Increased Intestinal Permeability and Stool Zonulin, Calprotectin and Beta-Defensin-2 Concentrations in Allogenic Hematopoietic Cell Transplantation Recipients
Abstract
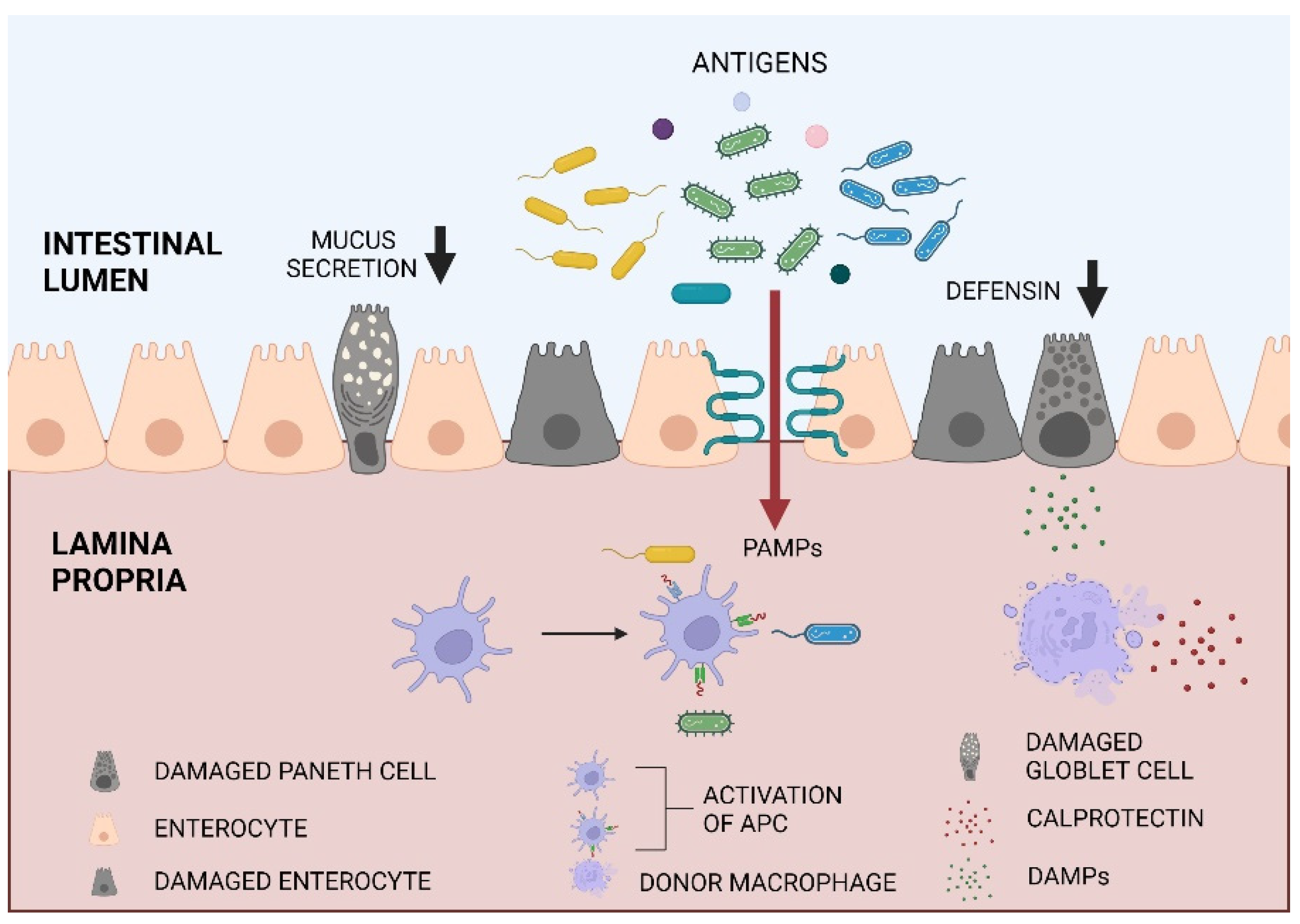
1. Introduction
2. Results
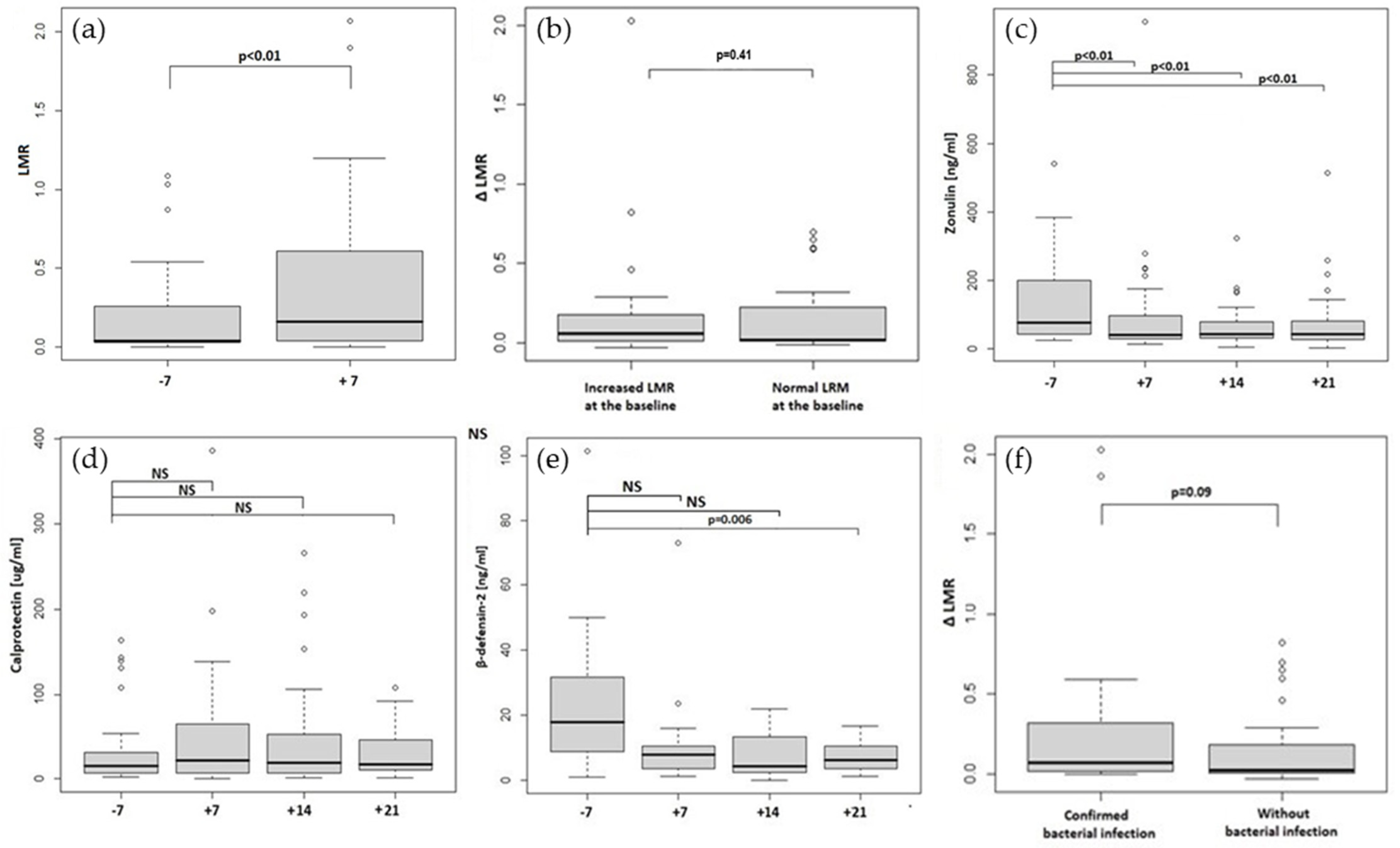
2.1. Increased Intestinal Permeability in Allo-HCT Recipients
2.2. Zonulin, Calprotectin and Beta-Defensin-2 Stool Concentrations
2.3. Intestinal Permeability and Stool Biomarker Associations
2.4. Correlations between the Transplantation Complications, Intestinal Permeability and Stool Biomarkers
2.4.1. Infectious Complications
2.4.2. aGVHD
2.4.3. Mucositis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Methods
4.2.1. Sugar Absorption Test
4.2.2. Stool Concentrations of Zonulin, Calprotectin and Beta-Defensin-2 Assays
4.2.3. Reference Limits
4.2.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenq, R.R.; Ubeda, C.; Taur, Y.; Menezes, C.C.; Khanin, R.; Dudakov, J.; Liu, C.; West, M.L.; Singer, N.V.; Equinda, M.J.; et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med. 2012, 209, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Taur, Y.; Xavier, J.; Lipuma, L.; Ubeda, C.; Goldberg, J.; Gobourne, A.; Lee, Y.J.; Dubin, K.A.; Socci, N.D.; Viale, A.; et al. Intestinal Domination and the Risk of Bacteremia in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Clin. Infect. Dis. 2012, 55, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Jenq, R.R.; Taur, Y.; Devlin, S.M.; Ponce, D.M.; Goldberg, J.D.; Ahr, K.F.; Littmann, E.R.; Ling, L.; Gobourne, A.C.; Miller, L.C.; et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2015, 21, 1373–1383. [Google Scholar] [CrossRef]
- Holler, E.; Butzhammer, P.; Schmid, K.; Hundsrucker, C.; Koestler, J.; Peter, K.; Zhu, W.; Sporrer, D.; Hehlgans, T.; Kreutz, M.; et al. Metagenomic Analysis of the Stool Microbiome in Patients Receiving Allogeneic Stem Cell Transplantation: Loss of Diversity Is Associated with Use of Systemic Antibiotics and More Pronounced in Gastrointestinal Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2014, 20, 640–645. [Google Scholar] [CrossRef]
- Shono, Y.; Docampo, M.D.; Peled, J.U.; Perobelli, S.M.; Velardi, E.; Tsai, J.J.; Slingerland, A.E.; Smith, O.M.; Young, L.F.; Gupta, J.; et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci. Transl. Med. 2016, 8, 339ra71. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, N.D.; Jenq, R.; Mathew, A.V.; Koenigsknecht, M.; Hanash, A.; Toubai, T.; Oravecz-Wilson, K.; Wu, S.-R.; Sun, Y.; Rossi, C.; et al. Gut microbiome–derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 2016, 17, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.; Morjaria, S.M.; Littmann, E.R.; Geyer, A.I.; Stover, D.E.; Barker, J.N.; Giralt, S.A.; Taur, Y.; Pamer, E.G. Gut Microbiota Predict Pulmonary Infiltrates after Allogeneic Hematopoietic Cell Transplantation. Am. J. Respir. Crit. Care Med. 2016, 194, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Taur, Y.; Jenq, R.; Perales, M.-A.; Littmann, E.R.; Morjaria, S.; Ling, L.; No, D.; Gobourne, A.; Viale, A.; Dahi, P.; et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014, 124, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Menard, S.; Cerf-Bensussan, N.; Heyman, M.B. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. 2010, 3, 247–259. [Google Scholar] [CrossRef]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of Oral Mucositis in Patients Who Have Cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef]
- Johansson, J.-E.; Ekman, T. Gastro-intestinal toxicity related to bone marrow transplantation: Disruption of the intestinal barrier precedes clinical findings. Bone Marrow Transplant. 1997, 19, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.E.; Brune, M.; Ekman, T. The gut mucosa barrier is preserved during allogeneic, haemopoietic stem cell trans-plantation with reduced intensity conditioning. Bone Marrow Transplant. 2001, 28, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Blijlevens, N.M.A.; Donnelly, J.P.; M’Rabet, L.; De Pauw, B.E.; Land, B.V. Measuring mucosal damage induced by cytotoxic therapy. Support. Care Cancer 2004, 12, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.-E.; Ekman, T. Gut Toxicity During Hemopoietic Stem Cell Transplantation May Predict Acute Graft-Versus-Host Disease Severity in Patients. Am. J. Dig. Dis. 2007, 52, 2340–2345. [Google Scholar] [CrossRef]
- Cooke, K.R.; Gerbitz, A.; Crawford, J.M.; Teshima, T.; Hill, G.R.; Tesolin, A.; Rossignol, D.P.; Ferrara, J.L. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J. Clin. Investig. 2001, 107, 1581–1589. [Google Scholar] [CrossRef]
- Peled, J.U.; Gomes, A.L.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef]
- Bates, D.W.; Parsonnet, J.; Ketchum, P.A.; Miller, E.B.; Novitsky, T.J.; Sands, K.; Hibberd, P.L.; Graman, P.S.; Lanken, P.N.; Schwartz, J.S.; et al. Limulus Amebocyte Lysate Assay for Detection of Endotoxin in Patients with Sepsis Syndrome. Clin. Infect. Dis. 1998, 27, 582–591. [Google Scholar] [CrossRef]
- Strutz, F.; Heller, G.; Krasemann, K.; Krone, B.; Müller, G.A. Relationship of antibodies to endotoxin core to mortality in medical patients with sepsis syndrome. Intensiv. Care Med. 1999, 25, 435–444. [Google Scholar] [CrossRef]
- Blijlevens, N.M.A.; Lutgens, L.C.H.W.; Schattenberg, A.V.M.B.; Donnelly, J.P. Citrulline: A potentially simple quantitative marker of intestinal epithelial damage following myeloablative therapy. Bone Marrow Transplant. 2004, 34, 193–196. [Google Scholar] [CrossRef]
- Crenn, P.; Coudray–Lucas, C.; Thuillier, F.; Cynober, L.; Messing, B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 2000, 119, 1496–1505. [Google Scholar] [CrossRef]
- Vreugdenhil, A.C.; Wolters, V.M.; Adriaanse, M.P.; Van den Neucker, A.M.; van Bijnen, A.A.; Houwen, R.; Buurman, W.A. Additional value of serum I-FABP levels for evaluating celiac disease activity in children. Scand. J. Gastroenterol. 2011, 46, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Mingrino, R.; Kaukinen, K.; Hayes, K.L.; Powell, R.M.; Macdonald, T.T.; Collins, J. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab. Investig. 2005, 85, 1139–1162. [Google Scholar] [CrossRef] [PubMed]
- Silberer, H.; Küppers, B.; Mickisch, O.; Baniewicz, W.; Drescher, M.; Traber, L.; Kempf, A.; Schmidt-Gayk, H. Fecal leukocyte proteins in inflammatory bowel disease and irritable bowel syndrome. Clin. Lab. 2005, 51, 117–126. [Google Scholar] [PubMed]
- Aasebo, A.T.; Gedde-Dahl, T.; Reims, H.M.; Baekkevold, E.S.; Jahnsen, F.L. Calprotectin Expressing Donor-Derived Macro-phages Increase in Acute Gastrointestinal Graft-Versus-Host Disease. Transplant. Cell. Ther. 2022, 28, 248-e1. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Physiological, Pathological, and Therapeutic Implications of Zonulin-Mediated Intestinal Barrier Modulation: Living Life on the Edge of the Wall. Am. J. Pathol. 2008, 173, 1243–1252. [Google Scholar] [CrossRef]
- Wang, W.; Uzzau, S.; Goldblum, S.E.; Fasano, A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell. Sci. 2000, 113 Pt 24, 4435–4440. [Google Scholar] [CrossRef]
- Fasano, A. Intestinal Permeability and Its Regulation by Zonulin: Diagnostic and Therapeutic Implications. Clin. Gastroenterol. Hepatol. 2012, 10, 1096–1100. [Google Scholar] [CrossRef]
- Fusco, A.; Savio, V.; Donniacuo, M.; Perfetto, B.; Donnarumma, G. Antimicrobial Peptides Human Beta-Defensin-2 and -3 Protect the Gut During Candida albicans Infections Enhancing the Intestinal Barrier Integrity: In Vitro Study. Front. Cell. Infect. Microbiol. 2021, 11, 486. [Google Scholar] [CrossRef]
- Han, F.; Zhang, H.; Xia, X.; Xiong, H.; Song, D.; Zong, X.; Wang, Y. Porcine β-Defensin 2 Attenuates Inflammation and Mucosal Lesions in Dextran Sodium Sulfate–Induced Colitis. J. Immunol. 2015, 194, 1882–1893. [Google Scholar] [CrossRef]
- Costa-Lima, C.; De Paula, E.V. ‘Leaky gut’ in hematological malignancies. Rev. Bras. De Hematol. E Hemoter. 2014, 36, 390–391. [Google Scholar] [CrossRef][Green Version]
- Sundström, G.M.; Wahlin, A.; Nordin-Andersson, I.; Suhr, O.B. Intestinal permeability in patients with acute myeloid leu-kemia. Eur. J. Haematol. 1998, 61, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.B.; Vilela, E.G.; Torres, H.O.D.G.; Ferrari, M.D.L.D.A.; da Cunha, A.S. Intestinal permeability in leukemic patients prior to chemotherapy. Rev. Bras. Hematol. Hemoter. 2014, 36, 409–413. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nocerino, R.; De Filippis, F.; Cecere, G.; Marino, A.; Micillo, M.; Di Scala, C.; de Caro, C.; Calignano, A.; Bruno, C.; Paparo, L.; et al. The therapeutic efficacy of Bifidobacterium animalis subsp. lactis BB-12(®) in infant colic: A randomised, double blind, placebo-controlled trial. Aliment. Pharmacol. Ther. 2020, 51, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Siljander, H.; Jason, E.; Ruohtula, T.; Selvenius, J.; Koivusaari, K.; Salonen, M.; Ahonen, S.; Honkanen, J.; Ilonen, J.; Vaarala, O.; et al. Effect of Early Feeding on Intestinal Permeability and Inflammation Markers in Infants with Genetic Susceptibility to Type 1 Diabetes: A Randomized Clinical Trial. J. Pediatr. 2021, 238, 305–311.e3. [Google Scholar] [CrossRef] [PubMed]
- Savilahti, E.M.; Kukkonen, A.K.; Tuure, T.; Kuitunen, M.; Haahtela, T. Intestinal defensin secretion in infancy is associated with the emergence of sensitization and atopic dermatitis. Clin. Exp. Allergy 2011, 42, 405–411. [Google Scholar] [CrossRef]
- Nocerino, R.; Paparo, L.; Terrin, G.; Pezzella, V.; Amoroso, A.; Cosenza, L.; Cecere, G.; De Marco, G.; Micillo, M.; Albano, F.; et al. Cow’s milk and rice fermented with Lactobacillus paracasei CBA L74 prevent infectious diseases in children: A randomized controlled trial. Clin. Nutr. 2017, 36, 118–125. [Google Scholar] [CrossRef]
- Malik, M.N.; Rafae, A.; Durer, C.; Durer, S.; Anwer, F. Fecal Calprotectin as a Diagnostic and Prognostic Biomarker for Gas-trointestinal Graft Versus Host Disease: A Systematic Review of Literature. Cureus 2019, 11, e4143. [Google Scholar]
- Legert, K.G.; Remberger, M.; Ringdén, O.; Heimdahl, A.; Dahllöf, G. Reduced intensity conditioning and oral care measures prevent oral mucositis and reduces days of hospitalization in allogeneic stem cell transplantation recipients. Support. Care Cancer 2014, 22, 2133–2140. [Google Scholar] [CrossRef]
- Haverman, T.M.; Raber-Durlacher, J.E.; Rademacher, W.M.H.; Vokurka, S.; Epstein, J.B.; Huisman, C.; Hazenberg, M.; De Soet, J.J.; De Lange, J.; Rozema, F.R. Oral Complications in Hematopoietic Stem Cell Recipients: The Role of Inflammation. Mediat. Inflamm. 2014, 2014, 378281. [Google Scholar] [CrossRef]
- Bjarnason, I.; Macpherson, A.; Hollander, D. Intestinal permeability: An overview. Gastroenterology 1995, 108, 1566–1581. [Google Scholar] [CrossRef]
- Zonulin Elisa. Available online: https://www.immundiagnostik.com/media/pages/testkits/k-5600/17c79275c91653271285/k5600_2022-02-16_idk-zonulin-elisa.pdf (accessed on 1 March 2022).
- ß-Defensin 2 ELISA. Available online: https://www.immundiagnostik.com/media/pages/testkits/k-6500/facd9e2d16-1653271285/k6500_2022-02-24_beta-defensin.pdf (accessed on 1 March 2022).
- Calprotectin Elisa. Available online: https://www.idkna.com/docs/KR6927_2022-02-15_IDK_Calprotectin_Stuhl_1h.pdf (accessed on 1 March 2022).
- Hałasa, M.; Maciejewska, D.; Ryterska, K.; Baśkiewicz-Hałasa, M.; Safranow, K.; Stachowska, E. Assessing the Association of Elevated Zonulin Concentration in Stool with Increased Intestinal Permeability in Active Professional Athletes. Medicina 2019, 55, 710. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, I. The Use of Fecal Calprotectin in Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2017, 13, 53–56. [Google Scholar]
| Patient or Transplant Characteristic | N | LMR Day −7, Median (IQR) | p | LMR Day +7 Median (IQR) | p | ΔLMR Median (IQR) | p |
|---|---|---|---|---|---|---|---|
| All | 45 | 0.04 (0.23) | 0.16 (0.57) | 0.02 (0.18) | x | ||
| Increased LMR on day −7 | 24 | 0.19 | <0.001 | 0.31 | <0.001 | 0.06 | 0.41 |
| Normal LMR on day −7 | 21 | 0.028 | 0.038 | 0.016 | |||
| Sex | |||||||
| woman | 22 | 0.040 (0.041) | 0.955 | 0.136 (0.588) | 0.865 | 0.025 (0.152) | 0.665 |
| men | 23 | 0.043 (0.302) | 0.271 (0.574) | 0.020 (0.196) | |||
| type of conditioning | |||||||
| RIC | 13 | 0.040 (0.038) | 0.853 | 0.161 (0.628) | 0.528 | 0.036 (0.280) | 0.665 |
| myeloablative | 32 | 0.040 (0.243) | 0.155 (0.527) | 0.020 (0.139) | |||
| age | |||||||
| <65 years old | 40 | 0.040 (0.067) | 0.367 | 0.108 (0.525) | 0.164 | 0.025 (0.185) | 0.639 |
| ≥65 years old | 5 | 0.026 (0.999) | 0.700 (0.783) | 0.020 (0.071) | |||
| HCT-CI | |||||||
| ≤2 | 25 | 0.041 (0.041) | 0.035 | 0.161 (0.301) | 0.364 | 0.036 (0.190) | 0.903 |
| >2 | 6 | 0.430 (0.821) | 0.670 (0.926) | 0.090 (0.185) | |||
| WHO | |||||||
| 0 | 28 | 0.037 (0.071) | 0.423 | 0.181 (0.583) | 0.737 | 0.028 (0.235) | 0.423 |
| >0 | 17 | 0.044 (0.350) | 0.110 (0.551) | 0.018 (0.065) |
| Parameter | −7 | +7 | +14 | +21 | p (−7 vs. +7) | p (−7:+21) |
|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||
| LMR | 0.04 (0.23) | 0.16 (0.57) | <0.001 | - | ||
| Zonulin [ng/mL] | 76.45 (157.2) | 39.95 (75.1) | 43.00 (47.75) | 41.45 (54.4) | <0.001 | <0.001 |
| Calprotectin [μg/mL] | 15.50 (25.2) | 21.50 (57.9) | 19.30 (44.9) | 16.90 (35.5) | 0.374 | 0.451 |
| Beta-defensin-2 [ng/mL] | 17.90 (22.8) | 7.80 (6.8) | 4.31 (11.0) | 6.20 (6.8) | 0.101 | 0.006 |
| Parameter | −7 | 7 | 14 | 21 | p |
|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||
| aGVHD | |||||
| Calprotectin [μg/mL] | 10.3 (24.2) | 64.8 (167.4) | 22.1 (26.0) | 29.7 (40.3) | p > 0.05 * |
| Zonulin [ng/mL] | 51.4 (31.9) | 42.8 (104.7) | 35.5 (21.0) | 30.6 (116.4) | p > 0.05 |
| without aGVHD | |||||
| Calprotectin [μg/mL] | 15.6 (22.6) | 14.1 (39.8) | 17.7 (45.9) | 14.9 (35.0) | p > 0.05 |
| Zonulin [ng/mL] | 86.7 (158.4) | 40 (63.1) | 45.2 (52.8) | 47.5 (51.4) | p > 0.05 |
| Age in years, median (range) | 54.0 (19–67) |
| Male sex, n (%) | 23 (45) |
| Performance Status, n (%) | |
| 0 | 30 (59) |
| 1 | 21 (41) |
| Conditioning treatment, n (%) | |
| myeloablative | 37 (73) |
| RIC | 14 (27) |
| GVHD prophylaxis regimen, n (%) | |
| CSA/MTX | 39 (76) |
| other | 12 (24) |
| Disease, n (%) | |
| Acute leukemia | 32 (63) |
| Aplastic anemia | 3 (6) |
| Myelodysplastic syndrome | 6 (12) |
| Chronic myeloid leukemia | 3 (6) |
| Others * | 7 (13) |
| Disease status before transplantation, n (%) | |
| CR | 28 (54) |
| CR, MRD(+) | 9 (18) |
| PR | 5 (10) |
| PD | 2 (4) |
| Chronic phase | 2 (4) |
| Transplantation upfront | 5 (10) |
| Complications, n (%): | |
| infections within 30 days (included FN): | 30 (58) |
| confirmed bacterial infection | 15 (29) |
| aGvHD | 16 (31) |
| aGvHD grade III and IV | 6 (12) |
| mucositis | 36 (71) |
| mucositis grade III and IV | 22 (43) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyszka, M.; Maciejewska-Markiewicz, D.; Biliński, J.; Lubas, A.; Stachowska, E.; Basak, G.W. Increased Intestinal Permeability and Stool Zonulin, Calprotectin and Beta-Defensin-2 Concentrations in Allogenic Hematopoietic Cell Transplantation Recipients. Int. J. Mol. Sci. 2022, 23, 15962. https://doi.org/10.3390/ijms232415962
Tyszka M, Maciejewska-Markiewicz D, Biliński J, Lubas A, Stachowska E, Basak GW. Increased Intestinal Permeability and Stool Zonulin, Calprotectin and Beta-Defensin-2 Concentrations in Allogenic Hematopoietic Cell Transplantation Recipients. International Journal of Molecular Sciences. 2022; 23(24):15962. https://doi.org/10.3390/ijms232415962
Chicago/Turabian StyleTyszka, Martyna, Dominika Maciejewska-Markiewicz, Jarosław Biliński, Arkadiusz Lubas, Ewa Stachowska, and Grzegorz W. Basak. 2022. "Increased Intestinal Permeability and Stool Zonulin, Calprotectin and Beta-Defensin-2 Concentrations in Allogenic Hematopoietic Cell Transplantation Recipients" International Journal of Molecular Sciences 23, no. 24: 15962. https://doi.org/10.3390/ijms232415962
APA StyleTyszka, M., Maciejewska-Markiewicz, D., Biliński, J., Lubas, A., Stachowska, E., & Basak, G. W. (2022). Increased Intestinal Permeability and Stool Zonulin, Calprotectin and Beta-Defensin-2 Concentrations in Allogenic Hematopoietic Cell Transplantation Recipients. International Journal of Molecular Sciences, 23(24), 15962. https://doi.org/10.3390/ijms232415962







