Ovarian Microbiota, Ovarian Cancer and the Underestimated Role of HPV
Abstract
1. Introduction
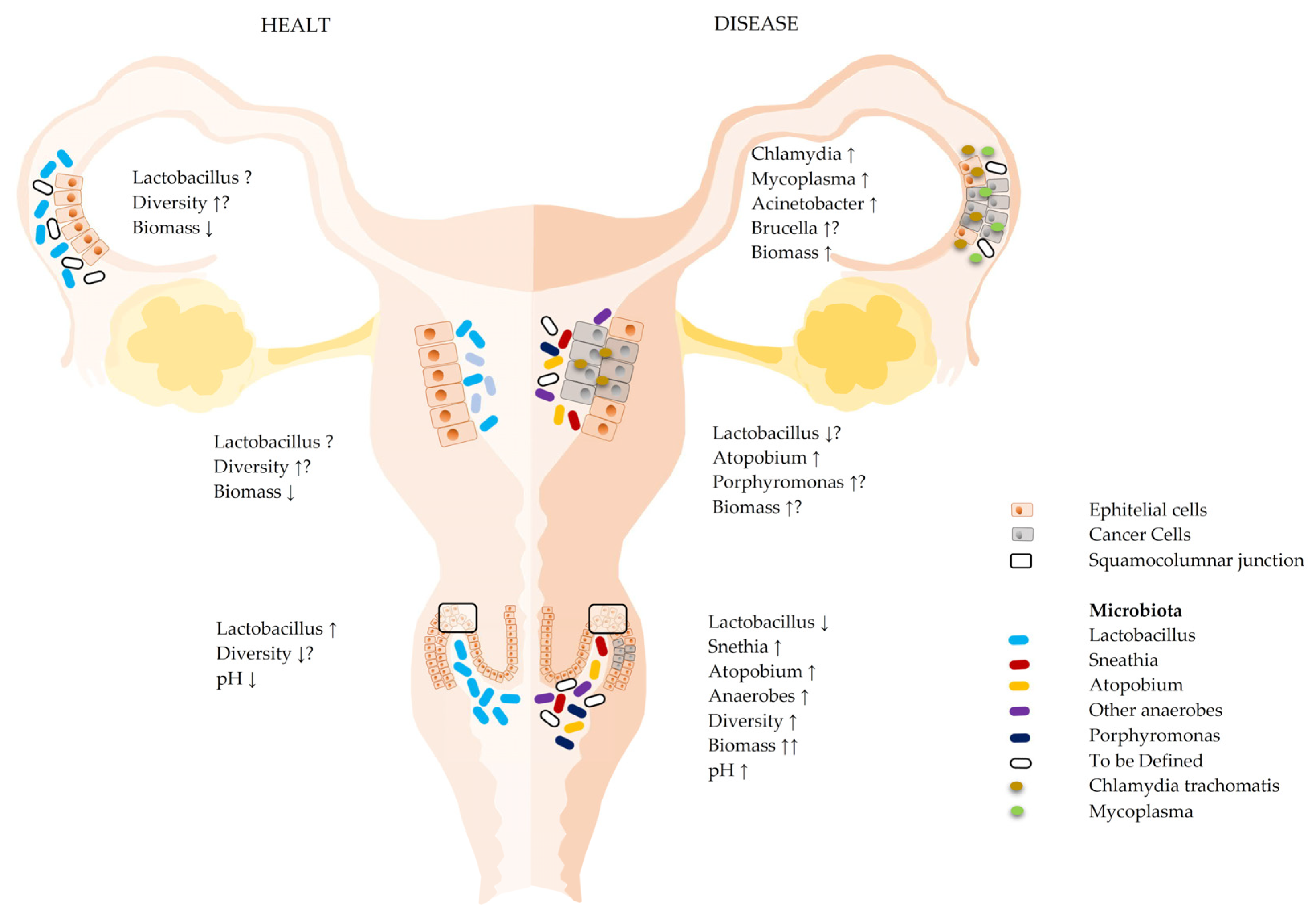
1.1. Microbiota of the Lower Female Reproductive Tract (Lower FRT)
1.2. Microbiota of the Upper Female Reproductive Tract (Upper FRT)
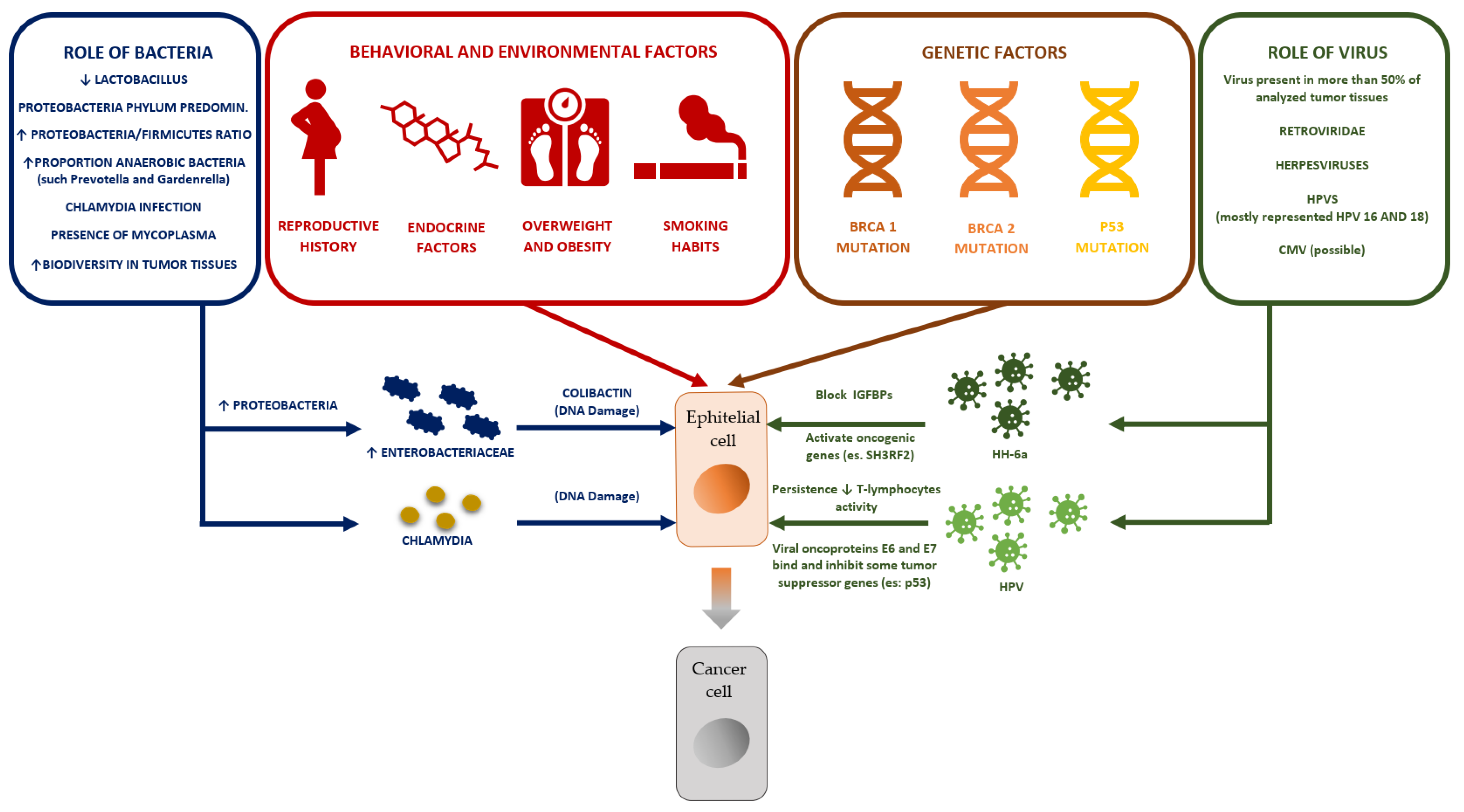
2. Microbiota and Ovarian Cancer
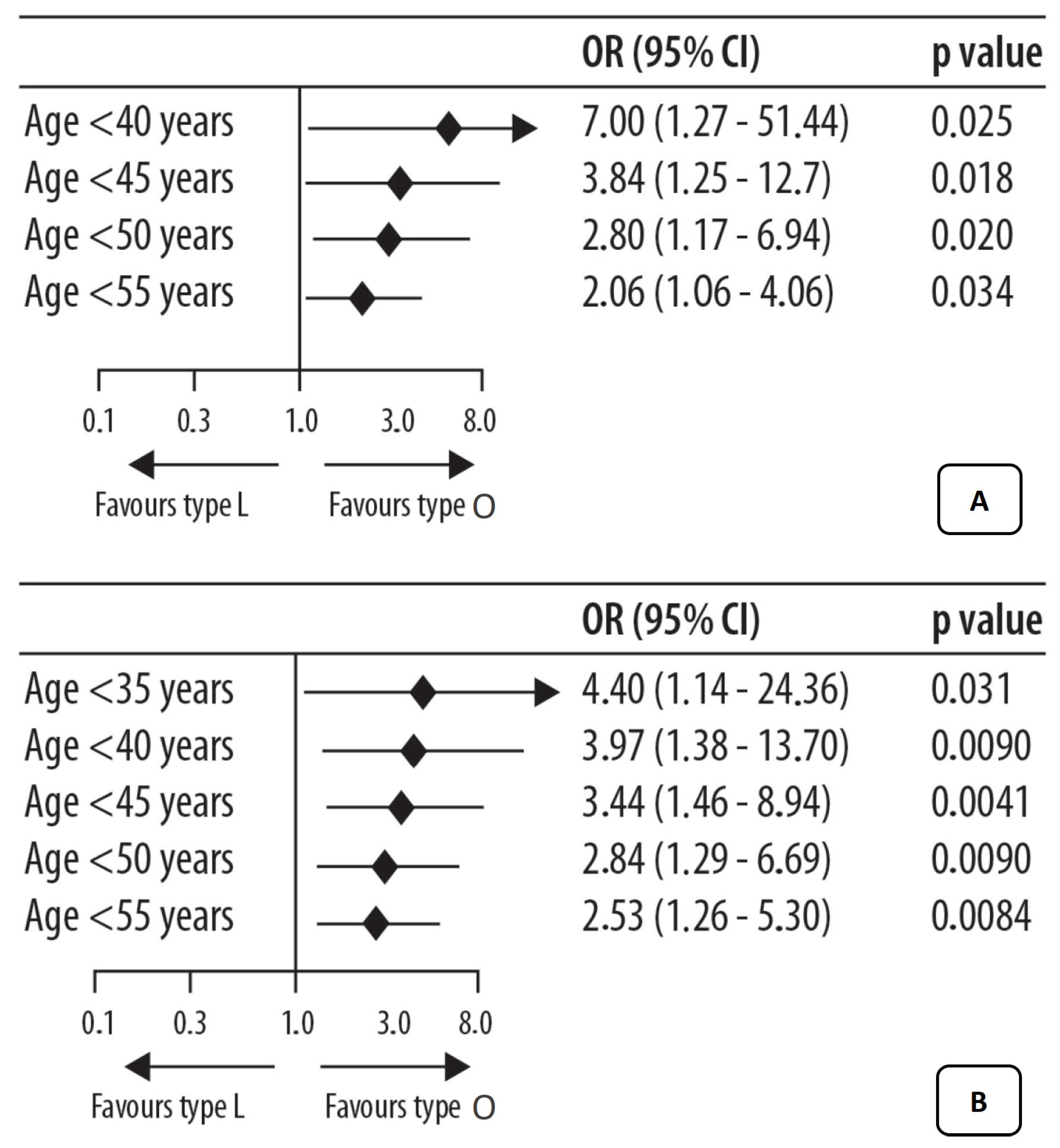
3. Vaginal Microbiota and Ovarian Tumor
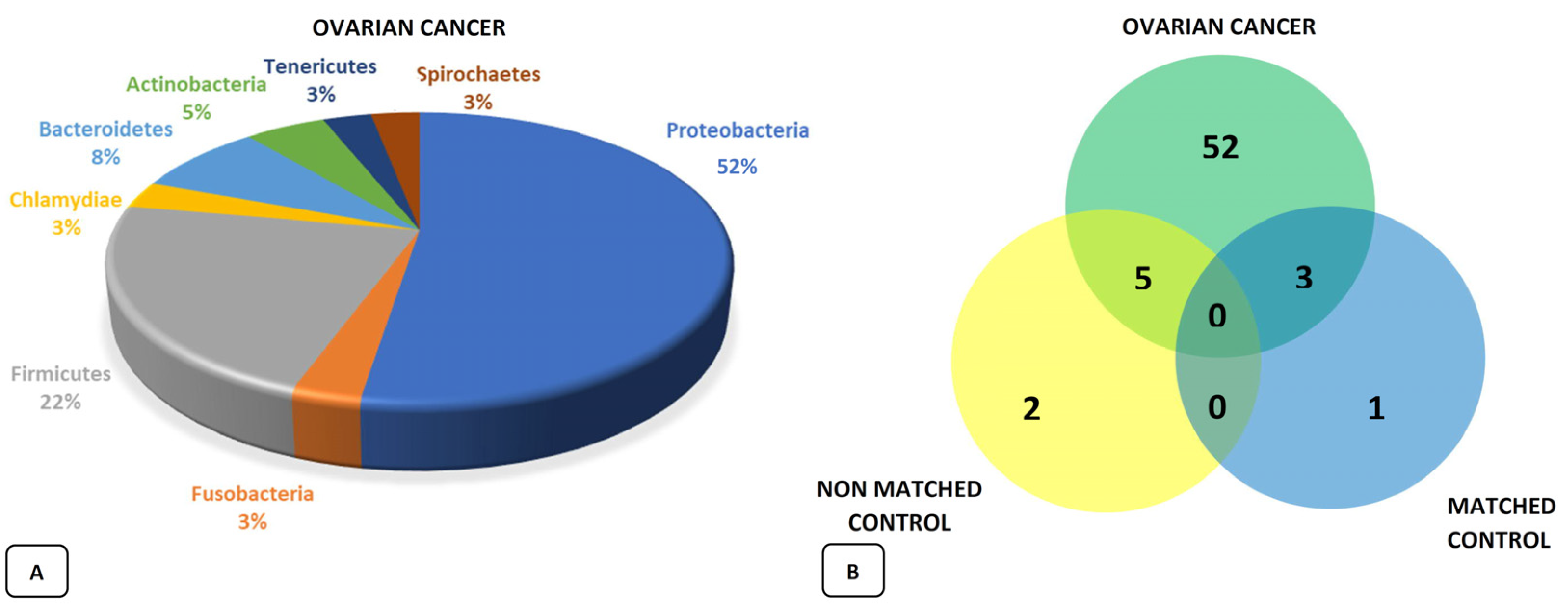
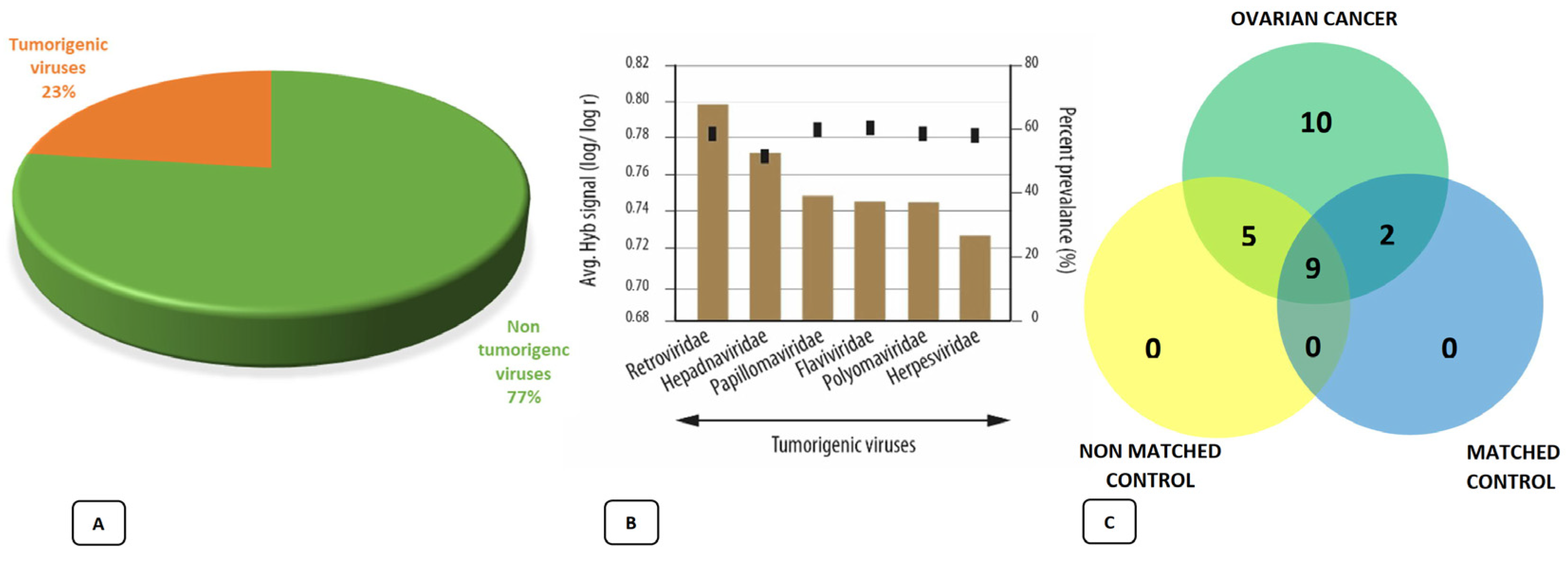
4. The Ovarian Microbiota and Ovarian Cancer
4.1. The Role of Bacteria
4.2. The Role of Viruses
5. The Possible Role of HHV-6a in Ovarian Cancer
6. The Possible Role of HPV in Ovarian Cancer
7. Possible Therapeutic Scenarios
- (1)
- Presence of an unfavorable vaginal CST (CST III or IV);
- (2)
- Presence and persistence of high-risk HPV with decreased p53 activity;
- (3)
- Reduction in immune activity expressed by the reduced proportion of T lymphocytes.
| Author | Ref. | Description |
|---|---|---|
| Laniewski et.al. | [4] | Introduces the concept of a different microbiota in relation to the various anatomical sites of the reproductive system and how it impacts the incidence and outcome of related oncological pathologies |
| Ravel | [6] | Introduction of the concept of vaginal CST |
| Bertuccioli | [15] | Collection of the essential characteristics of Lactobacillus crispatus M247 |
| Nene’ | [16] | First and most authoritative publication on the correlation between unfavorable CST genetic mutation and ovarian cancer risk |
| Benerjee | [22] | Thorough assessment and explanation of the ovarian microbiota |
| Zhang | [30] | Prognostic importance of the presence of intratumoral T-cells and indirect confirmation of the importance of the anti-tumor immune response |
| Di Pierro | [33] | Lactobacillus crispatus M247 oral administration in papillomavirus-infected women; results of a preliminary, uncontrolled, open trial |
| Smith | [38] | Confirmation of the action of AHCC in the eradication of persistent HPV thanks to the action of immune stimulation. |
8. Conclusions
- The presence of an unfavorable CST;
- The presence/persistence of HPV (especially of high-risk strains);
- Interference with the activity of some tumor suppressor genes (p53);
- Influence on the immune system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine Immunity and Microbiota: A Shifting Paradigm. Front. Immunol. 2019, 10, 2387. [Google Scholar] [CrossRef] [PubMed]
- Łaniewski, P.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. The microbiome and gynaecological cancer development, prevention and therapy. Nat. Rev. Urol. 2020, 17, 232–250. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Chunwei, Z.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Greenbaum, S.; Greenbaum, G.; Moran-Gilad, J.; Weintraub, A.Y. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am. J. Obstet. Gynecol. 2019, 220, 324–335. [Google Scholar] [CrossRef]
- Champer, M.; Wong, A.; Champer, J.; Brito, I.; Messer, P.; Hou, J.Y.; Wright, J.D. The role of the vaginal microbiome in gynaecological cancer. BJOG: Int. J. Obstet. Gynaecol. 2017, 125, 309–315. [Google Scholar] [CrossRef]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int. J. Gynecol. Obstet. 2021, 155 (Suppl. S1), 61–85. [Google Scholar] [CrossRef]
- La Vecchia, C. Ovarian cancer: Epidemiology and risk factors. Eur. J. Cancer Prev. 2017, 26, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kossaï, M.; Leary, A.; Scoazec, J.-Y.; Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2017, 85, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.M.; Bussies, P.; Vargas, R.; Esakov, E.; Tewari, S.; Reizes, O.; Michener, C. The Microbiome and Gynecologic Cancer: Current Evidence and Future Opportunities. Curr. Oncol. Rep. 2021, 23, 92. [Google Scholar] [CrossRef] [PubMed]
- Dellino, M.; Cascardi, E.; Laganà, A.S.; Di Vagno, G.; Malvasi, A.; Zaccaro, R.; Maggipinto, K.; Cazzato, G.; Scacco, S.; Tinelli, R.; et al. Lactobacillus crispatus M247 oral administration: Is it really an effective strategy in the management of papillomavirus-infected women? Infect. Agents Cancer 2022, 17, 53. [Google Scholar] [CrossRef]
- Bertuccioli, A.; Cardinali, M.; Zonzini, G.; Cazzaniga, M.; Di Pierro, F. Lactobacillus crispatus M247: Characteristics of a Precision Probiotic Instrument for Gynecological and Urinary Well-Being. Microbiol. Res. 2022, 13, 963–971. [Google Scholar] [CrossRef]
- Nené, N.R.; Reisel, D.; Leimbach, A.; Franchi, D.; Jones, A.; Evans, I.; Knapp, S.; Ryan, A.; Ghazali, S.; Timms, J.F.; et al. Association between the cervicovaginal microbiome, BRCA1 mutation status, and risk of ovarian cancer: A case-control study. Lancet Oncol. 2019, 20, 1171–1182. [Google Scholar] [CrossRef]
- Wahid, M.; Dar, S.A.; Jawed, A.; Mandal, R.K.; Akhter, N.; Khan, S.; Khan, F.; Jogaiah, S.; Rai, A.K.; Rattan, R. Microbes in gynecologic cancers: Causes or consequences and therapeutic potential. Semin. Cancer Biol. 2021, 86 Pt 2, 1179–1189. [Google Scholar] [CrossRef]
- Sipos, A.; Ujlaki, G.; Mikó, E.; Maka, E.; Szabó, J.; Uray, K.; Krasznai, Z.; Bai, P. The role of the microbiome in ovarian cancer: Mechanistic insights into oncobiosis and to bacterial metabolite signaling. Mol. Med. 2021, 27, 33. [Google Scholar] [CrossRef]
- Zhou, B.; Sun, C.; Huang, J.; Xia, M.; Guo, E.; Li, N.; Lu, H.; Shan, W.; Wu, Y.; Li, Y.; et al. The biodiversity Composition of Microbiome in Ovarian Carcinoma Patients. Sci. Rep. 2019, 9, 1691. [Google Scholar] [CrossRef]
- Shanmughapriya, S.; Senthilkumar, G.; Vinodhini, K.; Das, B.C.; Vasanthi, N.; Natarajaseenivasan, K. Viral and bacterial aetiologies of epithelial ovarian cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2311–2317. [Google Scholar] [CrossRef]
- Chan, P.; Seraj, I.M.; Kalugdan, T.H.; King, A. Prevalence of Mycoplasma Conserved DNA in Malignant Ovarian Cancer Detected Using Sensitive PCR–ELISA. Gynecol. Oncol. 1996, 63, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, M.D.; Alwine, J.C.; Coukos, G.; Robertson, E.S. The ovarian cancer oncobiome. Oncotarget 2017, 8, 36225–36245. [Google Scholar] [CrossRef] [PubMed]
- Gulve, N.; Rudel, T. Chlamydia trachomatis and human herpesvirus 6 infections in ovarian cancer—Casual or causal? PLOS Pathog. 2019, 15, e1008055. [Google Scholar] [CrossRef] [PubMed]
- Paradowska, E.; Jabłońska, A.; Studzińska, M.; Wilczyński, M.; Wilczyński, J.R. Detection and genotyping of CMV and HPV in tumors and fallopian tubes from epithelial ovarian cancer patients. Sci. Rep. 2019, 9, 19935. [Google Scholar] [CrossRef]
- Al-Shabanah, O.A.; Hafez, M.M.; Hassan, Z.K.; Sayed-Ahmed, M.M.; Abozeed, W.N.; Al-Rejaie, S.S.; Alsheikh, A.A. Human papillomavirus genotyping and integration in ovarian cancer Saudi patients. Virol. J. 2013, 10, 343. [Google Scholar] [CrossRef]
- Nakamura, M.; Obata, T.; Daikoku, T.; Fujiwara, H. The Association and Significance of p53 in Gynecologic Cancers: The Potential of Targeted Therapy. Int. J. Mol. Sci. 2019, 20, 5482. [Google Scholar] [CrossRef]
- Corney, D.C.; Flesken-Nikitin, A.; Choi, J.; Nikitin, A.Y. Role of p53 and Rb in Ovarian Cancer. Adv. Exp. Med. Biol. 2008, 622, 99–117. [Google Scholar] [CrossRef]
- Hung, C.-F.; Wu, T.C.; Monie, A.; Roden, R. Antigen-specific immunotherapy of cervical and ovarian cancer. Immunol. Rev. 2008, 222, 43–69. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef]
- Zeppa, S.D.; Sisti, D.; Amatori, S.; Gervasi, M.; Agostini, D.; Piccoli, G.; Bertuccioli, A.; Rocchi, M.B.; Stocchi, V.; Sestili, P. High-intensity Interval Training Promotes the Shift to a Health-Supporting Dietary Pattern in Young Adults. Nutrients 2020, 12, 843. [Google Scholar] [CrossRef] [PubMed]
- Bertuccioli, A.; Ninfali, P. The Mediterranean Diet in the era of globalization: The need to support knowledge of healthy dietary factors in the new socio-economical framework. Mediterr. J. Nutr. Metab. 2014, 7, 75–86. [Google Scholar] [CrossRef]
- DI Pierro, F.; Criscuolo, A.A.; Giudici, A.D.; Senatori, R.; Sesti, F.; Ciotti, M.; Piccione, E. Oral administration of Lactobacillus crispatus M247 to papillomavirus-infected women: Results of a preliminary, uncontrolled, open trial. Minerva Obstet. Gynecol. 2021, 73, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Polzonetti, V.; Patrone, V.; Morelli, L. Microbiological Assessment of the Quality of Some Commercial Products Marketed as Lactobacillus crispatus-Containing Probiotic Dietary Supplements. Microorganisms 2019, 7, 524. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Zacconi, P.; Bertuccioli, A.; Togni, S.; Eggenhoffner, R.; Giacomelli, L.; Scaltrini, S. A naturally-inspired, curcumin-based lecithin formulation (Meriva® formulated as the finished product Algocur®) alleviates the osteo-muscular pain conditions in rugby players. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4935–4940. [Google Scholar]
- Di Pierro, F.; Simonetti, G.; Petruzzi, A.; Bertuccioli, A.; Botta, L.; Bruzzone, M.G.; Cuccarini, V.; Fariselli, L.; Lamperti, E. A novel lecithin-based delivery form of Boswellic acids as complementary treatment of radiochemotherapy-induced cerebral edema in patients with glioblastoma multiforme: A longitudinal pilot experience. J. Neurosurg. Sci. 2019, 63, 286–291. [Google Scholar] [CrossRef]
- Smith, J.A.; Mathew, L.; Gaikwad, A.; Rech, B.; Burney, M.N.; Faro, J.P.; Lucci, J.A., III; Bai, Y.; Olsen, R.J.; Byrd, T.T. From Bench to Bedside: Evaluation of AHCC Supplementation to Modulate the Host Immunity to Clear High-Risk Human Papillomavirus Infections. Front. Oncol. 2019, 9, 173. [Google Scholar] [CrossRef]
- Smith, J.A.; Gaikwad, A.A.; Mathew, L.; Rech, B.; Faro, J.P.; Lucci, J.A.I.; Bai, Y.; Olsen, R.J.; Byrd, T.T. AHCC® Supplementation to Support Immune Function to Clear Persistent Human Papillomavirus Infections. Front. Oncol. 2022, 12, 881902. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, S.; Yun, S.-M.; Suh, D.H.; Kim, K.; No, J.H.; Jeong, E.-H.; Kim, Y.B. Active Hexose Correlated Compound (AHCC) Inhibits the Proliferation of Ovarian Cancer Cells by Suppressing Signal Transducer and Activator of Transcription 3 (STAT3) Activation. Nutr. Cancer 2017, 70, 109–115. [Google Scholar] [CrossRef]
- Di Pierro, F.; Bertuccioli, A.; Cavecchia, I. Possible therapeutic role of a highly standardized mixture of active compounds derived from cultured Lentinula edodes mycelia (AHCC) in patients infected with 2019 novel coronavirus. Minerva Gastroenterol. Dietol. 2020, 66, 172–176. [Google Scholar] [CrossRef]
| Abbreviation | Description |
|---|---|
| AHCC | Active xerose correlated compound |
| BRCA1 | Breast cancer tumor suppressor genes 1 |
| BRCA1 | Breast cancer tumor suppressor genes 2 |
| CMV | Cytomegalovirus |
| CST | Community state type |
| FRT | Female reproductive tract |
| HHV-6a | Human herpesvirus -6a |
| HPV | Human papillomavirus |
| IGFBPs | Insulin-like growth-factor-binding proteins |
| SH3RF2 | SH3 domain-containing ring finger 2 |
| General | Oncological |
|---|---|
| Patients with unfavorable CST | Affected patients (current or previous) |
| Patients HPV+ and/or with persistent HPV+ | High-risk women |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzaniga, M.; Cardinali, M.; Di Pierro, F.; Bertuccioli, A. Ovarian Microbiota, Ovarian Cancer and the Underestimated Role of HPV. Int. J. Mol. Sci. 2022, 23, 16019. https://doi.org/10.3390/ijms232416019
Cazzaniga M, Cardinali M, Di Pierro F, Bertuccioli A. Ovarian Microbiota, Ovarian Cancer and the Underestimated Role of HPV. International Journal of Molecular Sciences. 2022; 23(24):16019. https://doi.org/10.3390/ijms232416019
Chicago/Turabian StyleCazzaniga, Massimiliano, Marco Cardinali, Francesco Di Pierro, and Alexander Bertuccioli. 2022. "Ovarian Microbiota, Ovarian Cancer and the Underestimated Role of HPV" International Journal of Molecular Sciences 23, no. 24: 16019. https://doi.org/10.3390/ijms232416019
APA StyleCazzaniga, M., Cardinali, M., Di Pierro, F., & Bertuccioli, A. (2022). Ovarian Microbiota, Ovarian Cancer and the Underestimated Role of HPV. International Journal of Molecular Sciences, 23(24), 16019. https://doi.org/10.3390/ijms232416019








