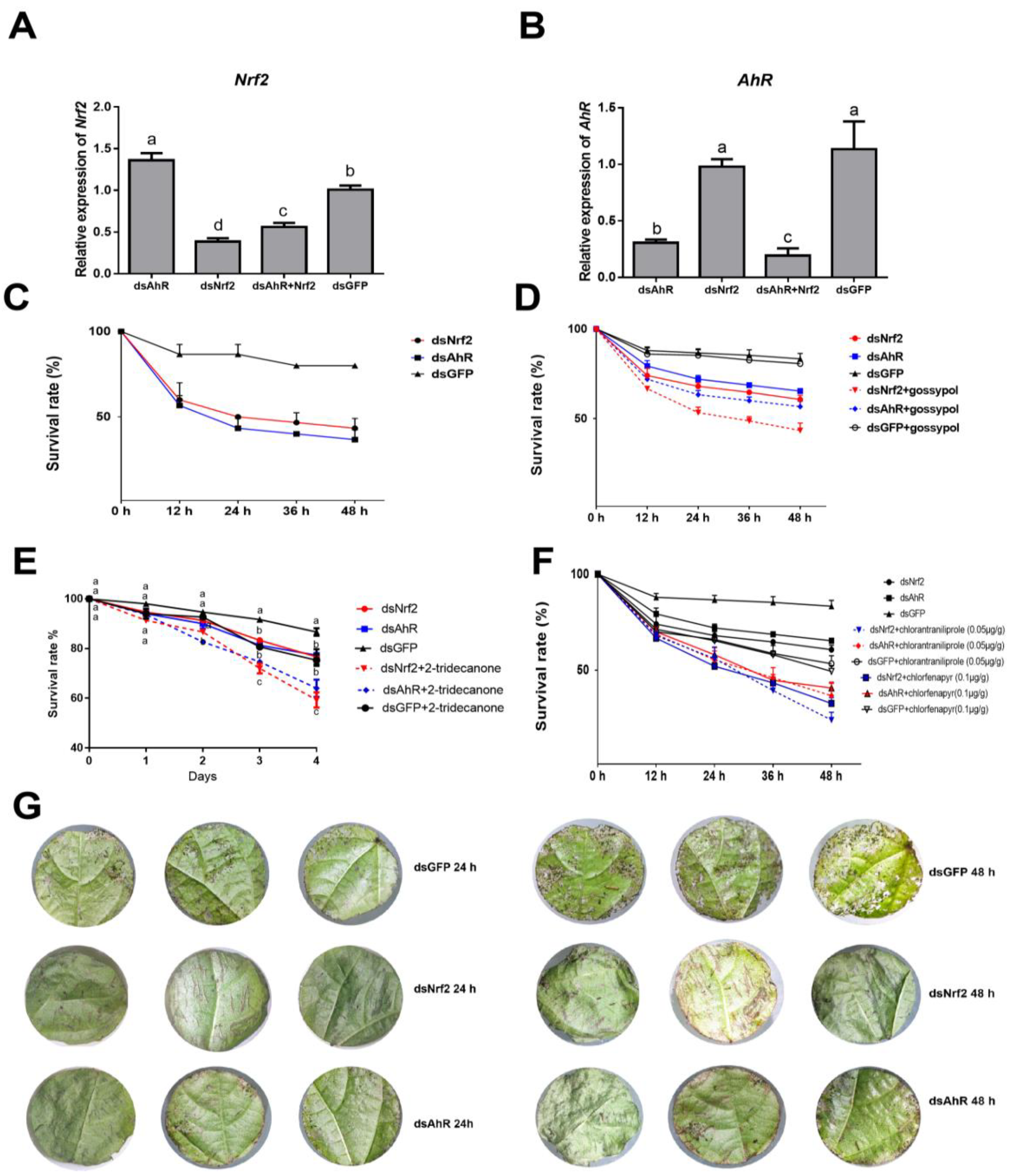
2.1. Effects of RNAi HaNrf2 and HaAhR Genes on Xenobiotics Susceptibility of H. armigera
2.2. RNA Sequencing and Analysis
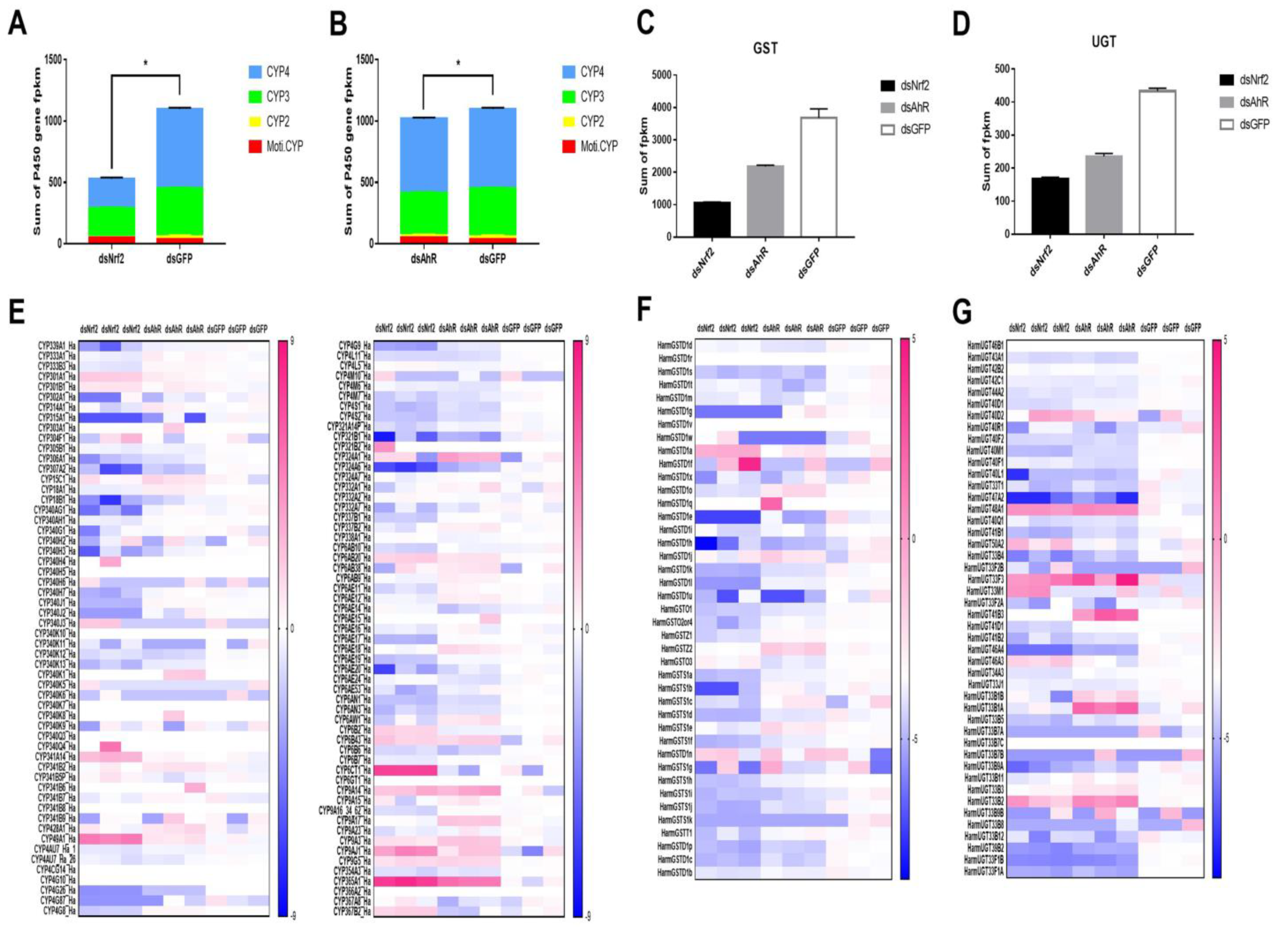
2.3. Analysis of P450s, UGTs and GSTs Genes Were Down-Regulated by RNAi HaNrf2 and HaAhR
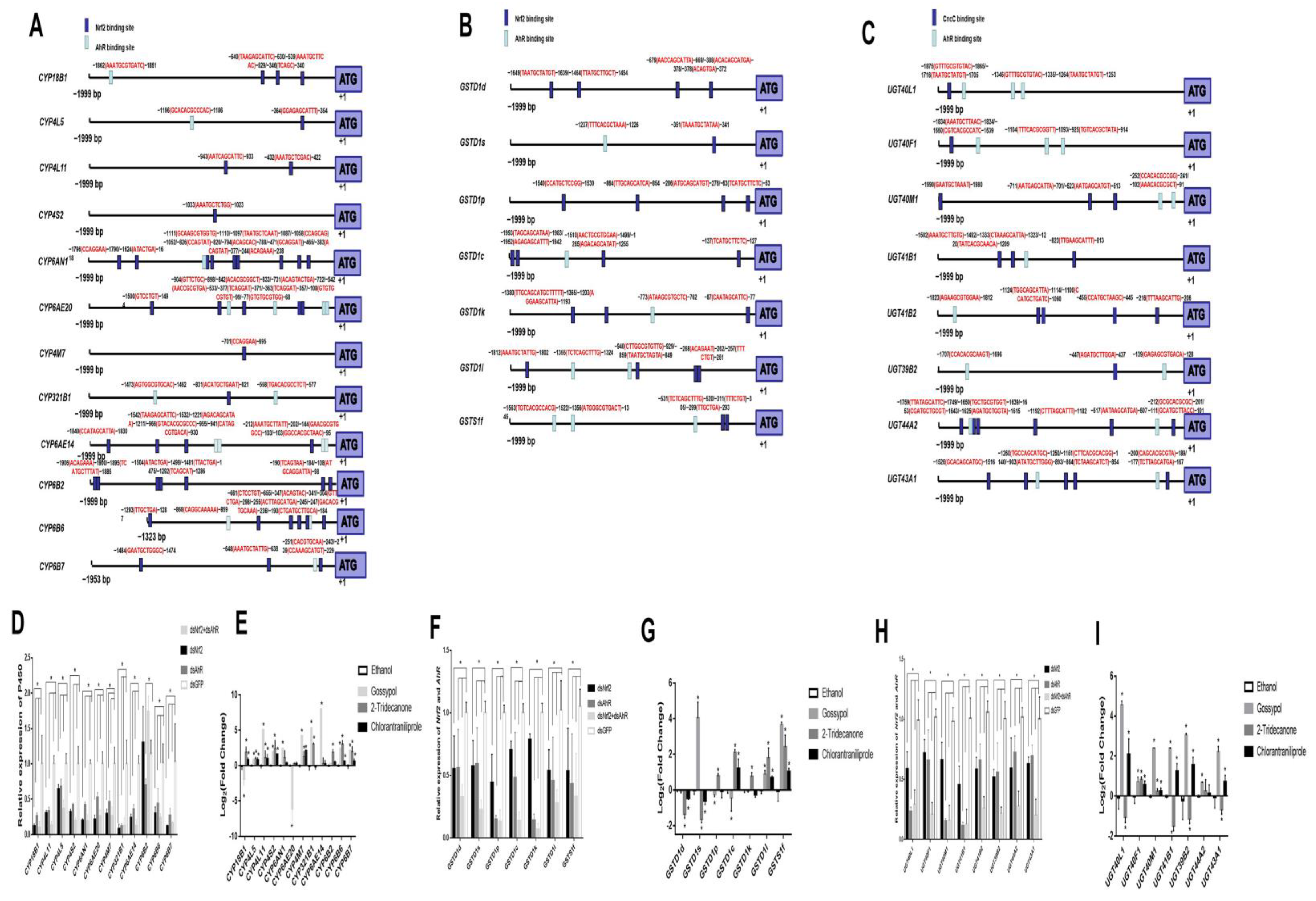
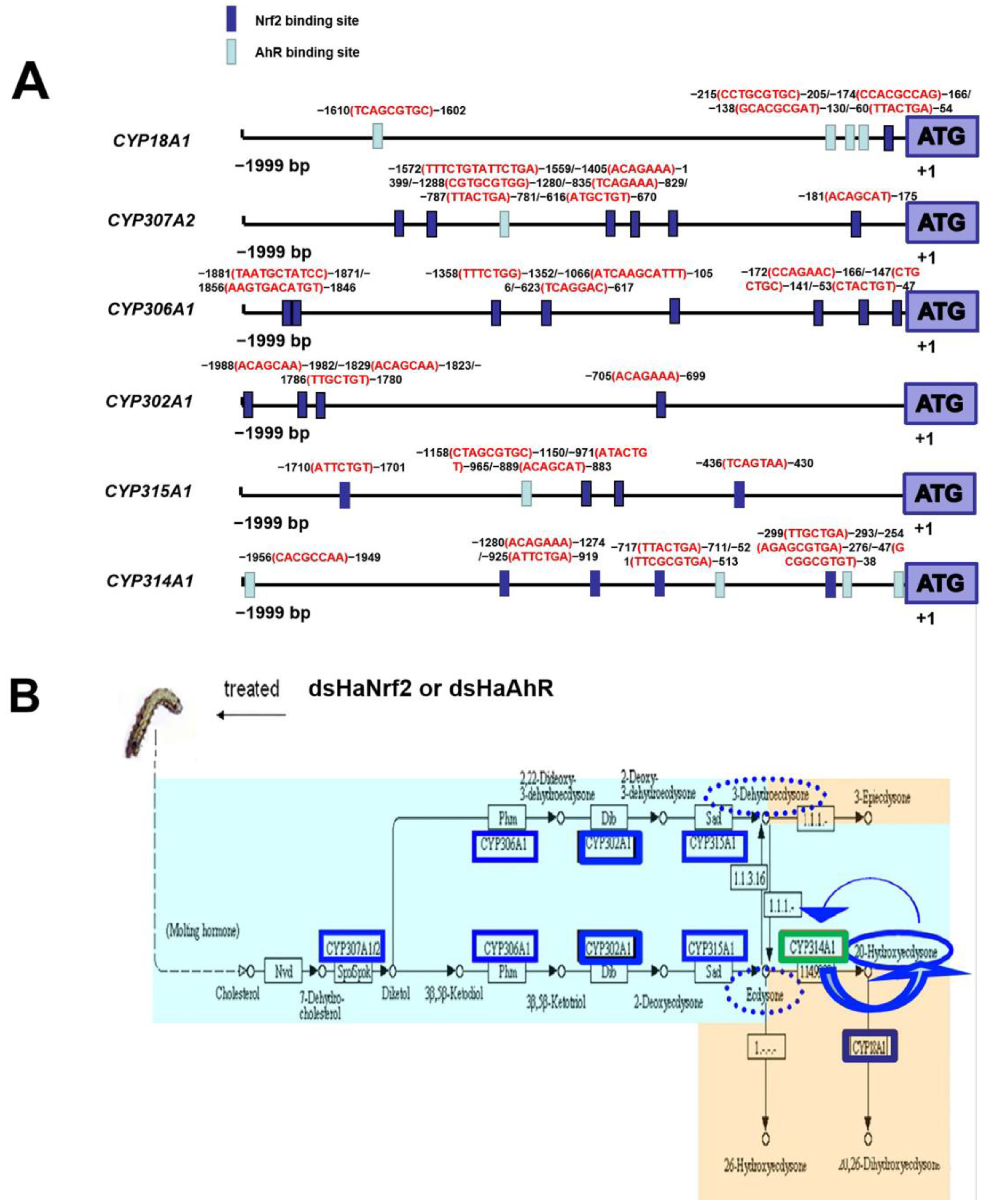
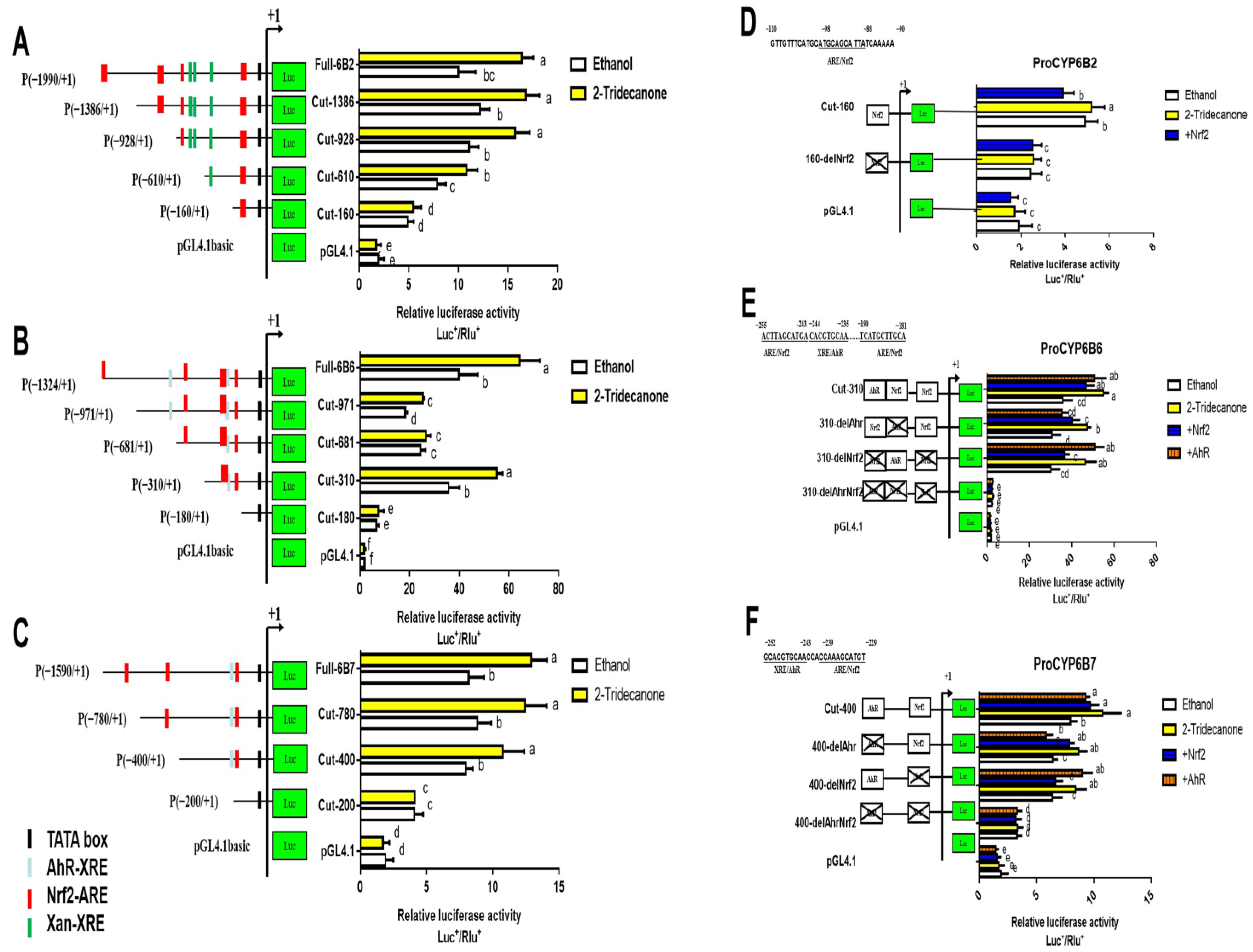
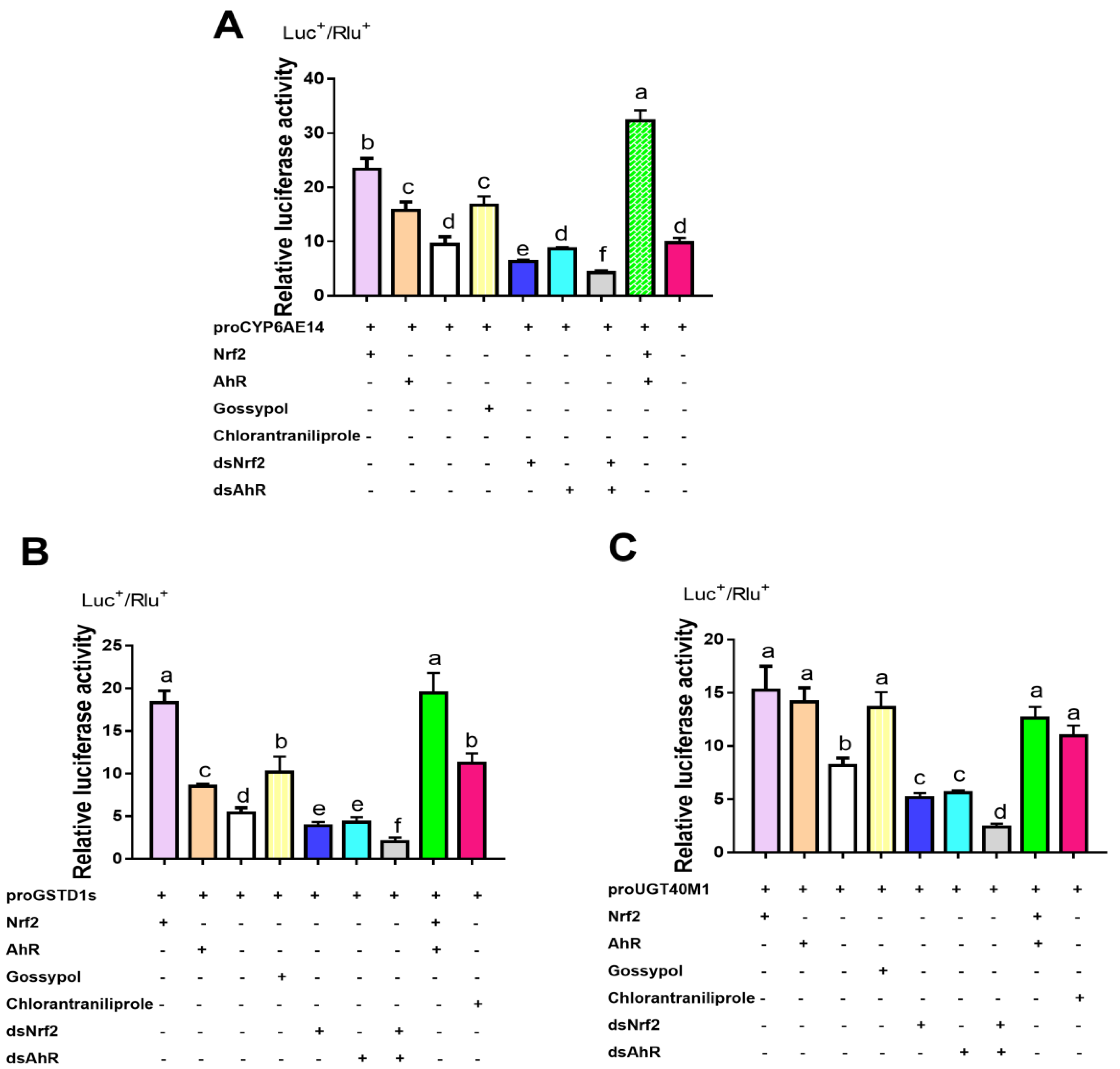
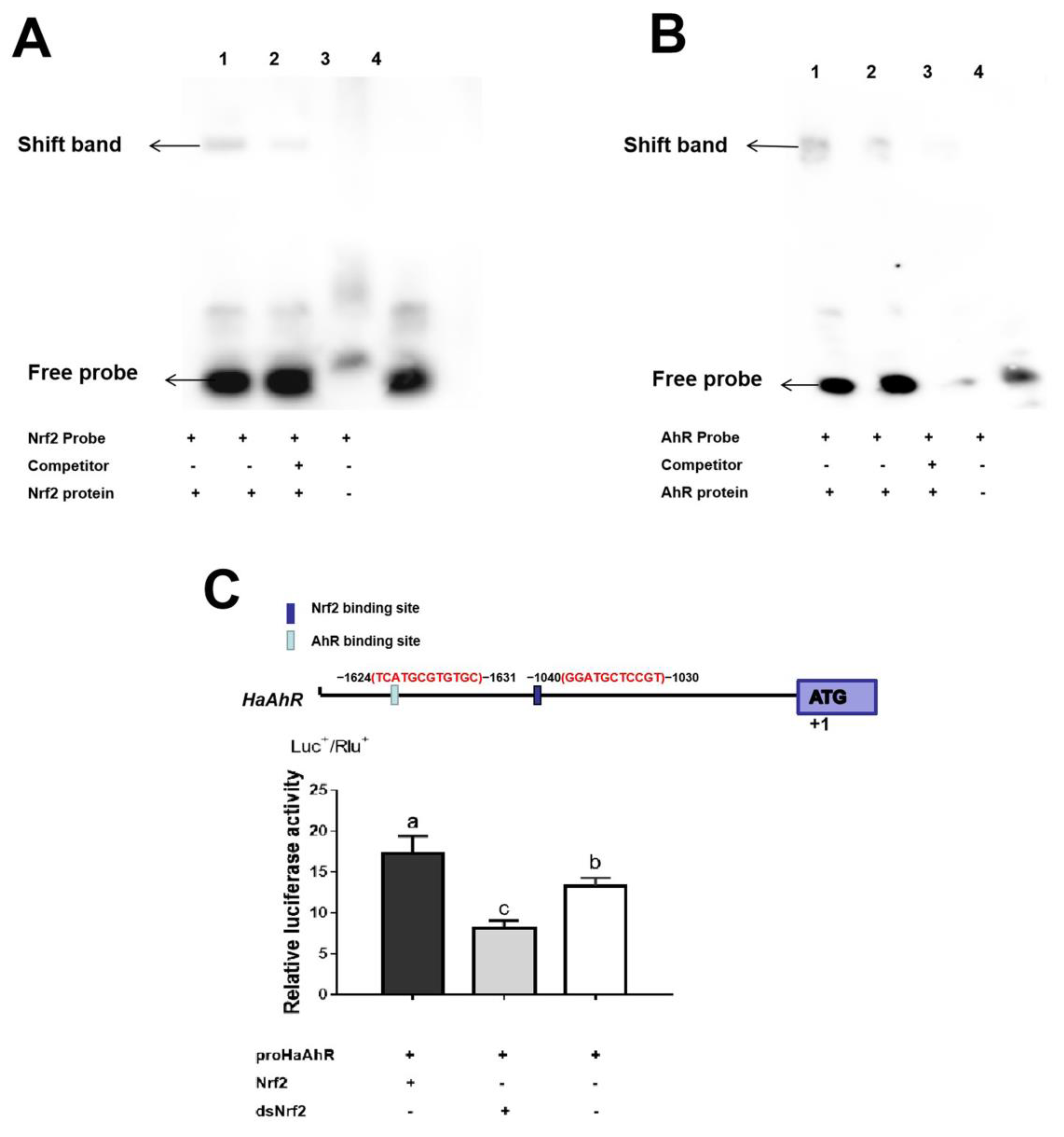
2.4. Transcription Factor Binding Sites Prediction and RT-qPCR Validation of Down-Regulated Genes
2.5. RNAi HaNrf2 and HaAhR Effects on the Synthesis of Insect Hormone
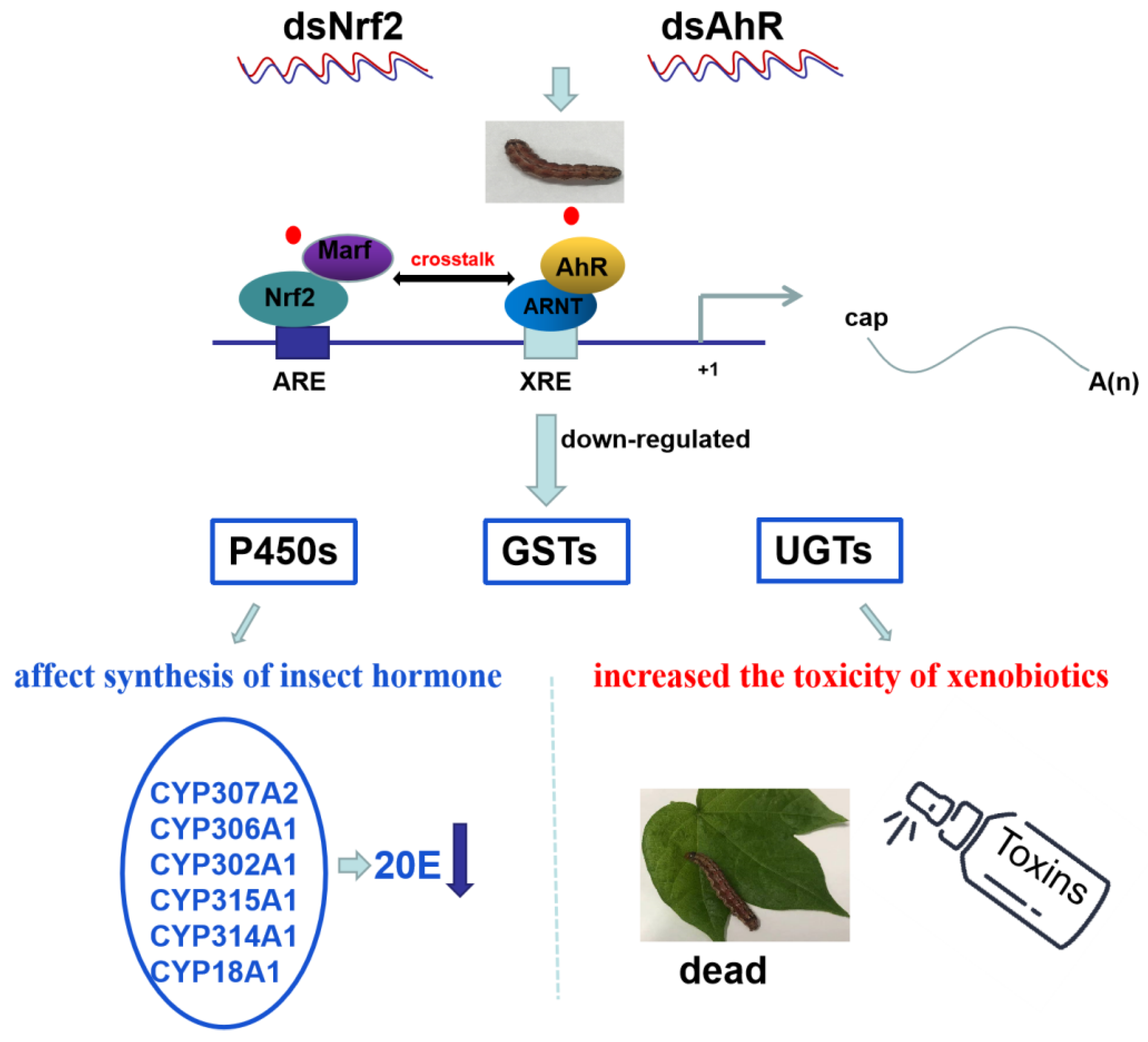
2.6. Detoxification Genes Regulated by HaNrf2 and HaAhR in H. armigera
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. RNA Isolation, Reverse Transcription, and Gene Cloning
4.3. HaNrf2 and HaAhR RNAi
4.4. Xenobiotics Treatment following HaNrf2 or HaAhR Knockdown
4.5. RNA Sequencing
4.6. Quantitative PCR Analysis of Down-Regulated Genes and Prediction of Transcription Factor Binding Sites
4.7. Transient Transfection and Dual Luciferase Assay
4.8. Super Electrophoretic Mobility Shift Assays (sEMSA)
4.9. Laser Confocal Light Microscopy
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heckel, D.G. Insect detoxification and sequestration strategies. In Annu. Plant Rev.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 77–114. [Google Scholar]
- Schuler, M.A. P450s in plant-insect interactions. Biochem. Biophys. Acta 2011, 1814, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Bass, C.; Feyereisen, R.; Vontas, J. The role of cytochrome P450s in insect toxicology and resistance. Annu. Rev. Entomol. 2022, 67, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Koirala, S.; Moural, T.; Zhu, F. Functional and structural diversity of insect Glutathione S-transferases in xenobiotic adaptation. Int. J. Biol. Sci. 2022, 18, 5713–5723. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.H.; Liao, C.Y.; Chakrabarty, S.; Zheng, W.G.; Wu, K.M.; Xiao, Y.T. Transcriptional response of ATPbinding cassette (ABC) transporters to insecticides in the cotton bollworm, Helicoverpa armigera. Pestic. Biochem. Physiol. 2019, 154, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Misra, J.R.; Lam, G.; Thummel, C.S. Constitutive activation of the Nrf2/Keap1 pathway in insecticide-resistant strains of Drosophila. Insect Biochem. Mol. 2013, 43, 1116–1124. [Google Scholar] [CrossRef]
- Cheng, T.; Wu, J.; Wu, Y.; Chilukuri, R.V.; Huang, L.; Yamamoto, K.; Feng, L.; Li, W.; Chen, Z.; Guo, H.; et al. Genomic adaptation to polyphagy and insecticides in a major East Asian noctuid pest. Nat. Ecol. Evol. 2017, 1, 1747. [Google Scholar] [CrossRef]
- Zhang, D.D.; Chapman, E. The role of natural products in revealing NRF2 function. Nat. Prod. Rep. 2022, 37, 797–826. [Google Scholar] [CrossRef]
- Afschar, S.; Toivonen, J.M.; Hoffmann, J.M.; Tain, L.S.; Wieser, D.; Finlayson, A.J.; Driege, Y.; Alic, N.; Emran, S.; Stinn, J.; et al. Nuclear hormone receptor DHR96 mediates the resistance to xenobiotics but not the increased lifespan of insulin-mutant Drosophila. Proc. Natl. Acad. Sci. USA 2016, 113, 1321–1326. [Google Scholar] [CrossRef]
- Yang, X.; Deng, S.; Wei, X.; Yang, J.; Zhao, Q.; Yin, C.; Du, T.; Guo, Z.; Xia, J.; Yang, Z.; et al. MAPK-directed activation of the whitefly transcription factor CREB leads to P450-mediated imidacloprid resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 10246–10253. [Google Scholar] [CrossRef]
- Hu, B.; Ren, M.; Fan, J.; Huang, S.; Wang, X.; Elzaki, M.E.A.; Bass, C.; Palli, S.R.; Su, J.Y. Xenobiotic transcription factors CncC and maf regulate expression of CYP321A16 and CYP332A1 that mediate chlorpyrifos resistance in Spodoptera exigua. J. Hazard. Mater. 2020, 398, 122971. [Google Scholar]
- Palli, S.R. CncC/Maf-mediated xenobiotic response pathway in insects. Arch. Insect Biochem. 2020, 104, e21674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lv, S.L.; Liu, Y.; Yang, L.W.; Liang, P.; Gao, X.W. Cellular redox-related transcription factor Nrf2 mediation of HaTrf response to host plant allelochemical 2-Tridecanone in Helicoverp armigera. J. Agric. Food Chem. 2020, 68, 6919–6926. [Google Scholar] [CrossRef] [PubMed]
- Sykiotis, G.P.; Bohmann, D. Stress-activated Cap’n’collar transcription factors in aging and human disease. Sci. Signal. 2010, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef] [PubMed]
- Denison, M.S.; Nagy, S.R. Activation of aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. 2003, 43, 309–334. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Y.; Xiang, M.; Shang, Q.; Gao, X. The retardant effect of 2-Tridecanone, mediated by cytochrome P450, on the development of cotton bollworm, Helicoverpa armigera. BMC Genom. 2016, 17, 954. [Google Scholar] [CrossRef]
- Celorio-Mancera, M.P.; Ahn, S.J.; Heiko, V.; David, G.H. Transcriptional responses underlying the hormetic and detrimental effects of the plant secondary metabolite gossypol on the generalist herbivore Helicoverpa armigera. BMC Genom. 2011, 2, 575. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Y.; Xiang, M.; Shang, Q.; Gao, X. A transferrin gene associated with development and 2-tridecanone tolerance in Helicoverpa armigera. Insect Mol. Biol. 2015, 24, 155–166. [Google Scholar] [CrossRef]
- Petryk, A.; Warren, J.T.; Marqués, G.; Jarcho, M.P.; Gilbert, L.I.; Kahler, J.; Parvy, J.-P.; Li, Y.; Dauphin-Villemant, C.; O’Connor, M.B. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc. Natl. Acad. Sci. USA 2003, 100, 13773–13778. [Google Scholar] [CrossRef]
- Gilbert, L.I.; Rybczynski, R.; Warren, J.T. Control and biochemical nature of the ecdysteroidogenic pathway. Annu. Rev. Entomol. 2002, 47, 883–916. [Google Scholar] [CrossRef]
- Kim, F.R.; Michael, B.O.C.; Lawrence, I.G. Molecular evolution of the insect Halloween family of cytochrome P450s: Phylogeny, gene organization and functional conservation. Insect Biochem. Mol. Biol. 2007, 37, 741–753. [Google Scholar]
- Kim, F.R.; Naoki, Y.; Michael, B.O.C. Steroid hormone inactivation is required during the juvenile-adult transition in Drosophila. Dev. Cell 2011, 19, 895–902. [Google Scholar]
- Jensen, N.B.; Zagrobelny, M.; Hjernø, K.; Olsen, C.E.; Houghton-Larsen, J.; Borch, J.; Møller, B.L.; Bak, S. Convergent evolution in biosynthesis of cyanogenic defence compounds in plants and insects. Nat. Commun. 2011, 2, 273. [Google Scholar] [CrossRef]
- Dermauw, W.; Van Leeuwen, T.; Feyereisen, R. Diversity and evolution of the P450 family in arthropods. Insect Biochem. Mol. 2020, 127, 103490. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, M.; Palli, S.R. Cap n collar transcription factor regulates multiple genes coding for proteins involved in insecticide detoxification in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. 2017, 90, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Gaddelapati, S.C.; Kalsi, M.; Roy, A.; Palli, S.R. Cap ‘n’ collar C regulates genes responsible for imidacloprid resistance in the Colorado potato beetle, Leptinotarsa decemlineata. Insect Biochem. Mol. 2018, 99, 54–62. [Google Scholar] [CrossRef]
- Chen, S.; Lu, M.; Zhang, N.; Zou, X.; Mo, M.; Zheng, S. Nuclear factor erythroid-derived 2-related factor 2 activates glutathione S-transferase expression in the midgut of Spodoptera litura (Lepidoptera: Noctuidae) in response to phytochemicals and insecticides. Insect Mol. Biol. 2018, 27, 522–532. [Google Scholar] [CrossRef]
- Lu, K.; Cheng, Y.; Li, W.; Li, Y.; Zeng, R.; Song, Y. Activation of CncC pathway by ROS burst regulates cytochrome P450 CYP6AB12 responsible for λ-cyhalothrin tolerance in Spodoptera litura. J. Hazard. Mater. 2020, 387, 121698. [Google Scholar] [CrossRef]
- Shi, L.; Shi, Y.; Liu, M.F.; Zhang, Y.; Liao, X.L. Transcription factor CncC potentially regulates the expression of multiple detoxification genes that mediate indoxacarb resistance in Spodoptera litura. Insect Sci. 2021, 28, 1426–1438. [Google Scholar] [CrossRef]
- Li, J.; Mao, T.; Wang, H.; Lu, Z.; Qu, J.; Fang, Y.; Chen, J.; Li, M.; Cheng, X.; Hu, J.; et al. The CncC/keap1 pathway is activated in high temperature-induced metamorphosis and mediates the expression of Cyp450 genes in silkworm, Bombyx mori. Biochem. Biophys. Res. Commun. 2019, 514, 1045–1050. [Google Scholar] [CrossRef]
- Wan, H.; Liu, Y.; Li, M.; Zhu, S.; Li, X.; Pittendrigh, B.R.; Qiu, X. Nrf2/Maf-binding-site-containing functional Cyp6a2 allele is associated with DDT resistance in Drosophila melanogaster. Pest Manag. Sci. 2014, 70, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Harada, N.; Hida, A.; Fujii-Kuriyama, Y.; Motohashi, H.; Yamamoto, M. Gene expression of detoxifying enzymes in AhR and Nrf2 compound null mutant mouse. Biochem. Biophys. Res. Commun. 2003, 303, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.P.; McDonnell, C.M.; Berenbaum, M.R.; Schuler, M.A. Regulation of an insect cytochrome P450 monooxygenase gene (CYP6B1) by aryl hydrocarbon and xanthotoxin response cascades. Gene 2005, 26, 39–52. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, C.M.; Brown, R.P.; Berenbaum, M.R.; Schuler, M.A. Conserved regulatory elements in the promoters of two allelochemical-inducible cytochrome P450 genes differentially regulate transcription. Insect Biochem. Mol. 2004, 34, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.P.; Berenbaum, M.R.; Schuler, M.A. Transcription of a lepidopteran cytochrome P450 promoter is modulated by multiple elements in its 5′-UTR and repressed by 20-hydroxyecdysone. Insect Mol. Biol. 2004, 13, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Chen, X.; Pan, Y.; Zheng, Z.; Wei, X.; Xi, J.; Zhang, J.; Gao, X.; Shang, Q. Transcription factor aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator is involved in regulation of the xenobiotic tolerance-related cytochrome P450 CYP6DA2 in Aphis gossypii Glover. Insect Mol. Biol. 2017, 26, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-B.; Cai, W.-J.; Wang, J.-W.; Hong, G.-J.; Tao, X.-Y.; Wang, L.-J.; Huang, Y.-P.; Chen, X.-Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Y.; Wang, L.; Liu, S.; Wu, S.; Yang, Y.; Feyereisen, R.; Wu, Y. CYP6AE gene cluster knockout in Helicoverpa armigera reveals role in detoxification of phytochemicals and insecticides. Nat. Commun. 2018, 9, 4820. [Google Scholar] [CrossRef]
- Tao, X.; Xue, X.; Huang, Y.; Chen, X.; Mao, Y. Gossypol-enhanced P450 gene pool contributes to cotton bollworm tolerance to a pyrethroid insecticide. Mol. Ecol. 2012, 21, 4371–4385. [Google Scholar] [CrossRef]
- Anwar-Mohamed, A.; Elshenawy, O.H.; Soshilov, A.A.; Denison, M.S.; Chris, L.X.; Klotz, L.O.; El-Kadi, A.O. Methylated pentavalent arsenic metabolites are bifunctional inducers, as they induce cytochrome P450 1A1 and NAD(P)H:quinone oxidoreductase through AhR- and Nrf2-dependent mechanisms. Free. Radic. Biol. Med. 2014, 67, 171–187. [Google Scholar] [CrossRef]
- Miao, W.M.; Hu, L.G.; Scrivens, P.J.; Batist, G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway direct crosstalk between phase I and II drug-metabolizing enzymes. J. Biol. Chem. 2005, 280, 20340–20348. [Google Scholar] [CrossRef] [PubMed]
- Horn, T.; Boutros, M. E-RNAi: A web application for the multi-species design of RNAi reagents-2010 update. Nucleic Acids Res. 2010, 38, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; Mccue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pearce, S.L.; Clarke, D.F.; East, P.D.; Elfekih, S.; Gordon, K.H.J.; Jermiin, L.S.; McGaughran, A.; Oakeshott, J.G.; Papanikolaou, A.; Perera, O.P.; et al. Genomic innovations, transcriptional plasticity and gene loss underlying the evolution and divergence of two highly polyphagous and invasive Helicoverpa pest Species. BMC Biol. 2017, 15, 63. [Google Scholar] [CrossRef]
- Mathelier, A.; Fornes, O.; Arenillas, D.J.; Chen, C.-Y.; Denay, G.; Lee, J.; Shi, W.; Shyr, C.; Tan, G.; Worsley-Hunt, R.; et al. JASPAR 2016: A major expansion and update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2016, 44, 110–115. [Google Scholar] [CrossRef]
- Lahiri, D.K.; Ge, Y.W. Electrophoretic mobility shift assay for the detection of specific DNA-protein complex in nuclear extracts from the cultured cells and frozen autopsy human brain tissue. Brain Res. Protoc. 2000, 5, 257–265. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Lv, S.; Li, M.; Gu, M.; Gao, X. A General Signal Pathway to Regulate Multiple Detoxification Genes Drives the Evolution of Helicoverpa armigera Adaptation to Xenobiotics. Int. J. Mol. Sci. 2022, 23, 16126. https://doi.org/10.3390/ijms232416126
Zhang L, Lv S, Li M, Gu M, Gao X. A General Signal Pathway to Regulate Multiple Detoxification Genes Drives the Evolution of Helicoverpa armigera Adaptation to Xenobiotics. International Journal of Molecular Sciences. 2022; 23(24):16126. https://doi.org/10.3390/ijms232416126
Chicago/Turabian StyleZhang, Lei, Shenglan Lv, Mingjian Li, Meng Gu, and Xiwu Gao. 2022. "A General Signal Pathway to Regulate Multiple Detoxification Genes Drives the Evolution of Helicoverpa armigera Adaptation to Xenobiotics" International Journal of Molecular Sciences 23, no. 24: 16126. https://doi.org/10.3390/ijms232416126
APA StyleZhang, L., Lv, S., Li, M., Gu, M., & Gao, X. (2022). A General Signal Pathway to Regulate Multiple Detoxification Genes Drives the Evolution of Helicoverpa armigera Adaptation to Xenobiotics. International Journal of Molecular Sciences, 23(24), 16126. https://doi.org/10.3390/ijms232416126





