Comprehensive Investigation of Parameters Influencing Fluorescence Lifetime Imaging Microscopy in Frequency- and Time-Domain Illustrated by Phasor Plot Analysis
Abstract
1. Introduction
2. Results
2.1. Setup Dependent Parameters
2.1.1. Wavelength
2.1.2. Modulation Frequency
2.1.3. Integration Time
2.2. Sample Dependent Parameter
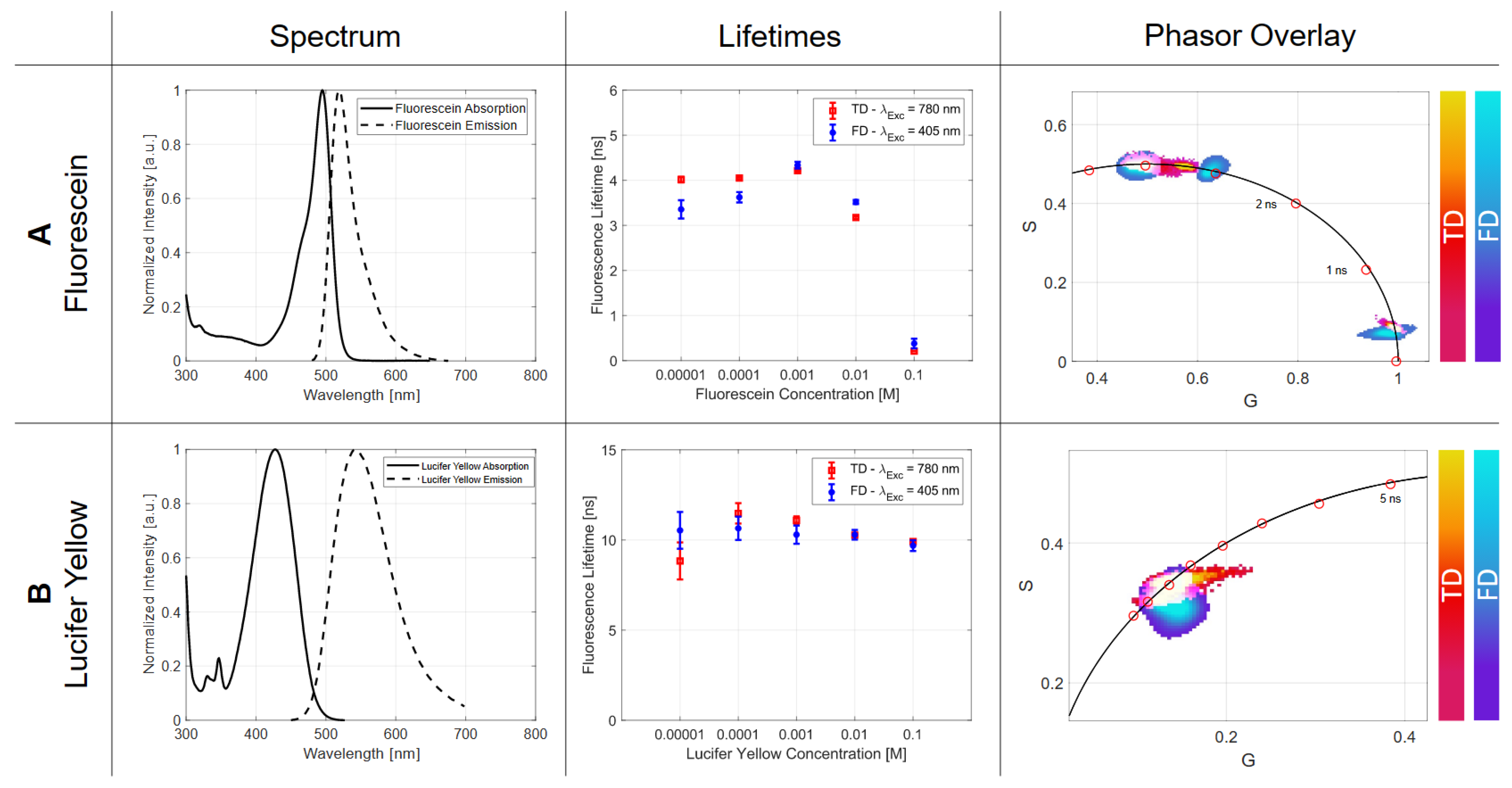
2.2.1. Concentration
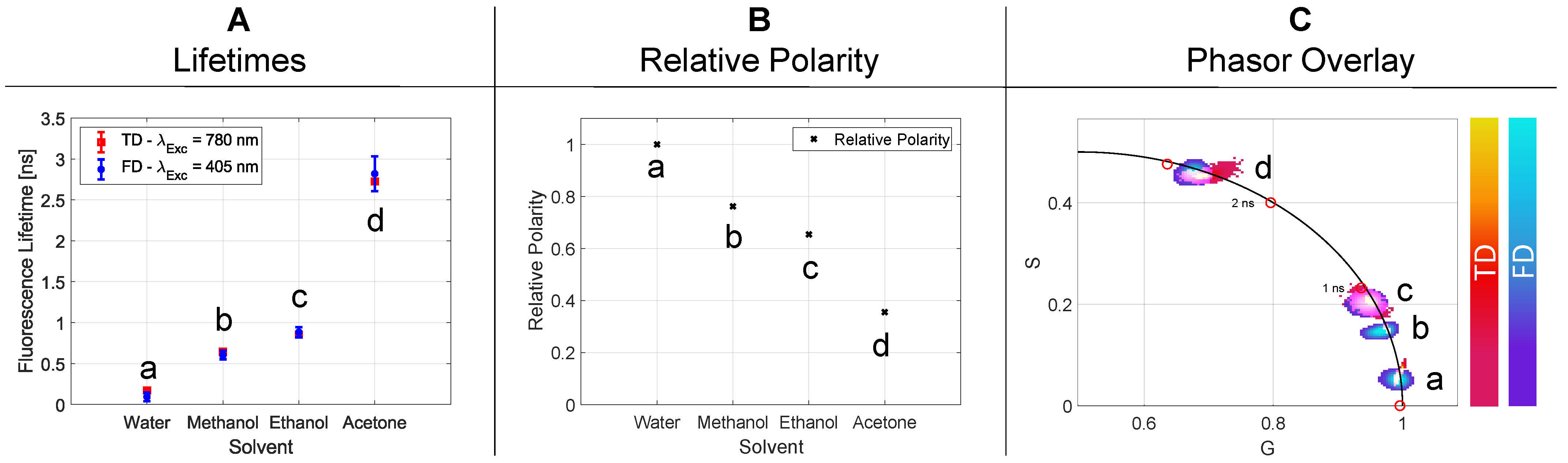
2.2.2. Solvent Polarity
2.2.3. Temperature
2.2.4. pH-Value
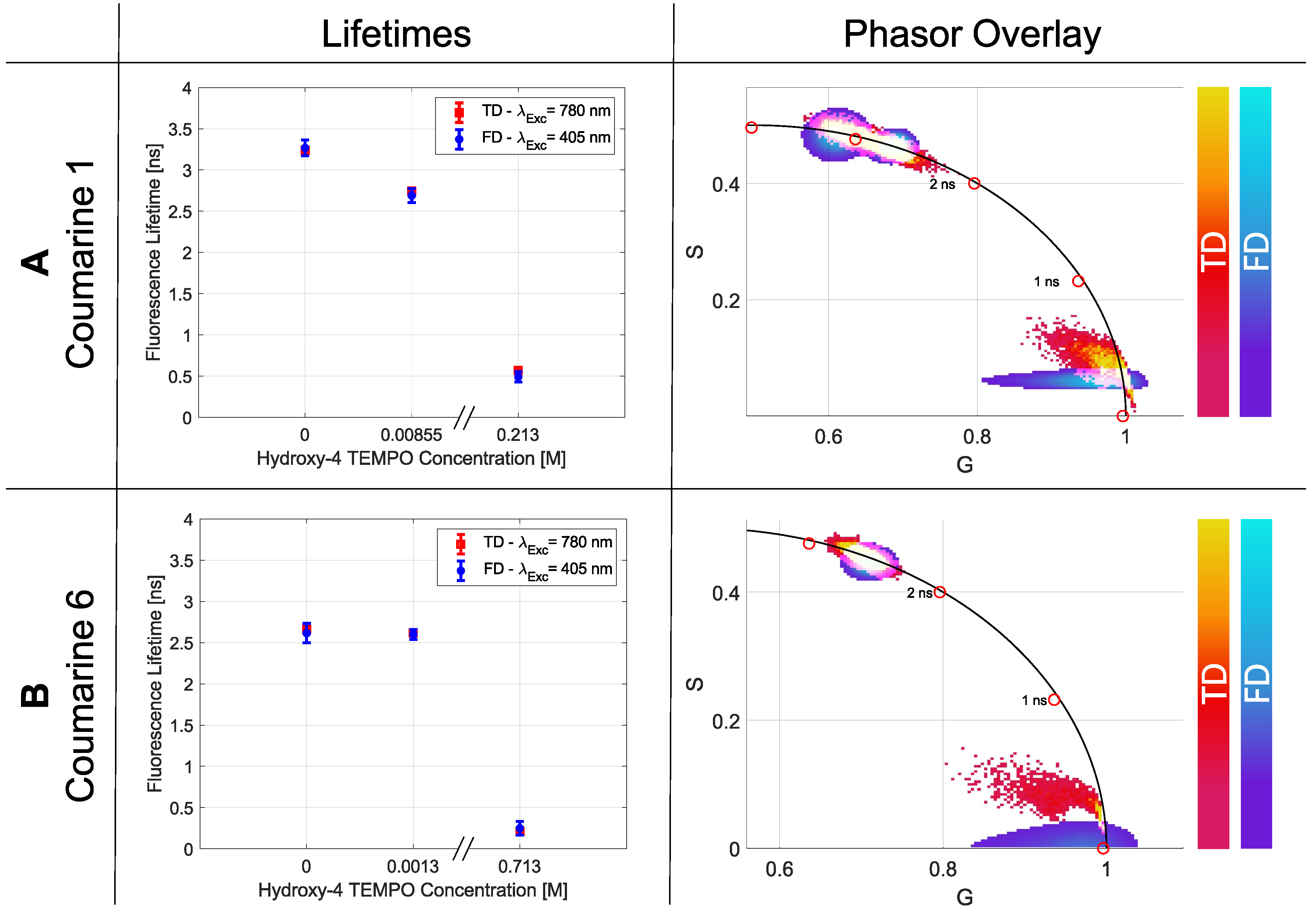
2.2.5. Quencher
3. Discussion
3.1. Setup Dependent Parameter
3.1.1. Wavelength
3.1.2. Modulation Frequency
3.1.3. Integration Time
3.2. Sample Dependent Parameter
3.2.1. Concentration
3.2.2. Solvent Polarity
3.2.3. Temperature
3.2.4. pH-Value
3.2.5. Quencher
3.3. Technique Comparison
4. Materials and Methods
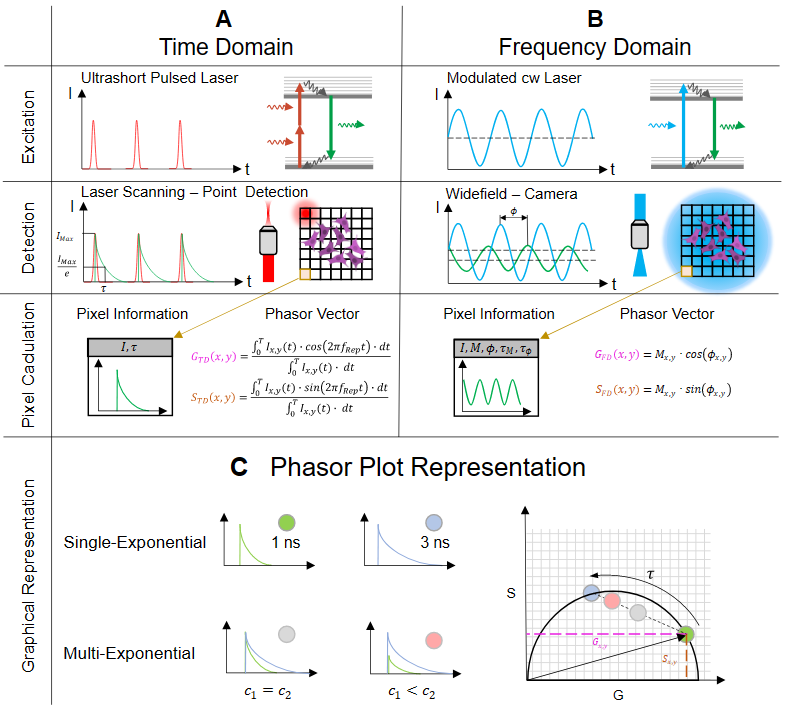
4.1. Theoretical Background
4.1.1. Time-Domain (TD)
4.1.2. Frequency Domain (FD)
4.1.3. Phasor Plot Approach
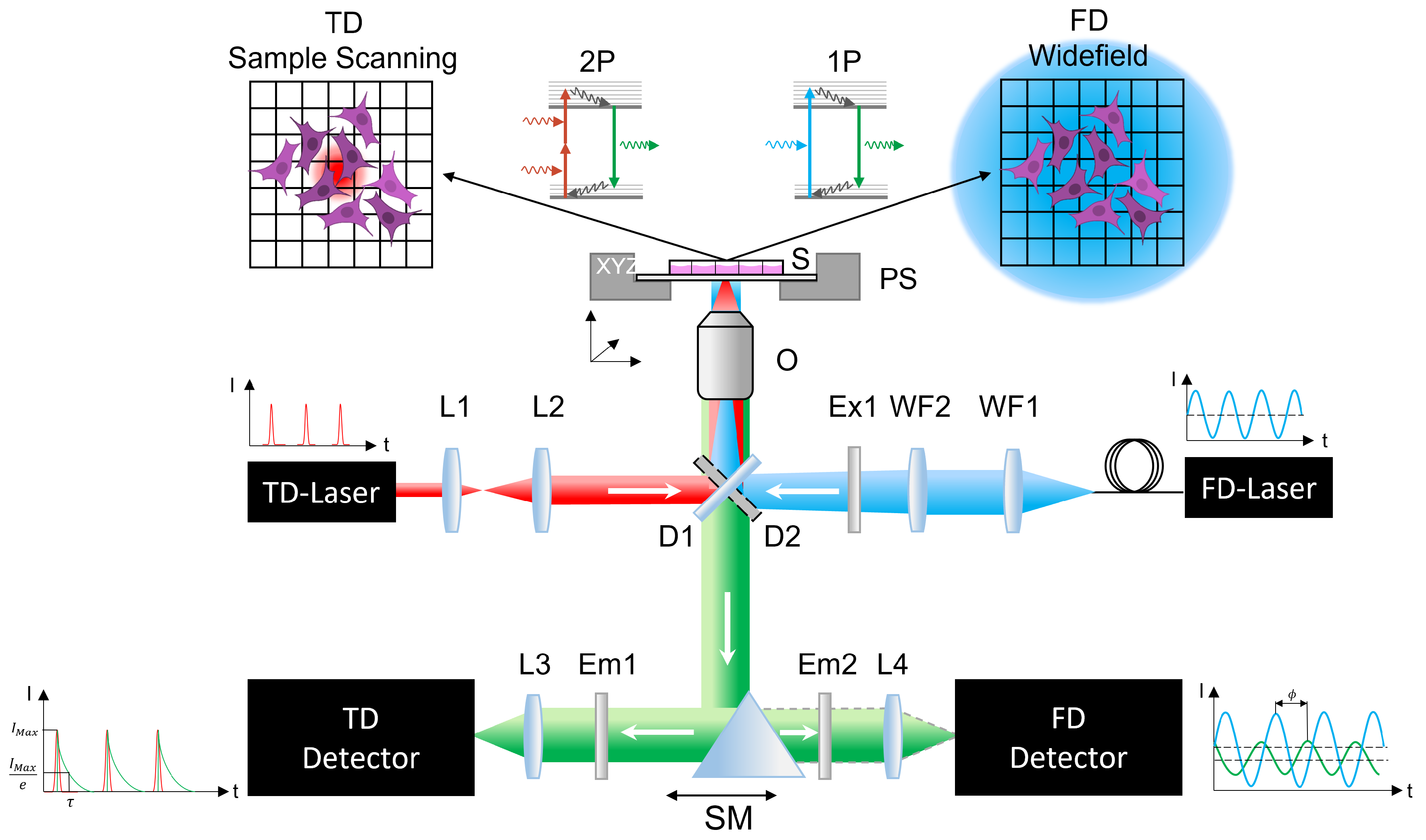
4.2. Experimental Setup
4.2.1. Time-Domain Experiment
4.2.2. Frequency-Domain Setup
4.3. Sample Preparation
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ettinger, A.; Wittmann, T. Fluorescence live cell imaging. Methods Cell Biol. 2014, 123, 77–94. [Google Scholar] [PubMed]
- Freudiger, C.W.; Min, W.; Saar, B.G.; Lu, S.; Holtom, G.R.; He, C.; Tsai, J.C.; Kang, J.X.; Xie, X.S. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 2008, 322, 1857–1861. [Google Scholar] [CrossRef] [PubMed]
- Ries, J.; Schwille, P. Fluorescence correlation spectroscopy. BioEssays 2012, 34, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Elson, E.L.; Magde, D. Fluorescence correlation spectroscopy. I. Conceptual basis and theory. Biopolym. Orig. Res. Biomol. 1974, 13, 1–27. [Google Scholar] [CrossRef]
- Gratton, E.; Breusegem, S.; Sutin, J.D.B.; Ruan, Q.; Barry, N.P. Fluorescence lifetime imaging for the two-photon microscope: Time-domain and frequency-domain methods. J. Biomed. Opt. 2003, 8, 381–390. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef]
- Rubart, M. Two-photon microscopy of cells and tissue. Circ. Res. 2004, 95, 1154–1166. [Google Scholar] [CrossRef]
- Icha, J.; Weber, M.; Waters, J.C.; Norden, C. Phototoxicity in live fluorescence microscopy, and how to avoid it. BioEssays 2017, 39, 1700003. [Google Scholar] [CrossRef]
- Boens, N.; Qin, W.; Basarić, N.; Hofkens, J.; Ameloot, M.; Pouget, J.; Lefevre, J.P.; Valeur, B.; Gratton, E.; VandeVen, M.; et al. Fluorescence lifetime standards for time and frequency domain fluorescence spectroscopy. Anal. Chem. 2007, 79, 2137–2149. [Google Scholar] [CrossRef]
- Casey, K.G.; Quitevis, E.L. Effect of solvent polarity on nonradiative processes in xanthene dyes: Rhodamine B in normal alcohols. J. Phys. Chem. 1988, 92, 6590–6594. [Google Scholar] [CrossRef]
- Gadella Jr, T.W.; Jovin, T.M.; Clegg, R.M. Fluorescence lifetime imaging microscopy (FLIM): Spatial resolution of microstructures on the nanosecond time scale. Biophys. Chem. 1993, 48, 221–239. [Google Scholar] [CrossRef]
- Kristoffersen, A.S.; Erga, S.R.; Hamre, B.; Frette, Ø. Testing fluorescence lifetime standards using two-photon excitation and time-domain instrumentation: Rhodamine B, coumarin 6 and lucifer yellow. J. Fluoresc. 2014, 24, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Fleming, G.; Knight, A.; Morris, J.; Morrison, R.; Robinson, G. Picosecond fluorescence studies of xanthene dyes. J. Am. Chem. Soc. 1977, 99, 4306–4311. [Google Scholar] [CrossRef]
- Periasamy, A.; Wodnicki, P.; Wang, X.F.; Kwon, S.; Gordon, G.W.; Herman, B. Time-resolved fluorescence lifetime imaging microscopy using a picosecond pulsed tunable dye laser system. Rev. Sci. Instruments 1996, 67, 3722–3731. [Google Scholar] [CrossRef]
- Krishnan, R.V.; Masuda, A.; Centonze, V.F.E.; Herman, B.A. Quantitative imaging of protein–protein interactions by multiphoton fluorescence lifetime imaging microscopy using a streak camera. J. Biomed. Opt. 2003, 8, 362–367. [Google Scholar] [CrossRef]
- Lakowicz, J. Principles of Fluorescence Spectroscopy, 2nd ed.; Plenum: New York, NY, USA, 1999. [Google Scholar]
- Andrade, J.; Estévez-Pérez, M. Statistical comparison of the slopes of two regression lines: A tutorial. Anal. Chim. Acta 2014, 838, 1–12. [Google Scholar] [CrossRef]
- Glasgow, B.J. Fluorescence lifetime imaging microscopy reveals quenching of fluorescein within corneal epithelium. Exp. Eye Res. 2016, 147, 12–19. [Google Scholar] [CrossRef]
- Love, L.C.; Upton, L.M.; Ritter, A.W. Solvent effects on fluorescence spectra decay times and quantum yields of atabrine and its homologs. Anal. Chem. 1978, 50, 2059–2064. [Google Scholar] [CrossRef]
- Freymüller, C.; Kalinina, S.; Rück, A.; Sroka, R.; Rühm, A. Quenched coumarin derivatives as fluorescence lifetime phantoms for NADH and FAD. J. Biophoton. 2021, 14, e202100024. [Google Scholar] [CrossRef]
- Hilborn, R.C. Einstein coefficients, cross sections, f values, dipole moments, and all that. Am. J. Phys. 1982, 50, 982–986. [Google Scholar] [CrossRef]
- Xu, C.; Webb, W.W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. JOSA B 1996, 13, 481–491. [Google Scholar] [CrossRef]
- Shibasaki, Y.; Suenobu, T.; Nakagawa, T.; Katoh, R. Effect of reabsorption of fluorescence on transient absorption measurements. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 220, 117127. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa-Ankerhold, H.C.; Ankerhold, R.; Drummen, G.P. Advanced fluorescence microscopy techniques—Frap, Flip, Flap, Fret and flim. Molecules 2012, 17, 4047–4132. [Google Scholar] [CrossRef] [PubMed]
- Wallrabe, H.; Periasamy, A. Imaging protein molecules using FRET and FLIM microscopy. Curr. Opin. Biotechnol. 2005, 16, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Loving, G.S.; Sainlos, M.; Imperiali, B. Monitoring protein interactions and dynamics with solvatochromic fluorophores. Trends Biotechnol. 2010, 28, 73–83. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Lee, H.; Akers, W.; Achilefu, S. Near infrared dyes as lifetime solvatochromic probes for micropolarity measurements of biological systems. Biophys. J. 2007, 93, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Spange, S.; Reuter, A.; Vilsmeier, E. On the determination of polarity parameters of silica by means of solvatochromic probe dyes. Colloid Polym. Sci. 1996, 274, 59–69. [Google Scholar] [CrossRef]
- Xiao, H.; Li, P.; Tang, B. Recent progresses in fluorescent probes for detection of polarity. Coord. Chem. Rev. 2021, 427, 213582. [Google Scholar] [CrossRef]
- Jue, T. Fundamental Concepts in Biophysics; Springer: Cham, Switzerland, 2009; Volume 1. [Google Scholar]
- Okabe, K.; Inada, N.; Gota, C.; Harada, Y.; Funatsu, T.; Uchiyama, S. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat. Commun. 2012, 3, 705. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, H.; He, S.; Zeng, H.; Pralle, A. Monodisperse magnetofluorescent nanoplatforms for local heating and temperature sensing. Nanoscale 2014, 6, 13463–13469. [Google Scholar] [CrossRef]
- Lee, J.; Kim, B.; Park, B.; Won, Y.; Kim, S.Y.; Lee, S. Real-time cancer diagnosis of breast cancer using fluorescence lifetime endoscopy based on the pH. Sci. Rep. 2021, 11, 16864. [Google Scholar] [CrossRef] [PubMed]
- Burgstaller, S.; Bischof, H.; Gensch, T.; Stryeck, S.; Gottschalk, B.; Ramadani-Muja, J.; Eroglu, E.; Rost, R.; Balfanz, S.; Baumann, A.; et al. pH-Lemon, a fluorescent protein-based pH reporter for acidic compartments. ACS Sensors 2019, 4, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, C. Joseph R. Lakowicz: Principles of Fluorescence Spectroscopy; Springer: Cham, Switzerland, 2008. [Google Scholar]
- Förster, T. Zwischenmolekulare energiewanderung und fluoreszenz. Ann. Der. Phys. 1948, 437, 55–75. [Google Scholar] [CrossRef]
- Doose, S.; Neuweiler, H.; Sauer, M. Fluorescence quenching by photoinduced electron transfer: A reporter for conformational dynamics of macromolecules. ChemPhysChem 2009, 10, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Parrish, J.A. The optics of human skin. J. Investig. Dermatol. 1981, 77, 13–19. [Google Scholar] [CrossRef]
- Conchello, J.A.; Lichtman, J.W. Optical sectioning microscopy. Nat. Methods 2005, 2, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Scipioni, L.; Wright, B.K.; Bartolec, T.K.; Zhang, J.; Masamsetti, V.P.; Gaus, K.; Gratton, E.; Cesare, A.J.; Hinde, E. Phasor histone FLIM-FRET microscopy quantifies spatiotemporal rearrangement of chromatin architecture during the DNA damage response. Proc. Natl. Acad. Sci. USA 2019, 116, 7323–7332. [Google Scholar] [CrossRef]
- Ranjit, S.; Datta, R.; Dvornikov, A.; Gratton, E. Multicomponent analysis of phasor plot in a single pixel to calculate changes of metabolic trajectory in biological systems. J. Phys. Chem. A 2019, 123, 9865–9873. [Google Scholar] [CrossRef]
- Orthaus-Mueller, S.; Kraemer, B.; Dowler, R.; Devaux, A.; Tannert, A.; Roehlicke, T.; Wahl, M.; Rahn, H.J.; Erdmann, R. RapidFLIM: The new and innovative method for ultra fast flim imaging. PicoQuant Appl. Note 2017, 2017, 1–8. [Google Scholar]
- Chen, H.; Holst, G.; Gratton, E. Modulated CMOS camera for fluorescence lifetime microscopy. Microsc. Res. Tech. 2015, 78, 1075–1081. [Google Scholar] [CrossRef]
- Esposito, A.; Oggier, T.; Gerritsen, H.; Lustenberger, F.; Wouters, F. All-solid-state lock-in imaging for wide-field fluorescence lifetime sensing. Opt. Express 2005, 13, 9812–9821. [Google Scholar] [CrossRef] [PubMed]
- Verveer, P.J.; Squire, A.; Bastiaens, P.I. Global analysis of fluorescence lifetime imaging microscopy data. Biophys. J. 2000, 78, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Franke, R.; Holst, G.A. Frequency-domain fluorescence lifetime imaging system (pco. flim) based on a in-pixel dual tap control CMOS image sensor. Imaging Manip. Anal. Biomol. Cells Tissues 2015, 9328, 241–259. [Google Scholar]
- Ranjit, S.; Malacrida, L.; Jameson, D.M.; Gratton, E. Fit-free analyss of fluorescence lifetime imaging data using the phasor approach. Nat. Protoc. 2018, 13, 1979–2004. [Google Scholar] [CrossRef]
- Sun, Y.; Liao, S.C. The Ultimate Phasor Plot and Beyond; ISS Inc.: San Antonio, TX, USA, 2014. [Google Scholar]
- Zhang, Y.; Hato, T.; Dagher, P.C.; Nichols, E.L.; Smith, C.J.; Dunn, K.W.; Howard, S.S. Automatic segmentation of intravital fluorescence microscopy images by K-means clustering of FLIM phasors. Opt. Lett. 2019, 44, 3928–3931. [Google Scholar] [CrossRef]
- Mannam, V.; Zhang, Y.; Yuan, X.; Ravasio, C.; Howard, S.S. Machine learning for faster and smarter fluorescence lifetime imaging microscopy. J. Phys. Photon. 2020, 2, 042005. [Google Scholar] [CrossRef]
- Datta, R.; Heylman, C.; George, S.C.; Gratton, E. Label-free imaging of metabolism and oxidative stress in human induced pluripotent stem cell-derived cardiomyocytes. Biomed. Opt. Express 2016, 7, 1690–1701. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Datta, R.; Gratton, E.; Hochbaum, A.I. Metabolic fingerprinting of bacteria by fluorescence lifetime imaging microscopy. Sci. Rep. 2017, 7, 3743. [Google Scholar] [CrossRef]
- Martin, M.M.; Lindqvist, L. The pH dependence of fluorescein fluorescence. J. Lumin. 1975, 10, 381–390. [Google Scholar] [CrossRef]
- Krzywinski, M.; Altman, N. Significance, p values and t-tests. Nat. Methods 2013, 10, 1041–1042. [Google Scholar] [CrossRef]
- Montero Llopis, P.; Senft, R.A.; Ross-Elliott, T.J.; Stephansky, R.; Keeley, D.P.; Koshar, P.; Marqués, G.; Gao, Y.S.; Carlson, B.R.; Pengo, T.; et al. Best practices and tools for reporting reproducible fluorescence microscopy methods. Nat. Methods 2021, 18, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yang, Z.; Wang, J.; Fan, J.; He, Y.; Song, F.; Wang, B.; Sun, S.; Qu, J.; Qi, J.; et al. Fluorescence ratiometry and fluorescence lifetime imaging: Using a single molecular sensor for dual mode imaging of cellular viscosity. J. Am. Chem. Soc. 2011, 133, 6626–6635. [Google Scholar] [CrossRef] [PubMed]


| Modulation Frequency | ||||||
|---|---|---|---|---|---|---|
| 1 MHz | 10 MHz | 20 MHz | 30 MHz | 40 MHz | Overall p-Value | |
| [ns] | 0.468 | 0.599 | 0.613 | 0.628 | 0.645 | 0.048 |
| [ns] | 1.387 | 0.056 | 0.053 | 0.04 | 0.052 | |
| [ns] | 0.501 | 0.589 | 0.602 | 0.631 | 0.645 | 0.046 |
| [ns] | 1.499 | 0.055 | 0.055 | 0.072 | 0.071 | |
| Single p-Values | 0.063 | 0.034 | 0.021 | 0.027 | 0 | |
| Integration Time (TD) | |||||||
|---|---|---|---|---|---|---|---|
| 5 s | 10 s | 20 s | 30 s | Overall p-Value | |||
| [ns] | 0.842 | 0.859 | 0.875 | 0.856 | 0.024 | ||
| [ns] | 0.020 | 0.015 | 0.013 | 0.011 | |||
| Exposure Time (FD) | |||||||
| 5 ms | 10 ms | 50 ms | 100 ms | 200 ms | 300 ms | Overall p-Value | |
| [ns] | 0.852 | 0.897 | 0.852 | 0.847 | 0.832 | 0.495 | 0.010 |
| [ns] | 0.181 | 0.166 | 0.180 | 0.348 | 0.368 | 1.636 | |
| [ns] | 0.832 | 0.895 | 0.842 | 0.877 | 0.904 | 0.595 | 0.038 |
| [ns] | 0.190 | 0.169 | 0.200 | 0.377 | 0.480 | 1.712 | |
| Single p-Values | 0.001 | 0.001 | 0.020 | 0.042 | 0.049 | 0.053 | |
| Lucifer Yellow | |||||
|---|---|---|---|---|---|
| Concentration | 0.00001 M | 0.0001 M | 0.001 M | 0.01 M | 0.1 M |
| [ns] | 8.837 | 11.476 | 11.087 | 10.265 | 9.907 |
| [ns] | 1.027 | 0.565 | 0.221 | 0.053 | 0.049 |
| [ns] | 10.532 | 10.646 | 10.294 | 10.288 | 9.685 |
| [ns] | 1.020 | 0.647 | 0.506 | 0.267 | 0.298 |
| Single p-Value | 0.052 | 0.499 | 0.045 | 0.042 | 0.049 |
| Fluorescein | |||||
| Concentration | 0.00001 M | 0.0001 M | 0.001 M | 0.01 M | 0.1 M |
| [ns] | 4.015 | 4.049 | 4.212 | 3.176 | 0.209 |
| [ns] | 0.064 | 0.025 | 0.018 | 0.028 | 0.002 |
| [ns] | 3.356 | 3.623 | 4.336 | 3.520 | 0.380 |
| [ns] | 0.203 | 0.115 | 0.075 | 0.045 | 0.106 |
| Single p-Value | 0.062 | 0.051 | 0.034 | 0.021 | 0.039 |
| Figure Declaration | Solvent | Relative Polarity | [ns] | [ns] | [ns] | [ns] | Single p-Value |
|---|---|---|---|---|---|---|---|
| a | Water | 1 | 0.127 | 0.014 | 0.093 | 0.052 | 0.050 |
| b | Methanol | 0.762 | 0.645 | 0.012 | 0.605 | 0.052 | 0.045 |
| c | Ethanol | 0.654 | 0.852 | 0.011 | 0.883 | 0.062 | 0.039 |
| d | Aceton | 0.355 | 2.725 | 0.014 | 2.819 | 0.214 | 0.021 |
| Fluorescein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature [ C] | 23 | 25 | 27 | 29 | 31 | 33 | 35 | 37 | 39 |
| [ns] | 3.857 | 3.813 | 3.830 | 3.769 | 3.789 | 3.783 | 3.781 | 3.796 | 3.763 |
| [ns] | 0.110 | 0.107 | 0.113 | 0.112 | 0.115 | 0.112 | 0.111 | 0.111 | 0.0122 |
| [ns] | 3.859 | 3.833 | 3.834 | 3.832 | 3.828 | 3.823 | 3.815 | 3.816 | 3.816 |
| [ns] | 0.110 | 0.110 | 0.120 | 0.130 | 0.130 | 0.120 | 0.110 | 0.120 | 0.120 |
| Single p-Values | 0.016 | 0.038 | 0.018 | 0.041 | 0.038 | 0.038 | 0.034 | 0.021 | 0.040 |
| Fluorescein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH-Value | 3 | 4.5 | 7.5 | 7.8 | 8 | 8.2 | 9.3 | 11.2 | 12 |
| [ns] | 3.107 | 3.330 | 3.944 | 3.945 | 4.144 | 4.154 | 4.345 | 4.424 | 4.514 |
| [ns] | 0.084 | 0.084 | 0.076 | 0.053 | 0.038 | 0.038 | 0.038 | 0.039 | 0.042 |
| pH-Value | 3 | 4.5 | 6.5 | 7 | 7.5 | 8 | 8.5 | 10.5 | 12 |
| [ns] | 3.104 | 3.303 | 3.565 | 3.832 | 3.885 | 3.985 | 4.327 | 4.445 | 4.615 |
| [ns] | 0.108 | 0.127 | 0.146 | 0.189 | 0.134 | 0.162 | 0.226 | 0.168 | 0.155 |
| Coumarine 1 | Coumarine 6 | |||||
|---|---|---|---|---|---|---|
| 4-hydroxy TEMPO | 0 M | 0.00855 M | 0.234 M | 0 M | 0.0013 M | 0.713 M |
| [ns] | 3.239 | 2.745 | 0.569 | 2.663 | 2.617 | 0.209 |
| [ns] | 0.017 | 0.016 | 0.011 | 0.013 | 0.014 | 0.002 |
| [ns] | 3.268 | 2.689 | 0.492 | 2.615 | 2.599 | 0.25 |
| [ns] | 0.097 | 0.086 | 0.063 | 0.118 | 0.060 | 0.082 |
| Single p-Value | 0.031 | 0.041 | 0.050 | 0.022 | 0.044 | 0.061 |
| Number | Fluorophore | Distributor | Article No. | Molar Weight (g/mol) | Concentration (M) |
|---|---|---|---|---|---|
| 1 | Rose Bengal | Sigma Aldrich | 330000 | 1017.64 | 10, 10, 10, 10 |
| 2 | Lucifer Yellow | Sigma Aldrich | LO144 | 521.57 | 10, 10, 10, 10, 810 |
| 3 | Fluorescein | Sigma Aldrich | 46955 | 332.31 | 10, 10, 10, 10 |
| 4 | Coumarin 1 | Sigma Aldrich | D87759 | 231.29 | 10, 10, 10, 10 |
| 5 | Coumarin 6 | Sigma Aldrich | 546283 | 350.43 | 10, 10, 10, 10 |
| Solvent | Distributor | Article No. | Fluorophores |
|---|---|---|---|
| Water | Merck | 1.15333 | 1, 2, 3 |
| Methanol | Merck | 1.06002 | 1, 2, 3, 4, 5 |
| Ethanol | Merck | 1.0098 | 1, 2 |
| Aceton | Sigma Aldrich | 270725-1L | 1, 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kellerer, T.; Janusch, J.; Freymüller, C.; Rühm, A.; Sroka, R.; Hellerer, T. Comprehensive Investigation of Parameters Influencing Fluorescence Lifetime Imaging Microscopy in Frequency- and Time-Domain Illustrated by Phasor Plot Analysis. Int. J. Mol. Sci. 2022, 23, 15885. https://doi.org/10.3390/ijms232415885
Kellerer T, Janusch J, Freymüller C, Rühm A, Sroka R, Hellerer T. Comprehensive Investigation of Parameters Influencing Fluorescence Lifetime Imaging Microscopy in Frequency- and Time-Domain Illustrated by Phasor Plot Analysis. International Journal of Molecular Sciences. 2022; 23(24):15885. https://doi.org/10.3390/ijms232415885
Chicago/Turabian StyleKellerer, Thomas, Janko Janusch, Christian Freymüller, Adrian Rühm, Ronald Sroka, and Thomas Hellerer. 2022. "Comprehensive Investigation of Parameters Influencing Fluorescence Lifetime Imaging Microscopy in Frequency- and Time-Domain Illustrated by Phasor Plot Analysis" International Journal of Molecular Sciences 23, no. 24: 15885. https://doi.org/10.3390/ijms232415885
APA StyleKellerer, T., Janusch, J., Freymüller, C., Rühm, A., Sroka, R., & Hellerer, T. (2022). Comprehensive Investigation of Parameters Influencing Fluorescence Lifetime Imaging Microscopy in Frequency- and Time-Domain Illustrated by Phasor Plot Analysis. International Journal of Molecular Sciences, 23(24), 15885. https://doi.org/10.3390/ijms232415885










