L-RAPiT: A Cloud-Based Computing Pipeline for the Analysis of Long-Read RNA Sequencing Data
Abstract
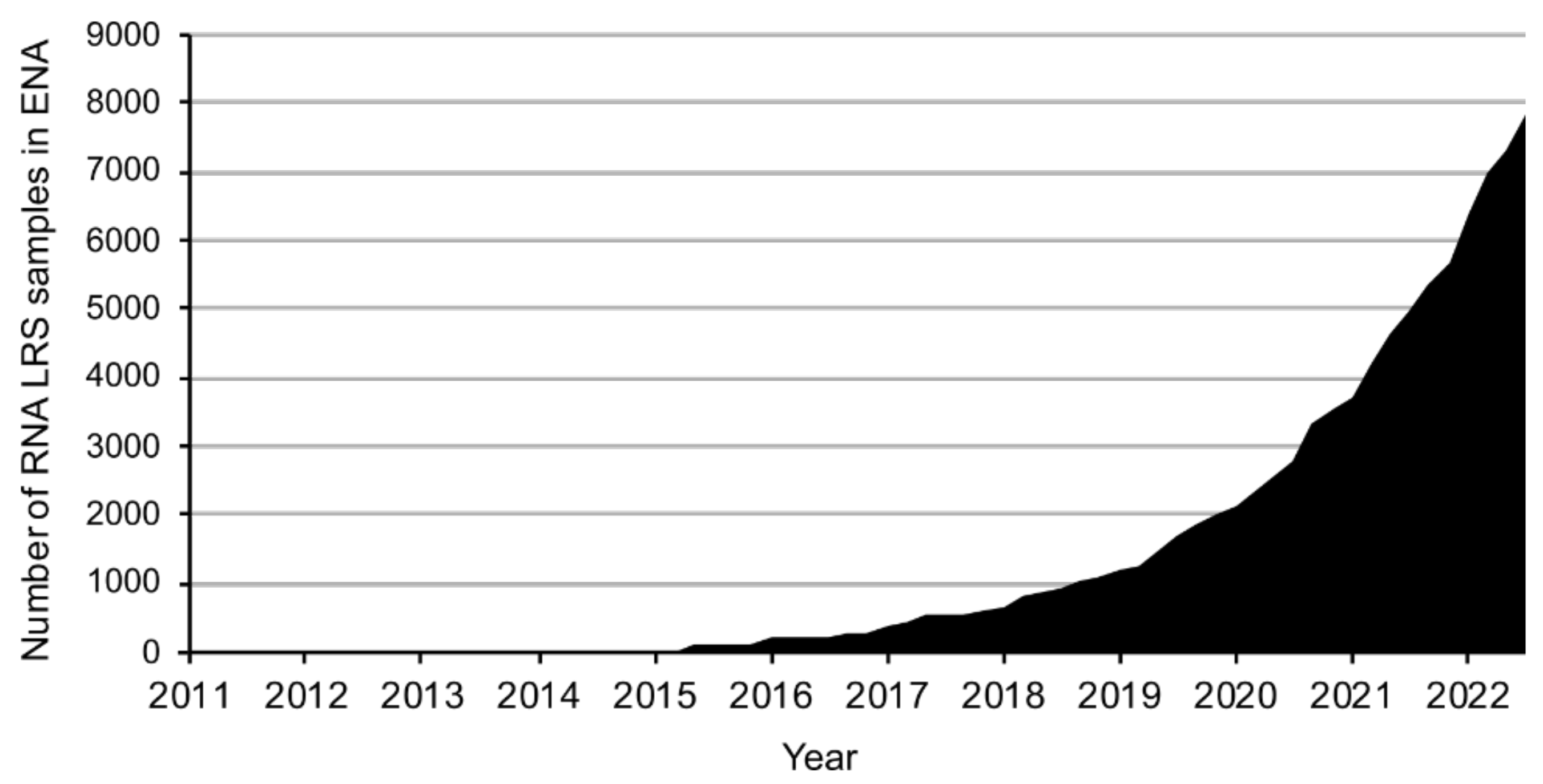
1. Introduction
2. Results
2.1. Conceptual Framework
2.2. User Experience
2.3. The Core Pipeline
2.4. Optional Pipeline Components
2.4.1. Source Data
2.4.2. Transcript Quantification and Characterization
2.4.3. Region-Specific Analysis
2.4.4. Region-Specific Visualization
2.4.5. Transcriptome-Wide Visualization
2.4.6. Quality Control
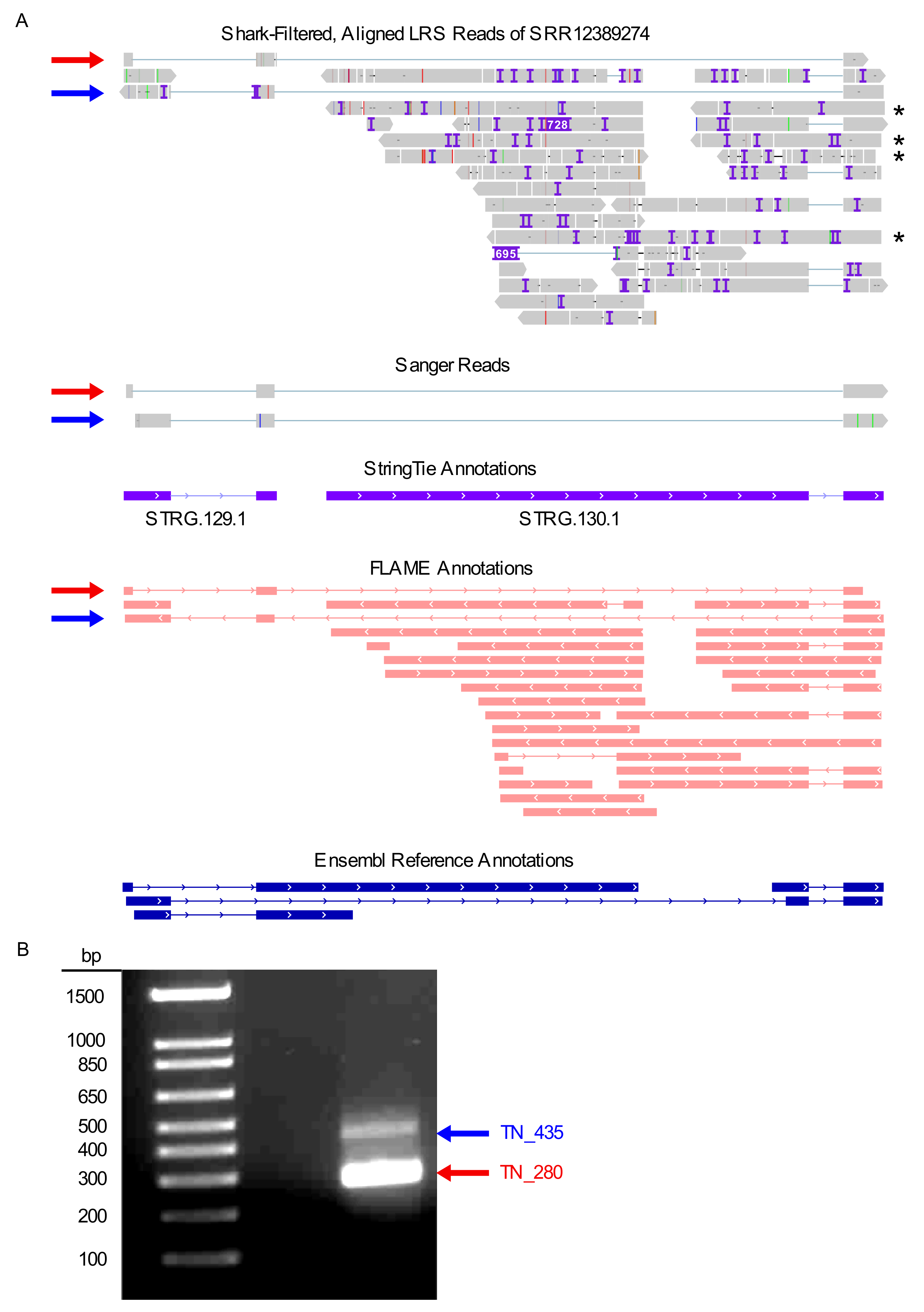
2.5. Use Case: Discovery and Validation of Novel Splice Variants
2.5.1. Background
2.5.2. Sample History
2.5.3. Input, Installation, and Data Retrieval
%env PIPELINE_FILE_PATH=/content
%env ACC=SRR12389274
%env INDEX_FILE_PATH=${PIPELINE_FILE_PATH}/long-read-sequencing-pipeline/prebuilt_indices/hg38.fa
%env ANNOTATION_FILE_PATH=${PIPELINE_FILE_PATH}/long-read-sequencing-pipeline/prebuilt_indices/hg38.ensGene.gtf
%env CHROMOSOME=chr12 %env CHROMOSOME_START=116533422 %env CHROMOSOME_FINISH=116536513
%env REGION_NAME=LINC00173
%env HUB_KEYWORD=LINC00173 %env HUB_NAME=“Human LINC00173” %env HUB_EMAIL=your@email.address
2.5.4. Read Filtering
2.5.5. Alignment
L-RAPiT Default Setting: Spliced Reads
Setting for High-Quality Sequences
Setting for Nanopore Direct RNA-seq
Use Case
Evaluation and Experimental Validation
2.5.6. Annotation
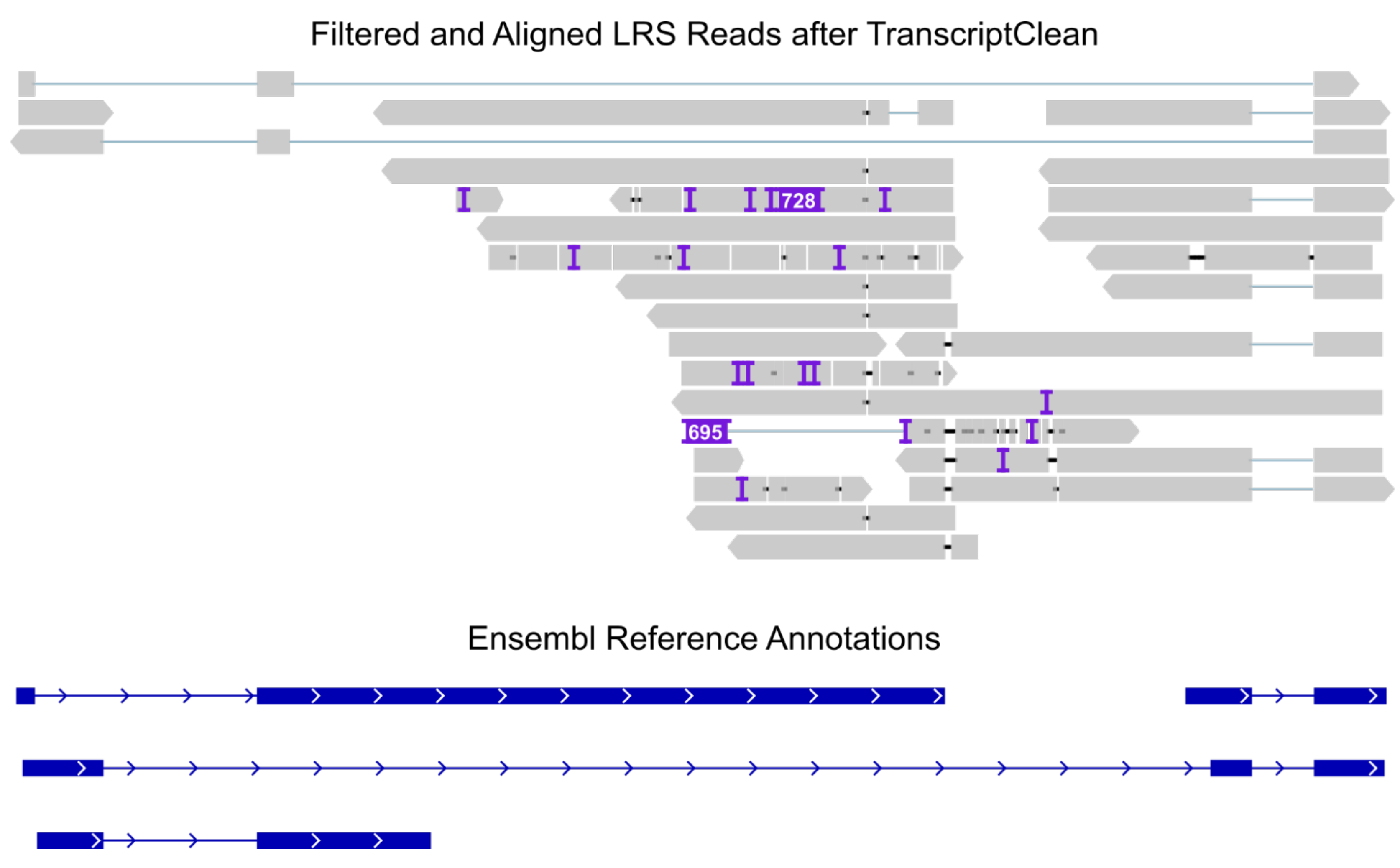
2.5.7. Read Correction
2.5.8. Read Counting
2.5.9. Visualization
2.5.10. Quality Control
3. Discussion
4. Materials and Methods
Experimental Validation of Novel Splice Variants in the Use Case
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mantere, T.; Kersten, S.; Hoischen, A. Long-Read Sequencing Emerging in Medical Genetics. Front. Genet. 2019, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulou, K.; Boti, M.A.; Adamopoulos, P.G.; Skourou, P.C.; Scorilas, A. Third-Generation Sequencing: The Spearhead towards the Radical Transformation of Modern Genomics. Life 2021, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, P.J.; Abood, A.; Farber, C.R.; Sheynkman, G.M. Bridging the Splicing Gap in Human Genetics with Long-Read RNA Sequencing: Finding the Protein Isoform Drivers of Disease. Hum. Mol. Genet. 2022, 31, ddac196. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, G.A.; Vollger, M.R.; Eichler, E.E. Long-Read Human Genome Sequencing and Its Applications. Nat. Rev. Genet. 2020, 21, 597–614. [Google Scholar] [CrossRef]
- Uszczynska-Ratajczak, B.; Lagarde, J.; Frankish, A.; Guigó, R.; Johnson, R. Towards a Complete Map of the Human Long Non-Coding RNA Transcriptome. Nat. Rev. Genet. 2018, 19, 535–548. [Google Scholar] [CrossRef]
- Glinos, D.A.; Garborcauskas, G.; Hoffman, P.; Ehsan, N.; Jiang, L.; Gokden, A.; Dai, X.; Aguet, F.; Brown, K.L.; Garimella, K.; et al. Transcriptome Variation in Human Tissues Revealed by Long-Read Sequencing. Nature 2022, 608, 353–359. [Google Scholar] [CrossRef]
- Chang, J.J.-Y.; Gleeson, J.; Rawlinson, D.; De Paoli-Iseppi, R.; Zhou, C.; Mordant, F.L.; Londrigan, S.L.; Clark, M.B.; Subbarao, K.; Stinear, T.P.; et al. Long-Read RNA Sequencing Identifies Polyadenylation Elongation and Differential Transcript Usage of Host Transcripts During SARS-CoV-2 In Vitro Infection. Front. Immunol. 2022, 13, 832223. [Google Scholar] [CrossRef]
- Dong, X.; Du, M.R.M.; Gouil, Q.; Tian, L.; Baldoni, P.L.; Smyth, G.K.; Amarasinghe, S.L.; Law, C.W.; Ritchie, M.E. Benchmarking Long-Read RNA-Sequencing Analysis Tools Using in Silico Mixtures. bioRxiv 2022. online preprint. [Google Scholar] [CrossRef]
- Križanović, K.; Echchiki, A.; Roux, J.; Šikić, M. Evaluation of Tools for Long Read RNA-Seq Splice-Aware Alignment. Bioinformatics 2018, 34, 748–754. [Google Scholar] [CrossRef]
- Kuo, R.I.; Tseng, E.; Eory, L.; Paton, I.R.; Archibald, A.L.; Burt, D.W. Normalized Long Read RNA Sequencing in Chicken Reveals Transcriptome Complexity Similar to Human. BMC Genom. 2017, 18, 323. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, J.; Zhao, S.; Hou, Y.; Li, F.; Tai, Y.; Wan, X.; Wei, C. Transcriptome Profiling Using Single-Molecule Direct RNA Sequencing Approach for In-Depth Understanding of Genes in Secondary Metabolism Pathways of Camellia Sinensis. Front. Plant Sci. 2017, 8, 1205. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, S.L.; Su, S.; Dong, X.; Zappia, L.; Ritchie, M.E.; Gouil, Q. Opportunities and Challenges in Long-Read Sequencing Data Analysis. Genome Biol. 2020, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, S.L.; Ritchie, M.E.; Gouil, Q. Long-Read-Tools.Org: An Interactive Catalogue of Analysis Methods for Long-Read Sequencing Data. GigaScience 2021, 10, giab003. [Google Scholar] [CrossRef] [PubMed]
- The Bioconda Team; Grüning, B.; Dale, R.; Sjödin, A.; Chapman, B.A.; Rowe, J.; Tomkins-Tinch, C.H.; Valieris, R.; Köster, J. Bioconda: Sustainable and Comprehensive Software Distribution for the Life Sciences. Nat. Methods 2018, 15, 475–476. [Google Scholar] [CrossRef]
- Woodcroft, B. Kingfisher. Available online: https://github.com/wwood/kingfisher-download (accessed on 10 January 2022).
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 10 January 2022).
- Denti, L.; Pirola, Y.; Previtali, M.; Ceccato, T.; Della Vedova, G.; Rizzi, R.; Bonizzoni, P. Shark: Fishing Relevant Reads in an RNA-Seq Sample. Bioinformatics 2021, 37, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Minimap2: Pairwise Alignment for Nucleotide Sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Wyman, D.; Mortazavi, A. TranscriptClean: Variant-Aware Correction of Indels, Mismatches and Splice Junctions in Long-Read Transcripts. Bioinformatics 2019, 35, 340–342. [Google Scholar] [CrossRef]
- Holmqvist, I.; Bäckerholm, A.; Tian, Y.; Xie, G.; Thorell, K.; Tang, K.-W. FLAME: Long-Read Bioinformatics Tool for Comprehensive Spliceome Characterization. RNA 2021, 27, 1127–1139. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Hu, Y.; Fang, L.; Chen, X.; Zhong, J.F.; Li, M.; Wang, K. LIQA: Long-Read Isoform Quantification and Analysis. Genome Biol. 2021, 22, 182. [Google Scholar] [CrossRef] [PubMed]
- Chen, M. FusionSeeker. Available online: https://github.com/Maggi-Chen/FusionSeeker (accessed on 26 August 2022).
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Pertea, G.; Pertea, M. GFF Utilities: GffRead and GffCompare. F1000Res 2020, 9, 304. [Google Scholar] [CrossRef]
- Egorov, A.A.; Sakharova, E.A.; Anisimova, A.S.; Dmitriev, S.E.; Gladyshev, V.N.; Kulakovskiy, I.V. Svist4get: A Simple Visualization Tool for Genomic Tracks from Sequencing Experiments. BMC Bioinform. 2019, 20, 113. [Google Scholar] [CrossRef] [PubMed]
- Hall, M. Pistis. Available online: https://github.com/mbhall88/pistis (accessed on 3 August 2022).
- Hoff, K.J. MakeHub: Fully Automated Generation of UCSC Genome Browser Assembly Hubs. Genom. Proteom. Bioinform. 2019, 17, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative Genomics Viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Cummins, C.; Ahamed, A.; Aslam, R.; Burgin, J.; Devraj, R.; Edbali, O.; Gupta, D.; Harrison, P.W.; Haseeb, M.; Holt, S.; et al. The European Nucleotide Archive in 2021. Nucleic Acids Res. 2022, 50, D106–D110. [Google Scholar] [CrossRef]
- Leinonen, R.; Sugawara, H.; Shumway, M.; on behalf of the International Nucleotide Sequence Database Collaboration. The Sequence Read Archive. Nucleic Acids Res. 2011, 39, D19–D21. [Google Scholar] [CrossRef]
- Holley, G.; Beyter, D.; Ingimundardottir, H.; Møller, P.L.; Kristmundsdottir, S.; Eggertsson, H.P.; Halldorsson, B.V. Ratatosk: Hybrid Error Correction of Long Reads Enables Accurate Variant Calling and Assembly. Genome Biol. 2021, 22, 28. [Google Scholar] [CrossRef]
- Karolchik, D. The UCSC Genome Browser Database. Nucleic Acids Res. 2003, 31, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The Human Genome Browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Frankish, A.; Diekhans, M.; Ferreira, A.-M.; Johnson, R.; Jungreis, I.; Loveland, J.; Mudge, J.M.; Sisu, C.; Wright, J.; Armstrong, J.; et al. GENCODE Reference Annotation for the Human and Mouse Genomes. Nucleic Acids Res. 2019, 47, D766–D773. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Lal, A. Long Noncoding RNAs in the P53 Network. Wiley Interdiscip Rev. RNA 2017, 8, e1410. [Google Scholar] [CrossRef] [PubMed]
- Goff, L.A.; Rinn, J.L. Linking RNA Biology to LncRNAs. Genome Res. 2015, 25, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.B.-T.; Ulitsky, I. The Functions of Long Noncoding RNAs in Development and Stem Cells. Development 2016, 143, 3882–3894. [Google Scholar] [CrossRef]
- Pircher, A.; Gebetsberger, J.; Polacek, N. Ribosome-Associated NcRNAs: An Emerging Class of Translation Regulators. RNA Biol. 2014, 11, 1335–1339. [Google Scholar] [CrossRef]
- Schwarzer, A.; Emmrich, S.; Schmidt, F.; Beck, D.; Ng, M.; Reimer, C.; Adams, F.F.; Grasedieck, S.; Witte, D.; Käbler, S.; et al. The Non-Coding RNA Landscape of Human Hematopoiesis and Leukemia. Nat. Commun. 2017, 8, 218. [Google Scholar] [CrossRef]
- Postler, T.S.; Pantry, S.N.; Desrosiers, R.C.; Ghosh, S. Identification and Characterization of a Long Non-Coding RNA up-Regulated during HIV-1 Infection. Virology 2017, 511, 30–39. [Google Scholar] [CrossRef]
- Mao, Y.; Fu, Z.; Zhang, Y.; Dong, L.; Zhang, Y.; Zhang, Q.; Li, X.; Liu, J. A Seven-LncRNA Signature Predicts Overall Survival in Esophageal Squamous Cell Carcinoma. Sci. Rep. 2018, 8, 8823. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, D.; Tang, Y.; Zhang, X.; Wei, Z.; Fu, H.; Xu, J.; Zhu, Z.; Cai, Q. Five-Long Non-Coding RNA Risk Score System for the Effective Prediction of Gastric Cancer Patient Survival. Oncol. Lett. 2019, 17, 4474–4486. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Tang, Y.; Tang, C.; Cong, H.; Wang, X.; Shen, X.; Ju, S. Diminished LINC00173 Expression Induced MiR-182-5p Accumulation Promotes Cell Proliferation, Migration and Apoptosis Inhibition via AGER/NF-ΚB Pathway in Non-Small-Cell Lung Cancer. Am. J. Transl. Res. 2019, 11, 4248–4262. [Google Scholar] [PubMed]
- Zeng, F.; Wang, Q.; Wang, S.; Liang, S.; Huang, W.; Guo, Y.; Peng, J.; Li, M.; Zhu, W.; Guo, L. Linc00173 Promotes Chemoresistance and Progression of Small Cell Lung Cancer by Sponging MiR-218 to Regulate Etk Expression. Oncogene 2020, 39, 293–307. [Google Scholar] [CrossRef]
- Fan, H.; Yuan, J.; Li, X.; Ma, Y.; Wang, X.; Xu, B.; Li, X. LncRNA LINC00173 Enhances Triple-Negative Breast Cancer Progression by Suppressing MiR-490-3p Expression. Biomed. Pharmacother. 2020, 125, 109987. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Zhu, H.; Liu, Y.; Cao, J.; Li, D.; Ding, B.; Yan, W.; Jin, H.; Wang, S. Identification of Potential Prognostic Long Non-Coding RNA Biomarkers for Predicting Recurrence in Patients with Cervical Cancer. Cancer Manag. Res. 2020, 12, 719–730. [Google Scholar] [CrossRef]
- Chen, J.; Liu, A.; Wang, Z.; Wang, B.; Chai, X.; Lu, W.; Cao, T.; Li, R.; Wu, M.; Lu, Z.; et al. LINC00173.v1 Promotes Angiogenesis and Progression of Lung Squamous Cell Carcinoma by Sponging MiR-511-5p to Regulate VEGFA Expression. Mol. Cancer 2020, 19, 98. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, X.; Yang, C.; Yin, F. Long Noncoding RNA LINC00173 Contributes to the Growth, Invasiveness and Chemo-Resistance of Colorectal Cancer Through Regulating MiR-765/PLP2 Axis. CMAR 2020, 12, 3363–3369. [Google Scholar] [CrossRef]
- Du, Q.; Liu, J.; Tian, D.; Zhang, X.; Zhu, J.; Qiu, W.; Wu, J. Long Noncoding RNA LINC00173 Promotes NUTF2 Expression Through Sponging MiR-765 and Facilitates Tumorigenesis in Glioma. CMAR 2020, 12, 7211–7217. [Google Scholar] [CrossRef]
- Hu, C.-H.; Yang, X.-J.; Yu, L.; Wang, L.-Y.; Zhao, X.-C.; Han, C.-H. Long Non-Coding RNA LINC00173 Serves as Sponge for MiR-338-3p to Promote Prostate Cancer Progression via Regulating Rab25. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9290–9302. [Google Scholar] [CrossRef]
- Li, Q.; Chen, X.; Chen, L.; Yan, H.; Li, J. LINC00173 Promotes the Apoptosis of Hypertrophic Scar Fibroblasts through Increasing β-Catenin Expression. Mol. Cell Biochem. 2021, 476, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, A.; Sun, S.; Ding, Y. Long Non-Coding RNA LINC00173 Enhances Cisplatin Resistance in Hepatocellular Carcinoma via the MicroRNA-641/RAB14 Axis. Oncol. Lett. 2021, 21, 371. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, X.; Yang, X.; Zhang, B.; Gu, Y.; Gu, G.; Xiong, J.; Li, Y.; Qian, Z. Long Intergenic Nonprotein Coding RNA 173 Inhibits Tumor Growth and Promotes Apoptosis by Repressing Sphingosine Kinase 1 Protein Expression in Pancreatic Cancer. DNA Cell Biol. 2021, 40, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, T.; Lu, X.; Zhang, H.; Liu, L.; Kong, X.; Li, S.; Wang, X.; Gao, H.; Wang, J.; et al. Identification of LINC00173 in Myasthenia Gravis by Integration Analysis of Aberrantly Methylated- Differentially Expressed Genes and CeRNA Networks. Front. Genet. 2021, 12, 726751. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhan, D.; Yang, Y.; Chong, Y.; Xue, H.; Zhu, P. LINC00173 Promotes Wilms’ Tumor Progression through MGAT1-Mediated MUC3A N-Glycosylation. Cell Cycle 2022, 21, 1795–1810. [Google Scholar] [CrossRef]
- May-Hau, D.I.; Bárcenas-López, D.A.; Núñez-Enríquez, J.C.; Bekker-Méndez, V.C.; Beltrán-Anaya, F.O.; Jiménez-Hernández, E.; Ortíz-Maganda, M.P.; Guerra-Castillo, F.X.; Medina-Sanson, A.; Flores-Lujano, J.; et al. Underexpression of LINC00173 in TCF3/PBX1-Positive Cases Is Associated With Poor Prognosis in Children with B-Cell Precursor Acute Lymphoblastic Leukemia. Front. Oncol. 2022, 12, 887766. [Google Scholar] [CrossRef]
- Brannan, K.W.; Chaim, I.A.; Marina, R.J.; Yee, B.A.; Kofman, E.R.; Lorenz, D.A.; Jagannatha, P.; Dong, K.D.; Madrigal, A.A.; Underwood, J.G.; et al. Robust Single-Cell Discovery of RNA Targets of RNA-Binding Proteins and Ribosomes. Nat. Methods 2021, 18, 507–519. [Google Scholar] [CrossRef]
- Baichoo, S.; Ouzounis, C.A. Computational Complexity of Algorithms for Sequence Comparison, Short-Read Assembly and Genome Alignment. Biosystems 2017, 156–157, 72–85. [Google Scholar] [CrossRef]
- Soneson, C.; Yao, Y.; Bratus-Neuenschwander, A.; Patrignani, A.; Robinson, M.D.; Hussain, S. A Comprehensive Examination of Nanopore Native RNA Sequencing for Characterization of Complex Transcriptomes. Nat. Commun. 2019, 10, 3359. [Google Scholar] [CrossRef]
- Depledge, D.P.; Srinivas, K.P.; Sadaoka, T.; Bready, D.; Mori, Y.; Placantonakis, D.G.; Mohr, I.; Wilson, A.C. Direct RNA Sequencing on Nanopore Arrays Redefines the Transcriptional Complexity of a Viral Pathogen. Nat. Commun. 2019, 10, 754. [Google Scholar] [CrossRef]
- Blankenberg, D.; Gordon, A.; Von Kuster, G.; Coraor, N.; Taylor, J.; Nekrutenko, A. The Galaxy Team Manipulation of FASTQ Data with Galaxy. Bioinformatics 2010, 26, 1783–1785. [Google Scholar] [CrossRef] [PubMed]
- de Koning, W.; Miladi, M.; Hiltemann, S.; Heikema, A.; Hays, J.P.; Flemming, S.; van den Beek, M.; Mustafa, D.A.; Backofen, R.; Grüning, B.; et al. NanoGalaxy: Nanopore Long-Read Sequencing Data Analysis in Galaxy. GigaScience 2020, 9, giaa105. [Google Scholar] [CrossRef] [PubMed]
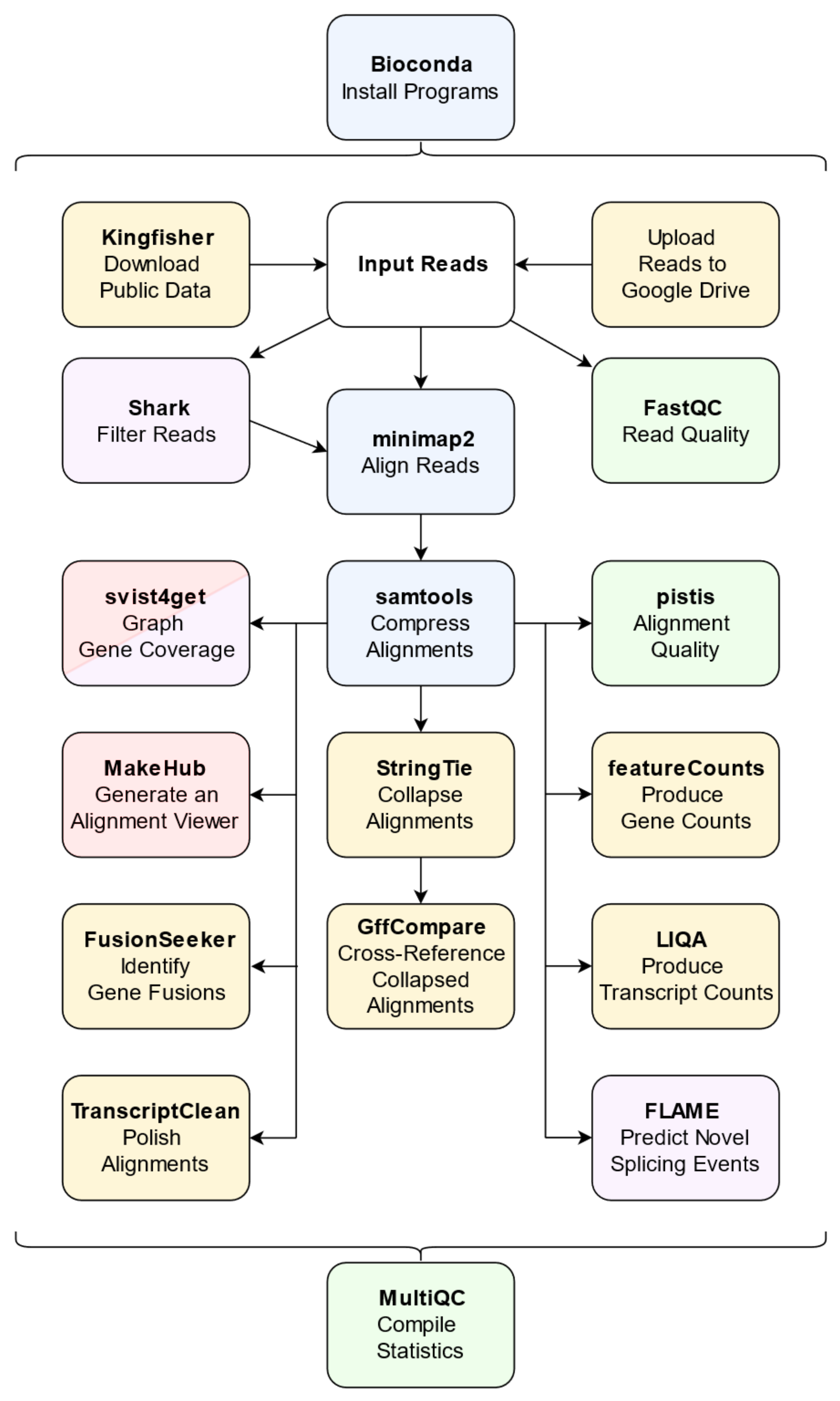


| Program | Function | Importance | Ref. |
|---|---|---|---|
| Google Drive | permanent cloud storage | optional | n/a |
| BioConda | bioinformatics software package manager | required | [14] |
| Kingfisher | download sequencing files from the European Nucleotide Archive and Sequence Read Archive | optional | [15] |
| FastQC | initial sequencing data quality report | optional | [16] |
| Shark | region-specific read filtering | optional | [17] |
| minimap2 | read alignment | required | [18] |
| SAMtools | sort and compress alignments | required | [19] |
| TranscriptClean | remove insertions/deletions from alignments | optional | [20] |
| FLAME | region-specific characterization of splicing patterns | optional | [21] |
| featureCounts | read quantification | optional | [22] |
| LIQA | transcript quantification | optional | [23] |
| FusionSeeker | detection of gene fusion events | optional | [24] |
| StringTie | transcript assembly | optional | [25] |
| GffCompare | transcript assembly statistics | optional | [26] |
| svist4get | region-specific coverage visualization | optional | [27] |
| Pistis | post-alignment quality control | optional | [28] |
| MakeHub | interactive read viewer | optional | [29] |
| MultiQC | combined quality-control output | optional | [30] |
| Transcript ID | Gene ID | Reference Gene| Reference Transcript | Code | Supporting Evidence |
|---|---|---|---|---|
| TCONS_00000106 | XLOC_000101 | ENSG00000196668|ENST00000489452 | = | q1:STRG.129|STRG.129.1|2|913.430603|754.408752|1.681929|273 |
| TCONS_00000107 | XLOC_000101 | ENSG00000196668|ENST00000477702 | = | q1:STRG.130|STRG.130.1|2|2806.789307|2318.147217|5.168231|2128 |
| Ensembl Gene ID | Ensembl Transcript ID | Transcript Count | % of Total Gene Count | Confidence |
|---|---|---|---|---|
| ENSG00000196668 | ENST00000477702 | 9 | 0.42857143 | 0.66666667 |
| ENSG00000196668 | ENST00000489452 | 0 | 0 | 0.66666667 |
| ENSG00000196668 | ENST00000480237 | 12 | 0.57142857 | 0.66666667 |
| ENSG00000196668 | ENST00000470091 | 0 | 0 | 0.66666667 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, T.M.; Ghosh, S.; Postler, T.S. L-RAPiT: A Cloud-Based Computing Pipeline for the Analysis of Long-Read RNA Sequencing Data. Int. J. Mol. Sci. 2022, 23, 15851. https://doi.org/10.3390/ijms232415851
Nelson TM, Ghosh S, Postler TS. L-RAPiT: A Cloud-Based Computing Pipeline for the Analysis of Long-Read RNA Sequencing Data. International Journal of Molecular Sciences. 2022; 23(24):15851. https://doi.org/10.3390/ijms232415851
Chicago/Turabian StyleNelson, Theodore M., Sankar Ghosh, and Thomas S. Postler. 2022. "L-RAPiT: A Cloud-Based Computing Pipeline for the Analysis of Long-Read RNA Sequencing Data" International Journal of Molecular Sciences 23, no. 24: 15851. https://doi.org/10.3390/ijms232415851
APA StyleNelson, T. M., Ghosh, S., & Postler, T. S. (2022). L-RAPiT: A Cloud-Based Computing Pipeline for the Analysis of Long-Read RNA Sequencing Data. International Journal of Molecular Sciences, 23(24), 15851. https://doi.org/10.3390/ijms232415851






