1. Introduction
Peripheral nerve injuries (PNIs) are a prevalent clinical issue, impacting the quality of life of those who are injured and providing a significant economic burden for those affected [
1]. Estimates suggest that some form of nerve injury, most commonly resulting from high-speed trauma, affects two million individuals in the United States alone (NIH Publication No. 18-NS-4853). Other age-associated etiologies include falls in the elderly and birth-related injuries in pediatric populations [
1]. Despite the ability of axons in injured nerves to regenerate after PNIs, only ~10% of patients experience some degree of recovery. This leaves many individuals permanently disabled, with irreversible symptoms such as impaired motor function, loss of sensation, and often pain [
2]. The extent to which functional recovery is not attained is often attributed to the fact that axon regeneration in injured nerves is slow and inefficient [
3] and that this sluggishness is exacerbated if repair of the injured nerve is delayed [
4]. Thus, novel treatments directed at enhancing axon regeneration are required to improve the outcomes for those with PNIs.
Based on the results of existing preclinical research, two experimental treatments, brief low-frequency (20 Hz) electrical stimulation [
5,
6] and moderate exercise [
3,
7,
8,
9], are especially effective in accelerating axon growth and improving functional recovery [
10]. We [
11,
12] and others [
5,
13] have shown previously that increasing the activity of injured neurons is both necessary and sufficient to improve axon regeneration when using these experimental therapies, so they have come to be known as activity-dependent therapies [
14]. Although these treatments are effective, there are barriers to their clinical use, such as delays prior to nerve repair surgery and co-morbidities that prevent their application [
8]. Additionally, not all nerve-injured patients can be stimulated or enrolled in an exercise program. Thus, we have sought an alternative to these two successful activity-dependent experimental therapies.
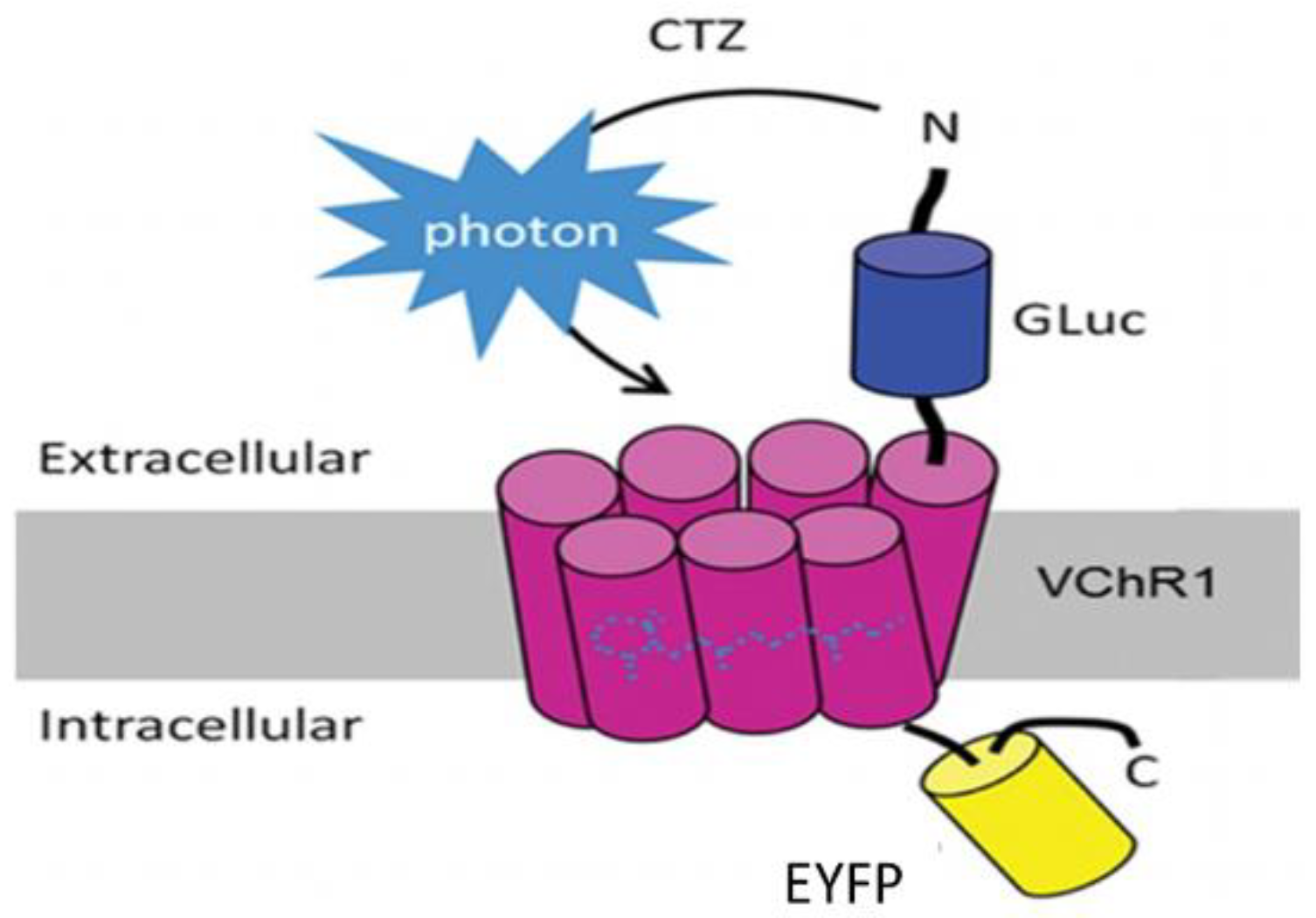
Bioluminescent optogenetics (BL-OG) could be such an alternative. BL-OG uses luminopsins—fusion proteins of light-sensing ion channels (opsins) and light-emitting luciferase (
Figure 1). The luminopsin used here is a red-shifted, highly sensitive channelrhodopsin variant from
Volvox carteri (
Figure 1: VChR1) fused with luciferase derived from
Gaussia princeps (
Figure 1: GLuc) through a short linker [
15,
16]. The resulting construct was slightly modified to include the trafficking signal from a neuronal potassium channel, for better membrane targeting, in front of an enhanced yellow fluorescent protein (
Figure 1: EYFP) tag (enhanced LMO3 or eLMO3) [
17]. When exposed to an appropriate luciferase substrate, such as coelenterazine (CTZ), bioluminescence is generated by the luciferase component and sensed by the opsin component, opening its pore and enabling an influx of cations. In this way, injured neurons induced to express an excitatory luminopsin could be activated directly to promote axon regeneration. Using transgenic mice engineered to express an excitatory luminopsin (eLMO3) exclusively in neurons, we showed previously [
18] that when treated once with CTZ after sciatic nerve transection and repair, motoneurons were excited for as long as three hours and subsequent axon regeneration was enhanced. The axons of more motoneurons regenerated and successfully reinnervated target muscles in animals treated with a fully functioning luminopsin than in wild-type controls and those treated with a non-functioning (R115A) luminopsin in which the opsin component was mutated to remove its ability to respond to the bioluminescence generated by CTZ treatment [
17,
18]. The aim of this study was to investigate whether similar results could be achieved using BL-OG when using a viral vector to induce luminopsin expression either before or after PNI. We also evaluated whether BL-OG treatment would have a similar effect on the regeneration of muscle sensory axons. The successful application of BL-OG in this manner could move it closer to translation to the clinical treatment of PNI.
3. Discussion
Poor recovery from peripheral nerve injuries remains a significant clinical problem, leaving many individuals with permanent symptoms and disabilities [
2]. As such, the development of novel treatments aimed at enhancing axon regeneration could improve outcomes for those affected [
3]. Increasing the activity of injured neurons is effective in promoting this enhancement [
11,
12]. Because some barriers exist to the clinical translation of experimental activity-dependent therapies, such as exercise or low-frequency electrical stimulation, we investigated BL-OG as an alternative to improve axon regeneration by directly stimulating injured neurons. In this study, we aimed to investigate the feasibility of enabling BL-OG via viral vector injection of a luminopsin construct.
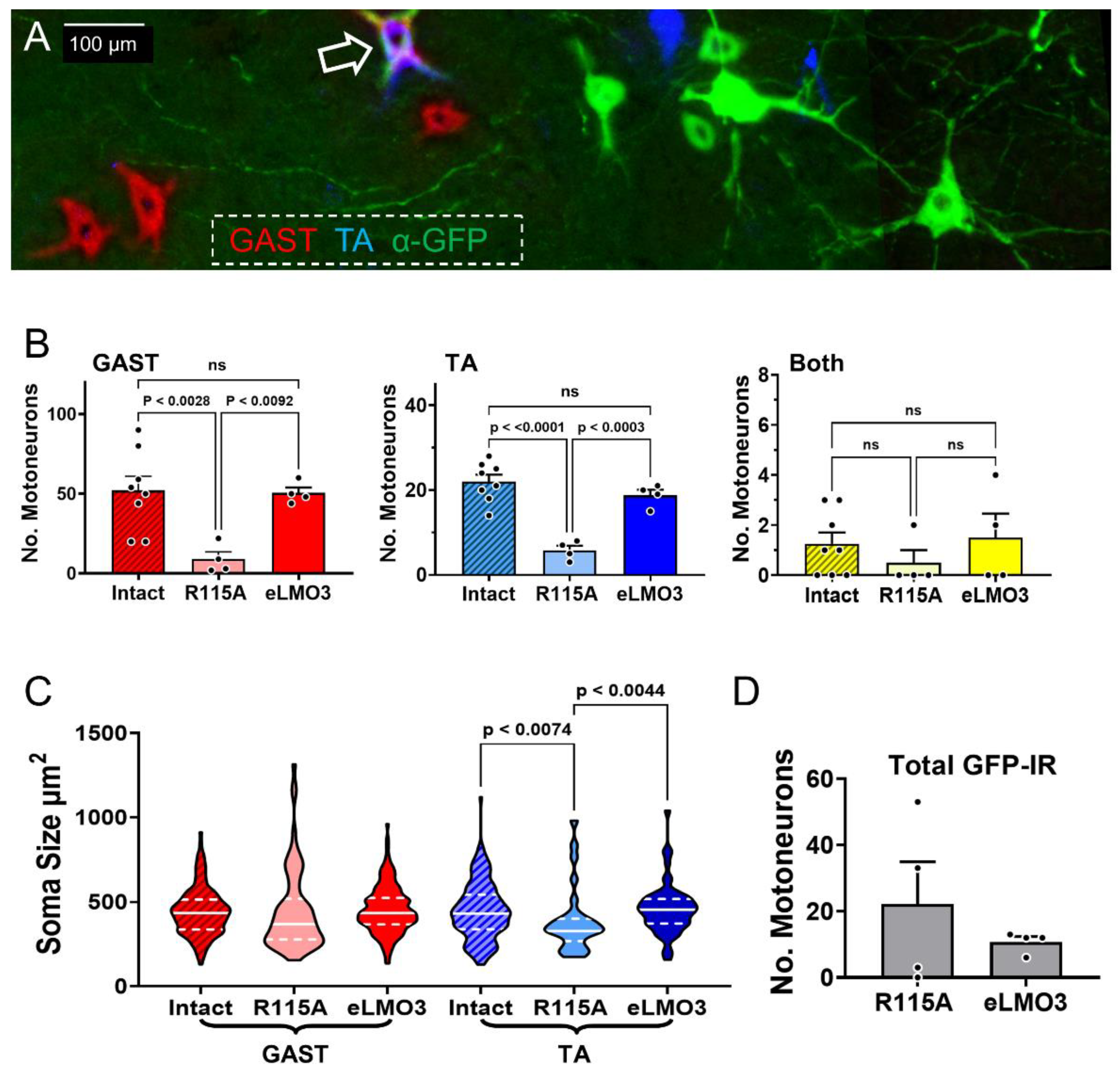
Experimental treatments to enhance axon regeneration after PNI are considered encouraging if they promote the effective regeneration of axons of more injured neurons leading to improved functional outcomes. One of the main findings of this study was an increased number of both motor and sensory neurons whose axons successfully regenerated and reinnervated their muscle targets in animals expressing the fully functioning eLMO3, compared to those induced to express the mutated R115A luminopsin. The increased number of motoneurons seen here was consistent with the results of our previous study investigating the use of BL-OG in transgenic mice [
18]. It is also consistent with the results of previous studies evaluating activity-dependent experimental therapies such as electrical stimulation [
13] or moderate exercise [
22]. However, this is the first study that investigates the effectiveness of BL-OG treatments using the viral induction of luminopsin in wild type mice, an important consideration for any future translation of BL-OG to clinical use. No significant difference in the number of sensory and motor neurons innervating two muscle targets exists between the injured and intact sides of the mice treated with BL-OG. This suggests a near complete reinnervation by
both sensory and motor neurons only four weeks after nerve injury in mice treated with BL-OG. An additional important finding is that BL-OG treatment in this manner resulted in a greater-than-four-fold restoration of neuromuscular function four weeks after injury than controls. Full muscle reinnervation after sciatic nerve injury in mice, based either upon retrograde labeling [
22] or restoration of M-wave amplitudes [
8,
18], takes considerably longer without treatment. It is now well established that at least some of the slowness of axon regeneration after PNI is due to a delay of days or weeks before some regenerating axons enter the distal nerve segment [
5]. The clear effect of BL-OG treatment in reducing this “temporal staggering” of regenerating motor [
5,
23] and sensory [
24] axons after PNI is remarkably similar to that demonstrated for the use of low-frequency electrical stimulation in rats. Based on these results, we consider BL-OG a promising potential therapy for enhancing axon regeneration after PNI.
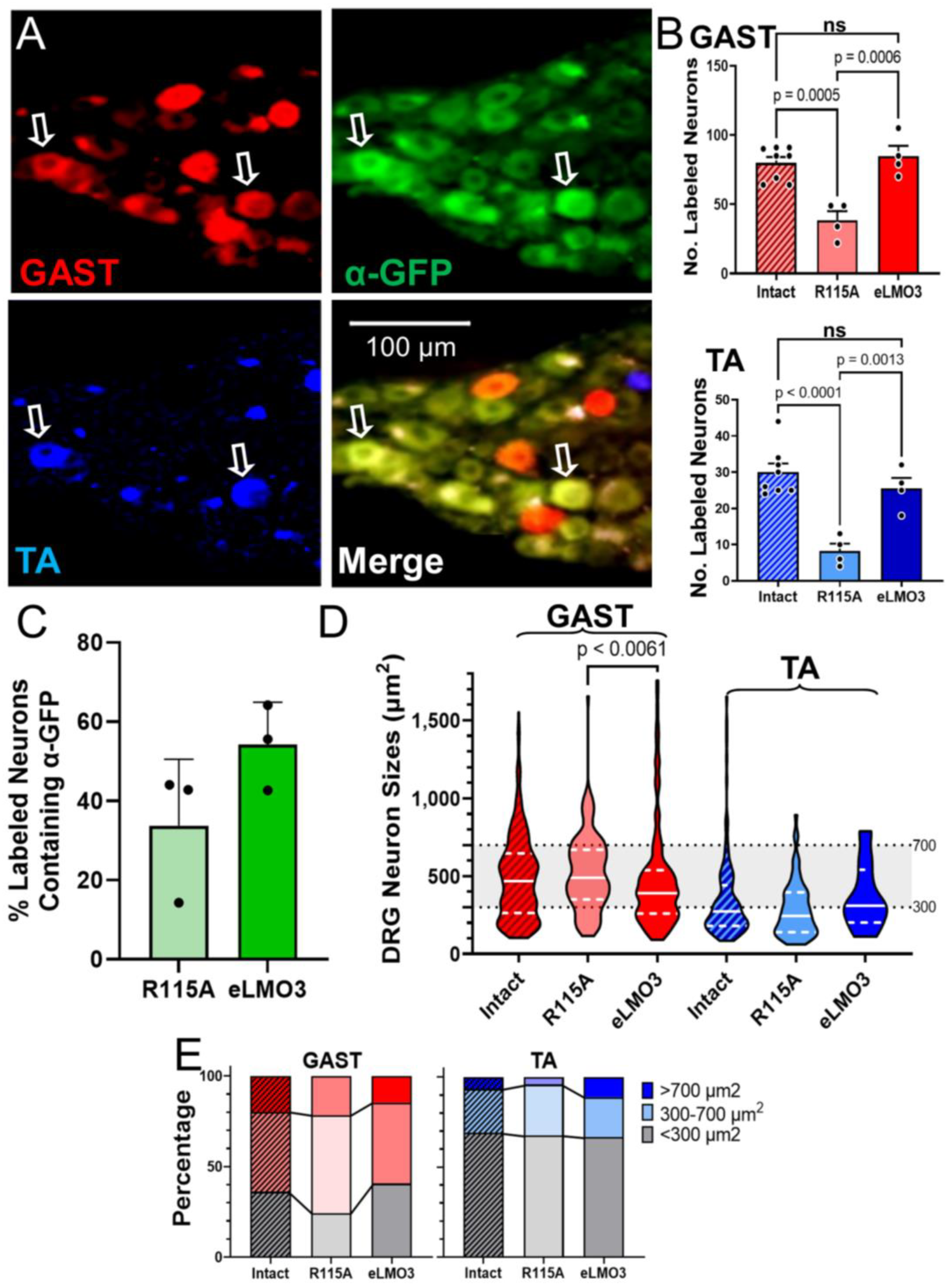
Because there was no significant difference between median sizes of successfully regenerating DRG neurons in BL-OG-treated and intact mice, this treatment seemingly does not alter the success of axon regeneration of sensory neurons of different sizes in either GAST or TA. By comparing the distribution of sensory neurons across three size groupings (<300 μm
2, 300–700 μm
2, and >700 μm
2) [
21], no significant differences were noted between the animals injected with the virus encoding the excitatory luminopsin and those injected with the virus encoding the R115A luminopsin. Significant differences were found in the proportions of DRG neurons in the different size classes between TA and GAST, but not between treatment groups. To the extent possible based only on size analysis, treatment with BL-OG after PNI restores the sensory neuron population to these two muscles appropriately.
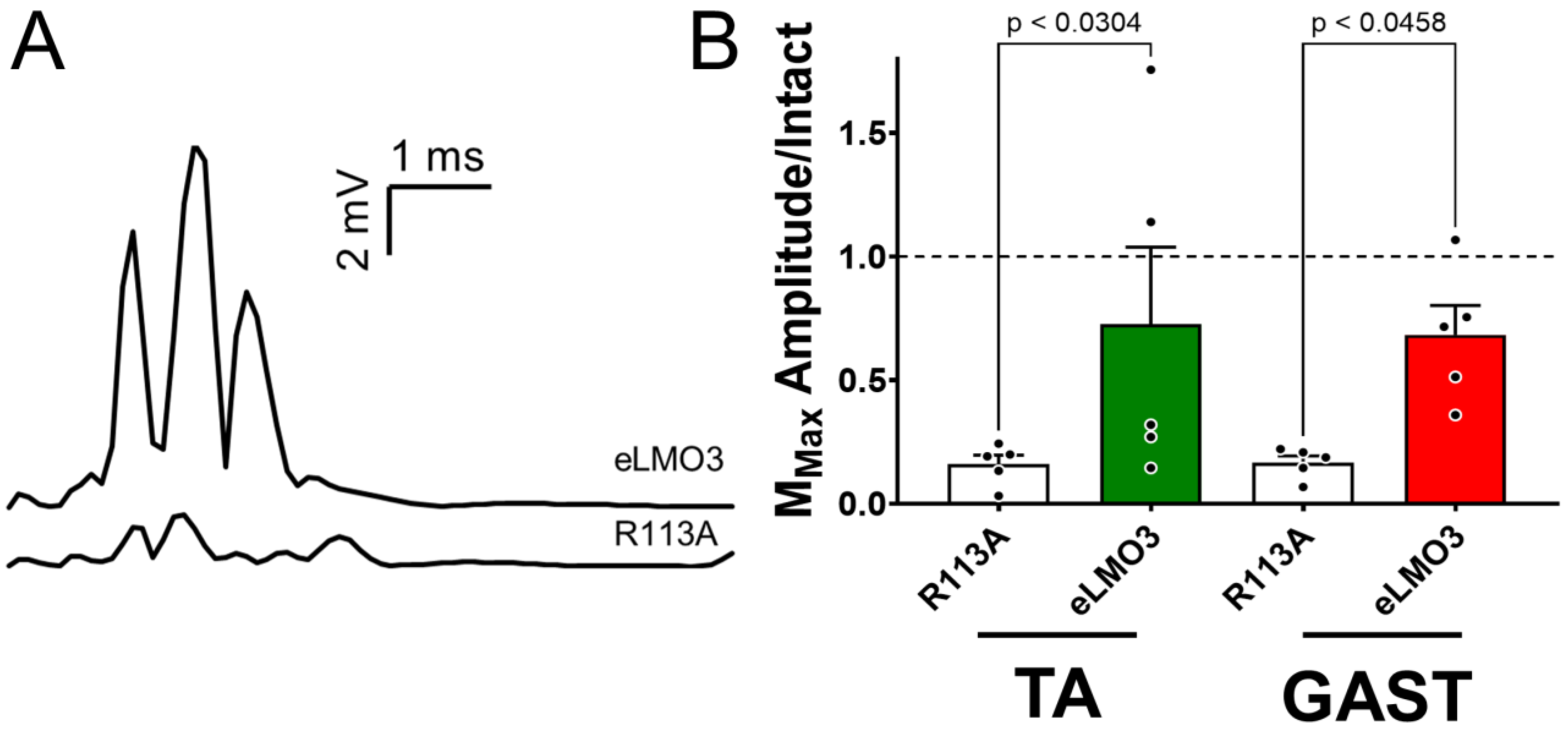
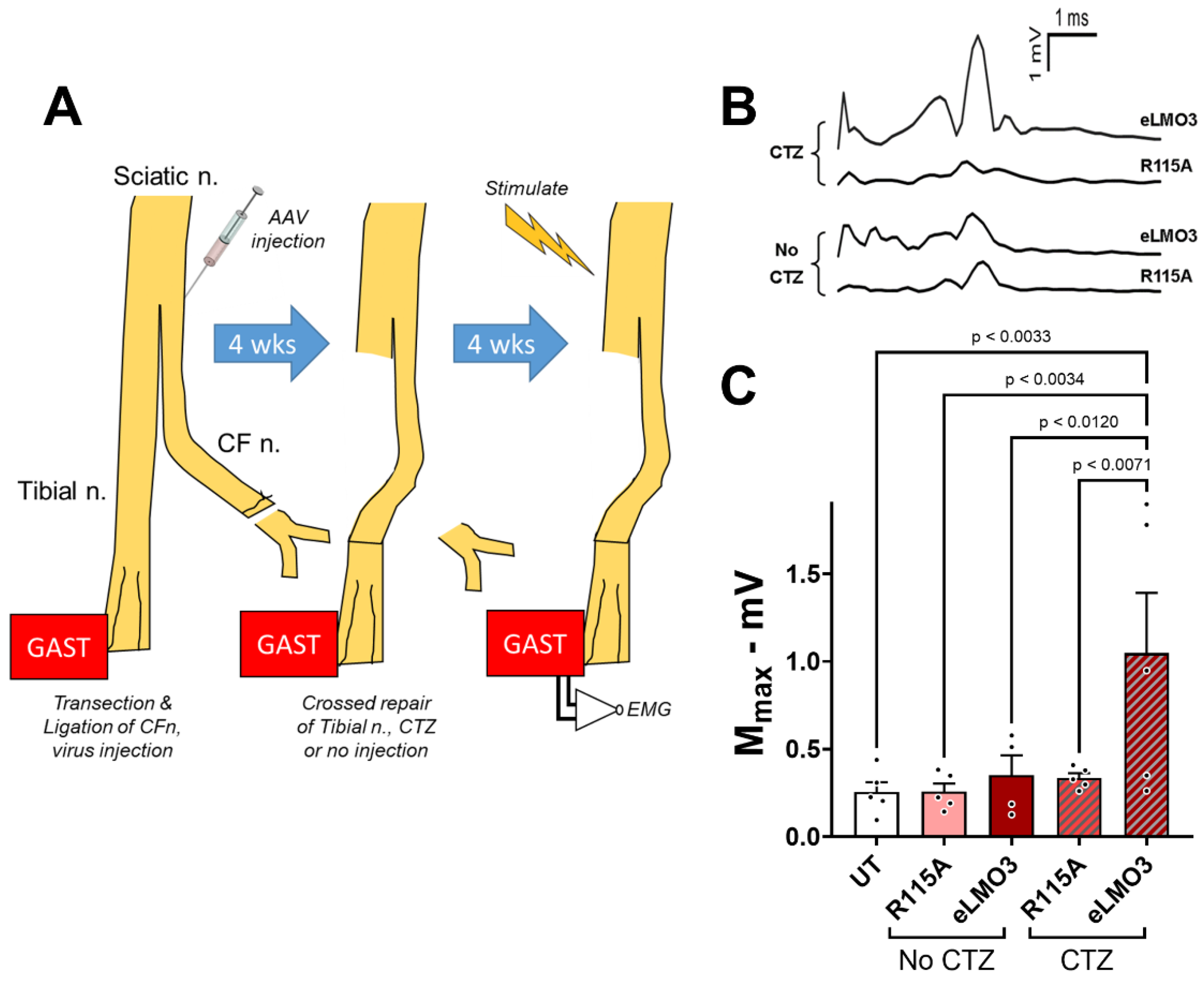
All of these studies were performed in mice in which luminopsin expression was induced prior to nerve injury. While these results support the feasibility of BL-OG to treat PNI, any proposed clinical application of BL-OG to treat PNI will require that luminopsin expression be induced after the injury. The results of our experiments, using delayed cross-reinnervation, addressed this concern. The induction of BL-OG by CTZ treatment, even after a four-week delay in the repair of a cut nerve to allow for post-injury induction of luminopsin expression, resulted in a 3.5-fold increase in M-wave amplitude, a commonly used clinical measure of neuromuscular function, relative to controls. While these results are very encouraging, they also must be considered as preliminary. More extensive study of dose and dosing of CTZ will be required in a clinically relevant model such as the one used here, but the current findings offer important evidence to support the feasibility of using BL-OG as a treatment for PNI.
While the increased number of successfully regenerating axons of motoneurons observed here after BL-OG treatment is promising, we were unable to attribute this positive effect specifically to luminopsin expression. The proportion of retrogradely labeled motoneurons expressing a luminopsin construct, as defined by immunoreactivity to GFP, was smaller than could account for the extent of improvement in motor axon regeneration produced by the BL-OG treatment. This lack of correlation between the extent of EYFP-expressing motoneurons and the increase in the numbers of retrogradely labeled motoneurons in BL-OG-treated mice significantly limits any mechanistic explanations of the enhancement of axon regeneration observed. It is possible that the level of luminopsin expression in many motoneurons was small and we were simply unable to detect the EYFP tag on it. Our difficulty in visualizing the luminopsin would have been further complicated if it became distributed throughout the membranes of the extensive dendritic arbors found in motoneurons. Future studies using methods such as PCR or further amplifying the GFP signal will need to be employed to improve the ability to detect the virally induced luminopsin in motoneurons. However, it is also possible that even a relatively weak luminopsin expression might be sufficient to promote motor axon regeneration when excited after CTZ administration.
The extent of luminopsin expression was much greater in dorsal root ganglion cells, which do not have the elaborate dendritic arbors found in motoneurons. Nearly half of the retrogradely labeled DRG neurons studied also expressed GFP immunoreactivity, a proportion that could be sufficient to account for the increase in sensory axon regeneration after BL-OG treatment. Given the disparity between the demonstrable identification of expression of the luminopsins in sensory and motor neurons, one might speculate that the effectiveness of the BL-OG treatment on sensory and motor axon regeneration could be due to different cellular mechanisms. Enhanced sensory axon regeneration could be the result of direct excitation of DRG neurons expressing eLMO3. Enhanced motor axon regeneration could be due to a combination of a direct excitation of those motoneurons expressing eLMO3 and an indirect excitation of motoneurons by eLMO3 expressing sensory neurons. The combination of direct excitation in motoneurons faintly expressing luminopsin and the excitation produced by CTZ-driven activity in DRG neurons projecting to them might be adequate to promote motor axon regeneration. At the time of administration of CTZ in our experiments, such excitatory connections continue to be functional [
25]. Although we showed previously, using optogenetics, that the activation of DRG neurons in the absence of increased motoneuron excitation was not sufficient to promote motor axon regeneration after PNI [
11], inducing excitation of motoneurons that might already be weakly excited, via direct connections from DRG neurons, remains a possibility.
In conclusion, we aimed to investigate whether, when inducing expression of an excitatory luminopsin either before or after PNI, treatment using BL-OG would promote subsequent axon regeneration. We report here that BL-OG treatment in such circumstances did significantly enhance the regeneration of the axons of motor and sensory neurons after peripheral nerve injury. We believe that the results presented here have moved the use of the therapy closer towards potential clinical applications, and the inclusion of sensory neurons and regenerating cell soma sizes provided valuable new information on the action of BL-OG. The use of BL-OG in enhancing regeneration after peripheral nerve injury remains a promising avenue through which those affected could regain function.
4. Materials and Methods
4.1. Animals and Surgeries
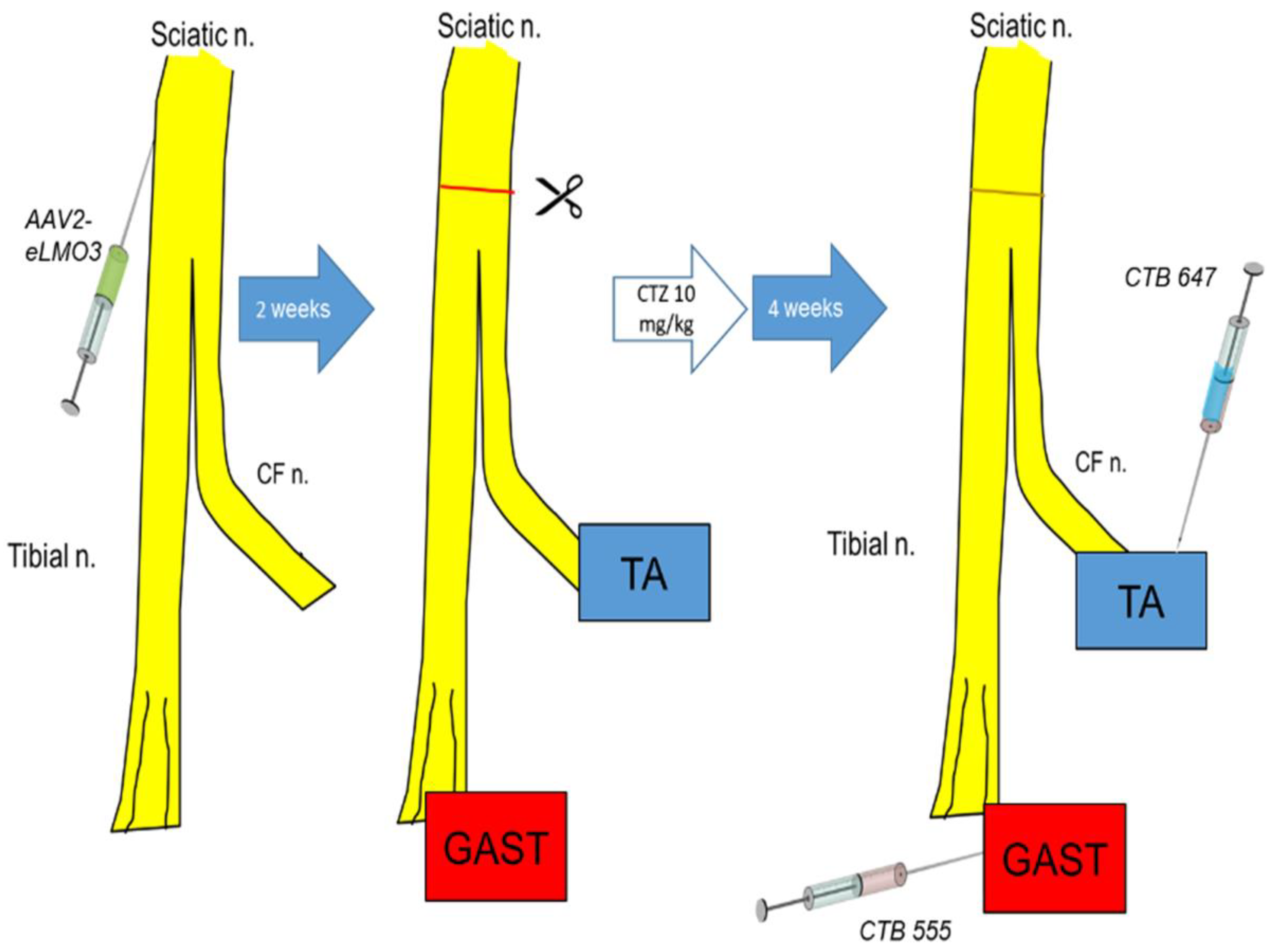
Eight intact C57B6/J mice (four female, four male) ranging from 6–13 weeks of age were used in the retrograde labeling experiments. Mice were anesthetized using isoflurane, and their right sciatic nerves were exposed in the mid-thigh. The exposed nerves were injected above the branching of the tibial and common fibular nerves, with 1–2 µL of an adeno-associated (AAV2/9) viral vector encoding either an excitatory luminopsin (eLMO3) (1.2 × 10
14 vg/mL) (four mice) or a luminopsin with a mutated opsin component (R115A) (3.5 × 10
14 vg/mL) [
18] (four mice). Both constructs were under the control of the human synapsin (Hsyn) promoter and were derived from
Volvox channelrhodopsin 1 (VChR1) fused with
Gaussia luciferase (GLuc) through a short linker [
15,
17] in front of the enhanced yellow fluorescent protein (EYFP) tag (
Figure 1).
The mutated luminopsin sequence is identical to that of the eLMO3 construct except for a single amino acid perturbation (arginine to alanine at location 115, R115A) in the VChR1 component. In this mutant, the luciferase component of the luminopsin generates bioluminescence in the presence of CTZ but the channelrhodopsin component does not respond to that light or activate neurons [
17,
18]. The use of this R115A luminopsin allowed us to ensure that any effect could be attributed to neuronal excitation, rather than other components of the BL-OG system [
18]. The use of this mutant also acts as a control for any effect of CTZ alone. CTZ has been shown to have antioxidant properties, so its presence might affect regeneration [
26]. Even though CTZ treatment alone did not enhance axon regeneration in transgenic mice [
18], it is important to control for this possibility.
After waiting two weeks for retrograde viral transport and neuronal transduction, injected sciatic nerves were cut in the mid-thigh and repaired by simple end-to-end anastomosis, as we have described in more detail elsewhere [
27]. The contralateral sciatic nerve in each mouse was not injected or injured and served as an intact control. Immediately after the repair surgery was completed, the mouse was administered a single dose of CTZ (10 mg/Kg, i.p.). The CTZ used was Inject-A-Lume (NanoLight Technologies), a form of native coelenterazine formulated for in vivo applications. In an earlier study [
18], we showed that an injection of this dose of CTZ resulted in a rapid and long-lasting increase in bioluminescence over the spinal cord and sciatic nerve of transgenic mice expressing the same luminopsin constructs. It did not increase spontaneous neuromuscular activity in these mice or in mice induced to express the luminopsin using the same viral vectors employed here, which would be associated with increased motoneuron firing, but it did lower the threshold for reflex excitation of luminopsin-expressing motoneurons, suggesting an increase in their excitability [
18]. This change in excitability reached a peak 45 min after CTZ injection and returned to baseline in three hours [
18]. Because of these previously published findings, we assumed that the CTZ treatments employed in the present study would result in a similar increase in neuronal excitability.
Four weeks after nerve repair and CTZ treatment, different retrograde fluorescent tracers were injected into the two heads of the gastrocnemius and the tibialis anterior muscles to mark motor and sensory neurons whose axons had reinnervated these muscles. This survival time was chosen to be compatible with our previous studies [
28,
29,
30] and others [
5,
8,
14] on activity-dependent experimental therapies to enhance peripheral axon regeneration after peripheral nerve injury. Two microliters of a 1% solution of the beta subunit of cholera toxin, conjugated to Alexa Fluor 555 (CTB 555), was injected into the gastrocnemius muscle (GAST) (4 µL total), and 2 µL of a similar reagent, but conjugated to Alexa Fluor 647 (CTB 647), was injected into the tibialis anterior muscle (TA). Injections were made at several sites in each muscle and the injection needle was left in place for five minutes at each site to prevent seepage of tracer out of the muscle along the needle track. Similar injections into the contralateral muscles served to mark motor and sensory innervation from intact sources. After the last injection, each surgical site was washed three times with normal saline and surgical wounds were closed in layers before animals were returned to their cages. Mice were euthanized using an intraperitoneal injection of Euthasol (pentobarbital sodium and phenytoin sodium, 150 mg/Kg) perfused with 4% paraformaldehyde solution five days after retrograde tracer injections. The entire sequence of experiments is shown diagrammatically in
Figure 2.
4.2. Tissue Processing and Immunohistochemistry
Following euthanasia, entire lumbar spinal cords, as well as right and left L4 and L5 DRGs, were collected from each animal and preserved in 20% sucrose solution. Spinal cords and DRGs were serially sectioned on a cryostat at 50 μm and 30 μm thickness, respectively, and mounted onto glass microscope slides. All sections were saved and used in analyses. Spinal cord tissue was reacted with antibodies to GFP to amplify visualization of the EYFP marker on the eLMO3 or R115A constructs. Slides were incubated with blocking solution containing 0.3% Triton X-100 and 10% normal goat serum for one hour before being incubated with primary antibody (Invitrogen, anti-GFP rabbit IgG, #A-11122, diluted 1:500 in phosphate-buffered solution (PBS), pH 7.4) at 4 °C overnight. Tissue was then washed three times with PBS before being incubated with secondary antibody (Invitrogen Goat anti-Rabbit 488, A-11008, 1:300) for 1 h, before being washed three more times.
4.3. Imaging
Images of sections of spinal cords were captured using a Leica DM6000 microscope, a Hamamatsu ORCA camera, and HCImage software. Images of DRG sections were captured using a Keyence BZ-X microscope. Motoneurons that were retrogradely labeled, indicating that their axons had successfully regenerated and reinnervated the gastrocnemius or tibialis anterior muscle, were counted and their sizes measured. Motoneurons were scored as retrogradely labeled if the fluorescent label filled the cell body and extended into the proximal dendrites, and contained a visible nuclear shadow (
Figure 3A) [
22]. A note was made of any neurons that contained both GFP (indicating presence of the luminopsin) and a retrograde label. Counts of DRG neurons, using the same criteria for inclusion, were conducted as well to determine the effect that the BL-OG treatment had on the regeneration of muscle sensory axons. Both motor and sensory neuron soma sizes were measured using Fiji software to determine whether the successfully regenerating neurons differed in size from those found on the intact sides of the animals. Images of adjacent microscope fields were stitched together using the Fiji plugin [
31].
4.4. Electrophysiology
To evaluate the extent of restoration of neuromuscular function after PNI, compound muscle action potentials (direct muscle responses or M waves) were recorded from the GAST and TA muscles in response to stimulation of the sciatic nerve. The methods used have been described in more detail elsewhere [
32]. In isoflurane-anesthetized mice, sciatic nerves were surgically exposed in the mid-thigh and paired needle electrodes (Ambu #74325-36/40, Columbia, MD, United States) were placed in contact with the nerve. Bipolar fine-wire EMG electrodes [
33] were inserted through the skin into the lateral gastrocnemius and tibialis anterior muscles. Ongoing activity recorded from these muscles was sampled at 10 KHz using a laboratory computer system running custom Labview software and, when activity over a 10 ms period was within a user-defined background range, the computer delivered a single brief (0.3 ms) constant voltage pulse to the nerve via the needle electrodes and recorded EMG activity for 50 ms. A range of stimulus intensities was applied, from subthreshold to supramaximal. To avoid fatigue, stimuli were delivered no more frequently than once every five seconds. Amplitudes of M waves were measured as the average full-wave rectified voltage between the onset and duration of the recorded triphasic action potential.
Two sets of experiments used M waves as outcome measures. In one set, M waves were recorded from GAST and TA in 10 intact mice and then viral vectors encoding either eLMO3 or R115A were injected into their sciatic nerves (five mice each), as described above for the experiments using retrograde labeling. Four weeks later, the sciatic nerves of these mice were cut and repaired, as described above, and the mice were treated once with CTZ (10 mg/Kg, i.p.). After four more weeks, M waves were recorded from the reinnervated GAST and TA muscles. The amplitude of the largest M wave (MMAX) recorded from a reinnervated muscle was expressed as a proportion of the MMAX recorded from that muscle in that mouse prior to injury. These scaled MMAX values from mice induced to express eLMO3 and mice induced to express R115A were then compared using a one-way ANOVA, with post hoc paired (Tukey) testing.
In a second set of experiments, M waves were used to evaluate the restoration of neuromuscular function when the induction of luminopsin expression was performed after the nerve injury. An outline of these experiments is shown in
Figure 6A. In isoflurane-anesthetized mice, the common fibular nerve was cut and ligated. Viral vectors encoding either eLMO3 or R115A (five mice each) were injected unilaterally into the proximal segment of the cut nerve near its bifurcation from the tibial nerve. In an additional five mice, nerve transection and ligation was performed but no viral injection was made. After allowing four weeks for retrograde transport and viral transduction, the injury site was exposed in isoflurane-anesthetized animals and the tibial nerve was transected. The ligated portion of the common fibular nerve was then trimmed with sharp scissors to remove the ligation and was aligned with the distal segment of the cut tibial nerve. The cross-repaired nerve was then secured in place using fibrin glue [
27]. Animals that had received virus injections were then either administered a single dose of CTZ (10 mg/Kg, i.p.) or left untreated. Animals that did not receive a viral injection remained untreated. Four weeks later, M waves were recorded from the reinnervated GAST muscles of all the mice. Comparisons of the amplitudes of M
MAX between mice in different groups were made using a one-way ANOVA, with post hoc paired (Tukey) testing.
4.5. Statistical Methods
Numbers of animals in all experimental groupings used were deemed adequate based on a post hoc power analysis performed using G * Power (Power = (1-β err prob) > 0.8). GraphPad Prism software was used for statistical analyses. Significance was set as p < 0.05 in all analyses.