Neuronal Nitric Oxide Synthase as a Shared Target for the Effects of Adiponectin and Resistin on the Mechanical Responses of the Mouse Gastric Fundus
Abstract
1. Introduction
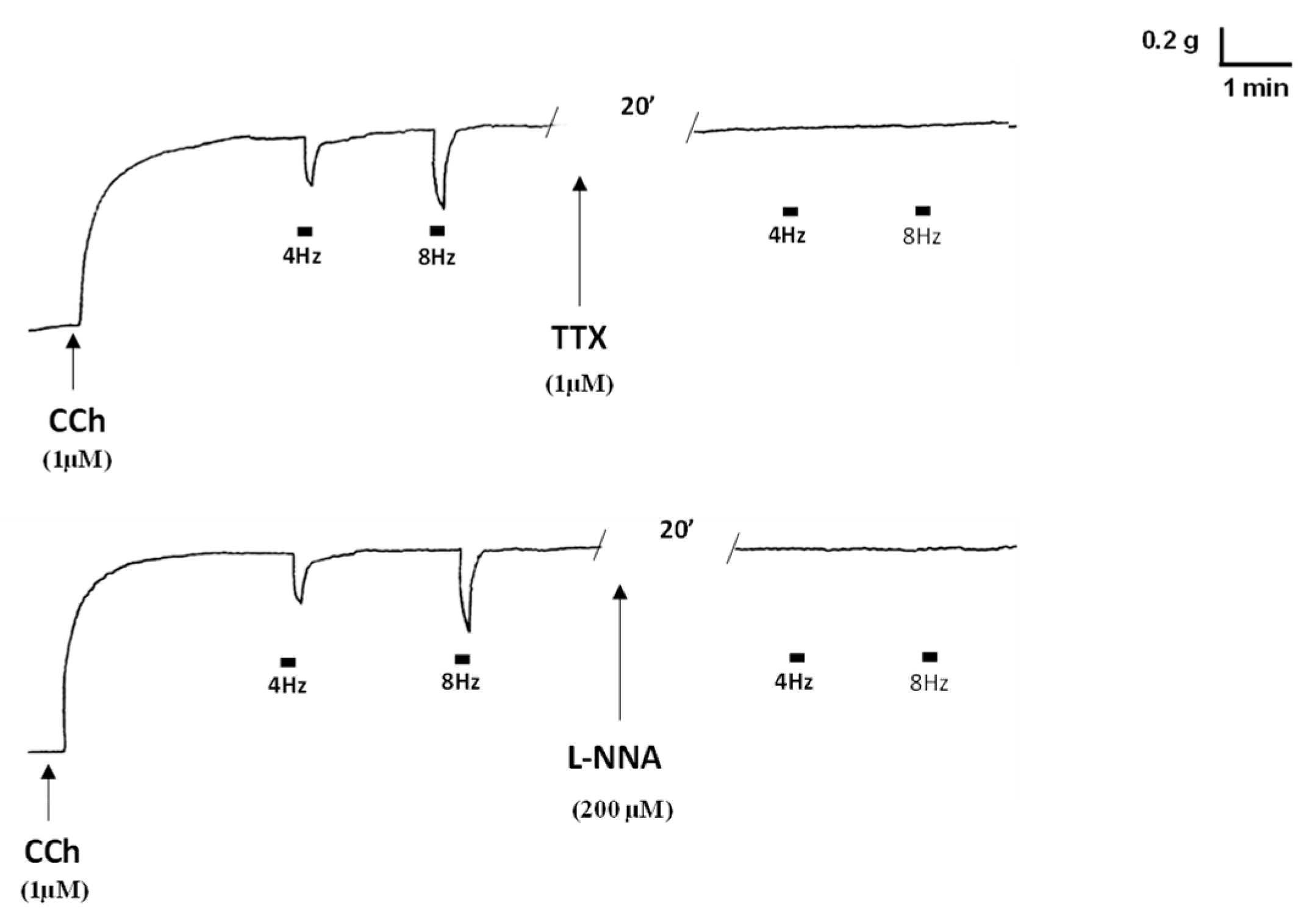
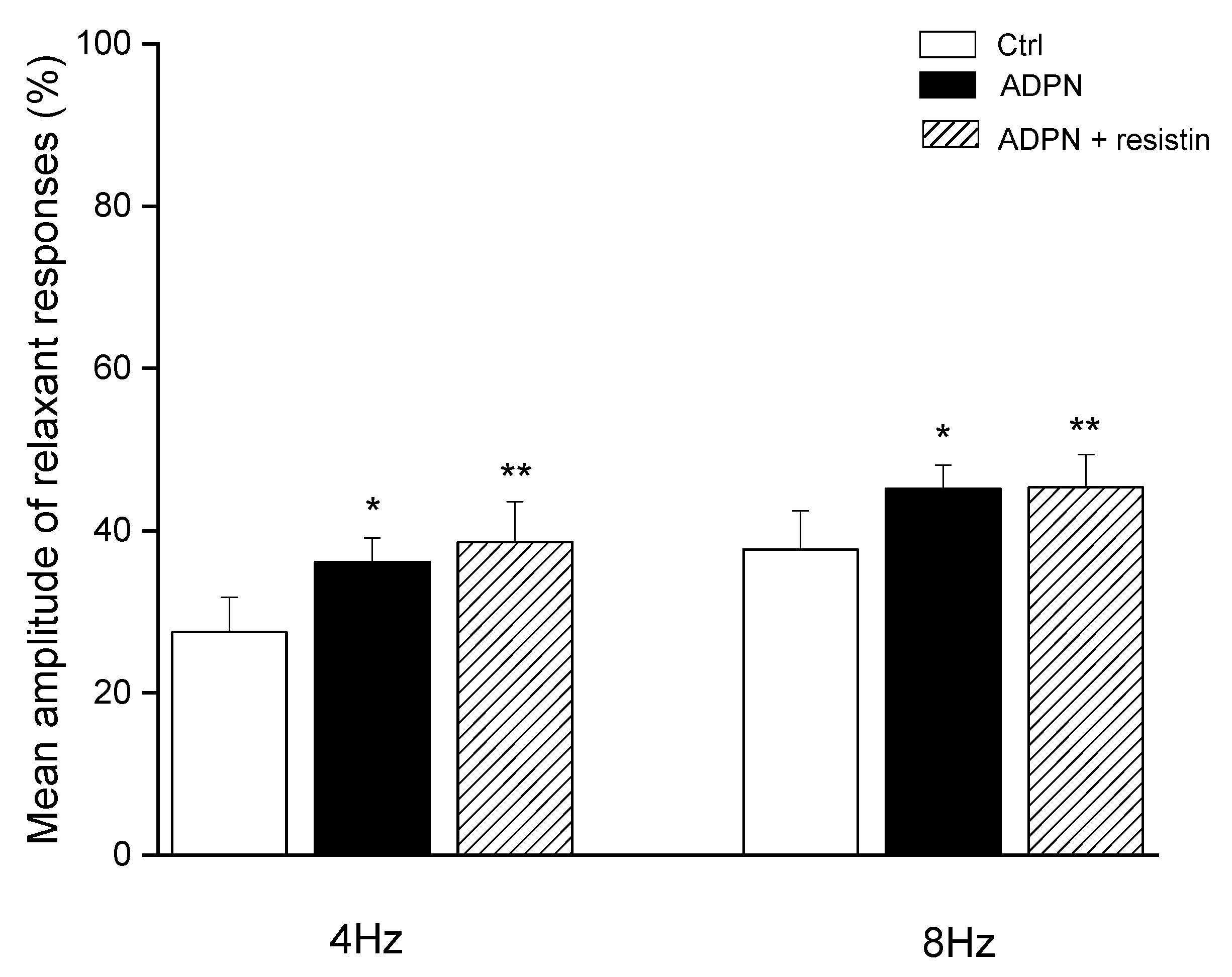
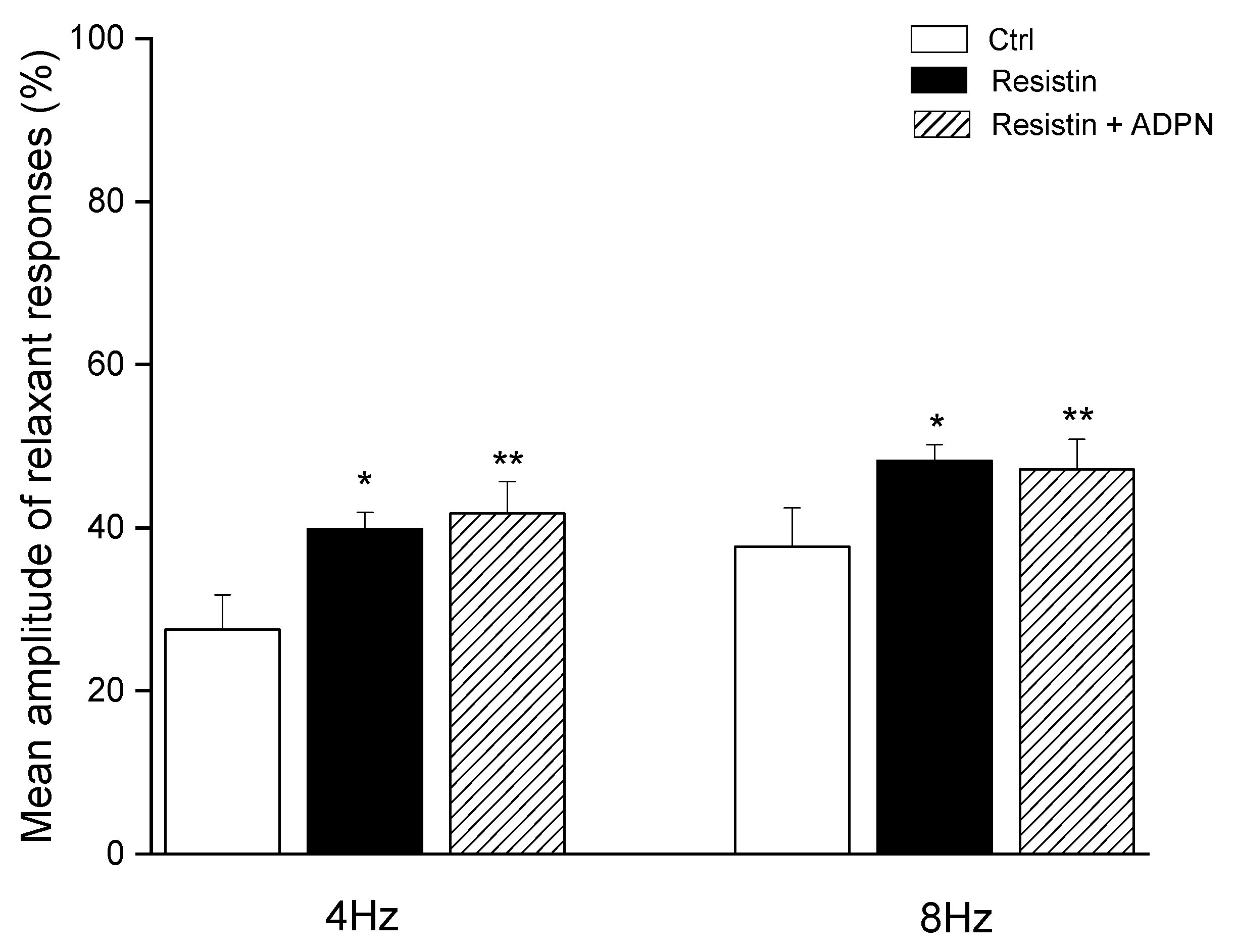
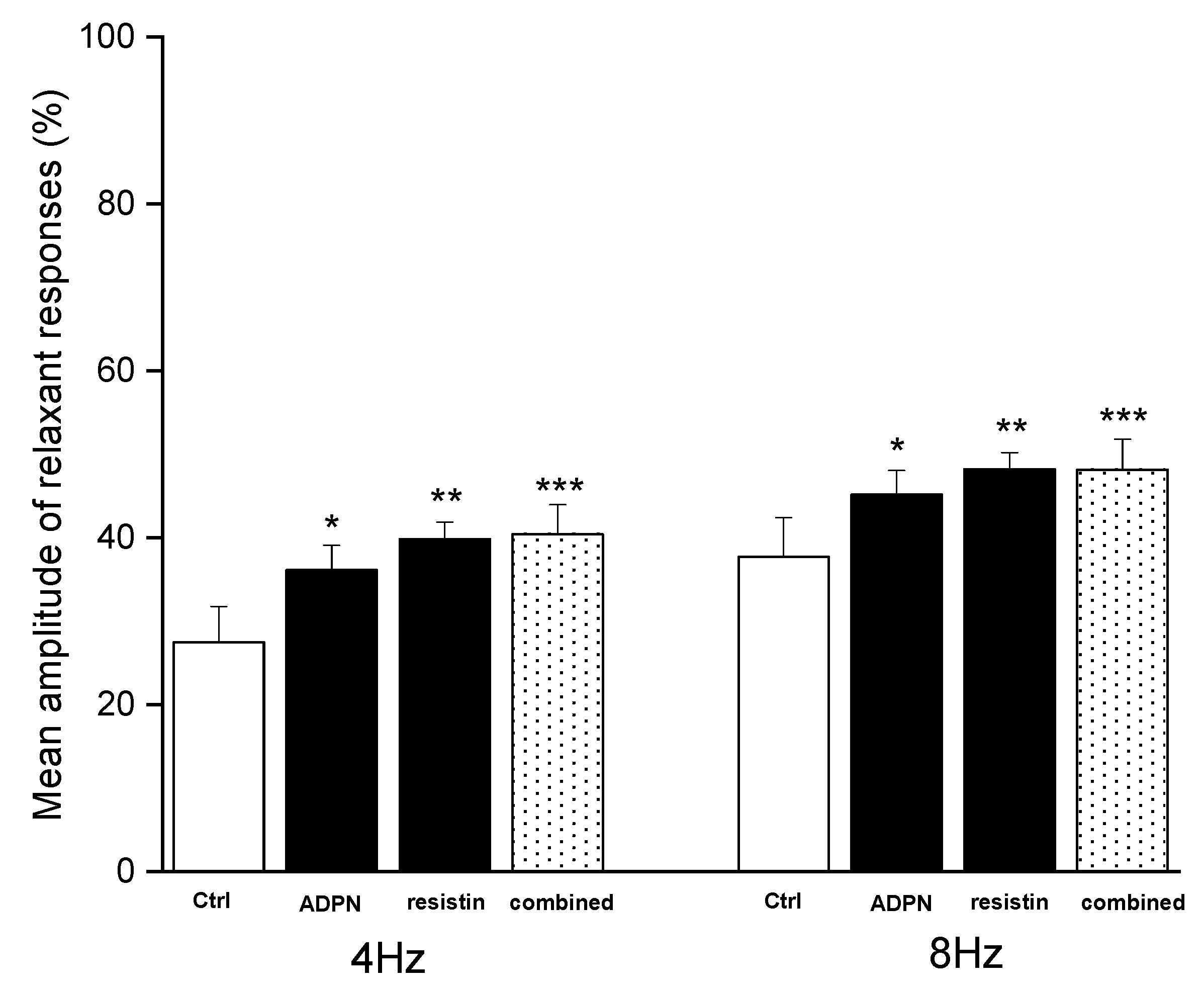
2. Results
2.1. Functional Experiments
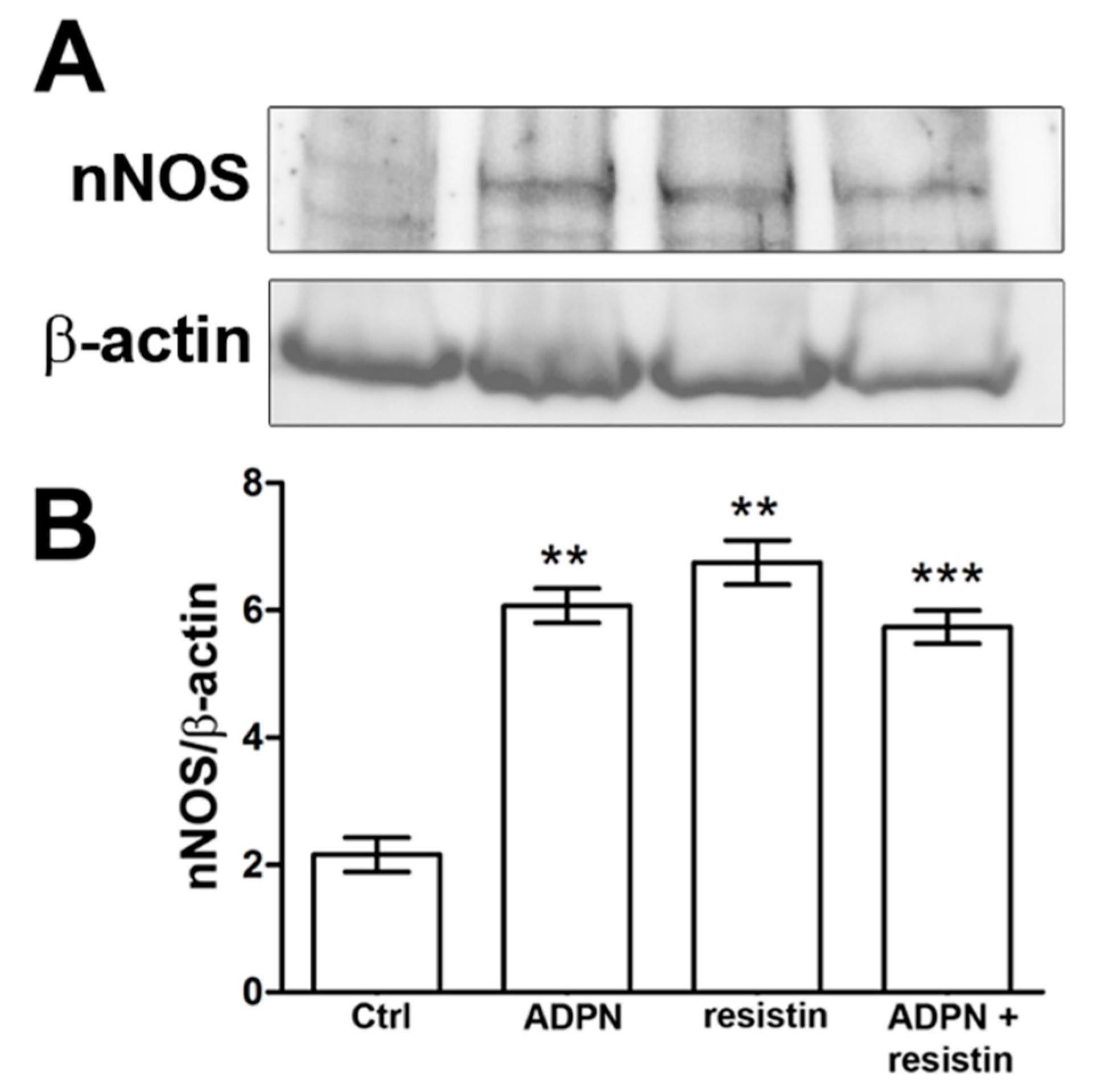
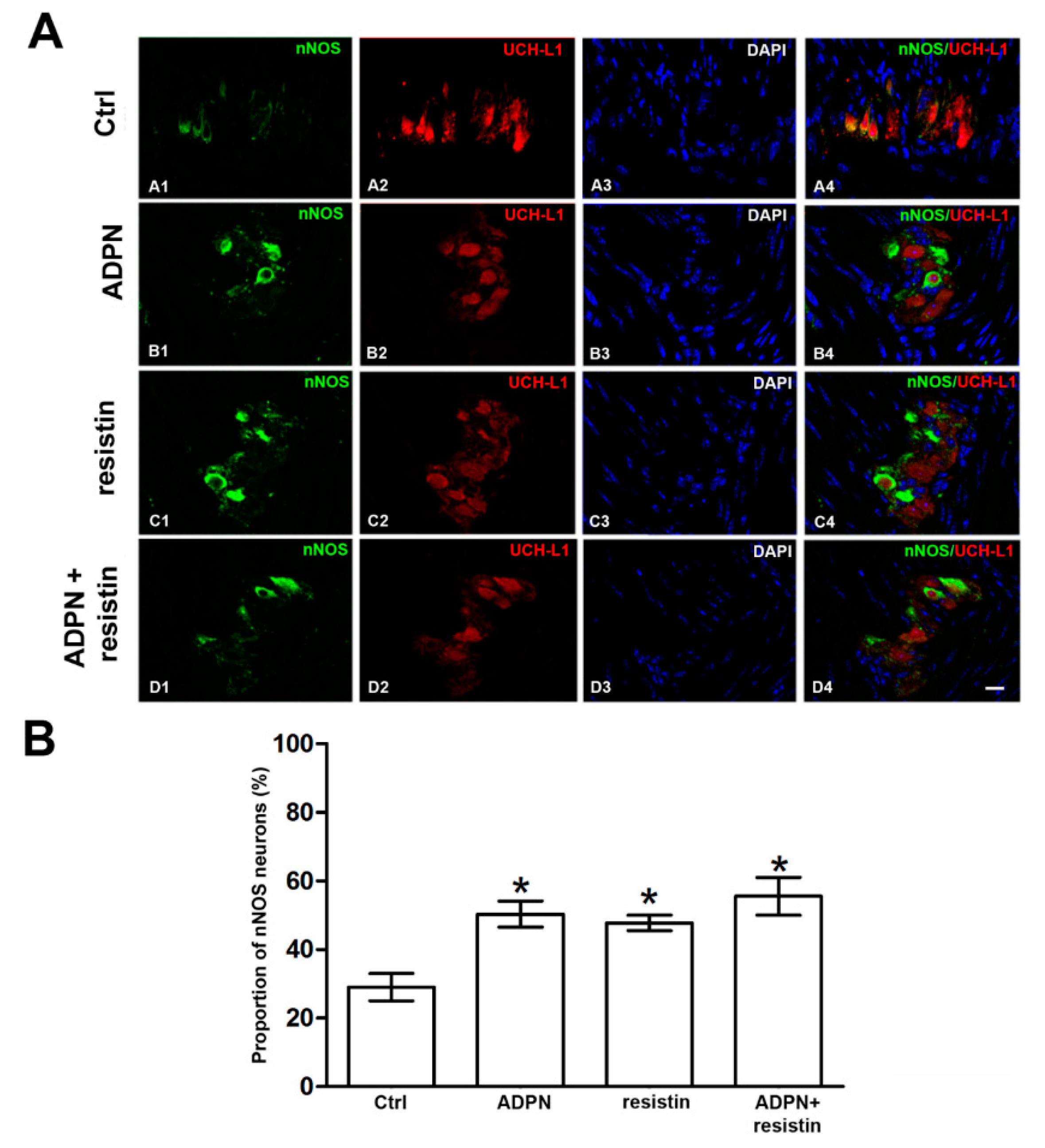
2.2. Western Blotting and Immunofluorescence Analysis
3. Discussion
4. Materials and Methods
4.1. Animals and Ethical Approval
4.2. Mechanical Recording
4.3. Western Blotting and Immunofluorescence Analysis
4.3.1. Western Blotting
4.3.2. Immunofluorescence Analysis
4.4. Drugs
4.5. Data Analysis and Statistical Tests
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADPN | Adiponectin |
| CCh | Carbachol |
| CTRL | Control |
| EFS | Electrical field stimulation |
| L-NNA | L-NG-nitro arginine |
| NANC | Non-adrenergic, non-cholinergic |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| nNOS | Neuronal nitric oxide synthase |
| TTX | Tetrodotoxin |
References
- Ahima, R.S. Adipose tissue as an endocrine organ. Obesity 2006, 5, 242S–249S. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Ezquerro, S.; Méndez-Giménez, L.; Becerril, S.; Frühbeck, G. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E691–E714. [Google Scholar] [CrossRef] [PubMed]
- Esmaili, S.; Hemmati, M.; Karamian, M. Physiological role of adiponectin in different tissues: A review. Arch. Physiol. Biochem. 2020, 126, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M. Leptin and the endocrine control of energy balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Harwood, H.J., Jr. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology 2012, 63, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Yarandi, S.S.; Hebbar, G.; Sauer, C.G.; Cole, C.R.; Ziegler, T.R. Diverse roles of leptin in the gastrointestinal tract: Modulation of motility, absorption, growth, and inflammation. Nutrition 2011, 27, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Estienne, A.; Bongrani, A.; Reverchon, M.; Ramé, C.; Ducluzeau, P.H.; Froment, P.; Dupont, J. Involvement of Novel Adipokines, Chemerin, Visfatin, Resistin and Apelin in Reproductive Functions in Normal and Pathological Conditions in Humans and Animal Models. Int. J. Mol. Sci. 2019, 20, 4431. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, X.; Chen, D.; Li, Z. The Controversial Role of Adiponectin in Appetite Regulation of Animals. Nutrients 2021, 13, 3387. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Bal, N.C. Adipokines from white adipose tissue in regulation of whole body energy homeostasis. Biochimie 2022, in press. [CrossRef] [PubMed]
- Czaja-Stolc, S.; Potrykus, M.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Pro-Inflammatory Profile of Adipokines in Obesity Contributes to Pathogenesis, Nutritional Disorders, and Cardiovascular Risk in Chronic Kidney Disease. Nutrients 2022, 14, 1457. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.B. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin. Sci. 2021, 135, 731–752. [Google Scholar] [CrossRef]
- Alipoor, E.; Mohammad Hosseinzadeh, F.; Hosseinzadeh-Attar, M.J. Adipokines in critical illness: A review of the evidence and knowledge gaps. Biomed. Pharmacother. 2018, 108, 1739–1750. [Google Scholar] [CrossRef]
- Musovic, S.; Shrestha, M.M.; Komai, A.M.; Olofsson, C.S. Resistin is co-secreted with adiponectin in white mouse adipocytes. Biochem. Biophys. Res. Commun. 2021, 534, 707–713. [Google Scholar] [CrossRef]
- Falcão-Pires, I.; Castro-Chaves, P.; Miranda-Silva, D.; Lourenço, A.P.; Leite-Moreira, A.F. Physiological, pathological and potential therapeutic roles of adipokines. Drug Discov. Today 2012, 17, 880–889. [Google Scholar] [CrossRef]
- Liu, L.; Shi, Z.; Ji, X.; Zhang, W.; Luan, J.; Zahr, T.; Qiang, L. Adipokines, adiposity, and atherosclerosis. Cell. Mol. Life Sci. 2022, 79, 272. [Google Scholar] [CrossRef]
- Tsai, Y.W.; Fu, S.H.; Dong, J.L.; Chien, M.W.; Liu, Y.W.; Hsu, C.Y.; Sytwu, H.K. Adipokine-Modulated Immunological Homeostasis Shapes the Pathophysiology of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2020, 21, 9564. [Google Scholar] [CrossRef]
- Idrizaj, E.; Garella, R.; Squecco, R.; Baccari, M.C. Adipocytes-released Peptides Involved in the Control of Gastrointestinal Motility. Curr. Protein Pept. Sci. 2019, 20, 614–629. [Google Scholar] [CrossRef]
- Wang, H.; Esemu-Ezewu, P.; Pan, J.; Ivanovska, J.; Gauda, E.B.; Belik, J. Adiponectin and the regulation of gastric content volume in the newborn rat. World J. Gastroenterol. 2021, 27, 5566–5574. [Google Scholar] [CrossRef] [PubMed]
- Idrizaj, E.; Garella, R.; Castellini, G.; Francini, F.; Ricca, V.; Baccari, M.C.; Squecco, R. Adiponectin Decreases Gastric Smooth Muscle Cell Excitability in Mice. Front. Physiol. 2019, 10, 1000. [Google Scholar] [CrossRef] [PubMed]
- Idrizaj, E.; Garella, R.; Castellini, G.; Mohr, H.; Pellegata, N.S.; Francini, F.; Ricca, V.; Squecco, R.; Baccari, M.C. Adiponectin affects the mechanical responses in strips from the mouse gastric fundus. World J. Gastroenterol. 2018, 24, 4028–4035. [Google Scholar] [CrossRef]
- Idrizaj, E.; Garella, R.; Nistri, S.; Squecco, R.; Baccari, M.C. Evidence that resistin acts on the mechanical responses of the mouse gastric fundus. Front. Physiol. 2022, 13, 930197. [Google Scholar] [CrossRef] [PubMed]
- Idrizaj, E.; Traini, C.; Vannucchi, M.G.; Baccari, M.C. Nitric Oxide: From Gastric Motility to Gastric Dysmotility. Int. J. Mol. Sci. 2021, 22, 9990. [Google Scholar] [CrossRef]
- Min, Y.W.; Hong, Y.S.; Ko, E.J.; Lee, J.Y.; Ahn, K.D.; Bae, J.M.; Rhee, P.L. Nitrergic Pathway Is the Main Contributing Mechanism in the Human Gastric Fundus Relaxation: An In Vitro Study. PLoS ONE 2016, 11, e0162146. [Google Scholar] [CrossRef][Green Version]
- Rand, M.J. Nitrergic transmission: Nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin. Exp. Pharmacol. Physiol. 1992, 19, 147–169. [Google Scholar] [CrossRef]
- Nowaczyk, A.; Kowalska, M.; Nowaczyk, J.; Grześk, G. Carbon Monoxide and Nitric Oxide as Examples of the Youngest Class of Transmitters. Int. J. Mol. Sci. 2021, 22, 6029. [Google Scholar] [CrossRef]
- Groneberg, D.; Voussen, B.; Friebe, A. Integrative Control of Gastrointestinal Motility by Nitric Oxide. Curr. Med. Chem. 2016, 23, 2715–2735. [Google Scholar] [CrossRef]
- Idrizaj, E.; Garella, R.; Nistri, S.; Dell’Accio, A.; Cassioli, E.; Rossi, E.; Castellini, G.; Ricca, V.; Squecco, R.; Baccari, M.C. Adiponectin Exerts Peripheral Inhibitory Effects on the Mouse Gastric Smooth Muscle through the AMPK Pathway. Int. J. Mol. Sci. 2020, 21, 9617. [Google Scholar] [CrossRef]
- Verbeure, W.; van Goor, H.; Mori, H.; van Beek, A.P.; Tack, J.; van Dijk, P.R. The Role of Gasotransmitters in Gut Peptide Actions. Front. Pharmacol. 2021, 12, 720703. [Google Scholar] [CrossRef] [PubMed]
- Bagyánszki, M.; Torfs, P.; Krecsmarik, M.; Fekete, E.; Adriaensen, D.; Van Nassauw, L.; Timmermans, J.P.; Kroese, A.B. Chronic alcohol consumption induces an overproduction of NO by nNOS- and iNOS-expressing myenteric neurons in the murine small intestine. Neurogastroenterol. Motil. 2011, 23, e237–e248. [Google Scholar] [CrossRef] [PubMed]
- Bódi, N.; Szalai, Z.; Bagyánszki, M. Nitrergic Enteric Neurons in Health and Disease-Focus on Animal Models. Int. J. Mol. Sci. 2019, 20, 2003. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Ward, S.M. Nitric oxide and its role as a non-adrenergic, non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract. Br. J. Pharmacol. 2019, 176, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, S.D.; Vergeer, M.; Heisterkamp, S.H.; Tytgat, G.N.; Boeckxstaens, G.E. Role of nitric oxide in gastric motor and sensory functions in healthy subjects. Gut 2002, 51, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J. Gastroenterol. 2003, 38, 421–430. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Gao, X.; Qian, J.; Yang, J.; Sun, W.; Wang, H.; Yang, Y. Resistin-like molecule beta augments phenotypic modulation of human aortic smooth muscle cell triggered by high glucose. Endocr. J. 2021, 68, 461–468. [Google Scholar] [CrossRef]
- Scott, T.A.; Babayeva, O.; Banerjee, S.; Zhong, W.; Francis, S.C. SGK1 is modulated by resistin in vascular smooth muscle cells and in the aorta following diet-induced obesity. Obesity 2016, 24, 678–686. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrizaj, E.; Nistri, S.; Zizi, V.; Baccari, M.C. Neuronal Nitric Oxide Synthase as a Shared Target for the Effects of Adiponectin and Resistin on the Mechanical Responses of the Mouse Gastric Fundus. Int. J. Mol. Sci. 2022, 23, 16113. https://doi.org/10.3390/ijms232416113
Idrizaj E, Nistri S, Zizi V, Baccari MC. Neuronal Nitric Oxide Synthase as a Shared Target for the Effects of Adiponectin and Resistin on the Mechanical Responses of the Mouse Gastric Fundus. International Journal of Molecular Sciences. 2022; 23(24):16113. https://doi.org/10.3390/ijms232416113
Chicago/Turabian StyleIdrizaj, Eglantina, Silvia Nistri, Virginia Zizi, and Maria Caterina Baccari. 2022. "Neuronal Nitric Oxide Synthase as a Shared Target for the Effects of Adiponectin and Resistin on the Mechanical Responses of the Mouse Gastric Fundus" International Journal of Molecular Sciences 23, no. 24: 16113. https://doi.org/10.3390/ijms232416113
APA StyleIdrizaj, E., Nistri, S., Zizi, V., & Baccari, M. C. (2022). Neuronal Nitric Oxide Synthase as a Shared Target for the Effects of Adiponectin and Resistin on the Mechanical Responses of the Mouse Gastric Fundus. International Journal of Molecular Sciences, 23(24), 16113. https://doi.org/10.3390/ijms232416113






