Elevated Glucagon-like Peptide-1 Receptor Level in the Paraventricular Hypothalamic Nucleus of Type 2 Diabetes Mellitus Patients
Abstract
1. Introduction
2. Results
2.1. GLP-1R mRNA Levels in Post Mortem Hypothalamic Samples of T2DM Patients
2.2. GLP-1R Protein Levels
2.3. GLP-1R in the Hypothalamus
2.4. GLP-1R Level in T2DM Patients in Various Parts of the Hypothalamus
2.5. GLP1 Fibres in the PVN
3. Discussion
3.1. The Presence of the GLP-1 System in the Human Hypothalamus
3.2. Elevated GLP-1R in T2DM Patients
3.3. Functional Implications of the PVN GLP-1 System
4. Materials and Methods
4.1. Human Brain Tissue Samples
4.2. Tissue Preparation
4.3. Gene Expressional Measurements with qRT-PCR
4.4. Protein Extraction for Western Blotting
4.5. Western Blot Analysis
4.6. Preparation of In Situ Hybridization Probes
4.7. In Situ Hybridization Histochemistry
4.8. Tissue Collection for Immunolabelling
4.9. Immunolabelling
4.10. Double Labelling of GLP-1 and Oxytocin
4.11. Microscopy and Photography
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Gaal, L.F.; Gutkin, S.W.; Nauck, M.A. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: Glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control? Eur. J. Endocrinol. 2008, 158, 773–784. [Google Scholar] [CrossRef][Green Version]
- De Leon, D.D.; Crutchlow, M.F.; Ham, J.Y.; Stoffers, D.A. Role of glucagon-like peptide-1 in the pathogenesis and treatment of diabetes mellitus. Int. J. Biochem. Cell Biol. 2006, 38, 845–859. [Google Scholar] [CrossRef]
- Van Bloemendaal, L.; Ten Kulve, J.S.; La Fleur, S.E.; Ijzerman, R.G.; Diamant, M. Effects of glucagon-like peptide 1 on appetite and body weight: Focus on the CNS. J. Endocrinol. 2014, 221, T1–T16. [Google Scholar] [CrossRef]
- Trapp, S.; Stanford, S.C. New developments in the prospects for GLP-1 therapy. Br. J. Pharmacol. 2022, 179, 489–491. [Google Scholar] [CrossRef]
- Muller, T.D.; Finan, B.; Bloom, S.R.; D’alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar]
- Theodorakis, M.J.; Carlson, O.; Michopoulos, S.; Doyle, M.E.; Juhaszova, M.; Petraki, K.; Egan, J.M. Human duodenal enteroendocrine cells: Source of both incretin peptides, GLP-1 and GIP. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E550–E559. [Google Scholar] [CrossRef]
- Merchenthaler, I.; Lane, M.; Shughrue, P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol. 1999, 403, 261–280. [Google Scholar] [CrossRef]
- Holt, M.K.; Richards, J.E.; Cook, D.R.; Brierley, D.I.; Williams, D.L.; Reimann, F.; Gribble, F.M.; Trapp, S. Preproglucagon Neurons in the Nucleus of the Solitary Tract Are the Main Source of Brain GLP-1, Mediate Stress-Induced Hypophagia, and Limit Unusually Large Intakes of Food. Diabetes 2019, 68, 21–33. [Google Scholar] [CrossRef]
- Vrang, N.; Hansen, M.; Larsen, P.J.; Tang-Christensen, M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res. 2007, 1149, 118–126. [Google Scholar] [CrossRef]
- Berthoud, H.R. Multiple neural systems controlling food intake and body weight. Neurosci. Biobehav. Rev. 2002, 26, 393–428. [Google Scholar] [CrossRef]
- Renner, E.; Puskas, N.; Dobolyi, A.; Palkovits, M. Glucagon-like peptide-1 of brainstem origin activates dorsomedial hypothalamic neurons in satiated rats. Peptides 2012, 35, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Grill, H.J.; Hayes, M.R. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012, 16, 296–309. [Google Scholar] [CrossRef]
- Larsen, P.J.; Tang-Christensen, M.; Holst, J.J.; Orskov, C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997, 77, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn-Smith, I.J.; Reimann, F.; Gribble, F.M.; Trapp, S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience 2011, 180, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Renner, É.; Szabó-Meltzer, K.I.; Puskás, N.; Tóth, Z.E.; Dobolyi, Á.; Palkovits, M. Activation of neurons in the hypothalamic dorsomedial nucleus via hypothalamic projections of the nucleus of the solitary tract following refeeding of fasted rats. Eur. J. Neurosci. 2010, 31, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Roland, B.; Tomaselli, K.; Dolman, C.S.; Lowe, C.; Heilig, J.S. Glucagon-like peptide-1 in the rat brain: Distribution of expression and functional implication. J. Comp. Neurol. 2013, 521, 2235–2261. [Google Scholar] [CrossRef]
- Biddinger, J.E.; Lazarenko, R.M.; Scott, M.M.; Simerly, R. Leptin suppresses development of GLP-1 inputs to the paraventricular nucleus of the hypothalamus. Elife 2020, 9, e59857. [Google Scholar] [CrossRef]
- Kabahizi, A.; Wallace, B.; Lieu, L.; Chau, D.; Dong, Y.; Hwang, E.S.; Williams, K.W. Glucagon-like peptide-1 (GLP-1) signalling in the brain: From neural circuits and metabolism to therapeutics. Br. J. Pharmacol. 2022, 179, 600–624. [Google Scholar] [CrossRef]
- Vrang, N.; Grove, K. The brainstem preproglucagon system in a non-human primate (Macaca mulatta). Brain Res. 2011, 1397, 28–37. [Google Scholar] [CrossRef]
- Heppner, K.M.; Kirigiti, M.; Secher, A.; Paulsen, S.J.; Buckingham, R.; Pyke, C.; Knudsen, L.B.; Vrang, N.; Grove, K.L. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology 2015, 156, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Cork, S.C.; Richards, J.E.; Holt, M.K.; Gribble, F.M.; Reimann, F.; Trapp, S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol. Metab. 2015, 4, 718–731. [Google Scholar] [CrossRef]
- Graham, D.L.; Durai, H.H.; Trammell, T.S.; Noble, B.L.; Mortlock, D.P.; Galli, A.; Stanwood, G.D. A novel mouse model of glucagon-like peptide-1 receptor expression: A look at the brain. J. Comp. Neurol. 2020, 528, 2445–2470. [Google Scholar] [CrossRef]
- Shughrue, P.J.; Lane, M.V.; Merchenthaler, I. Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinology 1996, 137, 5159–5162. [Google Scholar] [CrossRef]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Ahnfelt-Ronne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020, 5, e133429. [Google Scholar] [CrossRef]
- Farkas, E.; Szilvasy-Szabo, A.; Ruska, Y.; Sinko, R.; Rasch, M.G.; Egebjerg, T.; Pyke, C.; Gereben, B.; Knudsen, L.B.; Fekete, C. Distribution and ultrastructural localization of the glucagon-like peptide-1 receptor (GLP-1R) in the rat brain. Brain Struct. Funct. 2021, 226, 225–245. [Google Scholar] [CrossRef]
- Boland, B.B.; Laker, R.C.; O’brien, S.; Sitaula, S.; Sermadiras, I.; Nielsen, J.C.; Barkholt, P.; Roostalu, U.; Hecksher-Sorensen, J.; Sejthen, S.R.; et al. Peptide-YY3-36/glucagon-like peptide-1 combination treatment of obese diabetic mice improves insulin sensitivity associated with recovered pancreatic beta-cell function and synergistic activation of discrete hypothalamic and brainstem neuronal circuitries. Mol. Metab. 2022, 55, 101392. [Google Scholar] [CrossRef]
- Brierley, D.I.; Holt, M.K.; Singh, A.; De Araujo, A.; Mcdougle, M.; Vergara, M.; Afaghani, M.H.; Lee, S.J.; Scott, K.; Maske, C.; et al. Central and peripheral GLP-1 systems independently suppress eating. Nat. Metab. 2021, 3, 258–273. [Google Scholar] [CrossRef]
- Katsurada, K.; Yada, T. Neural effects of gut- and brain-derived glucagon-like peptide-1 and its receptor agonist. J. Diabetes Investig. 2016, 7 (Suppl. 1), 64–69. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Fortin, S.M.; Arnold, M.; Grill, H.J.; Hayes, M.R. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 2011, 152, 3103–3112. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, D.A.; Bagnol, D.; Woods, S.C.; D’alessio, D.A.; Seeley, R.J. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 2008, 57, 2046–2054. [Google Scholar] [CrossRef]
- Mcmahon, L.R.; Wellman, P.J. PVN infusion of GLP-1-(7-36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am. J. Physiol. 1998, 274, R23–R29. [Google Scholar] [CrossRef] [PubMed]
- Sisley, S.; Gutierrez-Aguilar, R.; Scott, M.; D’alessio, D.A.; Sandoval, D.A.; Seeley, R.J. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J. Clin. Investig. 2014, 124, 2456–2463. [Google Scholar] [CrossRef]
- Katsurada, K.; Maejima, Y.; Nakata, M.; Kodaira, M.; Suyama, S.; Iwasaki, Y.; Kario, K.; Yada, T. Endogenous GLP-1 acts on paraventricular nucleus to suppress feeding: Projection from nucleus tractus solitarius and activation of corticotropin-releasing hormone, nesfatin-1 and oxytocin neurons. Biochem. Biophys. Res. Commun. 2014, 451, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Navarrete, J.; Liang-Guallpa, J.; Lu, C.; Funderburk, S.C.; Chang, R.B.; Liberles, S.D.; Olson, D.P.; Krashes, M.J. Defined Paraventricular Hypothalamic Populations Exhibit Differential Responses to Food Contingent on Caloric State. Cell. Metab. 2019, 29, 681–694 e685. [Google Scholar] [CrossRef] [PubMed]
- Ten Kulve, J.S.; Van Bloemendaal, L.; Balesar, R.; Rg, I.J.; Swaab, D.F.; Diamant, M.; La Fleur, S.E.; Alkemade, A. Decreased Hypothalamic Glucagon-Like Peptide-1 Receptor Expression in Type 2 Diabetes Patients. J. Clin. Endocrinol. Metab. 2016, 101, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Andermann, M.L.; Lowell, B.B. Toward a Wiring Diagram Understanding of Appetite Control. Neuron 2017, 95, 757–778. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Conde, K.; Zhang, P.; Lilascharoen, V.; Xu, Z.; Lim, B.K.; Seeley, R.J.; Zhu, J.J.; Scott, M.M.; Pang, Z.P. Enhanced AMPA Receptor Trafficking Mediates the Anorexigenic Effect of Endogenous Glucagon-like Peptide-1 in the Paraventricular Hypothalamus. Neuron 2017, 96, 897–909 e895. [Google Scholar] [CrossRef]
- Koutcherov, Y.; Mai, J.K.; Ashwell, K.W.; Paxinos, G. Organization of the human paraventricular hypothalamic nucleus. J. Comp. Neurol. 2000, 423, 299–318. [Google Scholar] [CrossRef]
- Mai, J.K. Atlas of the Human Brain; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Alvarez, E.; Martinez, M.D.; Roncero, I.; Chowen, J.A.; Garcia-Cuartero, B.; Gispert, J.D.; Sanz, C.; Vazquez, P.; Maldonado, A.; De Caceres, J.; et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J. Neurochem. 2005, 92, 798–806. [Google Scholar] [CrossRef]
- Ke, J.; Liu, Y.; Yang, J.; Lu, R.; Tian, Q.; Hou, W.; Wang, G.; Wei, R.; Hong, T. Synergistic effects of metformin with liraglutide against endothelial dysfunction through GLP-1 receptor and PKA signalling pathway. Sci. Rep. 2017, 7, 41085. [Google Scholar] [CrossRef]
- Maida, A.; Lamont, B.J.; Cao, X.; Drucker, D.J. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-alpha in mice. Diabetologia 2011, 54, 339–349. [Google Scholar] [CrossRef]
- Labuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopien, B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [CrossRef]
- Li, N.; Zhou, T.; Fei, E. Actions of Metformin in the Brain: A New Perspective of Metformin Treatments in Related Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 8281. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Metabolic Messengers: Glucagon-like peptide 1. Nat. Metab. 2021, 3, 142–148. [Google Scholar] [CrossRef]
- Trapp, S.; Hisadome, K. Glucagon-like peptide 1 and the brain: Central actions-central sources? Auton. Neurosci. 2011, 161, 14–19. [Google Scholar] [CrossRef]
- Maejima, Y.; Yokota, S.; Nishimori, K.; Shimomura, K. The Anorexigenic Neural Pathways of Oxytocin and Their Clinical Implication. Neuroendocrinology 2018, 107, 91–104. [Google Scholar] [CrossRef]
- Rinaman, L.; Rothe, E.E. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R99–R106. [Google Scholar] [CrossRef]
- Wen, S.; Nguyen, T.; Xiao, W.Z.; Wang, C.X.; Gong, M.; Jin, J.L.; Zhou, L.G. Comparative study on anorexigenic effect of glucagon-like peptide-1 receptor agonists in rats. Sheng Li Xue Bao 2019, 71, 514–526. [Google Scholar]
- Palkovits, M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973, 59, 449–450. [Google Scholar] [CrossRef]
- Palkovits, M. Microdissection of Individual Brain Nuclei and Areas. In General Neurochemical Techniques; Boulton, A.A., Baker, G.B., Eds.; Humana Press: Totowa, NJ, USA, 1985; pp. 1–17. [Google Scholar]
- Dora, F.; Renner, E.; Keller, D.; Palkovits, M.; Dobolyi, A. Transcriptome Profiling of the Dorsomedial Prefrontal Cortex in Suicide Victims. Int. J. Mol. Sci. 2022, 23, 7067. [Google Scholar] [CrossRef]
- Likhite, N.; Warawdekar, U.M. A unique method for isolation and solubilization of proteins after extraction of RNA from tumor tissue using trizol. J. Biomol. Tech. 2011, 22, 37–44. [Google Scholar]
- Faber, C.A.; Dobolyi, A.; Sleeman, M.; Usdin, T.B. Distribution of tuberoinfundibular peptide of 39 residues and its receptor, parathyroid hormone 2 receptor, in the mouse brain. J. Comp. Neurol. 2007, 502, 563–583. [Google Scholar] [CrossRef]
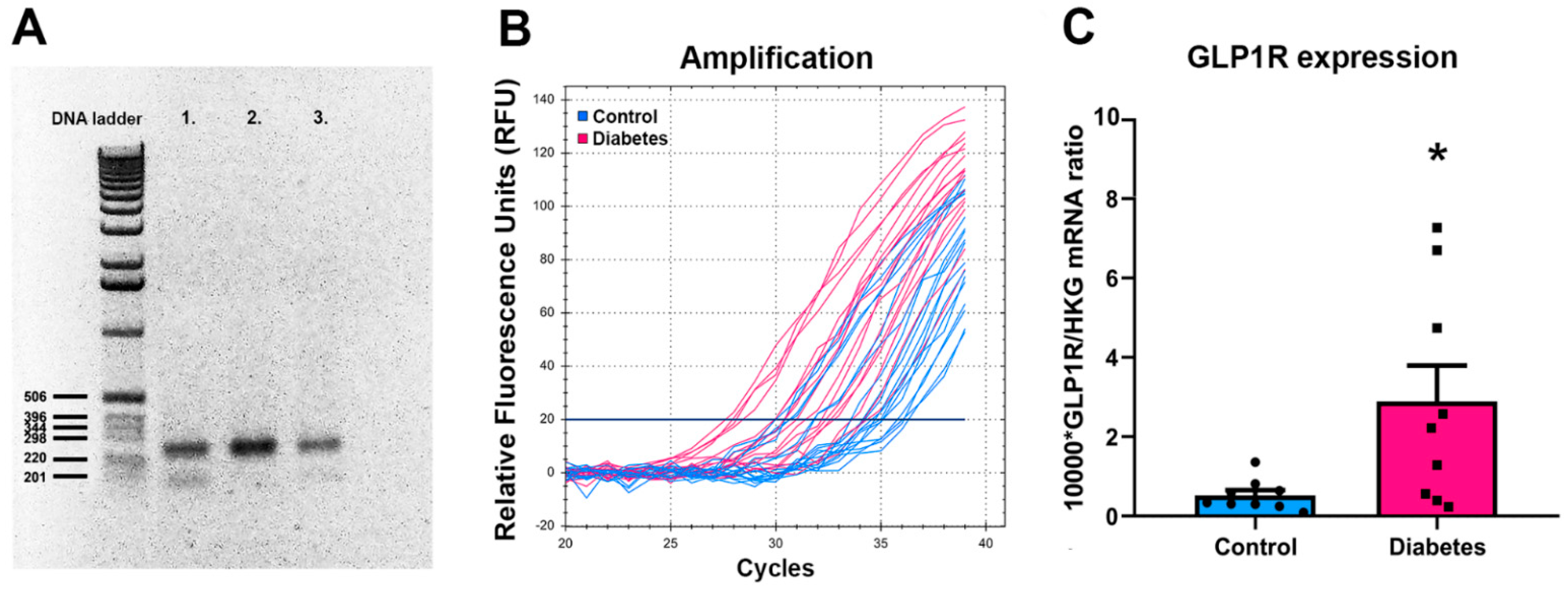

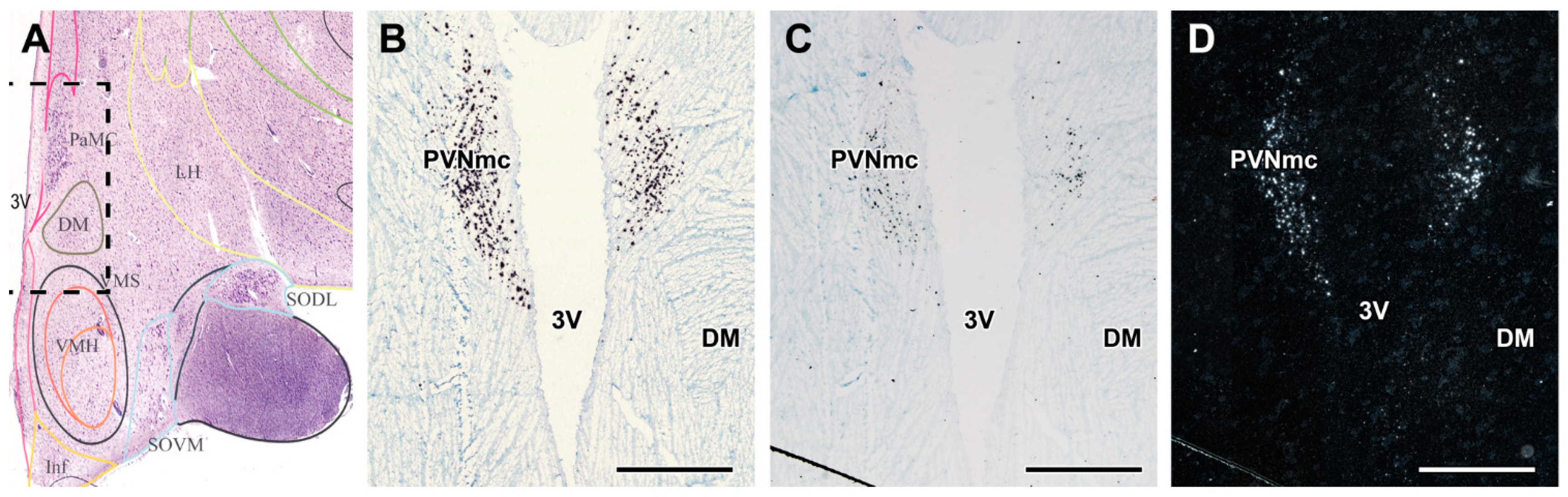
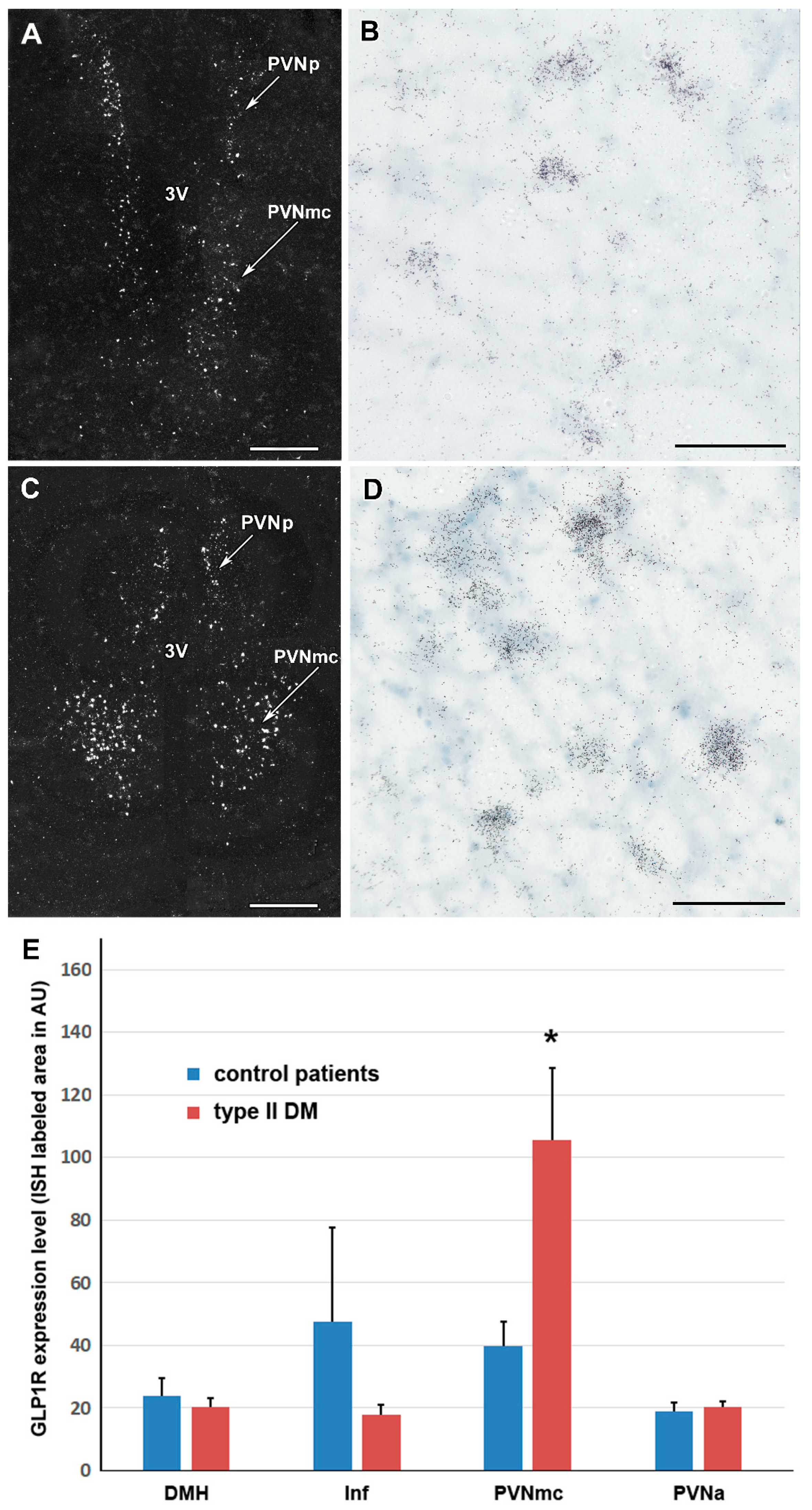
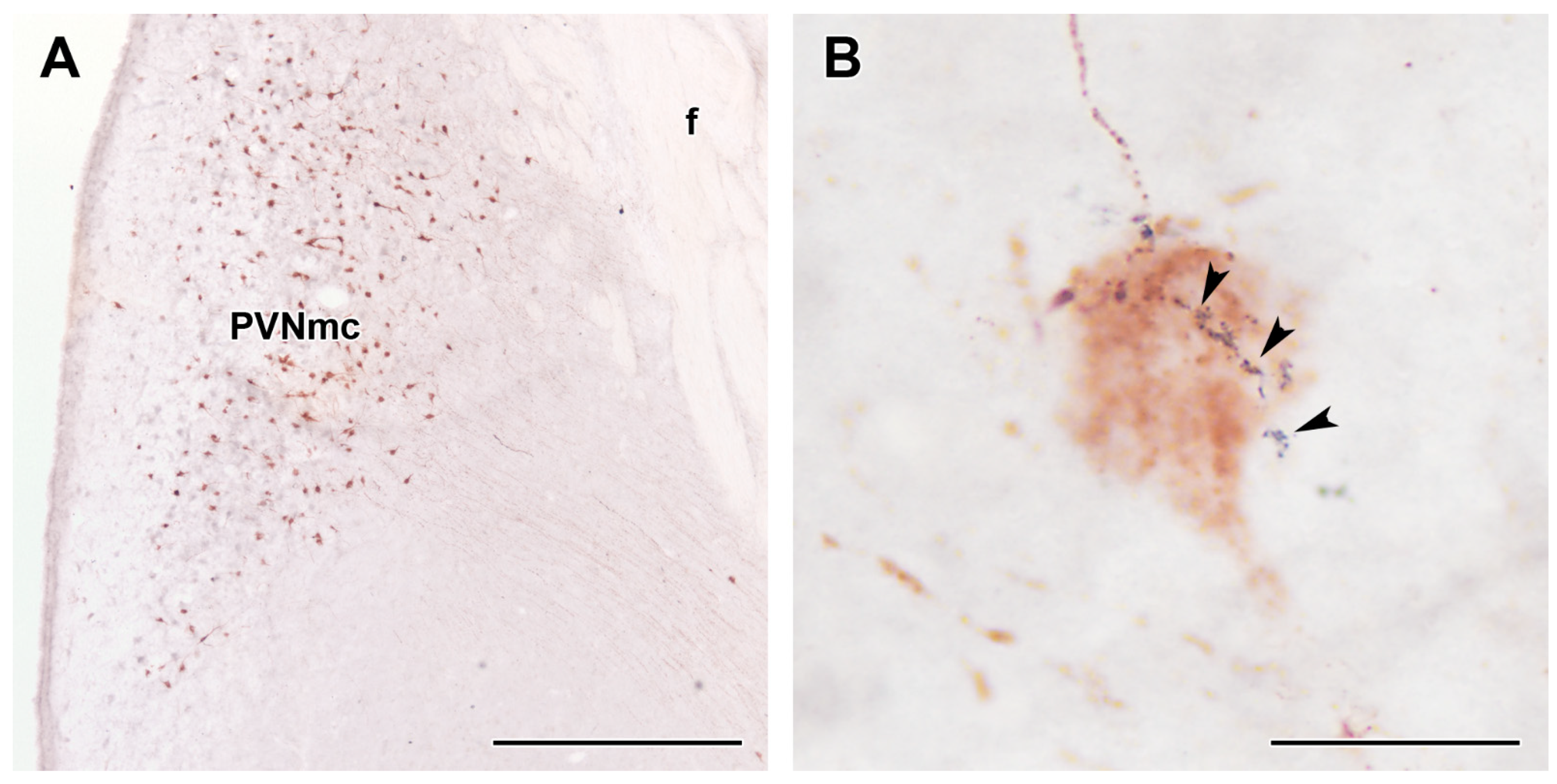

| Donor # | Experimental Group | Age | Sex | Clinical and Pathological Diagnosis (in Addition to T2DM in the Experimental Group) | Cause of Death | Post Mortem Interval/Hour |
|---|---|---|---|---|---|---|
| 1 | Control | 72 | female | Hypertonia | Acute cardiac insufficiency | 4.5 |
| 2 | Control | 67 | male | Hypertonia | Pulmonary embolism | 5 |
| 3 | Control | 81 | female | General atherosclerosis | SStroke (brain haemorrhage) | 5 |
| 4 | Control | 80 | male | Hypertonia, coronary sclerosis | Stroke (acute intracerebral haematoma) | 2 |
| 5 | Control | 70 | female | Hypertonia | Stroke | 7 |
| 6 | Control | 65 | male | Hypertonia, coronary sclerosis | Stroke, broncho-pneumonia | 10 |
| 7 | Control | 95 | female | Hypertonia, arteriosclerosis | Stroke | 4 |
| 8 | Control | 52 | male | Chronic alcoholism | No information | 8 |
| 9 | Control | 66 | male | Cardiorespiratory insufficiency | Stroke | 9 |
| 10 | T2DM | 69 | female | Pulmonary insufficiency, hypertonia | Stroke | 3 |
| 11 | T2DM | 67 | male | Duodenal ulcer | Pneumonia, cardiopulmonary insufficiency | 10 |
| 12 | T2DM | 75 | female | General atherosclerosis, mamma carcinoma | Stroke | 10 |
| 13 | T2DM | 79 | male | Hypertonia, cardiomyopathia | Stroke | 6 |
| 14 | T2DM | 92 | female | Cardiorespiratory insufficiency, arteriosclerosis universalis, renal insufficiency, hypertonia | oOedema cerebri | 4 |
| 15 | T2DM | 74 | male | Hypertonia, prostate carcinoma | Stroke, oedema cerebri | 3 |
| 16 | T2DM | 85 | female | Hypertonia, arteriosclerosis | Stroke | 8 |
| 17 | T2DM | 79 | male | Obesity, polyneuropathia | Cardiorespiratory insufficiency, stroke | 10 |
| 18 | T2DM | 77 | male | Chronic alcoholism, cardiorespiratory insufficiency | Stroke, bronchopneumonia | 5.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renner, É.; Dóra, F.; Oszwald, E.; Dobolyi, Á.; Palkovits, M. Elevated Glucagon-like Peptide-1 Receptor Level in the Paraventricular Hypothalamic Nucleus of Type 2 Diabetes Mellitus Patients. Int. J. Mol. Sci. 2022, 23, 15945. https://doi.org/10.3390/ijms232415945
Renner É, Dóra F, Oszwald E, Dobolyi Á, Palkovits M. Elevated Glucagon-like Peptide-1 Receptor Level in the Paraventricular Hypothalamic Nucleus of Type 2 Diabetes Mellitus Patients. International Journal of Molecular Sciences. 2022; 23(24):15945. https://doi.org/10.3390/ijms232415945
Chicago/Turabian StyleRenner, Éva, Fanni Dóra, Erzsébet Oszwald, Árpád Dobolyi, and Miklós Palkovits. 2022. "Elevated Glucagon-like Peptide-1 Receptor Level in the Paraventricular Hypothalamic Nucleus of Type 2 Diabetes Mellitus Patients" International Journal of Molecular Sciences 23, no. 24: 15945. https://doi.org/10.3390/ijms232415945
APA StyleRenner, É., Dóra, F., Oszwald, E., Dobolyi, Á., & Palkovits, M. (2022). Elevated Glucagon-like Peptide-1 Receptor Level in the Paraventricular Hypothalamic Nucleus of Type 2 Diabetes Mellitus Patients. International Journal of Molecular Sciences, 23(24), 15945. https://doi.org/10.3390/ijms232415945






