Abstract
Knee osteoarthritis (OA) is one of the most multifactorial joint disorders in adults. It is characterized by degenerative and inflammatory processes that are responsible for joint destruction, pain and stiffness. Despite therapeutic advances, the search for alternative strategies to target inflammation and pain is still very challenging. In this regard, there is a growing body of evidence for the role of several bioactive dietary molecules (BDMs) in targeting inflammation and pain, with promising clinical results. BDMs may be valuable non-pharmaceutical solutions to treat and prevent the evolution of early OA to more severe phenotypes, overcoming the side effects of anti-inflammatory drugs. Among BDMs, polyphenols (PPs) are widely studied due to their abundance in several plants, together with their benefits in halting inflammation and pain. Despite their biological relevance, there are still many questionable aspects (biosafety, bioavailability, etc.) that hinder their clinical application. This review highlights the mechanisms of action and biological targets modulated by PPs, summarizes the data on their anti-inflammatory and anti-nociceptive effects in different preclinical in vitro and in vivo models of OA and underlines the gaps in the knowledge. Furthermore, this work reports the preliminary promising results of clinical studies on OA patients treated with PPs and discusses new perspectives to accelerate the translation of PPs treatment into the clinics.
1. Introduction
Knee osteoarthritis (OA) is a complex clinical disorder that affects multiple joint tissues, including cartilage, subchondral bone, meniscus and synovium, with a tremendous impact on patients’ quality of life [1]. Metabolic alterations associated with obesity and diabetes, along with ageing and injuries, are among the main risk factors for OA. This heterogeneous framework reflects the variety of phenotypes in OA patients, which contributes to limiting the success of clinical trials on emerging drugs [2]. Joint inflammation is one of the main hallmarks of OA and, together with other pathological processes, ultimately leads to a disruption of joint homeostasis and biomechanics and causes joint pain and stiffness. The main goal of the treatments for knee OA is to act at an early stage in order to halt these degenerative and inflammatory aspects and improve mobility and function. Most of the current strategies include non-steroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase-2 (COX-2) inhibitors and intra-articular (IA) treatments with anti-inflammatory agents, viscosupplements and biologics agents, which provide only partial and temporary pain relief and entail several limitations and risks [3]. Therefore, there is an urgent need to find new, safer alternatives to counteract joint inflammation, degeneration and pain. Recently, bioactive dietary molecules (BDMs) have emerged as potential preventive and therapeutic options to complement conventional treatments in OA [4,5]. Indeed, several studies have emphasized their pleiotropic effects in modulating specific pathogenetic pathways implicated in joint dysfunction. In particular, BDMs belonging to the polyphenols (PPs) family have been described to modulate inflammatory [6] and pain-associated biological targets, making them good candidates to treat symptomatic OA [7,8]. Most of the evidence of their protective role comes from preclinical studies, while the number of clinical studies remains limited [9,10].
This review aims to provide an overview of past and current experimental research focusing on the anti-inflammatory and anti-nociceptive effects of PPs in OA by highlighting their molecular mechanisms of action and the data on their bioavailability, efficacy and safety (when available). Critical gaps in the knowledge and safety of PPs are discussed in order to guide future research and clinical efforts. Finally, this work examines the challenges and future perspectives to improve their applications and biological performance to treat OA.
Literature Search Strategy
PubMed and Web of Science literature searches were performed with the following keywords: “knee osteoarthritis,” “inflammation,” “pain,” “natural compounds,” “nutraceuticals” and “polyphenols”. Despite their biological relevance, articles focusing on extracts were excluded.
2. OA Description: Focus on Inflammation and Pain
2.1. Molecular Signalling Pathways during Inflammation in Knee OA
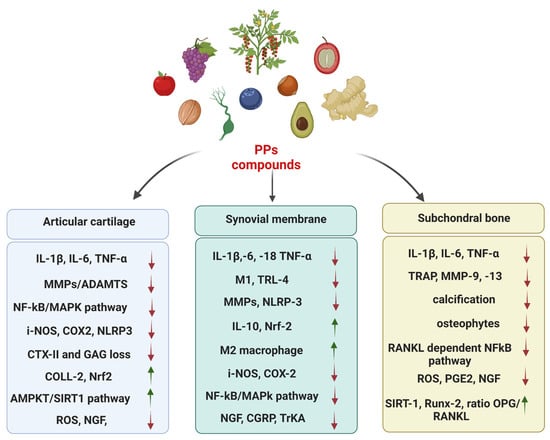
Despite the long-standing definition of OA as a wear disorder, the number of studies focusing on the link between inflammation and OA has been rapidly growing [11]. In this context, the interplay between cartilage and synovium plays a key role in fueling inflammatory responses by boosting the release of inflammatory cytokines, which are directly implicated in joint damage. Another key player and driver of inflammation in OA is the impairment of the immune system [12], particularly the imbalance of M1/M2 macrophages. The up-regulation of the M1 subset has harmful effects due to the synthesis of pro-inflammatory mediators and the release of tissue debris into the synovial cavity [13]. Moreover, this phlogistic circuit has important consequences on the subchondral bone via the up-regulation of osteoclastogenesis, bone resorption and angiogenesis [14]. The inflammation evokes catabolic responses, which result in the decrease in specific markers of cartilage homeostasis, i.e., collagen II, aggrecan, glycosaminoglycans (GAGs), SRY-box transcription factor 9 (Sox-9), β-integrins; and up-regulation of fibrotic and hypertrophic markers, i.e., collagen I, collagen X, runt-related transcription factor 2 (Runx-2), vascular endothelial growth factor (VEGF)-A, matrix metalloproteinase (MMP)-13.
In OA, one of the first pathological changes is the metabolic activation of chondrocytes and their phenotypic shift towards a degradative and hypertrophic-like state. These alterations determine the synthesis of inflammatory mediators and cartilage-degrading enzymes involved in the release of extracellular matrix (ECM) fragments from cartilage, known as damage-associated molecular patterns (DAMPs) [15,16]. Forms of DAMPs present in the OA joint include: (i) alarmins, i.e., high-mobility group box-1 (HMGB1) and S100 proteins; (ii) crystals, i.e., calcium pyrophosphate dihydrate (CPPD) and basic calcium phosphate (BCP). DAMPs interact with different pattern recognition receptors (PRRs): (i) toll-like receptors (TLRs) such as TLR2, and TLR4; (ii) NOD-like receptors (NLRs); and (iii) the receptor for advanced glycosylation end products (RAGEs) [17]. Their binding induces the synthesis of: (i) inflammatory cytokines such as interleukin (IL)-1β (IL-1β), tumor necrosis factor-α (TNF-α), IL-6/-8; and (ii) catabolic mediators (proteases) such as MMP-1,-3,-13, and a disintegrin and metalloprotease with thrombospondin motifs (ADAMTs)-4,-5. Among TLRs, TLR4 is overexpressed in chondrocytes from OA patients more than in non-OA patients [18]. Their binding with ligands induces the translocation of NF-κB into the nucleus, the activation of the MyD88 and the TIR domain-containing adaptor-inducing interferon-β (TRIF) pathways, resulting in the induction of inflammatory responses [19]. The imbalance between MMPs and their negative regulators (i.e., TIMPs, CITED2) is also implicated in cartilage breakdown in OA patients.
Other inflammatory mediators associated with OA include: nitric oxide (NO), catalyzed by inducible NO synthase (iNOS); cyclooxygenase-2 (COX-2), encoded by prostaglandin-endoperoxide synthase 2 (PTGS2); prostaglandin E2 (PGE2); and chemokines such as monocyte chemoattractant protein-1 (MCP-1, also called CCL2), C-C Motif Chemokine Ligand (CCL5); and C-X-C Motif Chemokine Ligand (CXCL)-1 [20].
In addition to inflammation, the production of reactive oxygen species (ROS) is closely associated with the evolution of the severity of OA. NADPH oxidase (NOX) is up-regulated during OA in chondrocytes, which is the major producer of ROS, especially hydrogen peroxide (H2O2) [21]. H2O2 induces mitochondrial damage, lipid peroxidation, and DNA damage, leading to the inhibition of ECM synthesis, ECM degradation, chondrocyte apoptosis, and inflammatory cytokines overproduction, which further leads to MMP formation. In turn, lipid peroxidation (of which MDA is a common indicator) activates the nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome by amplifying the inflammatory circuit via the release of IL-1β and IL-18. Notably, in OA synoviocytes, MDA is increased, while GPX, an anti-oxidant agent, is decreased [22]. Poly (ADP-ribose) polymerase (PARP)-1 is a nuclear enzyme activated during apoptosis (downstream to caspase-3 activation and DNA strand breaks), which plays a crucial role in oxidative stress-induced inflammation. It regulates the production of several inflammatory molecules, including transcription factors, cytokines, chemokines, COX-2, and iNOS [23]. Corroborating the link between ROS and inflammation in OA is the increasing evidence that NRF2, widely renowned as the master gene of anti-oxidant response, plays a pivotal role in the protection of joint cartilage during OA pathogenesis by modulating inflammation [24]. Nrf2 was found to be decreased after IL-1β stimulation [25] and in OA articular cartilage, along with glutathione s-transferase alpha (GSTA)-4, predisposing to cartilage degradation and synovium inflammation increasing hydroxynonenal (HNE) production [26]. Moreover, heme oxygenase (HO)-1, a downstream factor of Nrf2, is a critical factor in Nrf2-mediated NF-κB inhibition [27].
Several signaling pathways are implicated in inflammation during OA, in particular: (i) NF-κB; (ii) phosphoinositide 3-kinase/protein kinase B (PI3K/AKT); and (iii) mitogen-activated protein kinase (MAPK). The NF-κB pathway is widely recognized as the most important signaling regulating inflammation. It can be activated by several cytokines (TNF-α and IL1-β) and other signaling pathways (e.g., MAPK, Nrf2 signaling). The NF-κB protein normally forms a complex with a nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha protein (IκBα), which keeps it in an inactive state in the cytoplasm. During inflammation, the IκB kinase (IKK) complex phosphorylates IκB proteins (p65 NFkB, IκBα) and IκBα undergoes proteasomal degradation. The resulting activation of the NF-κB complex leads to the transcription of the genes involved in inflammation (i.e., immunomodulatory molecules, cytokines, COX-2, MMPs, and iNOS) and the imbalance between anabolism and catabolism [28,29]. In addition to its effects on inflammation, NF-κB can also modulate the expression of proteins implicated in apoptosis, such as c-caspase3, Cyto-c, Bax and Bcl-2, with contrasting literature about its role [30].
PI3K/Akt/mTOR signaling can promote both the anabolic and catabolic pathways via the activation of distinct downstream effectors, among which AKT and mammalian target the rapamycin complex 1 (mTORC1) [31]. The MAPK are intracellular Ser/Thr kinases classified into four sub-families: (i) extracellular signal-regulated kinase (ERK 1 and 2); (ii) c-Jun NH2-terminal kinase (JNK 1,2, and 3); (iii) p38 (α, β, γ, δ); and (iv) ERK5. MAPK has a crucial role in modulating multiple pathways implicated in joint destruction.
Several epigenetic factors (induced by various environmental factors i.e., pollutants, diet, exercise, stress) have been identified as playing a key role in OA inflammation and pain. They include DNA methylation, histone modifications and non-coding RNAs (i.e., small interfering RNA, miRNA, and long non-coding RNA) [21,32]. A growing body of research reported altered DNA methylation associated with specific genes involved in OA, such as MMP-3, -9, -13, and ADATMS-4 [33]. In this line, the aberrant expression of miRNAs, in terms of up-regulation (i.e.,miR146, miR34a, miR155, etc.) or down-regulation (i.e., miR124, miR-130, miR-140, miR-210, etc.), was documented in OA [34]. Similarly, histone-modifying proteins display important roles in the pathogenesis and progression of OA. In particular, sirtuins (SIRT-1,2,3,4,5,6,7) belonging to class III of histone deacetylases (HDACs) have a great impact in regulating chondrocyte differentiation and functions [35]. The silent information regulator of transcription 1 (SIRT1) is a longevity gene involved in the deacetylation of histones and transcription factors with pleiotropic activity [36]. Extremely relevant in the context of OA, SIRT1 is a negative regulator of inflammation, and in particular, inhibiting the P38-mediated inflammatory signaling pathway. The TLR4/MyD88/NF-κB signaling pathway reduces multiple proinflammatory cytokines and chemokines. Notably, the expression of the SIRT1 is poorly expressed in OA patients [37] and it is cleaved and inactivated following the treatment of chondrocytes with inflammatory stimuli [38,39,40].
Regarding the ageing and metabolic phenotypes of OA, patients display persistent low-grade systemic inflammation. During inflammaging, OA patients display increased levels of advanced glycation end-products (AGEs) and phlogistic mediators, which generate a senescence-associated secretory phenotype (SASP) [14]. Notably, ageing reduces the expression of HO-1, thus generating a reduction in anti-oxidant defenses and a pro-inflammatory tendency which generates ROS and MMPs, causing cartilage ECM degradation and joint dysfunction [41].
During obesity, metabolic dysfunction is the main pathophysiologic factor linked to inflammation (meta-inflammation). In this regard, several adipose-tissue-derived cytokines (adipokines) are secreted, among which leptin, which activates the JAK-STAT3 signaling pathway, along with increased expression of the interleukin-1 receptor-associated kinase (IRAK)-1 and IRAK4, activate tumor necrosis factor receptor-associated factor 6 (TRAF6) and NF-κB [28].
Recently, a body of research gave evidence of the presence of a gut-joint axis in the OA pathogenesis due to a disruption of gut homeostasis with the display of an inflammatory phenotype (dysbiosis), causing an alteration to the microbiota composition. The translocation of bacteria toward the joint would seem to be implicated in dysbiosis and contribute to the pathogenesis of OA, even if further investigations are still necessary [42].
Overall, the molecular understanding of factors driving OA is key to identifying alternative candidates for targeting inflammatory pathways as potential novel disease-modifying therapies to treat OA.
2.2. Biological Basis of Pain in Knee OA
Severe joint pain is one of the main symptoms of OA, limiting simple daily activities and negatively affecting patients’ mental well-being with a consequent impact on their quality of life. In the clinic, the most commonly used validated criteria to assess pre- and post-treatment of pain in OA include the visual analogue scale (VAS), the Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain subscale, and the Knee injury and Osteoarthritis Outcome Score (KOOS) [43]. These scores are aimed to track how OA patients experience pain in their daily (i.e., walking, using stairs, in bed, etc.), sports and recreational activities. Despite their widespread use, none of them can adequately describe the complex pain experienced by OA patients nor guide analgesic therapies [43]. The main structural changes associated with pain in OA, identified by MRI studies, include bone marrow lesions, synovitis, knee effusion, periarticular lesions, meniscal tears, and bone lesions [44].
Depending on the type of stimuli, pain is classified into three types: nociceptive, neuropathic and inflammatory. The aetiology of OA-related pain is still poorly understood for the relative paucity of mechanistic studies, even if the pathogenesis of pain is primarily thought to be inflammatory.
Nociceptive pain is a typical response of the nervous system to noxious stimuli, particularly following physical/mechanical injuries of tissues or chemical stimuli (i.e., heat, chemicals, inflammation, pressure). This is an acute form of pain, which resolves after tissue healing or the removal of noxious stimuli. Joint nociceptors (pain-sensing afferent neurons) are primarily located in the bone [45], synovial membrane [46], menisci [47] and soft tissues [48], while they are not present in the cartilage, which lacks nerve innervation unless ectopically innervated [49]. In response to noxious stimuli, nociceptor activation leads to the transduction of pain signals through interactions between the immune and nervous systems and the release of inflammatory mediators and neuropeptides [50]. There are several nociceptor-specific mediators involved in joint pain [51]. Among these, transient receptor potential (TRP) channels (ion channel receptors) mediate pain sensations by stimulating the neural secretion of calcitonin gene-related peptide (CGRP) and substance P (SP) secretion [52].
Neuropathic pain, often associated with allodynia (pain in response to normally non-noxious stimuli), is produced through damage to the central or peripheral nervous system. This is a chronic form of pain usually uncontrolled by analgesics. In peripheral neuropathic pain, the biological mechanisms underlying a nerve injury include the increased expression of voltage-gated sodium channels and new connections between adjacent neurons (“sprouting” or “ephaptic crosstalk”). These processes cause an increased release of: (i) neurotransmitters from the dorsal horn (i.e., glutamate, SP, gabaminergic, adenosine, 5 HT1, 5HT3, etc.); and (ii) immune and inflammatory mediators (i.e., catecholamine, histamine, cytokines, prostaglandins and ATP), which are responsible for nociceptor sensitization. In central neuropathic pain, the abnormal stimulation of neurons leads to an increase in N-methyl-D-aspartate receptor (NMDA) receptors and the up-regulation of cyclooxygenase and purinergic P2X3 receptors. Damage to the peripheral and central nervous system causes an abnormal generation and transmission of impulses. Inflammatory and metabolic disorders are among the major causes involved in neuropathic pain, which occur as a result of damaging stimuli [53].
Inflammatory pain is a response to chronic local inflammation in the joint and it is strongly interconnected to both nociceptive and neuropathic pain. Crosstalk and bidirectional interactions between the immune system and nociceptive neurons are central to inflammatory pain. Painful inflammatory stimuli can cause allodynia, hyperalgesia (neuronal hypersensitivity), mechanical sensitization and structural neuroplasticity of joint nociceptors.
Pain-related inflammatory mediators include IL-6 and TNF-α, which increase the serum levels of C-reactive protein (CRP), a circulating marker of systemic inflammation that correlates with increased pain in OA. Recently, Nees TA et al. provided a broad profile of inflammatory mediators of potential clinical relevance associated with joint pain in the synovial fluid, finding that IL-10, IL-12, IL-13, SCGF-β, and VEGF show clear correlations with joint pain and function [50]. Similarly, Liu et. Al. have indicated that blocking the IL-17 signaling pathway might contribute to treating OA pain due to the strong correlation between IL-17 levels and OA pain based on the WOMAC scale [54]. Increased levels of inflammatory mediators promote the expression of proteolytic enzymes, such as MMPs and ADAMTs [55].
Two key pain-sensitizing molecules implicated in OA pain are nerve growth factors (NGF) and CCL-2 [56]. NGF is produced by macrophages, mast cells, synoviocytes, and neutrophils and is up-regulated by cytokines such as TNF-α. The binding of NGF with the receptor tropomyosin receptor kinase (Trk)A leads to the up-regulation of the expression of the ion channels transient receptor potential vanilloid (TRPV)-1 and Nav1.8, CGRP, SP and brain-derived neurotrophic factor (BDNF) [57].
Among the main neurotransmitters implicated in joint pain, it is possible to distinguish between both: (i) inflammatory mediators, such as PGE2, PGI2, LTB4, NGF, proton, BK, ATP, adenosine, SP, neurokinin (NK)-A, B, 5-HT, histamine, glutamate, nor-epinephrine (NE) and NO; and (ii) non-inflammatory mediators, such as CGRP, gamma-aminobutyric acid (GABA), opioid peptides, glycine and cannabinoids. They determine the production of a second messenger that interacts with several ion channels by regulating pain [53].
MAPK and PI 3-K/AKT/mTOR, wingless-related integration site (Wnt) signaling pathways, are thought to be among the primary pathways implicated in chronic pain [31,58]. Under inflammatory and neuropathic pain, MAPK pathways are activated in neuron cells and regulate pain sensitization through central and peripheral mechanisms. Indeed, inhibitors of ERK, p-38 and JNK are effective in relieving pain symptoms, but cannot be used as drugs due to several issues [59]. the up-regulated expression of mTOR correlates with the decreased synovitis, strongly associated with pain [60]. The activation of canonical Wnt signaling has multiple effects on different tissues in the OA joint: (i) osteophyte formation; (ii) cartilage destruction; and (iii) synovitis, with consequent pain symptoms in OA patients [61]. However, how Wnt/β-catenin signaling regulates inflammatory cytokines and chemokines in the DRG and dorsal horn of the spinal cord, and the brain, and the crosstalk of Wnt/β-catenin signaling with the immune system is yet to be elucidated.
Other pathways closely associated with OA pain are the hypothalamic-mediated neuromodulation pathway (related to leptin, the neuropeptide Y (NPY) system and other neuropeptides [62] and the endocannabinoid (EC)-related pathway (which involves the frontal cortex, nucleus accumbens, striatum and hippocampus and is associated with chronic OA pain) [63].
Most of the progress in understanding the biological mechanisms of pain and its associated genes and molecules (neurotransmitters, receptors, intracellular messengers) comes from preclinical animal studies. To this end, rodent models have extensively contributed to the comprehension of several aspects related to acute and chronic pain states [64]. In particular, the mono-iodoacetate (MIA) model is often used to induce rapid pain-like responses as it induces the alteration of chondrocyte glycolysis and, consequently, cell death, vascularization processes, bone necrosis, bone collapse, and inflammation. The release of inflammatory mediators plays a crucial role as they fuel the pain response through their interaction with nociceptors upon tissue injury [65]. Other animal models of inflammatory pain include: (i) injection of capsaicin into the joint; (ii) carrageenan injection; (iii) complete Freund’s adjuvant (CFA) into the tail, paw and joint. Reflexive and non-reflexive pain tests are the two main types of outcome measures adopted in preclinical studies to assess pain [66,67,68]. Reflexive pain tests include the application of noxious stimuli (thermal/mechanical) at the site or outside the site of injury, with the consequent activation of nociceptors. In this regard, the assessment of paw withdrawal latency (PWL), paw withdrawal threshold (PWT), mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) are among the main indices considered in preclinical studies [66,67]. Non-reflexive pain tests include measures of spontaneous pain behavior, avoidance of evoked stimuli and quality assessment of life and function [68].
In addition, the establishment of the most appropriate animal model, which closely mimics the human experience of pain, would be a powerful tool to gain a full understanding of the potential use of future therapies to guide clinicians in the treatment of OA [69].
3. Traditional Therapeutic Strategies to Counteract Inflammation and Pain in OA: Pros and Cons
Although there have been successes in preclinical and early clinical studies, phase three clinical trials have failed so far and there are still no approved disease-modifying treatments (DMOAD) on the market.
The therapeutic strategies adopted for the treatment of OA are closely related to the staging of the disease. In the absence of DMOAD (targeting the underlying disease pathogenesis), the gold standard therapy for a mild-moderate grade of OA is still limited to the temporary systemic relief of symptoms through the use of analgesics, NSAIDs (meloxicam, diclofenac, naproxen), corticosteroids, paracetamol, COX-2 inhibitors (celecoxib); combined or not with the topical application of creams [70,71]. Traditional NSAIDs and COX-2-selective NSAIDs (coxibs) promote analgesic effects via the inhibition of the COX family of enzymes, implicated in the synthesis of prostaglandins, potent inflammatory and hyperalgesic mediators [72]. NSAIDs show beneficial effects through the inhibition of inflammation and pain; however, these oral medications often have side effects, including gastrointestinal disorders (e.g., irritation of the gastrointestinal tract, peptic ulcers, intestinal bleeding), immune reactions, toxicity, and cardiovascular effects [73]. As OA is a chronic condition that requires a long duration of treatment, this limits the success of these intervention approaches. Conversely, topical forms of NSAIDs may offer an alternative solution to relieve OA pain by overcoming the issues correlated to oral formulations; however, they only provide pain relief in patients with mild-to-moderate OA [74] and are recommended as an initial option, particularly for elderly patients [75]. Oral opioid administration is an option with limited application, not only due to its prolonged use often leading to physical dependence, but also because it exerts only minimal relief of OA symptoms within 12 weeks, as demonstrated by a recent meta-analysis of randomized controlled trials on patients with knee and/or hip OA [76,77]. Due to the localized nature of OA, injectable preparations are an attractive treatment approach to: (1) provide mechanical stability and lubricating mechanisms in the synovial fluid; (2) exert a direct effect on inflammation, oxidation and proteases production [78,79]; (3) mediate IA drug injection, minimizing side effects. IA therapies, include viscosupplements (hyaluronic acid, HA), corticosteroids (dexamethasone, methylprednisolone acetate, triamcinolone acetate) and blood-derived products to treat patients with mild to moderate symptomatic knee OA.
There are several formulations of HA (different sources, molecular weights, and purification methods) that are widely employed in clinics, even if they have shown some limitations, such as temporary pain relief and the need for repeated administrations. Its mechanism of action includes: (i) improvement of joint lubrication; (ii) reduction in collagen degradation; (iii) promotion of anabolic molecules; and (iv) reduction in several catabolic and inflammatory mediators [80,81,82]. Corticosteroids exert an immediate reduction in the patient’s pain with short-term effects, however, they have undesirable side effects when administered at high doses and frequency [83,84]. Platelet-rich plasma (PRP) IA injections improved short-term pain and knee function scores in a single-center prospective randomized controlled study with a one year follow-up and were safer than corticosteroids treatment [84]. However, the results of the use of PRP are extremely heterogenous, as its activity depends on the donor, number of platelets, type/quantity of growth factors, and the method of preparation, which makes it impossible to draw firm conclusions.
Despite all the progress in the standard conservative treatments, these approaches are often insufficient in controlling the patient’s symptoms, leading to the surgical indication of a knee joint replacement. This poses the need to search for alternative strategies to halt inflammation and pain with minimal side effects and more homogenous formulations to ensure repeatability in the clinical setting.
Among the new candidates as alternative strategies, dietary interventions are emerging as key non-pharmacological approaches for preventing and treating OA and have recently attracted increasing attention from nutritionists, food scientists, and even consumers, for their recognized roles in human health, particularly by inhibiting inflammatory processes.
4. Efficacy and Safety of PPs in the Management of OA
Role of PPs in Modulating Inflammation and Pain: Focus on Preclinical In Vitro and In Vivo Studies
PPs are natural compounds with phenolic structural features categorized into several classes according to their chemical composition, which influence their stability, bioavailability, and physiological functions [85]. The major known classes of PPs are phenolic acid, flavonoids, lignans, and stilbenes, which are shown in Figure 1, along with their main PPs constituents [85]. PPs are found abundantly in a wide variety of foods (fruits, vegetables, cereals, spices, herbs) and beverages (coffee, tea, wine, chocolate) and their use has been shown to protect against several chronic pathologies. Thanks to their pleiotropic effects, PPs exert several benefits on human health [86]. Moreover, they have recently been defined as epigenetically active dietary components that can be used as therapeutic interventions in alleviating persistent inflammation by targeting the epigenome [87].
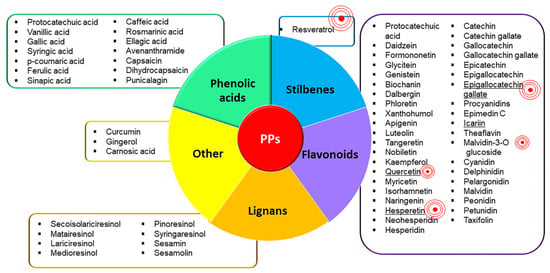
Figure 1.
PPs classification. Figure 1 shows the known classes of PPs and the main PPs which constitute each class. The two classes of PPs most represented are flavonoids and phenolic acids. The ‘red concentric symbol’ highlights the most studied PPs in the field of OA inflammation and pain; its size corresponds to the number of studies. Overall, the most studied is resveratrol, followed by epigallocatechin gallate, quercetin, icariin, and hesperetin. To the best of our knowledge the following PPs have never been investigated regarding OA inflammation and pain: syringic acid, cryptochlorogenic acid, neochlorogenic acid, glycitein, dalbergin, neohesperidin, taxifolin, catechin, catechin gallate, gallocatechin, gallocatechin gallate, epicatechin, epigallocatechin, pelargonidin, peonidin, petunidin, dihydrocapsaicin, secoisolariciresinol, matairesinol, lariciresinol, medioresinol, syringaresinol, sesamolin.
Several authors have investigated in vitro the effect of PPs on inflammation in OA [25,26,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197] (Table 1), particularly on articular chondrocytes obtained from OA joints, whereas a few studies have been carried out on peripheral blood mononuclear cells, osteoblasts, macrophages, and lymphocytes. Most studies have tested the preventive (treatment before OA induction- cell pre-treatment) or therapeutic (treatment after OA induction) effects of PPs on cell models mimicking OA inflammation. Among them, the most employed is the stimulation with IL-1β, due to its pivotal role in the pathogenesis of OA. In chondrocytes, IL-1β up-regulates the expression of several inflammatory mediators (e.g., NO, PGE2, IL-6) and inhibits ECM synthesis (collagen-II and aggrecan) by promoting the production of proteases (e.g., MMP-3, MMP-13, ADAMTS-4/5). Further in vitro models of inflammation include the use of other stimuli, such as AGE, TNF-α, H2O2, tert-butyl hydroperoxide (TBHP, a more stable form of H2O2 which activates ROS and ER), lipopolysaccharide (LPS, lipid A and polysaccharides which induce IL-1β and TNF-α expression). Notably, a few studies have tested PPs on co-culture systems between chondrocytes and synoviocytes or monocytes in order to study the crosstalk among joint cells. In general, all of the PP compounds displayed various anti-inflammatory, anti-oxidant and anti-catabolic effects, particularly via the modulation of NF-κB and MAPK signaling pathways, as shown in Table 1.

Table 1.
PPs effects on inflammation and pain: in vitro models of OA.
The most studied PP is resveratrol, belonging to the class of stilbenes found in the red variety of grapes (and consequently red wine), peanuts, blueberries, pines, rhubarb, and root extracts of the weed Polygonum cuspidatum. In chondrocytes, resveratrol exerts anti-inflammatory effects via the reduction in several inflammatory cytokines (IL-1β, -18, -6, TNF-α) and proteolytic enzymes (MMP-1, -3, -9, -13, ADAMTS4, ADAMTS5) and through the promotion of typical ECM proteins (collagen II, aggrecan, Sox-9, β1-integrin, GAG). Other molecules modulated by resveratrol are CXCL1, NOX4, PTGS2, and iNOS (Table 1). In the majority of studies, resveratrol administration has been linked to the inhibition of IkBα degradation, NF-kB activation and nuclear translocation. Several authors have demonstrated that resveratrol inhibits TLR4 and, consequently, TLR4/My88/NFkB signaling [88,89,90]. In particular, it targets IKK-NFKB and MAPK/AP-1 signaling pathways [91] and inhibits the production of mature IL-1β [92]. Interestingly, resveratrol modulates the epigenome by activating SIRT1 [93], which drives the inhibition of NFkB via SIRT1/FOXO signaling [94,95]; Yi and al. showed that resveratrol inhibits miR-210-5p, which targets LINC00654 and OGFRL1 [96]. Employing a co-culture model with macrophages and chondrocytes, Limagne et al. demonstrated that resveratrol can inhibit the inflammatory loop through the down-regulation of STAT3 in macrophages and the inhibition of NF kB in chondrocytes [97]. Further evidence of its anti-inflammatory role comes from the Siard et al. group, who demonstrated a decrease in INF-γ and TNF-α in lymphocytes treated with resveratrol [98].
Other PPs largely studied are epigallocatechin-3-gallate, quercetin, hesperitin and icariin, all belonging to the class of flavonoids.
Epigallocatechin-3-gallate is the major catechin found in green tea and exerts an anti-inflammatory effect by targeting: (i) in chondrocytes, the PTEN [99], MAPK [100,101], JNK [102] pathways; and (ii) in synoviocytes, the inflammatory cascades induced by DAMPS CPP crystals [103]. Moreover, it modulates miRNA: it down-regulates miR-29b-3p [99] and up-regulates miRNA-199a-3p [101].
Quercetin is ubiquitously present in fruits and vegetables; it exerts an anti-inflammatory effect through SIRT1/AMPK activation and ER stress inhibition [104], suppressing IRAK1/NLRP3 [105] and p38/MAPK [106] in chondrocytes. Notably, Hu et.al. showed, in a co-culture model of monocytes and chondrocytes, that quercetin: (1) drives the M2 polarization of monocytes; (2) induces anti-inflammatory, anti-catabolic, and anti-apoptotic effects on chondrocytes; (3) promotes the pro-chondrogenic environment via the release of transforming growth factor β (TGF-β) and insulin growth factor in monocytes [107]. The main effector of these processes is the suppression of AKT/NF-κB signaling [107]. Li et. al. showed that quercetin could suppress the expression of the IRAK1/NLRP3 pathway and decrease the levels of pro-inflammatory cytokines (IL-18, and TNF-α) in IL-1β-induced rat chondrocytes [105]. Gain-of-function assays showed that the molecular overexpression of NLRP3 or IRAK1 significantly reversed the anti-inflammatory and anti-apoptotic effects of quercetin in IL-1β-induced rat chondrocytes, whereas NLRP3 knockdown restored its protective function [105].
Hesperetin can be found in some citrus species and exerts an anti-inflammatory response through NF-κB inhibition [108], particularly via the down-regulation of TLR-2 [109]. Interestingly, hesperidin inhibits the expression of IL-17, one of the main cytokines involved in OA pain [110].
Icariin, found in Epimedii herba, Epimedium sagittatum plants, exerts an inflammatory response through NF-κB inhibition by acting on several pathways: (i) NLRP3 inflammasome-mediated caspase-1 signaling [111]; (ii) TLR4/Myd88 [112,113]; and (iii) p38/ERK/JNK [114]. Liu et al. found that the effects of icariin are associated with the activation of miR-206 in chondrocytes [115]. In LPS-treated synoviocytes, icariin inhibited ferroptosis through the activation of the Xc-/GPX4 and NRF2 axis [116]. Notably, icariin modulates OA pain by: (i) decreasing NPY, NPY1R, SP R and 5-HT1B R neuropeptides; (ii) increasing VIP neuropeptides; and (iii) enhancing the expression of sensory-related genes [117]. To our knowledge, this is one of the few shreds of evidence of PPs modulating key molecular pathways of pain.
As for the other PPs, they act similarly to those above-mentioned in inhibiting inflammation. In particular, several other PPs inhibit NFkB signaling: i.e., carnosol [118], CAPE [119], tangeretin [120], nobiletin [121], naringenin [122], theaflavin-3-3′-digallate [123], cyanidin [124,125], ellagic acid [126]. Similarly to resveratrol and quercetin, other PPs modulate epigenome by acting as SIRT1 inductors: i.e., ferulic acid [127], fisetin [128], procyanidins [129]; while cyanidin was showed to increase SIRT6 [125]. Moreover, other PPs modulate miRNA: carnosic acid (CA) up-regulates miR140 as shown by Ishitobi et. al.; curcumin up-regulates miR-124 and miR-143 [130].
Similarly to quercetin, vanillic acid [131], chlorogenic acid [132], carnosol [118], biochanin [133] and pinoresinol diglucoside [134] induce the expression of TIMP-1. Some PPs such as sesamin [135], and carnosol [118] can suppress the autocrine signaling of IL-1β in chondrocytes.
Moreover, one of the most documented pathways up-regulated by PPs is the NRF2/HO1; i.e., sinapic acid [136,137], xanthohumol [138], myricetin [139], lutein [140], theaflavin-3-3′-digallate [123], delphinidin [141], 6-gingerol [26], CAPE [119], genistein [25] and tangeretin [120]. In particular, it has been reported that Nrf2 and HO-1 could alleviate inflammation through the inhibition of p65 translocation, thus inhibiting NFKB pathways [27,119,120]. Notably, CAPE and lutein directly interact with the Keap1-NRF2 complex by inhibiting ubiquitination, resulting in Nrf2 stabilization and promoting the nuclear translocation of NRF2 [25,140]. Moreover, xanthohumol promotes the binding of HO-1 and C/EBPβ and inhibits C/EBPβ nuclear translocation [142]. The relevance of Nrf2 and/or HO1 signaling, upstream to NF-κB signaling inhibition, was proven by loss of function experiments using silencing [119,120,123,138,142]. Notably, Zhou et al. depicted the interaction among NRF2 and AMPK signaling upon luteolin stimulation [140]. Luteolin exerts anti-inflammatory and anti-catabolic effects on H2O2 chondrocytes, mediated by Nrf2 and AMPK cascade activation [140]. Similarly, Pan et. al. showed that myricetin activates Nrf2/HO-1 downstream to PI3K/Akt [139].
Other authors have shown the activation of several pathways downstream to PPs activation in chondrocytes. Interestingly, Shi et. Al. found that tangeretin induces an anti-inflammatory effect and prevents OA, due to NF-κB inhibition, by simultaneously modulating Nrf2-HO-1/NF-κB and MAPK/NF-κB signaling pathways [120]. Teng et al. showed that theaflavin-3-3′-gallate inhibited the PI3K/AKT/NF-κB and MAPK pathways while promoting the Nrf2/HO-1 pathway [123]. Chuntakaruk et. al. showed that several anthocyanins can exert anti-inflammatory effects upon AGE stimulation by targeting NF-κB and MAPK signaling [143]. Huang et. al. showed that vanillic acid exerted an anti-inflammatory effect on IL-1β by targeting both MAPK and PI3K/AKT/NF-κB [125]. Shi et. al. showed that tangeretin activates both Nrf2 and MAPK signaling [120]; Chen et al. demonstrated that rosmarinic acid activates both NF-κB and MAPK [144]. Moreover, Kim et al. showed that fisetin activates both the JNK and NF-κB pathways in monocytes [145]. However, the reciprocal interaction among the pathways was not analyzed.
Overall, most of these in vitro studies have provided evidence of the anti-inflammatory effects of the different classes of PPs, particularly after stimulation with IL-1β, as well as their benefits in reducing catabolic factors and up-regulating anabolic factors to avoid cartilage breakdown with minor evidence for pain process [133,135,146].
Biological benefits observed in the in vitro studies were confirmed in different preclinical animal models of OA (Table 2). In vivo models adopted to study the effect of PPs on inflammation and pain included: (i) post-traumatic OA models through surgical approaches (destabilization of the medial meniscus (DMM), anterior cruciate ligament transection (ACLT), etc.) to assess regeneration; and (ii) pain models (MIA-induced OA model, injection of capsaicin or carrageenan injection, complete Freund’s adjuvant) to explore pain response. Most studies have been conducted in mouse and rat models with different ranges of dosages/intervals of PPs and follow-ups to test the safety and efficacy of PPs in counteracting OA. In addition to the monotherapeutic approach, several studies investigated different combinations of PPs with cell-based therapies, or with other classes of BDMs or novel delivery systems (Table 2). Among PPs, resveratrol, quercetin, formononetin, protocatechuic and hesperetin combined with several delivery systems reported chondroprotective (reduced OARSI score, increased collagen II), anti-inflammatory (reduction in synovitis, reduction in inflammatory mediators) and immunomodulatory (polarization towards M2 macrophage subset) effects, thus representing valid tools to reduce pain and inflammatory responses. The oral gavage of punicalagin has been shown to inhibit inflammatory injury in an OA rat model by activating the Foxo1/Prg4/HIF3α axis and the modulation of Foxo1-autophagy-related gene (ULK1, Beclin1, LC3 and p62) [147]. Repeated IA and intraperitoneal injections of quercetin in surgical models of OA showed that it exerts chondroprotective and anti-inflammatory effects in terms of: (i) reduced OARSI score, apoptotic and phlogistic mediators; and (ii) increased anabolic markers (COLL-2, aggrecan) [105,107]. Moreover, Hui et al. [107] validated the immunomodulatory effect of resveratrol in a rat model and described its potential to drive M2 polarization in the synovial membrane. Further evidence of the chondroprotective role of PPs comes from the study of Wei B. et al. group, who reported, in a post-traumatic rabbit model of OA, the inhibition of cartilage degeneration via a decreased ratio of MMP-13/TIMP-1 [148]. To overcome the limitations related to the low bioavailability of quercetin in the articular joint, some authors explored the IA injection of quercetin encapsulated in an intelligent smart gel (thermogel). They described its prolonged activity in situ, associated with the relief of pain symptoms and the delay of OA progression [149]. Similarly, Britti D et al. employed a novel nanocomposite formulation of palmitoylethanolamide and quercetin to ensure a long-lasting effect in carrageenan paw oedema and MIA-induced OA models in rats. In particular, the authors demonstrated that quercetin counteracts inflammatory and pain responses by reducing: (i) inflammatory and catabolic mediators; (ii) paw oedema, thermal hyperalgesia, and mechanical allodynia; and (iii) neurotrophins, such as NGF [150]. In addition to IA and intragastric deliveries, the topic administration of quercetin combined with nanoparticles also reported benefits in inhibiting OA progression via the decrease in proteases such as MMP-9, -13 and ADAMTs-5 [149].

Table 2.
PPs effects on inflammation and pain: in vivo models of OA.
Similar to quercetin, an IA injection of resveratrol into post-traumatic and pain models of OA reported chondroprotective effects [95]. In both models, resveratrol increased the levels of SIRT-1, severely decreased during OA progression, and was involved in the shift towards a hypertrophic phenotype of chondrocytes [95]. In particular, Deng Z et al. demonstrated that the overexpression of SIRT-1 leads to the repression of i-NOS and MMP-13 in the articular cartilage, in part through the modulation of the NF-kB signaling pathway [95]. Regardless of the route of administration (IA, intragastric, oral supplementation), resveratrol displayed anti-inflammatory and anti-nociceptive effects in the MIA-induced OA models [95].
Several studies with different curcumin formulations have demonstrated its role in slowing down OA progression. The oral administration of curcumin counteracted OA progression, but could not affect OA-related symptoms in a DMM-induced OA model. Notably, the topic administration of curcumin encapsulated in customized nanoparticles provided the first evidence of its effect in ameliorating OA-related pain through the reduction in: (i) adipokines and inflammatory mediators; (ii) synovitis and; (iii) tactile hypersensitivity [151]. Taken together, these results suggest that orally delivered curcumin cannot reach biologically/pharmacologically active concentrations in the serum, synovial fluid, or joint tissues to attenuate OA-related pain. In contrast, the combination of curcumin with nanoparticles provided greater benefits in slowing down OA than oral administration. Along this line, Yabas M et al. proposed a highly bioavailable formulation of curcumin, known as Next Generation Ultrasol Curcumin (NGUC) and explored its impact on pain response in the MIA-induced OA model [152]. The authors demonstrated that NGUC promoted anti-nociceptive effects and reduced the levels of antioxidant enzymes SOD, CAT, and GPX, improving the pathophysiology of OA [152]. Recently, Qiu et al. showed the significant benefits, in a mouse model, of OA after treatment with exosomes derived from curcumin-treated MSCs. In particular, this treatment led to the up-regulation of miR-124 and miR-143, which modulate the ROCK1/TLR9 and NF-kB signaling pathway [130].
Both malvidin and naringenin using MIA-induced OA models described anti-nociceptive and pain-relieving effects [122,153]. Notably, naringenin treatment showed pain alleviation from the fourth day of the treatment and showed a dose-dependent effect (the pain alleviation was more pronounced at a dose of 40 mg/kg rather than 20 mg/kg), as assessed by PWL and PWT in MIA-induced OA rats [122]. In general, all the evaluated PPs in post-traumatic models of OA displayed a significant reduction in inflammatory and catabolic genes and an up-regulation of anabolic markers. The treatment of PPs in MIA-induced OA models, carrageenan paw oedema, and obesity-related models allowed OA pain alleviation and a reduction in inflammatory mediators, implicated in the onset of neuropathic and inflammatory pain (Table 2). Indeed, combing PPs with new technological approaches showed a high bioavailability at the site of injury and an improvement in the biological response to halt OA-associated processes.
Overall, these animal studies have produced further indications of the protective role of PPs in halting OA progression through various mechanisms of action. Furthermore, they provided more evidence of their anti-nociceptive properties through the use of reflex tests combined with non-stimulus evoked methods, such as weight bearing and gait analysis. However, the different conditions used in preclinical in vivo studies, including various severities of OA, different models, routes of administration, doses, dosing intervals and follow-up, offer a multifaceted scenario in which clear recommendations to better guide clinical choices have not yet been defined (Table 2 and Figure 2).
5. From Basic Research to Translational Applications of PPs
5.1. Clinical Studies on PPs for the Management of OA
Recently, interest in natural compounds has grown tremendously worldwide, leading to an increase in the consumption of these products as an alternative to treating OA in the early stages of the disease. The BDMs market has recorded staggering sales rates due to increased patient awareness of the benefits of taking natural compounds with minimal side effects compared to conventional drugs, such as NSAIDs [217,218]. The aforementioned preclinical in vitro and in vivo studies have shown that PPs may represent adjuvant strategies to treat OA by acting on several signaling pathways involved in the release of inflammatory and catabolic mediators (Table 1 and Table 2). However, only a few studies have been performed, thus hindering their widespread clinical application (Table 3). Among the PPs class, curcumin, resveratrol, quercetin, Pycnogenol® and capsaicin have been included in several clinical studies for the treatment of OA. Most of these studies have shown that their supplementation by different routes of administration (IA, topic, oral) can improve pain symptoms by reducing inflammatory mediators. In particular, OA patients treated with resveratrol monotherapy (oral dose of 500 mg per day) reported a reduction in the VAS and KOOS scores and good safety and tolerability, based on liver and renal function tests and haematological indices [219]. Similarly, studies on pycnogenol® and quercetin showed benefits in pain relief (VAS score) thanks to their ability to reduce all the major inflammatory actors involved in OA. The feasibility of combining PPs with other types of molecules, such as other families of BDMs or classic anti-inflammatory drugs, is growing with the aim of improving their efficacy and reducing drug-related side effects. It has been proven that PPs can work as adjuvants by reducing the number of doses of drugs, with enormous benefits for OA patients. In this regard, the synergistic effect of resveratrol, added to meloxicam therapy for knee OA, has been shown to reduce pain more effectively than meloxicam alone [220]. Moreover, a significant reduction in serum biomarkers of inflammation implicated in pain response, such as TNF-α, IL-1β, IL-6, and complement proteins has been noticed [220] (Table 3). Despite the promising results on the use of PPs, the heterogeneity of the existing literature and the scarcity of randomized, controlled clinical studies in humans make it difficult to propose specific recommendations on the amount of PPs to use as nutritional recommendations in personalized therapeutic approaches.

Table 3.
Clinical studies of PPs on OA patients.
5.2. PPs: Research and Clinical Gaps in OA
Considering both the upsides and downsides, the future use of BDMs seems promising, as they would ideally ensure a high safety profile and significant therapeutic benefits to treat OA patients. In this context, the strong interaction between the knowledge derived from basic research and clinical needs is key to promoting solutions capable of ensuring the well-being of patients. Basic research is crucial to substantiate the biological efficacy of BDMs, providing evidence-based medicine. On the other hand, the clinicians’ perspective is fundamental to focus on the patients’ needs and identify potential limitations of BDMs. To bridge the gap between research and the clinic, and to overcome the current shortcomings that limit their effectiveness, several key aspects need to be refined.
First, the development of products with safe and effective roles is necessary to ensure successful clinical outcomes. In particular, the absence of local and systemic toxicity (cytotoxicity, mutagenicity and genotoxicity), and non-immunogenic (structural features, contaminants, dose and length of treatment) reactions are among the main gaps in this field of research. Despite much evidence on the benefits of PPs in OA, the number of studies focused on their safety profile is low and sometimes with little statistical evidence due to the unavailability of large-scale evaluations in clinical trials [227,228]. Apart from the beneficial effects of PPs, there are controversial data on their role at high dosages, which require more detailed efficacy studies [229]. Pesticides represent one of the most harmful contaminants in BDMs because of their severe toxicity following their ingestion (skin rash, respiratory neurological disorders).
Second, the lack of standardization of products is another major roadblock, which may alter their biological properties and introduce certain risk factors that could undermine their stability. Differences in the region of origin, harvest period and cultivation of PPs may be elements involved in the heterogeneity of products and, consequently, in their altered efficacy. Moreover, the presence of different formulations (tablets, beverages, etc.), and batch-to-batch variations have not allowed us to obtain clear indications of their therapeutic value for the treatment of this disorder.
Third, in terms of their exert biological effects, BDMs need to reach the affected joints; this highlights their main limitation: oral bioaccessibility and bioavailability. Bioaccessibility (fraction of a compound released from food by dietary supplementation to ensure absorption) and bioavailability (fraction of a compound that reaches its site of action) are key prerequisites to ensure the presence of BDMs at the site of action. Ideally, these two properties should be maximized to obtain their best efficacy. Bioaccessibility and bioavailability depend on several factors: route of administration, chemical stability, microenvironment conditions, matrix interactions, and gut microbiota. Regarding the route of administration, topical delivery has advantages over conventional routes (dietary supplementation); in particular, it avoids first-pass metabolism and is a non-invasive mode of drug delivery with a sustained and controlled release profile. Furthermore, the targeting of cartilage via oral administration has been questioned due to the systemic application of a therapeutic agent to the relatively avascular nature of articular cartilage [227]. In this regard, capsaicin, a polyphenol compound, is not suitable for oral administration due to its high first-pass metabolism and gastric irritation. Aqueous aloe vera gel and Carbopol 934 with capsaicin in clove oil emulsion improved the permeability properties [228,229]. To date, the low bioavailability of the most promising natural compounds has determined the low effectiveness of the treatments: curcuminoids—0.47% [230], pterostilbene—35–80% [231], and resveratrol—20% [232]. PCA is produced following the ingestion of an anthocyanin-rich diet and is distributed through blood circulation to body tissues, where it remains longer than its parent compound [233]. The study of the anthocyanin bioavailability after ingestion and metabolism in humans stated that C3G, Pg-3-glc, P3G and their metabolite, PCA, were found in circulating blood [233,234]. Most studies have reported that the use of resveratrol via oral supplementation is limited by its poor solubility, rapid metabolism and low bioavailability [235]. Resveratrol is absorbed through epithelial diffusion in the gastrointestinal tract, forms complexes with transporter proteins, and is rapidly excreted from the body through the urinary tract. The half-life of resveratrol in the plasma of human volunteers has been reported to be 9.2–0.6 h after an oral dose of 25 mg [235]. Moreover, the local injection of resveratrol can overcome its low oral bioavailability and rapid first-pass metabolism [95]. To improve the low aqueous solubility of resveratrol, various methodological approaches, including liposomes, nanoparticles, and micelles, can be used to reduce the excretion speed and thus increase bioavailability. Moreover, dysbiosis is another critical factor that can alter the bioavailability of the site of interest. PPs have a low oral bioavailability, mainly due to extensive biotransformation mediated by phase I and phase II reactions in enterocytes and liver, but also by gut microbiota. Total PPs absorption in the small intestine is relatively low, being mainly bio-transformed by gut microbiota, followed by absorption in the bloodstream. Moreover, some PPs are not absorbed completely through the enterocyte due to their high hydrophilicity and molecular weight.
A major limitation of IA drug injection is that free drugs are cleared from the joint cavity rapidly, resulting in reduced drug bioavailability and the inconvenience of frequent injections. Moreover, some drugs that are highly insoluble in aqueous media form a crystal suspension that introduces the risk of crystal deposition. Furthermore, there is great variability in PP response due to intra-individual differences related to metabolic state, gut microbiota composition, and genetic profile [236]. Therefore, the stratification of patients according to their metabolic and lipidomic profiles is mandatory for improving efficacy [237].
Fourth, the complex and heterogeneous framework of OA disorder is another key aspect that has limited the application in the clinics of several promising molecules, described as the main reason for the failure of phase three clinical trials. In the clinic, OA is classified using the Kellgren-Lawrence (KL) classification [238], which identifies five grades of OA, ranging between 0 (no signs of OA) and 4 (severe OA). Usually, OA is defined as present starting from grade 2 upwards, however, the clinical presentation can be extremely heterogeneous with difficulties in selecting the most effective therapy.
Fifth, the lack of a universal regulatory framework among countries is critical to ensure product quality and reproducible results worldwide. This generates disorganization, with a lack of conformity to a global standard. The harmonization of the regulatory framework for BDMs across countries is mandatory to ensure better translation and to achieve global standards [239]. In contrast to in the US, in Europe, the European Food Safety Authority (EFSA) has outlined rules to ensure their pre-market safety [240] (Figure 3).
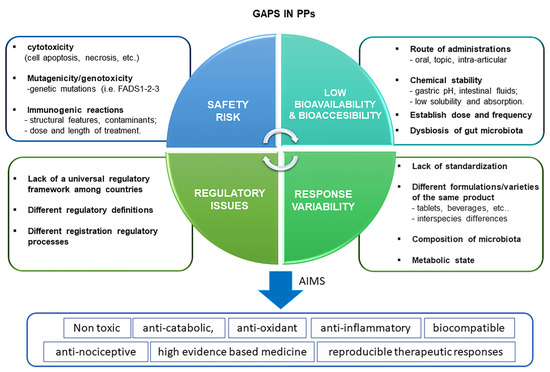
Figure 3.
Schematic representation of main knowledge gaps for the use of PPs for the treatment of OA. The main limitations of their use include mainly the consideration of four aspects: safety, bioavailability, response variability and regulatory aspects. A safety profile should be devoid of cytotoxicity (absence of cell apoptosis, death, etc.), mutagenicity and genotoxicity and immunogenic reactions at a local and systemic level. Bioavailability depends on a certain number of factors, including the route of administration, chemical stability, dose and frequency of BDMs, and gut microbiota (dysbiosis). Response variability of BDMs is closely dependent on the lack of standardization, different formulations batch variations, metabolic state, and microbiota composition which render unreproducible therapeutic effects among OA patients. Finally, the regulatory aspect is another critical limitation due to the lack of universal rules which govern the BDMs process worldwide.
Finally, to better target new BDM-based strategies for OA it is crucial to further elucidate their molecular mechanisms beyond the BDM effect.
5.3. From Knowledge Gaps to New Opportunities: Perspectives of PPs in OA
Considering the current shortcomings in the biology of PPs, several perspectives can be envisaged to improve their safety and efficacy profiles, thanks to the increasing knowledge in basic and clinical research, and technological advances [6,8,241,242]. Most studies focused on the safety profiles of PPs on various cell systems in different formulations and with different delivery systems are necessary to provide more evidence-based medicine for the management of OA. Given the limited availability of different PPs compounds, there is an urgent medical need to develop novel delivery systems to guarantee their prolonged activity in the joint space, overcoming gastrointestinal side effects.
To tackle this challenge, a bulk of research has focused on the development of advanced nanoencapsulation and nanofabricated systems as suitable tools to deliver BDMs into the articular joint via IA delivery. Common bio-based nano-delivery systems for PPs primarily include protein-based systems, polysaccharide-based systems, and lipid-based systems [243]. These systems can include different formulations: liposomes (which efficiently entrap hydrophobic molecules), nanocrystals (which control the delivery of poorly water-soluble molecules), and nanoparticles based on polymers such as polylactic acid (PLA), lipids, or metals. The nano-encapsulation of PPs with different carriers can be a valid strategy to boost their bioavailability and efficiency [244]. Studies on DMM and MIA-induced OA models have proven that quercetin-loaded nanoparticle gel counteracts the typical degenerative OA features, such as proteoglycan degradation and the up-regulation of MMP-9, MMP-13, and ADAMTS-5 [208,209]. Recently, Wang et al. demonstrated that incorporating quercetin into nano-octahedral ceria allows the polarization of M1 toward the anti-inflammatory phenotype, M2, with important implications for the treatment of pain in OA [106]. Along this line, further studies have demonstrated the benefits of an IA injection of quercetin-loaded polycaprolactone microspheres to reduce the degenerative effects in a rat model, ensuring controlled release for over 30 days [245]. Concerning formononetin (FMN), which belongs to the 7-hydroxyisoflavones class, the formulation of a poly (ethylene glycol) (PEG)-formononetin (FMN) nano drug showed longer joint permanence and better anti-inflammatory effects than FMN alone [204]. All of the above-reported studies have proven that the use of new technological approaches can overcome three main gaps in the systemic administration of PPs: (1) minimizing safety risks; (2) improving the low bioavailability due to their rapid absorption, metabolization and excretion; (3) enhancing their healing effects. Further research studies are mandatory to guarantee the safety profile of the nanofabricated delivery systems, as their use in food applications remains scarce. Regarding the regulatory aspects of nanomaterials in the medical field, there are no specific rules to guarantee the safety of encapsulated products worldwide [241]. Moreover, the sterilization processes of these systems must be considered to ensure the chemical stability of PPs and their safety in humans, which is essential for the clinical use of drugs [244]. Given the low bioavailability of several PPs, there is a high medical need to develop BDM delivery systems to avoid the burst release and rapid clearance of BDMs in the joint space. Furthermore, this might be useful in the case of some BDMs with known side effects, e.g., gastrointestinal adverse effects of vanillic acid in vivo. To tackle this challenge, several approaches can be considered that have been used to promote IA drug delivery [246]: liposomes, nanocrystals, nanoparticles, lipids or metal, and micro-encapsulation by spray. Moreover, there is an urgent need for adequate and controlled release to ensure lasting effects. The release of quercetin from MPEG-PA hydrogel could be sustained for over 28 days, which means approximately 30% of quercetin remained in the hydrogel on day 28 [149]. Quercetin was encapsulated in polycaprolactone by the solvent evaporation method; quercetin release showed a biphasic nature due to the initial burst effect, followed by a controlled release [245]. Sheu et al. obtained an injectable hydrogel based on oxidized hyaluronic acid (oxi-HA) and resveratrol. First, sodium periodate was used to create the oxi-HA, whose functional group was further crosslinked with resveratrol [154]. Then, articular mouse chondrocytes were cultured within the gel and confirmed its good viability, as well as its good potential for reducing inflammatory reaction and damage [154,247]. Xiong et. al. demonstrated that metal–organic frameworks (MOFs) are materials with dense and large pores and abundant metal sites, with pH-responsive properties but poor water stability. They combined the most used MOFs, MIL-100, with HA, to improve its stability and dispersibility; they then combined it with protocatechuic acid to obtain MOF@HA@PCA. The protocatechuic acid was released in small amounts at pH 7.4, while the release amount increased with the reduction in the pH value (pH 5.6). It was released quickly in the first few hours, then there was a sustained and controlled release that reached a maximum within 24 h [157].
Notably, Ouyang et al. developed a delivery system capable of delivering the targeted release of hesperetin to chondrocytes, by using nanoparticles modified with cartilage affinity peptide (CAP, DWRVIIPPRPSA). They showed the cartilage-binding ability of nanoparticles that smartly released hesperetin, attenuating chondrocyte apoptosis and inflammation and articular cartilage degeneration [109].
Another innovative aspect to be studied is the coupling of BDMs with existing anti-inflammatory drugs, such as NSAIDs, or with other classes of BDMs. As already mentioned, this aims to reduce the dosage or number of drug administrations and, consequently, their side effects. PPs have been shown to suppress inflammation by inhibiting inflammatory cytokines and other catabolic and ROS mediators, while NSAIDs inhibit the pro-inflammatory enzyme COX. Considering their different mechanisms of action, their combination may be useful to enhance their healing potential. In this regard, the synergic effect of ferulic acid with other vitamins or active ingredients has provided better results, performance, and stability of the compounds [248]. Similarly, the co-injection of kaempferol and apigenin seems to increase efficacy [197]. Despite these promising findings, further studies are needed to examine the use of different classes of BDMs that could simultaneously target multiple cellular signaling pathways. Indeed, better-designed preclinical studies and larger clinical studies, using rigorous design and controls, are needed to reach clear-cut conclusions [249].
Another critical aspect to be taken into consideration for preclinical and clinical studies is the stratification of patients to minimize the issues related to heterogeneity in OA. In particular, an initial screening of the patient’s genetic, lipid, and metabolic pattern is of paramount importance to select which natural compounds may be most effective for personalized therapy. Unravelling the emerging factors influencing susceptibility/resistance to inflammation (genetic background, microbiota composition, metabolomic profiles) will be crucial to provide precise nutritional and lifestyle recommendations for specific groups of OA sufferers (e.g., personalized approaches based on inflammatory epigenetic signatures) [249,250]. In this context, the gut is a new and intriguing target for OA, as gut dysbiosis is one of the factors contributing to the pathophysiology of OA [251,252], where PPs have important roles. Lan et al. showed that the daily intragastric administration of quercetin in the MIA-induced OA model, from day 1 to day 28, partially reversed intestinal flora disorder [253]. Moreover, a more controlled and uniform regulatory management, by different countries, could help to reduce the large variability observed between different research and clinical studies worldwide, which makes it difficult to obtain clear-cut results and dietary recommendations.
In general, the challenges in this field are varied and, certainly, the interaction between a multidisciplinary team is necessary to speed up the development of future treatments for the management of OA (Figure 4).
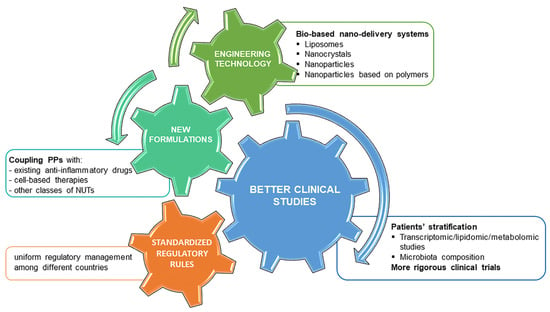
Figure 4.
Overview of main perspectives to improve future research approaches studying PPs. The main challenges in this research field are addressed to improve their safety and efficacy profile. We distinguished four main research areas, engineering technology, new formulations, better clinical studies and standardized regulatory rules, which are closely interconnected and require different expertise. Engineering technology can represent a valid tool thanks to the new bio-based delivery systems to preserve PPs efficacy and ensure long-lasting effects in the joint. Better clinical studies should include previous patients’ stratification and more rigorous clinical trials. Combinatorial therapies with cell-based therapies, other BDMs or anti-inflammatory drugs can have synergistic benefits in reducing inflammation and pain. Finally, more standardized regulatory rules among countries could facilitate to reach and speed up of clear-cut conclusions on BDMs.
6. Conclusions
In conclusion, PPs may have great potential for the treatment of OA due to their anti-inflammatory and anti-nociceptive properties. Several preclinical studies have reported how these natural compounds interfere with various inflammation and pain pathways and inhibit the release of inflammatory mediators, as well as molecules involved in matrix degradation. Results from clinical studies, although still limited in number, report the benefits of some compounds, such as resveratrol and curcumin, thus providing preliminary indications of their therapeutic potential. More recent advances in tissue engineering may allow the development of promising new formulations with greater stability and bioavailability at the damaged site of interest. Certainly, more and better-designed clinical trials, together with integrative transcriptomic, lipidomic, and metabolomic analyses, might provide a basis for precision treatments with PPs in OA patients.
Author Contributions
Conceptualization, L.G. and G.D.; methodology, L.G., A.P., A.C. and G.D; software, L.G. and A.C; validation, L.G., G.D. and F.G.; formal analysis, L.G., A.C., A.P. and G.D.; investigation, L.G., A.P., A.R. and G.D.; resources, L.G., A.C., F.G., B.G., A.P., A.R., C.F. and G.D; data curation, L.G., A.C. and G.D.; writing—original draft preparation, L.G., A.P. and G.D; writing—review and editing, L.G., A.C., F.G., B.G., A.P., A.R., C.F. and G.D; visualization, L.G., A.C., F.G., B.G., A.P., A.R., C.F. and G.D.; supervision, L.G., A.C., F.G., B.G., A.P., A.R., C.F. and G.D.; project administration, L.G. and G.D; funding acquisition, L.G, A.R and G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health, Grant number GR-2019-12370030.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This manuscript is part of the research conducted within the funded project by the Italian Ministry of Health, Grant number GR-2019-12370030.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Acronym | Full-Extensive name |
| ACAN | Aggrecan |
| ADAMTs | a disintegrin and metalloproteinase with thrombospondin motifs |
| AGEs | advanced glycation end-products |
| Arg1 | arginase-1 |
| BCP | basic calcium phosphate |
| BDNF | brain-derived neurotrophic factor |
| BDMs | bioactive dietary molecules |
| BDNF | brain-derived neurotrophic factor |
| CCL | Chemokine (C-C motif) ligand |
| CFA | complete Freund’s adjuvant |
| CGRP | calcitonin gene-related peptide |
| ChPF | Chondroitin Polymerizing Factor |
| CHSY-1 | Chondroitin sulfate synthase 1 |
| COX-2 | cyclooxygenase-2 |
| CPPD | Calcium pyrophosphate dihydrate |
| CRP | C-reactive protein |
| CSPG | Chondroitin sulfate proteoglycans |
| CXCL | C-X-C Motif Chemokine Ligand |
| DAMPs | damage-associated molecular patterns |
| DEC | Decorin core protein |
| DRG | dorsal root ganglia |
| ECM | extracellular matrix |
| EFSA | European Food Safety Authority |
| ERK | extracellular signal regulated kinase |
| FMN | Formononetin |
| GABA | Gamma-aminobutyric acid |
| GAGs | Glycosamminoglycans |
| GSH | reduced glutathione |
| GSSG | oxidized disulfide form glutathione |
| GSTA4-4 | glutathione-s-tranferase A4-4 |
| H2O2 | produces hydrogen peroxide |
| HA | hyaluronic acid |
| HMGB1 | high-mobility group box-1 |
| HNE | 4-hydroxynonenal |
| HNE | 4-hydroxynonenal |
| IA | intra-articular |
| IL | Interleukin |
| IL1-R | interleukin-1 receptor |
| iNOS | inducible NO synthase |
| JNK | c-Jun NH2-terminal kinase |
| KOOS | Knee injury and Osteoarthritis Outcome Score |
| LGI | low-grade inflammation |
| MAPK | mitogen-activated protein kinase |
| MCP-1, CCL2 | monocyte chemoattractant protein-1 |
| MIA | mono-iodoacetate |
| miR | miRNA |
| MMPs | matrix metalloproteinases |
| MWT | mechanical withdrawal threshold |
| NE | nor-epinephrine |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGF | nerve growth factor |
| NK | neurokinin |
| NLRP3 | nucleotide-binding oligomerization domain-like receptor protein 3 |
| NLRs | NOD-like receptors |
| NMDA | N-methyl-D-aspartate |
| NO | nitric oxide |
| NOX | NADPH Oxidase |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| OA | osteoarthritis |
| OSM | oncostatin M |
| PG | Proteoglycans |
| PGE2 | prostaglandin E2 |
| PI3K/ AKT | phosphoinositide 3-kinase/protein kinase B |
| PLGA | poly lactic-co-glycolic acid |
| PPs | polyphenols |
| PRP | Platelet-rich plasma |
| PRRs | pattern recognition receptors |
| PTGS2 | prostaglandin-endoperoxide synthase 2 |
| PWL | paw withdrawal latency |
| PWT | paw withdrawal threshold |
| RAGEs | receptor for advanced glycosylation end products |
| ReHo | Regional homogeneity |
| ROM | Range of motion |
| ROS | Reactive oxygen species |
| Rsv | Resveratol |
| Runx-2 | runt-related transcription factor 2 |
| SASP | senescence-associated secretory phenotype |
| Sox-9 | SRY-box transcription factor 9 |
| SP | substance P |
| TGF- β | transforming growth factor β |
| TBHP | tert-butylhydroperoxide |
| TKA | total knee arthroplasty |
| TLR | toll-like receptors |
| TNF-α | tumor necrosis factor-α |
| TRIF | TIRdomain-containing adaptor-inducing interferon-β |
| TrK | tropomyosin receptor kinase |
| TRP | Transient receptor potential |
| TrpV1 | Transient receptor potential cation channel subfamily V member 1 |
| TWL | thermal withdrawal latency |
| VAS | Visual Analogh scale |
| VEGF | vascular endothelial growth factor |
| WOMAC | Western Ontario and McMaster Universities Arthritis Index |
| XT | Xylosyltransferase |
| Ym1 | chitinase 3-like protein 3 |
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Van Spil, W.E.; Kubassova, O.; Boesen, M.; Bay Jensen, A.C.; Mobasheri, A. Osteoarthritis phenotypes and novel therapeutic targets. Biochem. Pharmacol. 2019, 165, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Conaghan, P.G.; Cook, A.D.; Hamilton, J.A.; Tak, P.P. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat. Rev. Rheumatol. 2019, 15, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, M. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Imeneo, M.; Luzza, F. Potential role of nutraceutical compounds in inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 2483–2492. [Google Scholar] [CrossRef]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Tayab, M.A.; Islam, M.N.; Chowdhury, K.A.A.; Tasnim, F.M. Targeting neuroinflammation by polyphenols: A promising therapeutic approach against inflammation-associated depression. Biomed. Pharmacother. 2022, 147, 112668. [Google Scholar]
- Iqubal, A.; Ahmed, M.; Iqubal, M.K.; Pottoo, F.; Haque, S.E. Polyphenols as Potential Therapeutics for Pain and Inflammation in Spinal Cord Injury. Curr. Mol. Pharmacol. 2021, 14, 714–730. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Panichi, V.; Mariani, E.; Flamigni, F.; Borzì, R.M.B. Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells 2020, 9, 1232. [Google Scholar] [CrossRef]
- Liu, X.; Machado, G.C.; Eyles, J.P.; Ravi, V.; Hunter, D.J. Dietary supplements for treating osteoarthritis: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 167–175. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Ebata, T.; Yokota, S.; Takahashi, D.; Endo, T.; Matsumae, G.; Shimizu, T.; Kadoya, K.; Iwasaki, N. Low-Grade Inflammation in the Pathogenesis of Osteoarthritis: Cellular and Molecular Mechanisms and Strategies for Future Therapeutic Intervention. Biomedicines 2022, 10, 1109. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zuo, Z.; Kuang, Y. An Emerging Target in the Battle against Osteoarthritis: Macrophage Polarization. Int. J. Mol. Sci. 2020, 21, 8513. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yu, Y.; Zhuang, Q.; Wang, L.; Zhan, B.; Du, S.; Liu, Y.; Huang, J.; Hao, J.; Zhu, X. Bone erosion in inflammatory arthritis is attenuated by Trichinella spiralis through inhibiting M1 monocyte/macrophage polarization. iScience 2022, 25, 103979. [Google Scholar] [CrossRef] [PubMed]
- Frevert, C.W.; Felgenhauer, J.; Wygrecka, M.; Nastase, M.V.; Schaefer, L. Danger-Associated Molecular Patterns Derived From the Extracellular Matrix Provide Temporal Control of Innate Immunity. J. Histochem. Cytochem. 2018, 66, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Sofat, N. Analysing the role of endogenous matrix molecules in the development of osteoarthritis. Int. J. Exp. Pathol. 2009, 90, 463–479. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Schelbergen, R.F.; Blom, A.B.; van den Bosch, M.H.; Sloetjes, A.; Abdollahi-Roodsaz, S.; Schreurs, W.; Mort, J.S.; Vogl, T.; Roth, J.; van den Berg, W.B.; et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 2012, 64, 1477–1487. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Yang, S.; Kim, J.; Ryu, J.H.; Oh, H.; Chun, C.H.; Kim, B.J.; Min, B.H.; Chun, J.S. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 2010, 16, 687–693. [Google Scholar] [CrossRef]
- Shen, J.; Abu-Amer, Y.; O’Keefe, R.J.; McAlinden, A. Inflammation and epigenetic regulation in osteoarthritis. Connect. Tissue Res. 2017, 58, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Grigolo, B.; Roseti, L.; Fiorini, M.; Facchini, A. Enhanced lipid peroxidation in synoviocytes from patients with osteoarthritis. J. Rheumatol. 2003, 30, 345–347. [Google Scholar] [PubMed]
- Boehme, K.A.; Rolauffs, B. Onset and Progression of Human Osteoarthritis-Can Growth Factors, Inflammatory Cytokines, or Differential miRNA Expression Concomitantly Induce Proliferation, ECM Degradation, and Inflammation in Articular Cartilage? Int. J. Mol. Sci. 2018, 19, 2282. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Ferrandiz, M.L. Relevance of Nrf2 and heme oxygenase-1 in articular diseases. Free Radic. Biol. Med. 2020, 157, 83–93. [Google Scholar] [CrossRef]
- Liu, F.C.; Wang, C.C.; Lu, J.W.; Lee, C.H.; Chen, S.C.; Ho, Y.J.; Peng, Y.J. Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation. Nutrients 2019, 11, 1180. [Google Scholar] [CrossRef]
- Abusarah, J.; Benabdoune, H.; Shi, Q.; Lussier, B.; Martel-Pelletier, J.; Malo, M.; Fernandes, J.C.; de Souza, F.P.; Fahmi, H.; Benderdour, M. Elucidating the Role of Protandim and 6-Gingerol in Protection Against Osteoarthritis. J. Cell Biochem. 2017, 118, 1003–1013. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-kappaB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Roberti, A.; Chaffey, L.E.; Greaves, D.R. NF-kappaB Signaling and Inflammation-Drug Repurposing to Treat Inflammatory Disorders? Biology 2022, 11, 372. [Google Scholar] [CrossRef]
- Rigoglou, S.; Papavassiliou, A.G. The NF-kappaB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Jimenez, S.A. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef]
- Xie, J.; Lin, J.; Wei, M.; Teng, Y.; He, Q.; Yang, G.; Yang, X. Sustained Akt signaling in articular chondrocytes causes osteoarthritis via oxidative stress-induced senescence in mice. Bone Res. 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Penas, C.; Navarro, X. Epigenetic Modifications Associated to Neuroinflammation and Neuropathic Pain After Neural Trauma. Front. Cell. Neurosci. 2018, 12, 158. [Google Scholar] [CrossRef]
- den Hollander, W.; Meulenbelt, I. DNA Methylation in Osteoarthritis. Curr. Genomics 2015, 16, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, P.K.; Lambrou, G.I. The Involvement of MicroRNAs in Osteoarthritis and Recent Developments: A Narrative Review. Mediterr. J. Rheumatol. 2018, 29, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Zhang, F.; Yao, H.; Li, H.; Tuan, R.S. Histone Modifications and Chondrocyte Fate: Regulation and Therapeutic Implications. Front. Cell Dev. Biol. 2021, 9, 626708. [Google Scholar] [CrossRef]
- Deng, Z.; Li, Y.; Liu, H.; Xiao, S.; Li, L.; Tian, J.; Cheng, C.; Zhang, G.; Zhang, F. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Oppenheimer, H.; Gabay, O.; Meir, H.; Haze, A.; Kandel, L.; Liebergall, M.; Gagarina, V.; Lee, E.J.; Dvir-Ginzberg, M. 75-kd sirtuin 1 blocks tumor necrosis factor alpha-mediated apoptosis in human osteoarthritic chondrocytes. Arthritis Rheum. 2012, 64, 718–728. [Google Scholar] [CrossRef]
- Dvir-Ginzberg, M.; Gagarina, V.; Lee, E.J.; Booth, R.; Gabay, O.; Hall, D.J. Tumor necrosis factor alpha-mediated cleavage and inactivation of SirT1 in human osteoarthritic chondrocytes. Arthritis Rheum. 2011, 63, 2363–2373. [Google Scholar] [CrossRef]
- Takayama, K.; Ishida, K.; Matsushita, T.; Fujita, N.; Hayashi, S.; Sasaki, K.; Tei, K.; Kubo, S.; Matsumoto, T.; Fujoka, H. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum. 2009, 60, 2731–2740. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, W.; Wu, P.; Deng, Z.; Zeng, C.; Li, H.; Yang, T.; Lei, G. The expression of SIRT1 in articular cartilage of patients with knee osteoarthritis and its correlation with disease severity. J. Orthop. Surg. Res. 2016, 11, 144. [Google Scholar] [CrossRef]
- Greene, M.A.; Loeser, R.F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Chisari, E.; Wouthuyzen-Bakker, M.; Friedrich, A.W.; Parvizi, J. The relation between the gut microbiome and osteoarthritis: A systematic review of literature. PLoS ONE 2021, 16, e0261353. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, T.W.; Felson, D.T. Mechanisms of Osteoarthritis (OA) Pain. Curr. Osteoporos. Rep. 2018, 16, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Gronblad, M.; Liesi, P.; Korkala, O.; Karaharju, E.; Polak, J. Innervation of human bone periosteum by peptidergic nerves. Anat. Rec. 1984, 209, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Mapp, P.I. Innervation of the synovium. Ann. Rheum. Dis. 1995, 54, 398–403. [Google Scholar] [CrossRef]
- Ashraf, S.; Wibberley, H.; Mapp, P.I.; Hill, R.; Wilson, D.; Walsh, D.A. Increased vascular penetration and nerve growth in the meniscus: A potential source of pain in osteoarthritis. Ann. Rheum. Dis. 2011, 70, 523–529. [Google Scholar] [CrossRef]
- Hirasawa, Y.; Okajima, S.; Ohta, M.; Tokioka, T. Nerve distribution to the human knee joint: Anatomical and immunohistochemical study. Int. Orthop. 2000, 24, 1–4. [Google Scholar] [CrossRef]
- Suri, S.; Gill, S.E.; Massena de Camin, S.; Wilson, D.; McWilliams, D.F.; Walsh, D.A. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann. Rheum. Dis. 2007, 66, 1423–1428. [Google Scholar] [CrossRef]
- Nees, T.A.; Rosshirt, N.; Zhang, J.A.; Reiner, T.; Sorbi, R.; Tripel, E.; Walker, T.; Schiltenwolf, M.; Hagmann, S.; Moradi, B. Synovial Cytokines Significantly Correlate with Osteoarthritis-Related Knee Pain and Disability: Inflammatory Mediators of Potential Clinical Relevance. J. Clin. Med. 2019, 8, 1343. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Takayama, Y.; Derouiche, S.; Maruyama, K.; Tominaga, M. Emerging Perspectives on Pain Management by Modulation of TRP Channels and ANO1. Int. J. Mol. Sci. 2019, 20, 3411. [Google Scholar] [CrossRef]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, H.; Meng, Z.; Wei, M. Correlation of IL-17 Level in Synovia and Severity of Knee Osteoarthritis. Med. Sci. Monit. 2015, 21, 1732–1736. [Google Scholar]
- Pan, F.; Tian, J.; Cicuttini, F.; Jones, G. Prospective Association Between Inflammatory Markers and Knee Cartilage Volume Loss and Pain Trajectory. Pain Ther. 2022, 11, 107–119. [Google Scholar] [CrossRef]
- Vincent, T.L. Peripheral pain mechanisms in osteoarthritis. Pain 2020, 161 (Suppl. S1), S138–S146. [Google Scholar] [CrossRef]
- Hirose, M.; Kuroda, Y.; Murata, E. NGF/TrkA Signaling as a Therapeutic Target for Pain. Pain Pract. 2016, 16, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, Y.; Liu, R.; Li, W.; Hua, B.; Bao, Y. Wnt Signaling Pathways: A Role in Pain Processing. Neuromolecular Med. 2022, 24, 233–249. [Google Scholar] [CrossRef]
- Ji, R.R.; Gereau, R.W.T.; Malcangio, M.; Strichartz, G.R. MAP kinase and pain. Brain Res. Rev. 2009, 60, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Luo, J.; Guo, J.; Yao, X.; Jing, X.; Guo, F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr. Cartil. 2020, 28, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Lories, R.J.; Monteagudo, S. Review Article: Is Wnt Signaling an Attractive Target for the Treatment of Osteoarthritis? Rheumatol. Ther. 2020, 7, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Houweling, P.; Kulkarni, R.N.; Baldock, P.A. Neuronal control of bone and muscle. Bone 2015, 80, 95–100. [Google Scholar] [CrossRef]
- Mlost, J.; Wasik, A.; Michaluk, J.T.; Antkiewicz-Michaluk, L.; Starowicz, K. Changes in Monoaminergic Neurotransmission in an Animal Model of Osteoarthritis: The Role of Endocannabinoid Signaling. Front. Mol. Neurosci. 2018, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.M.; Wilcox, G.L.; Fairbanks, C.A. The Study of Pain in Rats and Mice. Comp. Med. 2019, 69, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Dubner, R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010, 16, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Xu, B.X.; Fan, K.J.; Li, Y.W.; Wu, J.; Wang, T.Y. Dexamethasone-Loaded Thermosensitive Hydrogel Suppresses Inflammation and Pain in Collagen-Induced Arthritis Rats. Drug Des. Devel. Ther. 2020, 14, 4101–4113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Lu, S.; Yu, A.; Fu, Z. Increased pain in response to mechanical or thermal stimulation in a rat model of incision-induced pain with nicotine dependence and withdrawal. Exp. Ther. Med. 2013, 5, 1063–1066. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gregory, N.S.; Harris, A.L.; Robinson, C.R.; Dougherty, P.M.; Fuchs, P.N.; Sluka, K.A. An overview of animal models of pain: Disease models and outcome measures. J. Pain 2013, 14, 1255–1269. [Google Scholar]
- Piel, M.J.; Kroin, J.S.; van Wijnen, A.J.; Kc, R.; Im, H.J. Pain assessment in animal models of osteoarthritis. Gene 2014, 537, 184–188. [Google Scholar] [CrossRef]
- Deng, Z.H.; Zeng, C.; Yang, Y.; Li, Y.S.; Wei, J.; Yang, T.; Li, H.; Lei, G.H. Topical diclofenac therapy for osteoarthritis: A meta-analysis of randomized controlled trials. Clin. Rheumatol. 2016, 35, 1253–1261. [Google Scholar] [CrossRef]
- da Costa, B.R.; Reichenbach, S.; Keller, N.; Nartey, L.; Wandel, S.; Jüni, P.; Trelle, S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: A network meta-analysis. Lancet 2017, 390, e21–e33. [Google Scholar] [CrossRef] [PubMed]
- Burian, M.; Geisslinger, G. COX-dependent mechanisms involved in the antinociceptive action of NSAIDs at central and peripheral sites. Pharmacol. Ther. 2005, 107, 139–154. [Google Scholar] [CrossRef]
- Mukherjee, D.; Nissen, S.E.; Topol, E.J. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001, 286, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Honvo, G.; Leclercq, V.; Geerinck, A.; Thomas, T.; Veronese, N.; Charles, A.; Rabenda, V.; Beaudart, C.; Cooper, C.; Reginster, J.Y.; et al. Safety of Topical Non-steroidal Anti-Inflammatory Drugs in Osteoarthritis: Outcomes of a Systematic Review and Meta-Analysis. Drugs Aging 2019, 36, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Osani, M.C.; Lohmander, L.S.; Bannuru, R.R. Is There Any Role for Opioids in the Management of Knee and Hip Osteoarthritis? A Systematic Review and Meta-Analysis. Arthritis Care Res. 2021, 73, 1413–1424. [Google Scholar] [CrossRef]
- Abdel Shaheed, C.; Awal, W.; Zhang, G.; Gilbert, S.E.; Gallacher, D.; McLachlan, A.; Day, R.O.; Ferreira, G.E.; Jones, C.M.P.; Ahedi, H.; et al. Efficacy, safety, and dose-dependence of the analgesic effects of opioid therapy for people with osteoarthritis: Systematic review and meta-analysis. Med. J. Aust. 2022, 216, 305–311. [Google Scholar] [CrossRef]
- Presti, D.; Scott, J.E. Hyaluronan-mediated protective effect against cell damage caused by enzymatically produced hydroxyl (OH.) radicals is dependent on hyaluronan molecular mass. Cell Biochem. Funct. 1994, 12, 281–288. [Google Scholar] [CrossRef]
- Julovi, S.M.; Yasuda, T.; Shimizu, M.; Hiramitsu, T.; Nakamura, T. Inhibition of interleukin-1beta-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis Rheum. 2004, 50, 516–525. [Google Scholar] [CrossRef]
- Moreland, L.W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: Mechanisms of action. Arthritis Res. Ther. 2003, 5, 54–67. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Huang, H.T.; Ho, C.J.; Shih, C.L.; Chen, C.H.; Cheng, T.L.; Wang, Y.C.; Lin, S.Y. Molecular Weight of Hyaluronic Acid Has Major Influence on Its Efficacy and Safety for Viscosupplementation in Hip Osteoarthritis: A Systematic Review and Meta-Analysis. Cartilage 2021, 13, 169S–184S. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Di Matteo, B.; Tentoni, F.P.; Selleri, F.; Cenacchi, A.; Kon, E.; Filardo, G. Platelet-Rich Plasma Versus Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis: Results at 5 Years of a Double-Blind, Randomized Controlled Trial. Am. J. Sports Med. 2019, 47, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Danial, C.M.; Braun, H.J.; Pouliot, M.A.; Kim, H.J. The chondrotoxicity of single-dose corticosteroids. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1809–1814. [Google Scholar] [CrossRef] [PubMed]
- Elksnins-Finogejevs, A.; Vidal, L.; Peredistijs, A. Intra-articular platelet-rich plasma vs. corticosteroids in the treatment of moderate knee osteoarthritis: A single-center prospective randomized controlled study with a 1-year follow up. J. Orthop. Surg. Res. 2020, 15, 257. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomas-Barberan, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Milagro, F.I.; Riezu-Boj, J.I.; Martinez, J.A. Epigenetic signatures underlying inflammation: An interplay of nutrition, physical activity, metabolic diseases, and environmental factors for personalized nutrition. Inflamm. Res. 2021, 70, 29–49. [Google Scholar] [CrossRef]
- Liu, L.; Gu, H.; Liu, H.; Jiao, Y.; Li, K.; Zhao, Y.; An, L.; Yang, J. Protective effect of resveratrol against IL-1beta-induced inflammatory response on human osteoarthritic chondrocytes partly via the TLR4/MyD88/NF-kappaB signaling pathway: An “in vitro study”. Int. J. Mol. Sci. 2014, 15, 6925–6940. [Google Scholar] [CrossRef]
- Gu, H.; Jiao, Y.; Yu, X.; Li, X.; Wang, W.; Ding, L.; Liu, L. Resveratrol inhibits the IL-1beta-induced expression of MMP-13 and IL-6 in human articular chondrocytes via TLR4/MyD88-dependent and -independent signaling cascades. Int. J. Mol. Med. 2017, 39, 734–740. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Yang, Y.; He, J.; Jiang, M.; Huang, Y.; Liu, X.; Liu, L.; Gu, H. Resveratrol Exerts Anti-Osteoarthritic Effect by Inhibiting TLR4/NF-kappaB Signaling Pathway via the TLR4/Akt/FoxO1 Axis in IL-1beta-Stimulated SW1353 Cells. Drug Des. Devel. Ther. 2020, 14, 2079–2090. [Google Scholar] [CrossRef]
- Liu, F.C.; Hung, L.F.; Wu, W.L.; Chang, D.M.; Huang, C.Y.; Lai, J.H.; Ho, L.J. Chondroprotective effects and mechanisms of resveratrol in advanced glycation end products-stimulated chondrocytes. Arthritis Res. Ther. 2010, 12, R167. [Google Scholar] [CrossRef] [PubMed]
- Csaki, C.; Keshishzadeh, N.; Fischer, K.; Shakibaei, M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. 2008, 75, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Braun, H.J.; Dragoo, J.L. The effect of resveratrol on normal and osteoarthritic chondrocyte metabolism. Bone Joint. Res. 2014, 3, 51–59. [Google Scholar] [CrossRef]
- Liang, C.; Xing, H.; Wang, C.; Xu, X.; Hao, Y.; Qiu, B. Resveratrol protection against IL-1beta-induced chondrocyte damage via the SIRT1/FOXO1 signaling pathway. J. Orthop. Surg. Res. 2022, 17, 406. [Google Scholar] [CrossRef]
- Li, W.; Cai, L.; Zhang, Y.; Cui, L.; Shen, G. Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating SIRT1 and thereby silencing HIF-2alpha. J. Orthop. Res. 2015, 33, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Zhang, W.; Cui, S.Y.; Fan, J.B.; Zhu, X.H.; Liu, W. Identification and validation of key long non-coding RNAs in resveratrol protect against IL-1beta-treated chondrocytes via integrated bioinformatic analysis. J. Orthop. Surg. Res. 2021, 16, 421. [Google Scholar] [CrossRef] [PubMed]
- Limagne, E.; Lancon, A.; Delmas, D.; Cherkaoui-Malki, M.; Latruffe, N. Resveratrol Interferes with IL1-beta-Induced Pro-Inflammatory Paracrine Interaction between Primary Chondrocytes and Macrophages. Nutrients 2016, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Siard, M.H.; McMurry, K.E.; Adams, A.A. Effects of polyphenols including curcuminoids, resveratrol, quercetin, pterostilbene, and hydroxypterostilbene on lymphocyte pro-inflammatory cytokine production of senior horses in vitro. Vet. Immunol. Immunopathol. 2016, 173, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Cao, G.; Ba, X.; Jiang, H. Epigallocatechin-3-O-gallate promotes extracellular matrix and inhibits inflammation in IL-1beta stimulated chondrocytes by the PTEN/miRNA-29b pathway. Pharm. Biol. 2022, 60, 589–599. [Google Scholar] [CrossRef]
- Akhtar, N.; Haqqi, T.M. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res. Ther. 2011, 13, R93. [Google Scholar] [CrossRef]
- Rasheed, Z.; Rasheed, N.; Al-Shobaili, H.A. Epigallocatechin-3-O-gallate up-regulates microRNA-199a-3p expression by down-regulating the expression of cyclooxygenase-2 in stimulated human osteoarthritis chondrocytes. J. Cell Mol. Med. 2016, 20, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ahmed, S.; Malemud, C.J.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin-3-gallate selectively inhibits interleukin-1beta-induced activation of mitogen activated protein kinase subgroup c-Jun N-terminal kinase in human osteoarthritis chondrocytes. J. Orthop. Res. 2003, 21, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, F.; Sfriso, P.; Scanu, A.; Fiocco, U.; Spinella, P.; Punzi, L. Epigallocatechin-3-gallate reduces inflammation induced by calcium pyrophosphate crystals in vitro. Front. Pharmacol. 2013, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Chen, Z.; Pengcheng, L.; Zhang, S.; Wang, X. Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J. Cell Physiol. 2019, 234, 18192–18205. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Tang, Y.; Lu, H.; Qi, Y.; Li, G.; He, H.; Lu, F.; Yang, Y.; Sun, H. Quercetin Alleviates Osteoarthritis Progression in Rats by Suppressing Inflammation and Apoptosis via Inhibition of IRAK1/NLRP3 Signaling. J. Inflamm. Res. 2021, 14, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Xie, W.P.; Bi, Y.F.; Wang, B.A.; Song, H.B.; Wang, S.L.; Bi, R.X. Quercetin suppresses apoptosis of chondrocytes induced by IL-1beta via inactivation of p38 MAPK signaling pathway. Exp. Ther. Med. 2021, 21, 468. [Google Scholar] [CrossRef]
- Hu, Y.; Gui, Z.; Zhou, Y.; Xia, L.; Lin, K.; Xu, Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic. Biol. Med. 2019, 145, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Fu, C.; Yan, Z.; Wu, Y.; Zhan, J.; Lou, Z.; Liao, X.; Pan, J. The protective effect of hesperetin in osteoarthritis: An in vitro and in vivo study. Food Funct. 2020, 11, 2654–2666. [Google Scholar] [CrossRef]
- Ouyang, Z.; Tan, T.; Liu, C.; Duan, J.; Wang, W.; Guo, X.; Zhang, Q.; Li, Z.; Huang, Q.; Dou, P.; et al. Targeted delivery of hesperetin to cartilage attenuates osteoarthritis by bimodal imaging with Gd2(CO3)3@PDA nanoparticles via TLR-2/NF-kappaB/Akt signaling. Biomaterials 2019, 205, 50–63. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, Z.; Xie, Q.; Lei, H.; Xiang, S. Hesperidin protects against IL-1beta-induced inflammation in human osteoarthritis chondrocytes. Exp. Ther. Med. 2018, 16, 3721–3727. [Google Scholar]
- Zu, Y.; Mu, Y.; Li, Q.; Zhang, S.T.; Yan, H.J. Icariin alleviates osteoarthritis by inhibiting NLRP3-mediated pyroptosis. J. Orthop. Surg. Res. 2019, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Y.; Li, H.; Jia, L.; Wang, J.; Liang, S.; Cai, A.; Tan, X.; Wang, L.; Wang, X.; et al. Verification of pain-related neuromodulation mechanisms of icariin in knee osteoarthritis. Biomed. Pharmacother. 2021, 144, 112259. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, L.; Wang, S.; Wang, L.; Wang, X.; Xu, H.; Li, X.; Ye, H. Confirmation of inhibiting TLR4/Myd88/NFkB signalling pathway by Duhuo Jisheng decoction on osteoarthritis: A network pharmacology approach-integrated experimental study. Front. Pharmacol. 2022, 12, 784822. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Rong, X.F.; Li, R.H.; Wu, X.Y. Icariin inhibits MMP1, MMP3 and MMP13 expression through MAPK pathways in IL1betastimulated SW1353 chondrosarcoma cells. Mol. Med. Rep. 2017, 15, 2853–2858. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, T.; Cao, B.R.; Luan, F.Y.; Liu, R.X.; Yin, H.R.; Wang, W.B. Icariin possesses chondroprotective efficacy in a rat model of dexamethasone-induced cartilage injury through the activation of miR-206 targeting of cathepsin K. Int. J. Mol. Med. 2018, 41, 1039–1047. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, R. Icariin enhances cell survival in lipopolysaccharide-induced synoviocytes by suppressing ferroptosis via the Xc-/GPX4 axis. Exp. Ther. Med. 2021, 21, 72. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Zhao, S.; Xu, H.; Peng, Z.; Zhang, B.; Cai, W.; Mo, Y.; Zhao, W. Evaluation of the effectiveness and safety of icariin in the treatment of knee osteoarthritis: A protocol for a systematic review and meta-analysis. Medicine 2021, 17, e28277. [Google Scholar] [CrossRef]
- Li, J.; Luo, M.; Wang, S.; Jin, G.; Han, Z.; Ma, Y.; Tang, G.; Guo, Z. Icariin Ameliorates Lower Back Pain in Rats via Suppressing the Secretion of Cytokine-Induced Neutrophil Chemoatractant-1. Biomed Res Int. 2020, 2020, 4670604. [Google Scholar] [CrossRef]
- Schwager, J.; Richard, N.; Fowler, A.; Seifert, N.; Raederstorff, D. Carnosol and Related Substances Modulate Chemokine and Cytokine Production in Macrophages and Chondrocytes. Molecules 2016, 21, 465. [Google Scholar] [CrossRef]
- Sun, W.; Xie, W.; Huang, D.; Cui, Y.; Yue, J.; He, Q.; Jiang, L.; Xiong, J.; Sun, W.; Yi, Q. Caffeic acid phenethyl ester attenuates osteoarthritis progression by activating NRF2/HO1 and inhibiting the NFkappaB signaling pathway. Int. J. Mol. Med. 2022, 50, 1–14. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Li, S.; Wu, Y.; Yu, C.; Ni, L.; Xiao, J.; Shao, Z.; Zhu, H.; Wang, J.; et al. Tangeretin suppresses osteoarthritis progression via the Nrf2/NF-kappaB and MAPK/NF-kappaB signaling pathways. Phytomedicine 2022, 98, 153928. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, D.; Huang, L.; Jiang, C.; Pan, T.; Kang, X.; Pan, J. Nobiletin Inhibits IL-1beta-Induced Inflammation in Chondrocytes via Suppression of NF-kappaB Signaling and Attenuates Osteoarthritis in Mice. Front. Pharmacol. 2019, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Guo, L.; Tian, F.D.; An, N.; Luo, L.; Hao, R.H.; Wang, B.; Zhou, Z.H. Naringenin regulates production of matrix metalloproteinases in the knee-joint and primary cultured articular chondrocytes and alleviates pain in rat osteoarthritis model. Braz. J. Med. Biol. Res. 2017, 50, e5714. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Jin, Z.; Ren, W.; Lu, M.; Hou, M.; Zhou, Q.; Wang, W.; Yang, H.; Zou, J. Theaflavin-3,3’-Digallate Protects Cartilage from Degradation by Modulating Inflammation and Antioxidant Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 3047425. [Google Scholar] [CrossRef] [PubMed]
- Chuntakaruk, H.; Kongtawelert, P.; Pothacaroen, P. Chondroprotective effects of purple corn anthocyanins on advanced glycation end products induction through suppression of NFkB and MAPK signalling. Sci. Rep. 2021, 11, 1895. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, Z.M.; Hu, J.N.; Jin, Y.; Guo, Q.; Xu, J.J.; Chen, Z.X.; Jiang, R.H.; Wu, Y.S. Cyanidin ameliorates the progression of osteoarthritis via the Sirt6/NF-kappaB axis in vitro and in vivo. Food Funct. 2019, 10, 5873–5885. [Google Scholar] [CrossRef]
- Lin, Z.; Lin, C.; Fu, C.; Lu, H.; Jin, H.; Chen, Q.; Pan, J. The protective effect of Ellagic acid (EA) in osteoarthritis: An in vitro and in vivo study. Biomed. Pharmacother. 2020, 125, 109845. [Google Scholar] [CrossRef]
- Du, K.; Fang, X.; Li, Z. Ferulic acid suppresses interleukin-1beta-induced degeneration of chondrocytes isolated from patients with osteoarthritis through the SIRT1/AMPK/PGC-1alpha signaling pathway. Immun. Inflamm. Dis. 2021, 9, 710–720. [Google Scholar] [CrossRef]
- Zheng, W.; Feng, Z.; You, S.; Zhang, H.; Tao, Z.; Wang, Q.; Chen, H.; Wu, Y. Fisetin inhibits IL-1beta-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. Int. Immunopharmacol. 2017, 45, 135–147. [Google Scholar] [CrossRef]
- Wang, K.; Chen, X.; Chen, Y.; Sheng, S.; Huang, Z. Grape seed procyanidins suppress the apoptosis and senescence of chondrocytes and ameliorates osteoarthritis via the DPP4-Sirt1 pathway. Food Funct. 2020, 11, 10493–10505. [Google Scholar] [CrossRef]
- Qiu, B.; Xu, X.; Yi, P.; Hao, Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J. Cell. Mol. Med. 2020, 24, 10855–10865. [Google Scholar] [CrossRef] [PubMed]
- Ziadlou, R.; Barbero, A.; Martin, I.; Wang, X.; Qin, L.; Alini, M.; Grad, S. Anti-Inflammatory and Chondroprotective Effects of Vanillic Acid and Epimedin C in Human Osteoarthritic Chondrocytes. Biomolecules 2020, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Tang, J.L.; Bao, J.P.; Hu, P.F.; Shi, Z.L.; Wu, L.D. Anti-arthritic effects of chlorogenic acid in interleukin-1beta-induced rabbit chondrocytes and a rabbit osteoarthritis model. Int. Immunopharmacol. 2011, 11, 23–28. [Google Scholar] [CrossRef]
- Wu, D.Q.; Zhong, H.M.; Ding, Q.H.; Ba, L. Protective effects of biochanin A on articular cartilage: In vitro and in vivo studies. BMC Complement. Altern. Med. 2014, 14, 444. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Zhang, Y.; Fang, J.; Jin, Y. Network Pharmacology-Based Prediction and Verification of the Potential Targets of Pinoresinol Diglucoside for OA Treatment. Evid.-Based Complementary Altern. Med. 2022, 2022, 9733742. [Google Scholar] [CrossRef] [PubMed]
- Srisuthtayanont, W.; Pruksakorn, D.; Kongtawelert, P.; Pothacharoen, P. Effects of sesamin on chondroitin sulfate proteoglycan synthesis induced by interleukin-1beta in human chondrocytes. BMC Complement. Altern. Med. 2017, 17, 286. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Ding, X.; Xuan, J.; Hu, Z.; Wu, D.; Zhu, X.; Feng, Z.; Ni, W.; Wu, A. The protective effect of sinapic acid in osteoarthritis: In vitro and in vivo studies. J. Cell. Mol. Med. 2019, 23, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Huff, T.W.; Liu, J.; Yuan, T.; Wei, Z.; Qin, J. Alleviation of Cartilage Destruction by Sinapic Acid in Experimental Osteoarthritis. Biomed. Res. Int. 2019, 2019, 5689613. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Hong, H.; Wang, N.; Chen, J.; Lu, S.; Zhang, H.; Zhang, X.; Bei, C. Xanthohumol suppresses inflammation in chondrocytes and ameliorates osteoarthritis in mice. Biomed. Pharmacother. 2021, 137, 111238. [Google Scholar] [CrossRef]
- Pan, X.; Chen, T.; Zhang, Z.; Chen, X.; Chen, C.; Chen, L.; Wang, X.; Ying, X. Activation of Nrf2/HO-1 signal with Myricetin for attenuating ECM degradation in human chondrocytes and ameliorating the murine osteoarthritis. Int. Immunopharmacol. 2019, 75, 105742. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, L.; Liu, Y.; Huang, C.; Xia, W.; Zhou, H.; Zhou, Z.; Zhou, X. Luteolin Protects Chondrocytes from H2O2-Induced Oxidative Injury and Attenuates Osteoarthritis Progression by Activating AMPK-Nrf2 Signaling. Oxid. Med. Cell. Longev. 2022, 2022, 5635797. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, Y.J.; Song, M.G.; Kim, D.R.; Zada, S.; Kim, D.H. Cytoprotective Effects of Delphinidin for Human Chondrocytes against Oxidative Stress through Activation of Autophagy. Antioxidants 2020, 9, 83. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, R.; Zheng, T.; Chen, Z.; Ji, G.; Peng, F.; Wang, W. Xanthohumol Attenuated Inflammation and ECM Degradation by Mediating HO-1/C/EBPbeta Pathway in Osteoarthritis Chondrocytes. Front. Pharmacol. 2021, 12, 680585. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xi, Y.; Mao, Z.; Chu, X.; Zhang, R.; Ma, X.; Ni, B.; Cheng, H.; You, H. Vanillic acid attenuates cartilage degeneration by regulating the MAPK and PI3K/AKT/NF-kappaB pathways. Eur. J. Pharmacol. 2019, 859, 172481. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Jin, G.J.; Xiong, Y.; Hu, P.F.; Bao, J.P.; Wu, L.D. Rosmarinic acid down-regulates NO and PGE2 expression via MAPK pathway in rat chondrocytes. J. Cell. Mol. Med. 2018, 22, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kang, S.H.; Jeong, S.J.; Kim, S.H.; Ko, H.S.; Kim, S.H. Inhibition of c-Jun N-terminal kinase and nuclear factor kappa B pathways mediates fisetin-exerted anti-inflammatory activity in lipopolysccharide-treated RAW264.7 cells. Immunopharmacol. Immunotoxicol. 2012, 34, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Gomis-Ruth, F.X.; Maskos, K.; Betz, M.; Betz, M.; Bergner, A.; Huber, R.; Suzuki, K.; Yoshida, N.; Nagase, H.; Brew, K.; et al. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature 1997, 389, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, H.; Li, D.; Wu, X.; Han, Q. Punicalagin attenuates osteoarthritis progression via regulating Foxo1/Prg4/HIF3alpha axis. Bone 2021, 152, 116070. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Zhang, Y.; Tang, L.; Ji, Y.; Yan, C.; Zhang, X. Protective effects of quercetin against inflammation and oxidative stress in a rabbit model of knee osteoarthritis. Drug Dev. Res. 2019, 80, 360–367. [Google Scholar] [CrossRef]
- Mok, S.W.; Fu, S.C.; Cheuk, Y.C.; Chu, I.M.; Chan, K.M.; Qin, L.; Yung, S.H.; Kevin Ho, K.W. Intra-Articular Delivery of Quercetin Using Thermosensitive Hydrogel Attenuate Cartilage Degradation in an Osteoarthritis Rat Model. Cartilage 2020, 11, 490–499. [Google Scholar] [CrossRef]
- Britti, D.; Crupi, R.; Impellizzeri, D.; Gugliandolo, E.; Fusco, R.; Schievano, C.; Morittu, V.M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models. BMC Vet. Res. 2017, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Yabas, M.; Orhan, C.; Er, B.; Tuzcu, M.; Durmus, A.S.; Ozercan, I.H.; Sahin, N.; Bhanuse, P.; Morde, A.A.; Padigaru, M.; et al. A Next Generation Formulation of Curcumin Ameliorates Experimentally Induced Osteoarthritis in Rats via Regulation of Inflammatory Mediators. Front. Immunol. 2021, 12, 609629. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Shi, K.; Chen, G.; Shen, Y.; Pan, T. Malvidin attenuates pain and inflammation in rats with osteoarthritis by suppressing NF-kappaB signaling pathway. Inflamm. Res. 2017, 66, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.Y.; Chen, W.S.; Sun, J.S.; Lin, F.H.; Wu, T. Biological characterization of oxidized hyaluronic acid/resveratrol hydrogel for cartilage tissue engineering. J. Biomed. Mater. Res. A 2013, 101, 3457–3466. [Google Scholar] [CrossRef]
- Shakibaei, M.; John, T.; Seifarth, C.; Mobasheri, A. Resveratrol inhibits IL-1 beta-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann. N. Y. Acad. Sci. 2007, 1095, 554–563. [Google Scholar] [CrossRef]
- Wendling, D.; Abbas, W.; Godfrin-Valnet, M.; Guillot, X.; Khan, K.A.; Cedoz, J.P.; Baud, L.; Prati, C.; Herbein, G. Resveratrol, a sirtuin 1 activator, increases IL-6 production by peripheral blood mononuclear cells of patients with knee osteoarthritis. Clin. Epigenetics 2013, 5, 10. [Google Scholar] [CrossRef]
- Xiong, F.; Qin, Z.; Chen, H.; Lan, Q.; Wang, Z.; Lan, N.; Yang, Y.; Zheng, L.; Zhao, J.; Kai, D. pH-responsive and hyaluronic acid-functionalized metal-organic frameworks for therapy of osteoarthritis. J. Nanobiotechnol. 2020, 18, 139. [Google Scholar] [CrossRef]
- Wen, L.; Qu, T.B.; Zhai, K.; Ding, J.; Hai, Y.; Zhou, J.L. Gallic acid can play a chondroprotective role against AGE-induced osteoarthritis progression. J. Orthop. Sci. 2015, 20, 734–741. [Google Scholar] [CrossRef]
- Huang, X.; You, Y.; Xi, Y.; Ni, B.; Chu, X.; Zhang, R.; You, H. p-Coumaric Acid Attenuates IL-1beta-Induced Inflammatory Responses and Cellular Senescence in Rat Chondrocytes. Inflammation 2020, 43, 619–628. [Google Scholar] [CrossRef]
- Chen, M.P.; Yang, S.H.; Chou, C.H.; Yang, K.C.; Wu, C.C.; Cheng, Y.H.; Lin, F.H. The chondroprotective effects of ferulic acid on hydrogen peroxide-stimulated chondrocytes: Inhibition of hydrogen peroxide-induced pro-inflammatory cytokines and metalloproteinase gene expression at the mRNA level. Inflamm. Res. 2010, 59, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Pan, Q.; Mao, Z.; Zhang, R.; Ma, X.; Xi, Y.; You, H. Sinapic Acid Inhibits the IL-1beta-Induced Inflammation via MAPK Downregulation in Rat Chondrocytes. Inflammation 2018, 41, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xi, Y.; Pan, Q.; Mao, Z.; Zhang, R.; Ma, X.; You, H. Caffeic acid protects against IL-1beta-induced inflammatory responses and cartilage degradation in articular chondrocytes. Biomed. Pharmacother. 2018, 107, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Zhang, Y.; Dai, B.L.; Ma, Y.J.; Zhang, Q.; Wang, Y.; Yang, H. Chlorogenic acid prevents inflammatory responses in IL1betastimulated human SW1353 chondrocytes, a model for osteoarthritis. Mol. Med. Rep. 2017, 16, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Wu, L.D. Chlorogenic acid suppresses interleukin-1beta-induced inflammatory mediators in human chondrocytes. Int. J. Clin. Exp. Pathol. 2014, 7, 8797–8801. [Google Scholar]
- Huh, J.E.; Seo, D.M.; Baek, Y.H.; Choi, D.Y.; Park, D.S.; Lee, J.D. Biphasic positive effect of formononetin on metabolic activity of human normal and osteoarthritic subchondral osteoblasts. Int. Immunopharmacol. 2010, 10, 500–507. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Q.; Guo, P.; Huang, Y.; Ye, Z.; Hu, J. Antichondrocyte apoptosis effect of genistein in treating inflammationinduced osteoarthritis. Mol. Med. Rep. 2020, 22, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Cho, I.A.; Kang, K.R.; You, J.S.; Yu, S.J.; Lee, G.J.; Seo, Y.S.; Kim, C.S.; Kim, D.K.; Kim, S.G.; et al. Biochanin-A antagonizes the interleukin-1beta-induced catabolic inflammation through the modulation of NFkappaB cellular signaling in primary rat chondrocytes. Biochem. Biophys. Res. Commun. 2016, 477, 723–730. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, C.; Zhang, C.; Cai, L.; Chen, H. The protective effect of phloretin in osteoarthritis: An in vitro and in vivo study. Food Funct. 2018, 9, 263–278. [Google Scholar] [CrossRef]
- Cho, C.; Kang, L.J.; Jang, D.; Jeon, J.; Lee, H.; Choi, S.; Han, S.J.; Oh, E.; Nam, J.; Kim, C.S.; et al. Cirsium japonicum var. maackii and apigenin block Hif-2alpha-induced osteoarthritic cartilage destruction. J. Cell. Mol. Med. 2019, 23, 5369–5379. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, D.K.; Shin, H.D.; Lee, H.J.; Jo, H.S.; Jeong, J.H.; Choi, Y.L.; Lee, C.J.; Hwang, S.C. Apigenin Regulates Interleukin-1beta-Induced Production of Matrix Metalloproteinase Both in the Knee Joint of Rat and in Primary Cultured Articular Chondrocytes. Biomol. Ther. 2016, 24, 163–170. [Google Scholar] [CrossRef]
- Xue, J.; Ye, J.; Xia, Z.; Cheng, B. Effect of luteolin on apoptosis, MAPK and JNK signaling pathways in guinea pig chondrocyte with osteoarthritis. Cell. Mol. Biol. 2019, 65, 91–95. [Google Scholar] [CrossRef]
- Kang, B.J.; Ryu, J.; Lee, C.J.; Hwang, S.C. Luteolin Inhibits the Activity, Secretion and Gene Expression of MMP-3 in Cultured Articular Chondrocytes and Production of MMP-3 in the Rat Knee. Biomol. Ther. 2014, 22, 239–245. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, L.; Zheng, Y.; Liu, W.; Xu, Y. Kaempferol attenuates the effects of XIST/miR-130a/STAT3 on inflammation and extracellular matrix degradation in osteoarthritis. Future Med. Chem. 2021, 13, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Ye, G.; Huang, B. Kaempferol Alleviates the Interleukin-1beta-Induced Inflammation in Rat Osteoarthritis Chondrocytes via Suppression of NF-kappaB. Med. Sci. Monit. 2017, 23, 3925–3931. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.S.; Tseng, C.Y.; Lee, C.H.; Su, S.L.; Lee, H.S. Effects of (-)-epigallocatechin-3-gallate on cyclooxygenase 2, PGE(2), and IL-8 expression induced by IL-1beta in human synovial fibroblasts. Rheumatol. Int. 2010, 30, 1197–1203. [Google Scholar] [CrossRef]
- Li, J.; Wu, R.; Qin, X.; Liu, D.; Lin, F.; Feng, Q. Isorhamnetin inhibits IL1betainduced expression of inflammatory mediators in human chondrocytes. Mol. Med. Rep. 2017, 16, 4253–4258. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qian, Y.; Chen, C.; Feng, F.; Pan, L.; Yang, L.; Wang, C. Hesperetin Exhibits Anti-Inflammatory Effects on Chondrocytes via the AMPK Pathway to Attenuate Anterior Cruciate Ligament Transection-Induced Osteoarthritis. Front. Pharmacol. 2021, 12, 735087. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Ding, H.; Zhuang, C.; Fan, W. Effects of Hesperidin on H2O2-Treated Chondrocytes and Cartilage in a Rat Osteoarthritis Model. Med. Sci. Monit. 2018, 24, 9177–9186. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mi, B.; Lv, H.; Liu, J.; Xiong, Y.; Hu, L.; Xue, H.; Panayi, A.C.; Liu, G.; Zhou, W. Shared KEGG pathways of icariin-targeted genes and osteoarthritis. J. Cell Biochem. 2018, 120, 7741–7750. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhang, Y.; Chen, N.; Yang, L. Icariin Regulates Cellular Functions and Gene Expression of Osteoarthritis Patient-Derived Human Fibroblast-Like Synoviocytes. Int. J. Mol. Sci. 2017, 18, 2656. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Chen, D.; Haqqi, T.M. Delphinidin inhibits IL-1beta-induced activation of NF-kappaB by modulating the phosphorylation of IRAK-1(Ser376) in human articular chondrocytes. Rheumatology 2013, 52, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Song, W.H.; Lee, G.; Kim, H.S.; Park, D.; Huh, Y.H.; Ryu, J.H. Avenanthramide C as a novel candidate to alleviate osteoarthritic pathogenesis. BMB Rep. 2021, 54, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Csaki, C.; Nebrich, S.; Mobasheri, A. Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. 2008, 76, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Mobasheri, A.; Buhrmann, C. Curcumin synergizes with resveratrol to stimulate the MAPK signaling pathway in human articular chondrocytes in vitro. Genes Nutr. 2011, 6, 171–179. [Google Scholar] [CrossRef]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009, 11, R165. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, D.; Tang, J. Identification of the Resveratrol Potential Targets in the Treatment of Osteoarthritis. Evid.-Based Complementary Altern. Med. 2021, 2021, 9911286. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Zhang, W.; Cui, Z.M.; Cui, S.Y.; Fan, J.B.; Zhu, X.H.; Liu, W. Resveratrol alleviates the interleukin-1beta-induced chondrocytes injury through the NF-kappaB signaling pathway. J. Orthop. Surg. Res. 2020, 15, 424. [Google Scholar] [CrossRef]
- Ma, P.; Yue, L.; Yang, H.; Fan, Y.; Bai, J.; Li, S.; Yuan, J.; Zhang, Z.; Yao, C.; Lin, M.; et al. Chondroprotective and anti-inflammatory effects of amurensin H by regulating TLR4/Syk/NF-kappaB signals. J. Cell. Mol. Med. 2020, 24, 1958–1968. [Google Scholar] [CrossRef]
- Dave, M.; Attur, M.; Palmer, G.; Al-Mussawir, H.E.; Kennish, L.; Patel, J.; Abramson, S.B. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 2008, 58, 2786–2797. [Google Scholar] [CrossRef]
- Wongwichai, T.; Teeyakasem, P.; Pruksakorn, D.; Kongtawelert, P.; Pothacharoen, P. Anthocyanins and metabolites from purple rice inhibit IL-1beta-induced matrix metalloproteinases expression in human articular chondrocytes through the NF-kappaB and ERK/MAPK pathway. Biomed. Pharmacother. 2019, 112, 108610. [Google Scholar] [CrossRef] [PubMed]
- Garbacki, N.; Angenot, L.; Bassleer, C.; Damas, J.; Tits, M. Effects of prodelphinidins isolated from Ribes nigrum on chondrocyte metabolism and COX activity. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2002, 365, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z.; Anbazhagan, A.N.; Akhtar, N.; Ramamurthy, S.; Voss, F.R.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res. Ther. 2009, 11, R71. [Google Scholar] [CrossRef]
- Singh, R.; Ahmed, S.; Islam, N.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: Suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB. Arthritis Rheum. 2002, 46, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rahman, A.; Hasnain, A.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic. Biol. Med. 2002, 33, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Ishitobi, H.; Sanada, Y.; Kato, Y.; Ikuta, Y.; Shibata, S.; Yamasaki, S.; Lotz, M.K.; Matsubara, K.; Miyaki, S.; Adachi, N. Carnosic acid attenuates cartilage degeneration through induction of heme oxygenase-1 in human articular chondrocytes. Eur. J. Pharmacol. 2018, 830, 1–8. [Google Scholar] [CrossRef]
- Phitak, T.; Pothacharoen, P.; Settakorn, J.; Poompimol, W.; Caterson, B.; Kongtawelert, P. Chondroprotective and anti-inflammatory effects of sesamin. Phytochemistry 2012, 80, 77–88. [Google Scholar] [CrossRef]
- Estakhri, F.; Reza Panjehshahin, M.; Tanideh, N.; Gheisari, R.; Azarpira, N.; Gholijani, N. Efficacy of Combination Therapy with Apigenin and Synovial Membrane-Derived Mesenchymal Stem Cells on Knee Joint Osteoarthritis in a Rat Model. Iran, J. Med. Sci. 2021, 46, 383–394. [Google Scholar]
- Estakhri, F.; Panjehshahin, M.R.; Tanideh, N.; Gheisari, R.; Mahmoodzadeh, A.; Azarpira, N.; Gholijani, N. The effect of kaempferol and apigenin on allogenic synovial membrane-derived stem cells therapy in knee osteoarthritic male rats. Knee 2020, 27, 817–832. [Google Scholar] [CrossRef]
- Kalff, K.M.; El Mouedden, M.; van Egmond, J.; Veening, J.; Joosten, L.; Scheffer, G.J.; Meert, T.; Vissers, K. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur. J. Pharmacol. 2010, 641, 108–113. [Google Scholar] [CrossRef]
- Huang, H.T.; Cheng, T.L.; Yang, C.D.; Chang, C.F.; Ho, C.J.; Chuang, S.C.; Li, J.Y.; Huang, S.H.; Lin, Y.S.; Shen, H.Y.; et al. Intra-Articular Injection of (-)-Epigallocatechin 3-Gallate (EGCG) Ameliorates Cartilage Degeneration in Guinea Pigs with Spontaneous Osteoarthritis. Antioxidants 2021, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.T.; Cheng, T.L.; Ho, C.J.; Huang, H.H.; Lu, C.C.; Chuang, S.C.; Li, J.Y.; Lee, T.C.; Chen, S.T.; Lin, Y.S.; et al. Intra-Articular Injection of (-)-Epigallocatechin 3-Gallate to Attenuate Articular Cartilage Degeneration by Enhancing Autophagy in a Post-Traumatic Osteoarthritis Rat Model. Antioxidants 2020, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xu, P. The articular cartilage preservative effects of genistein in an experimental model of knees osteoarthritis. Appl. Physiol. Nutr. Metab. 2021, 46, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, B.; Chen, X.; Chen, D.; Yang, H. Protocatechuic acid attenuates anterior cruciate ligament transection-induced osteoarthritis by suppressing osteoclastogenesis. Exp. Ther. Med. 2020, 19, 232–240. [Google Scholar] [CrossRef]
- Xiong, W.; Lan, Q.; Liang, X.; Zhao, J.; Huang, H.; Zhan, Y.; Qin, Z.; Jiang, X.; Zheng, L. Cartilage-targeting poly(ethylene glycol) (PEG)-formononetin (FMN) nanodrug for the treatment of osteoarthritis. J. Nanobiotechnol. 2021, 19, 197. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, J.; Zhao, D.; Wang, C.; Zhang, Y.; Wang, Y.; Li, T. Therapeutic effect and mechanism of action of quercetin in a rat model of osteoarthritis. J. Int. Med. Res. 2020, 48, 300060519873461. [Google Scholar] [CrossRef]
- Heydari Nasrabadi, M.; Parsivand, M.; Mohammadi, N.; Asghari Moghaddam, N. Comparison of Elaeagnus angustifolia L. extract and quercetin on mouse model of knee osteoarthritis. J. Ayurveda Integr. Med. 2022, 13, 100529. [Google Scholar] [CrossRef]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother. 2018, 103, 1585–1591. [Google Scholar] [CrossRef]
- Permatasari, D.A.; Karliana, D.; Iskandarsyah, I.; Arsianti, A.; Bahtiar, A. Quercetin prevent proteoglycan destruction by inhibits matrix metalloproteinase-9, matrix metalloproteinase-13, a disintegrin and metalloproteinase with thrombospondin motifs-5 expressions on osteoarthritis model rats. J. Adv. Pharm. Technol. Res. 2019, 10, 2–8. [Google Scholar]
- Zhou, Z.; Deng, Z.; Liu, Y.; Zheng, Y.; Yang, S.; Lu, W.; Xiao, D.; Zhu, W. Protective Effect of SIRT1 Activator on the Knee with Osteoarthritis. Front. Physiol. 2021, 12, 661852. [Google Scholar] [CrossRef]
- Wang, Z.M.; Chen, Y.C.; Wang, D.P. Resveratrol, a natural antioxidant, protects monosodium iodoacetate-induced osteoarthritic pain in rats. Biomed. Pharmacother. 2016, 83, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.J.; Liu, C.C.; Chen, L.M.; Wu, G.H.; Wang, X.P. Reduction of SIRT1 Mediates Monosodium Iodoacetate-Induced Osteoarthritic Pain by Upregulating p53 Expression in Rats. Pain Physician 2021, 24, E1025–E1035. [Google Scholar] [PubMed]
- Long, Z.; Xiang, W.; Li, J.; Yang, T.; Yu, G. Exploring the Mechanism of Resveratrol in Reducing the Soft Tissue Damage of Osteoarthritis Based on Network Pharmacology and Experimental Pharmacology. Evid.-Based Complementary Altern. Med. 2021, 2021, 9931957. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Li, X.; Yu, X.; Liu, X.; Xu, X.; He, J.; Gu, H.; Liu, L. Oral Administration of Resveratrol Alleviates Osteoarthritis Pathology in C57BL/6J Mice Model Induced by a High-Fat Diet. Mediat. Inflamm. 2017, 2017, 7659023. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Xu, Z.; Zhao, X.; Yuan, M.; Pan, L.; Lu, T.; Du, A.; Qin, L. In Vivo Effect of Resveratrol-Cellulose Aerogel Drug Delivery System to Relieve Inflammation on Sports Osteoarthritis. Gels 2022, 8, 544. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, Z.; Zhang, L.; Li, X.; Xu, B.; Xiao, Y.; Shi, X.; Zhang, H.; Liao, T.; Wang, P. Vanillic Acid Reduces Pain-Related Behavior in Knee Osteoarthritis Rats Through the Inhibition of NLRP3 Inflammasome-Related Synovitis. Front. Pharmacol. 2020, 11, 599022. [Google Scholar] [CrossRef]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Efficacy and safety of combination of curcuminoid complex and diclofenac versus diclofenac in knee osteoarthritis: A randomized trial. Medicine 2020, 99, e19723. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Efficacy and safety of Meriva(R), a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern. Med. Rev. 2010, 15, 337–344. [Google Scholar]
- Marouf, B.H. Effect of Resveratrol on Serum Levels of Type II Collagen and Aggrecan in Patients with Knee Osteoarthritis: A Pilot Clinical Study. Biomed. Res. Int. 2021, 2021, 3668568. [Google Scholar] [CrossRef]
- Marouf, B.H.; Hussain, S.A.; Ali, Z.S.; Ahmmad, R.S. Resveratrol Supplementation Reduces Pain and Inflammation in Knee Osteoarthritis Patients Treated with Meloxicam: A Randomized Placebo-Controlled Study. J. Med. Food 2018, 21, 1253–1259. [Google Scholar] [CrossRef]
- Matsuno, H.; Nakamura, H.; Katayama, K.; Hayashi, S.; Kano, S.; Yudoh, K.; Kiso, Y. Effects of an oral administration of glucosamine-chondroitin-quercetin glucoside on the synovial fluid properties in patients with osteoarthritis and rheumatoid arthritis. Biosci. Biotechnol. Biochem. 2009, 73, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, N.; Saito, K.; Maeda, A.; Kitagawa, Y.; Kiso, Y.; Watanabe, K.; Tomonaga, A.; Nagaoka, I.; Yamaguchi, H. Effect of a dietary supplement containing glucosamine hydrochloride, chondroitin sulfate and quercetin glycosides on symptomatic knee osteoarthritis: A randomized, double-blind, placebo-controlled study. J. Sci. Food Agric. 2012, 92, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.S.M.; Stocks, J.; Sarmanova, A.; Fernandes, G.; Walsh, D.A.; Doherty, M.; Zhang, W. Individual responses to topical ibuprofen gel or capsaicin cream for painful knee osteoarthritis: A series of n-of-1 trials. Rheumatology 2021, 60, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.M.; Ervin, J.; Nezzer, J.; Nieves, Y.; Guedes, K.; Burges, R.; Hanson, P.D.; Campbell, J.N. Randomized, Double-Blind, Placebo-Controlled Trial of Intraarticular Trans-Capsaicin for Pain Associated With Osteoarthritis of the Knee. Arthritis Rheumatol. 2019, 71, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Farid, R.; Mirfeizi, Z.; Mirheidari, M.; Hatef, M.R.; Mirheidari, M.; Mansouri, H.; Esmaelli, H.; Bentley, G.; Lu, Y.; Foo, Y.; et al. Pycnogenol supplementation reduces pain and stiffness and improves physical function in adults with knee osteoarthritis. Nutr. Res. 2007, 27, 692–697. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Errichi, S.; Zulli, C.; Errichi, B.M.; Vinciguerra, G.; Ledda, A.; Di Renzo, A.; Stuard, S.; Dugall, M.; et al. Variations in C-reactive protein, plasma free radicals and fibrinogen values in patients with osteoarthritis treated with Pycnogenol. Redox Rep. 2008, 13, 271–276. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Daliri, E.B.M.; Elahi, F.; Chelliah, R.; Lee, B.H.; Oh, D.H. New Insights on the Use of Polyphenols as Natural Preservatives and Their Emerging Safety Concerns. Front. Sustain. Food Syst. 2020, 4, 525810. [Google Scholar] [CrossRef]
- Ochi, M.; Uchio, Y.; Tobita, M.; Kuriwaka, M. Current concepts in tissue engineering technique for repair of cartilage defect. Artif. Organs 2001, 25, 172–179. [Google Scholar] [CrossRef]
- Rompicherla, N.C.; Joshi, P.; Shetty, A.; Sudhakar, K.; Amin, H.I.M.; Mishra, Y.; Mishra, V.; Albutti, A.; Alhumeed, N. Design, Formulation, and Evaluation of Aloe vera Gel-Based Capsaicin Transemulgel for Osteoarthritis. Pharmaceutics 2022, 14, 1812. [Google Scholar] [CrossRef]
- Gutierres, V.O.; Campos, M.L.; Arcaro, C.A.; Assis, R.P.; Baldan-Cimatti, H.M.; Peccinini, R.G.; Paula-Gomes, S.; Kettelhut, I.C.; Baviera, A.M.; Brunetti, I.L. Curcumin Pharmacokinetic and Pharmacodynamic Evidences in Streptozotocin-Diabetic Rats Support the Antidiabetic Activity to Be via Metabolite(s). Evid.-Based Complementary Altern. Med. 2015, 2015, 678218. [Google Scholar] [CrossRef]
- Azzolini, M.; La Spina, M.; Mattarei, A.; Paradisi, C.; Zoratti, M.; Biasutto, L. Pharmacokinetics and tissue distribution of pterostilbene in the rat. Mol. Nutr. Food Res. 2014, 58, 2122–2132. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Horio, F.; Osawa, T. Absorption and metabolism of cyanidin 3-O-beta-D-glucoside in rats. FEBS Lett. 1999, 449, 179–182. [Google Scholar] [CrossRef] [PubMed]
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Blanchard, O. Enhancing the delivery of resveratrol in humans: If low bioavailability is the problem, what is the solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Eseberri, I.; Trepiana, J.; Leniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; Portillo, M.P. Variability in the Beneficial Effects of Phenolic Compounds: A Review. Nutrients 2022, 14, 1925. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteoarthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ Novel Formulations: The Good, the Bad, the Unknown and Patents Involved. Recent Pat. Drug Deliv. Formul. 2019, 13, 105–156. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals—Shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef]
- Sirse, M. Effect of Dietary Polyphenols on Osteoarthritis-Molecular Mechanisms. Life 2022, 12, 436. [Google Scholar] [CrossRef]
- Puttasiddaiah, R.; Lakshminarayana, R.; Somashekar, N.L.; Gupta, V.K.; Inbaraj, B.S.; Usmani, Z.; Raghavendra, V.; Sridhar, K.; Sharma, M. Advances in Nanofabrication Technology for Nutraceuticals: New Insights and Future Trends. Bioengineering 2022, 9, 478. [Google Scholar] [CrossRef]
- Jayusman, P.A.; Nasruddin, N.S.; Mahamad Apandi, N.I.; Ibrahim, N.; Budin, S.B. Therapeutic Potential of Polyphenol and Nanoparticles Mediated Delivery in Periodontal Inflammation: A Review of Current Trends and Future Perspectives. Front. Pharmacol. 2022, 13, 847702. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.; Krithica, N.; Madhan, B.; Sehgal, P.K. Formulation and evaluation of quercetin polycaprolactone microspheres for the treatment of rheumatoid arthritis. J. Pharm. Sci. 2011, 100, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef] [PubMed]
- Maudens, P.; Jordan, O.; Allemann, E. Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug Discov. Today 2018, 23, 1761–1775. [Google Scholar] [CrossRef]
- Le Clanche, S.; Cheminel, T.; Rannou, F.; Bonnefont-Rousselot, D.; Borderie, D.; Charrueau, C. Use of Resveratrol Self-Emulsifying Systems in T/C28a2 Cell Line as Beneficial Effectors in Cellular Uptake and Protection Against Oxidative Stress-Mediated Death. Front. Pharmacol. 2018, 9, 538. [Google Scholar] [CrossRef]
- Cavalcanti, G.R.; Duarte, F.I.C.; Converti, A.; de Lima, A.A.N. Ferulic Acid Activity in Topical Formulations: Technological and Scientific Prospecting. Curr. Pharm. Des. 2021, 27, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A. Intersection of inflammation and herbal medicine in the treatment of osteoarthritis. Curr. Rheumatol. Rep. 2012, 14, 604–616. [Google Scholar] [CrossRef]
- Oskouie, M.N.; Aghili Moghaddam, N.S.; Butler, A.E.; Butler, A.E.; Zamani, P.; Sahebkar, A. Therapeutic use of curcumin-encapsulated and curcumin-primed exosomes. J. Cell Physiol. 2019, 234, 8182–8191. [Google Scholar] [CrossRef]
- Gleason, B.; Chisari, E.; Parvizi, J. Osteoarthritis Can Also Start in the Gut: The Gut-Joint Axis. Indian J. Orthop. 2022, 56, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Ramires, L.C.; Santos, G.S.; Ramires, R.P.; da Fonseca, L.F.; Jeyaraman, M.; Muthu, S.; Lana, A.V.; Azzini, G.; Smith, C.S.; Lana, J.F. The Association between Gut Microbiota and Osteoarthritis: Does the Disease Begin in the Gut? Int. J. Mol. Sci. 2022, 23, 1494. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Hong, W.; Qian, D.; Peng, F.; Li, H.; Liang, C.; Du, M.; Gu, J.; Mai, J.; Bai, B.; et al. Quercetin modulates the gut microbiota as well as the metabolome in a rat model of osteoarthritis. Bioengineered 2021, 12, 6240–6250. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).