Implications of Post-Translational Modifications in Autoimmunity with Emphasis on Citrullination, Homocitrullination and Acetylation for the Pathogenesis, Diagnosis and Prognosis of Rheumatoid Arthritis
Abstract
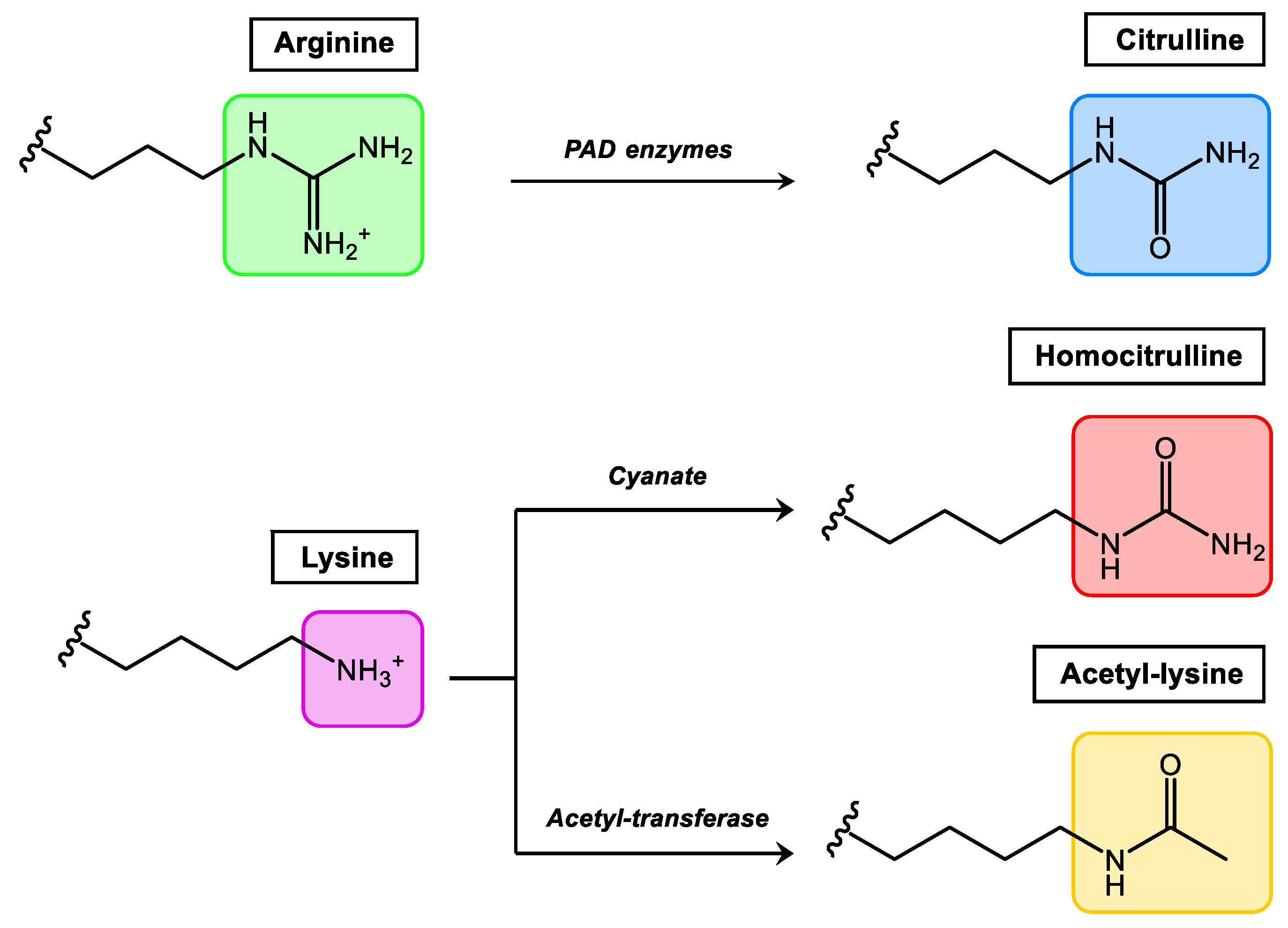
1. Post-Translational Modifications in Proteins
2. PTMs in the Generation of Autoimmunity
3. PTMs in Inflammatory Immune-Mediated Rheumatic Diseases
4. AMPAs in the Pathogenesis, Diagnosis and Prognosis of RA
5. Synthetic Post-Translationally Modified Peptides for the Specific Detection of Autoantibodies in RA Patients
| Protein | Sequence | PTM Peptide | References |
|---|---|---|---|
| Filaggrin | (306–324) (304–324) | SHQESTCitGRSRGRSGRSGS (cyclic) HQCESTCitGRSRGRCGRSGS (cyclic) HQCHQESTCitGRSRGRCGRSGS (cyclic) HSGIGHGQASSAVRDSGHCitGYS HSTSQEGQDTIHGHCitGS | [81,93] [94,95,96,97,98] [81,91,99] [55] [55] |
| Profilaggrin | (293–310) | TIHAHPGSCitCitGGRHGYHH TIHAHPGSCitRGGCitHGYHH | [100] |
| Fibrinogen (α-chain) | (27–43) (31–50) (36–50) (41–60) (84–96) (211–230) (556–575) (563–583) (580–600) (616–636) (617–631) (621–635) | FLAEGGGVCitGPRVVERH GGGVCitGPRVVERHQSACKDS GPCitVVECitHQSASKDS ECitHQSACKDSDWPFCSDEDW (cyclic) TSSTSYNCitGDSTF DLLPSCitDRQHLPLIKMKPVP (cyclic) NTKESSSHHPGIAEFPSCitGK (cyclic) HHPGIAEFPSCitGKSSSYSKQF SKQFTSSTSYNCitGDSTFESKS THSTKCitGHAKSCitPVCitDCDDVL (cyclic) HSTKRGHAKSRPVCitG CitGHAKSCitPVCitGIHTS | [56,95] [100] [86,91,93,94,98,101] [102] [95] [102] [97,102] [91,93,94,96,98,103] [91,93,94,98,103] [100,102] [83] [86,91,93,94,96,98,101] |
| Fibrinogen (β-chain) | (36–52) (43–62) (60–74) (54–80) (62–81) (62–81) | NEEGFFSACitGHRPLDKK ARGHCitPLDKKREEAPSLCitPA CitPAPPPISGGGYCitACit EEAPSLCitPAPPPISGGGYCitACitPAKAAA APPPISGGGYCitARPAKAAAT APPPISGGGYRACitPAKAAAT | [56,91,93,94,96,97,98] [83] [86,91,93,94,96,98,101] [95] [94,96,97,103] [94,96,97,103] |
| Fibronectin | (1029–1042) (2350–2362) | LTVGLTCitCitGQPRQY YNQYSQCitYHQRTN | [95] [95] |
| Vimentin | (2–17) (47–72) (59–70) (60–75) (265–278) (420–435) | STCitSVSSSSYCitCitMFGG STSRSLYASSPGGVYATRSSAVRLCitS GRVYATCitSSAVR VYATRSSAVRLCitSSVP LTAALCitDVCitQQYES SLNLCitETNLDSLPLVD | [56,93,94,96,97,98] [84] [104,105] [56,93,94,96,97,98] [95] [95] |
| α-enolase | (5–21) | CKIHACitEIFDSCitGNPTVEC (cyclic) | [56,85,91,93,94,95,96,97,98] |
| Collagen type II Collagen type II (citC1III) | (282–298) (359–369) | GLPGVKGHCitGYPGLDGA (GPO)5-GACitGLTGCitPGDA(GPO)2-GKKYG | [95] [91,96,97,98] |
| Biglycan | (247–266) | EDLLCitYSKLYCitLGLGHNQIR (cyclic) | [90,102] |
| Clusterin | (221–240) (334–353) | PKSCitIVCitSLMPFSPYEPLNF (cyclic) AECitLTCitKYNELLKSYQWKML | [90,102] [100] |
| β-actin | (191–216) | MKILTECitGYSFTTAECitEIVCitDIKEKL | [95] |
| MNDA | (121–135) | KLTSEACitGCitIPVAQK | [95] |
| Histone 2A Histone 2B | (1–20) (62–81) | MSGCitGKTGGKACitAKAKSCitSS (cyclic) IMNSFVNDIFECitIAGEASCitL (cyclic) | [90,102] [90,102] |
| Histone 4 | (33–47) | AIRRLACitCitGGVLRIS | [62] |
| Tenascin C | (2176–2200) | EHSIQFAEMKLCitPSNFCitNLEGCitCitKR | [106] |
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uy, R.; Wold, F. Posttranslational covalent modification of proteins. Science 1977, 198, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Posttranslational Modifications of Proteins in Health and Disease; Cecilio, J.V., Ed.; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-6381-9. [Google Scholar]
- Huang, K.-Y.; Lee, T.-Y.; Kao, H.-J.; Ma, C.-T.; Lee, C.-C.; Lin, T.-H.; Chang, W.-C.; Huang, H.-D. dbPTM in 2019: Exploring disease association and cross-talk of post-translational modifications. Nucleic Acids Res. 2019, 47, D298–D308. [Google Scholar] [CrossRef] [PubMed]
- Farley, A.R.; Link, A.J. Identification and quantification of protein posttranslational modifications. Methods Enzymol. 2009, 463, 725–763. [Google Scholar] [CrossRef]
- Ramazi, S.; Zahiri, J. Post-translational modifications in proteins: Resources, tools and prediction methods. Database 2021, 2021, baab012. [Google Scholar] [CrossRef]
- de Breven, A.G.; Rebehmed, J. Current status of PTMs structural databases: Applications, limitations and prospects. Amino Acids 2022, 54, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.J.; Dammer, E.B.; Wang, G.; Seyfried, N.T.; Levey, A.I. Proteomics of protein post-translational modifications implicated in neurodegeneration. Transl. Neurodegener. 2014, 3, 23. [Google Scholar] [CrossRef]
- Sharma, B.S.; Prabhakaran, V.; Desai, A.P.; Bajpai, J.; Verma, R.J.; Swain, P.K. Post-translational modifications (PTMs), from a cancer perspective: An overview. Oncogene 2019, 2, 12. [Google Scholar] [CrossRef]
- Harauz, G.; Ishiyama, N.; Hill, C.M.D.; Bates, I.R.; Libich, D.S.; Farès, C. Myelin basic protein-diverse conformational states of an intrinsically unstructured protein and its roles in myelin assembly and multiple sclerosis. Micron 2004, 35, 503–542. [Google Scholar] [CrossRef]
- Wegner, N.; Lundberg, K.; Kinloch, A.; Fisher, B.; Malmström, V.; Feldmann, M.; Venables, P.J. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol. Rev. 2010, 233, 34–54. [Google Scholar] [CrossRef]
- Trouw, L.A.; Theo Rispens, T.; Toes, R.E.M. Beyond citrullination: Other post-translational protein modifications in rheumatoid arthritis. Nat. Rev. Rheumatol. 2017, 13, 331–339. [Google Scholar] [CrossRef]
- Sanmartí, R.; Haro, I.; Cañete, J.D. Palindromic rheumatism: A unique and enigmatic entity with a complex relationship with rheumatoid arthritis. Expert. Rev. Clin. Immunol. 2021, 17, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Sanmartí, R.; Frade-Sosa, B.; Morlà, R.; Castellanos-Moreira, R.; Cabrera-Villalba, S.; Ramirez, J.; Salvador, G.; Haro, I.; Cañete, J.D. Palindromic Rheumatism: Just a Pre-rheumatoid Stage or Something Else? Front. Med. 2021, 8, 657983. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-L.; Sodre, F.M.C.; Mamula, M.J.; Overbergh, L. Citrullination and PAD Enzyme biology in type 1 diabetes—regulators of inflammation, autoimmunity, and pathology. Front. Immunol. 2021, 12, 678953. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lin, J.; Chen, W. Post-translational modifications in T cells in systemic erythematosus lupus. Rheumatology 2021, 60, 2502–2516. [Google Scholar] [CrossRef]
- Chapman, E.A.; Lyon, M.; Simpson, D.; Mason, D.; Beynon, R.J.; Moots, R.J.; Wright, H.L. Caught in a trap? Proteomic analysis of neutrophil extracellular traps in rheumatoid arthritis and systemic lupus erythematosus. Front. Immunol. 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Fresneda Alarcon, M.; McLaren, Z.; Wright, H.L. Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O. Front. Immunol. 2021, 12, 649693. [Google Scholar] [CrossRef]
- Arentz-Hansen, H.; Körner, R.; Molberg, O.; Quarsten, H.; Vader, W.; Kooy, Y.M.C.; Lundin, K.E.A.; Koning, F.; Roepstorff, P.; Sollid, L.M.; et al. The intestinal T-cell response to α-gliadin in adult celiac disease is focused on a single deaminated glutamine targeted by tissue transglutaminase. J. Exp. Med. 2000, 91, 603–612. [Google Scholar] [CrossRef]
- Choo, J.; Heo, G.; Pothoulakis, C.; Im, E. Posttranslational modifications as therapeutic targets for intestinal disorders. Pharmacol. Res. 2021, 165, 105412. [Google Scholar] [CrossRef]
- Ishida-Yamamoto, A.; Senshu, T.; Takahashi, H.; Akiyama, K.; Nomura, K.; Iizuka, H. Decreased deiminated Keratin K1 in psoriatic hyperproliferative epidermis. J. Investig. Dermatol. 2000, 114, 701–705. [Google Scholar] [CrossRef]
- Frasca, L.; Palazzo, R.; Chimenti, M.S.; Alivernini, S.; Tolusso, B.; Bui, L.; Botti, E.; Giunta, A.; Bianchi, L.; Petricca, L.; et al. Anti-LL37 Antibodies Are Present in Psoriatic Arthritis (PsA) Patients: New Biomarkers in PsA. Front. Immunol. 2018, 9, 1936. [Google Scholar] [CrossRef]
- Buttari, B.; Profumo, E.; Capozzi, A.; Saso, L.; Sorice, M.; Riganò, R. Post-translational modifications of proteins in antiphospholipid antibody syndrome. Crit. Rev. Clin. Lab. Sci. 2019, 56, 511–525. [Google Scholar] [CrossRef]
- Zavala-Cerna, M.G.; Martínez-García, E.A.; Torres-Bugarín, O.; Rubio-Jurado, B.; Carlos Riebeling, C.; Nava, A. The clinical significance of posttranslational modification of autoantigens. Clinic. Rev. Allerg. Immunol. 2014, 47, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Dragoni, G.; De Hertogh, G.; Vermeire, S. The role of citrullination in inflammatory bowel disease: A neglected player in triggering inflammation and fibrosis? Inflamm. Bowel Dis. 2021, 27, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Nezos, A.; Cinoku, I.; Mavragani, C.P.; Moutsopoulos, H.M. Antibodies against citrullinated alpha enolase peptides in primary Sjogren’s syndrome. Clin. Immunol. 2017, 183, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Doyle, H.A.; Mamula, M.J. Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol. 2001, 22, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Doyle, H.A.; Mamula, M.J. Autoantigenesis: The evolution of protein modifications in autoimmune disease. Curr. Opin. Immunol. 2012, 24, 112–118. [Google Scholar] [CrossRef]
- Utz, P.J.; Gensler, T.J.; Anderson, P. Death, autoantigen modifications, and tolerance. Arthritis Res. 2000, 2, 101–114. [Google Scholar] [CrossRef]
- Valesini, G.; Gerardi, M.C.; Iannuccelli, C.; Pacucci, V.A.; Pendolin, M.; Shoenfeld, Y. Citrullination and autoimmunity. Autoimmun. Rev. 2015, 14, 490–497. [Google Scholar] [CrossRef]
- Carrasco-Marin, E.; Paz-Miguel, J.; Lopez-Mato, P.; Alvarez-Dominguez, C.; Leyva-Cobian, F. Oxidation of defined antigens allows protein unfolding and increases both proteolytic processing and exposes peptide epitopes which are recognized by specific T cells. Immunology 1998, 95, 314–321. [Google Scholar] [CrossRef]
- Opdenakker, G.; El-Asrar, A.A.; Van Damme, J. Remnant epitopes generating autoimmunity: From model to useful paradigm. Trends Immunol. 2020, 41, 367–378. [Google Scholar] [CrossRef]
- Opdenakker, G.; Van Damme, J. Cytokine-regulated proteases in autoimmune diseases. Immunol. Today 1994, 15, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, M.; Brown, C.D.; Maranville, J.C. A catalog of GWAS fine-mapping efforts in autoimmune disease. Am. J. Hum. Genet. 2021, 108, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Opdenakker, G.; Proost, P.; Van Damme, J. Microbiomic and posttranslational modifications as preludes to autoimmune diseases. Trends Mol. Med. 2016, 22, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Goulas, T.; Mizgalska, D.; Garcia-Ferrer, I.; Kantyka, T.; Guevara, T.; Szmigielski, B.; Sroka, A.; Millán, C.; Usón, I.; Veillard, F.; et al. Tructure and mechanism of a bacterial host-protein citrullinating virulence factor, Porphyromonas gingivalis peptidylarginine deiminase. Sci. Rep. 2015, 5, 11969. [Google Scholar] [CrossRef]
- Chaplin, C.; Pozzilli, P. SARS-CoV-2 induced post-translational protein modifications: A trigger for developing autoimmune diabetes? Diabetes Metab. Res. Rev. 2022, 38, e3508. [Google Scholar] [CrossRef]
- de Pablo, P.; Chapple, I.L.; Buckley, C.D.; Dietrich, T. Periodontitis in systemic rheumatic diseases. Nat. Rev. Rheumatol. 2009, 5, 218–224. [Google Scholar] [CrossRef]
- Maresz, K.J.; Hellvard, A.; Sroka, A.; Adamowicz, K.; Bielecka, E.; Koziel, J.; Gawron, K.; Mizgalska, D.; Marcinska, K.A.; Benedyk, M.; et al. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 2013, 9, e1003627. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.-S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.-S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef]
- Sangha, O. Epidemiology of rheumatic diseases. Rheumatology 2000, 39, 3–12. [Google Scholar] [CrossRef]
- Haro, I.; Sanmarti, R. Rheumatoid arthritis: Current advances in pathogenesis, diagnosis and therapy. Curr. Top. Med. Chem. 2013, 13, 697. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League against rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Thiele, G.M.; Duryee, M.J.; Anderson, D.R.; Klassen, L.W.; Mohring, S.M.; Young, K.A.; Benissan-Messan, D.; Sayles, H.; Dusad, A.; Hunter, C.D.; et al. Malondialdehyde-acetaldehyde adducts and anti-malondialdehyde-acetaldehyde antibodies in rheumatoid arthritis. Arthritis Rheumatol. 2015, 67, 645–655. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, L.; Regueiro, C.; Amhaz-Escanlar, S.; Pena, C.; Herbello-Hermelo, P.; Moreda-Piñeiro, A.; Rodriguez-Garcia, J.; Mera-Varela, A.; Pérez-Pampín, E.; González, A. Antibodies against 4 atypical post-translational protein modifications in patients with rheumatoid arthritis. Diagnostics 2022, 12, 352. [Google Scholar] [CrossRef]
- Mikuls, T.R.; Duryee, M.J.; England, B.R.; Anderson, D.R.; Hearth-Holmes, M.; Su, K.; Michaud, K.; Payne, J.B.; Sayles, H.; Hunter, C.; et al. Malondialdehyde–acetaldehyde antibody concentrations in rheumatoid arthritis and other rheumatic conditions. Int. Immunopharmacol. 2018, 56, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R.; Duryee, M.J.; Shurmur, S.W.; Um, J.Y.; Bussey, W.D.; Hunter, C.D.; Garvin, R.P.; Sayles, H.R.; Mikuls, T.R.; Klassen, L.W.; et al. Unique antibody responses to malondialdehyde-acetaldehyde (MAA)-protein adducts predict coronary artery disease. PLoS ONE 2014, 9, e107440. [Google Scholar] [CrossRef]
- Rolla, R.; Vay, D.; Mottaran, E.; Parodi, M.; Traverso, N.; Aricó, S.; Sartori, M.; Bellomo, G.; Klassen, L.W.; Thiele, G.M.; et al. Detection of circulating antibodies against malondialdehyde-acetaldehyde adducts in patients with alcohol-induced liver disease. Hepatology 2000, 31, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Vehkala, L.; Ukkola, O.; Kesaniemi, Y.A.; Kahonen, M.; Nieminen, M.; Salomaa, V.; Jula, A.S.; Horkko, S. Plasma IgA antibody levels to malondialdehyde acetaldehyde-adducts are associated with inflammatory mediators, obesity and type 2 diabetes. Ann. Med. 2013, 45, 501–510. [Google Scholar] [CrossRef]
- McCaskill, M.L.; Kharbanda, K.K.; Tuma, D.J.; Reynolds, J.D.; DeVasure, J.M.; Sisson, J.H.; Todd, A.; Wyatt, T.A. Hybrid malondialdehyde and acetaldehyde protein adducts form in the lungs of mice exposed to alcohol and cigarette smoke. Alcohol. Clin. Exp. Res. 2011, 35, 1106–1113. [Google Scholar] [CrossRef]
- Parekh, R.B.; Dwek, R.A.; Sutton, B.J.; Fernandes, D.L.; Leung, A.; Stanworth, D.; Rademacher, T.W.; Mizuochi, T.; Taniguchi, T.; Matsuta, K.; et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 1985, 316, 452–457. [Google Scholar] [CrossRef]
- Ercan, A.; Cui, J.; Chatterton, D.E.; Deane, K.D.; Hazen, M.M.; Brintnell, W.; O’Donnell, C.I.; Derber, L.A.; Weinblatt, M.E.; Shadick, N.A.; et al. Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2239–2248. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Ou, J.; Nandakumar, K.S. Autoantibodies as diagnostic markers and mediator of joint inflammation in Arthritis. Mediat. Inflamm. 2019, 2019, 6363086. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.-J.; Ju, J.H. Impact of posttranslational modification in pathogenesis of rheumatoid arthritis: Focusing on citrullination, carbamylation, and acetylation. Int. J. Mol. Sci. 2021, 22, 10576. [Google Scholar] [CrossRef]
- Tilvawala, R.; Nguyen, S.H.; Maurais, A.J.; Nemmara, V.V.; Mitesh Nagar, M.; Salinger, A.J.; Nagpal, S.; Weerapana, E.; Thompson, P.R. The Rheumatoid Arthritis-Associated Citrullinome. Cell Chem. Biol. 2018, 25, 691–704. [Google Scholar] [CrossRef]
- Union, A.; Meheus, L.; Humbel, R.L.; Conrad, K.; Steiner, G.; Moereels, H.; Pottel, H.; Serre, G.; De Keyser, F. Identification of citrullinated rheumatoid arthritis-specific epitopes in natural filaggrin relevant for antifilaggrin autoantibody detection by line immunoassay. Arthritis Rheum. 2002, 46, 1185–1195. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A.; Willemze, A.; Robinson, D.B.; Peschken, C.A.; Markland, J.; van der Woude, D.; Elias, B.; Ménard, H.A.; Newkirk, M.; Fritzler, M.J.; et al. Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease. Arthritis Rheum. 2008, 58, 3000–3008. [Google Scholar] [CrossRef] [PubMed]
- Snir, O.; Widhe, M.; Von Spee, C.; Lindberg, J.; Padyukov, L.; Lundberg, K.; Engström, A.; Venables, P.J.; Lundeberg, J.; Holmdahl, R.; et al. Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: Association with HLA-DRB1 alleles. Ann. Rheum. Dis. 2008, 68, 736–743. [Google Scholar] [CrossRef]
- Van Beers, J.J.; Willemze, A.; Stammen-Vogelzangs, J.; Drijfhout, J.W.; Toes, R.E.; Pruijn, G.J.M. Anti-citrullinated fibronectin antibodies in rheumatoid arthritis are associated with human leukocyte antigen-DRB1 shared epitope alleles. Arthritis Res. Ther. 2012, 14, R35. [Google Scholar] [CrossRef]
- Van Beers, J.J.; Schwarte, C.M.; Stammen-Vogelzangs, J.; Oosterink, E.; Bozic, B.; Pruijn, G.J.M. The rheumatoid arthritis synovial fluid citrullinome reveals novel citrullinated epitopes in apolipoprotein E, myeloid nuclear differentiation antigen, and β-actin. Arthr. Rheum. 2013, 65, 69–80. [Google Scholar] [CrossRef]
- Shoda, H.; Fujio, K.; Shibuya, M.; Okamura, T.; Sumitomo, S.; Okamoto, A.; Sawada, T.; Yamamoto, K. Detection of autoantibodies to citrullinated BiP in rheumatoid arthritis patients and pro-inflammatory role of citrullinated BiP in collagen-induced arthritis. Arthritis Res. Ther. 2011, 13, R191. [Google Scholar] [CrossRef]
- Meng, X.; Ezzati, P.; Smolik, I.; Bernstein, C.N.; Hitchon, C.A.; El-Gabalawy, H.S. Characterization of autoantigens targeted by anti-citrullinated protein antibodies in vivo: Prominent role for epitopes derived from Histone 4 proteins. PLoS ONE 2016, 11, e0165501. [Google Scholar] [CrossRef] [PubMed]
- Goëb, V.; Thomas-L’Otellier, M.; Daveau, R.; Charlionet, R.; Fardellone, P.; Le Loët, X.; Tron, F.; Gilbert, D.; Vittecoq, O. Candidate autoantigens identified by mass spectrometry in early rheumatoid arthritis are chaperones and citrullinated glycolytic enzymes. Arthritis Res. Ther. 2009, 11, R38. [Google Scholar] [CrossRef] [PubMed]
- Konig, M.F.; Giles, J.T.; Nigrovic, P.A.; Andrade, F. Antibodies to native and citrullinated RA33 (hnRNP A2/B1) challenge citrullination as the inciting principle underlying loss of tolerance in rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.; Moots, R.; Edwards, S. The multifactorial role of neutrophils in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Giannini, D.; Antonucci, M.; Petrelli, F.; Bilia, S.; Alunno, A.; Puxeddu, I. One year in review 2020: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2020, 38, 387–397. [Google Scholar] [PubMed]
- Stastny, P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N. Engl. J. Med. 1978, 298, 869–871. [Google Scholar] [CrossRef]
- Ollier, W.E.R.; MacGregor, A. Genetic epidemiology of rheumatoid disease. Br. Med. Bull. 1995, 51, 267–285. [Google Scholar] [CrossRef]
- Raychaudhuri, S.; Sandor, C.; Stahl, E.A.; Freudenberg, J.; Lee, H.-S.; Jia, X.; Alfredsson, L.; Padyukov, L.; Klareskog, L.; Worthington, J.; et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat. Genet. 2012, 44, 291–296. [Google Scholar] [CrossRef]
- Van Der Helm-Van Mil, A.H.M.; Verpoort, K.N.; Breedveld, F.C.; Huizinga, T.W.J.; Toes, R.E.M.; De Vries, R.R.P. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 2006, 54, 1117–1121. [Google Scholar] [CrossRef]
- Liu, Y.; Aryee, M.J.; Padyukov, L.; Fallin, M.D.; Hesselberg, E.; Runarsson, A.; Reinius, L.; Acevedo, N.; Taub, M.; Ronninger, M.; et al. Epigenome-wide association data implicate DNA methylation as an intermediary of genetic risk in rheumatoid arthritis. Nat. Biotechnol. 2013, 31, 142–147. [Google Scholar] [CrossRef]
- van Wesemael, T.J.; Ajeganova, S.; Humphreys, J.; Terao, C.; Muhammad, A.; Symmons, D.P.M.; MacGregor, A.L.; Hafström, I.; Trouw, L.A.; van der Helm-van Mil, A.H.M.; et al. Smoking is associated with the concurrent presence of multiple autoantibodies in rheumatoid arthritis rather than with anticitrullinated protein antibodies per se: A multicenter cohort study. Arthritis Res. Ther. 2016, 18, 285. [Google Scholar] [CrossRef] [PubMed]
- Klareskog, L.; Stolt, P.; Lundberg, K.; Kallberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Erlandsson Harris, E.; Ulfgren, A.-K.; Rantapää-Dahlqvist, S.; et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006, 54, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Holers, V.M.; Demoruelle, M.K.; Kuhn, K.A.; Buckner, J.H.; Robinson, W.H.; Okamoto, Y.; Norris, J.M.; Deane, K.D. Rheumatoid arthritis and the mucosal origins hypothesis: Protection turns to destruction. Nat. Rev. Rheumatol. 2018, 14, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Catrina, A.; Krishnamurthy, A.; Rethi, B. Current view on the pathogenic role of anti-citrullinated protein antibodies in rheumatoid arthritis. RMD Open 2021, 7, e001228. [Google Scholar] [CrossRef] [PubMed]
- Van Delft, M.A.M.; Huizinga, T.W.J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef]
- Tan, E.M.; Smolen, J.S. Historical observations contributing insights on etiopathogenesis of rheumatoid arthritis and role of rheumatoid factor. J. Exp. Med. 2016, 213, 1937–1950. [Google Scholar] [CrossRef]
- Falkenburg, W.J.J.; van Schaardenburg, D. Evolution of autoantibody responses in individuals at risk of rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 42–52. [Google Scholar] [CrossRef]
- Gomara, M.J.; Haro, I. Citrullinated Peptides in the Diagnosis of Rheumatoid Arthritis. Curr. Top. Med. Chem. 2013, 13, 743–751. [Google Scholar] [CrossRef]
- Schellekens, G.A.; de Jong, B.A.; van den Hoogen, F.H.; van de Putte, L.B.; van Venrooij, W.J. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J. Clin. Investig. 1998, 101, 273–278. [Google Scholar] [CrossRef]
- Schellekens, G.A.; Visser, H.; de Jong, B.A.; van den Hoogen, F.H.; Hazes, J.M.; Breedveld, F.C.; van Venrooij, W.J. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000, 43, 155–163. [Google Scholar] [CrossRef]
- Perez, M.L.; Gomara, M.J.; Kasi, D.; Alonso, A.; Vinas, O.; Ercilla, G.; Sanmarti, R.; Haro, I. Synthesis of overlapping fibrin citrullinated peptides and their use for diagnosing rheumatoid arthritis. Chem. Biol. Drug Des. 2006, 68, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.L.; Gomara, M.J.; Ercilla, G.; Sanmartí, R.; Haro, I. Antibodies to citrullinated human fibrinogen synthetic peptides in diagnosing rheumatoid arthritis. J. Med. Chem. 2007, 50, 3573–3584. [Google Scholar] [CrossRef] [PubMed]
- Malakoutikhah, M.; Gomara, M.J.; Gomez-Puerta, J.A.; Sanmartí, R.; Haro, I. The use of chimeric vimentin citrullinated peptides for the diagnosis of rheumatoid arthritis. J. Med. Chem. 2011, 54, 7486–7492. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, K.; Kinloch, A.; Fisher, B.A.; Wegner, N.; Wait, R.; Charles, P.; Mikuls, T.R.; Venables, P.J. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008, 58, 3009–3019. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, M.; Moinard, N.; Auger, I.; Clavel, C.; Arnaud, J.; Nogueira, L.; Roudier, J.; Serre, G. Epitopes of human fibrin recognized by the rheumatoid arthritis-specific autoantibodies to citrullinated proteins. Eur. J. Immunol. 2006, 36, 2250–2263. [Google Scholar] [CrossRef]
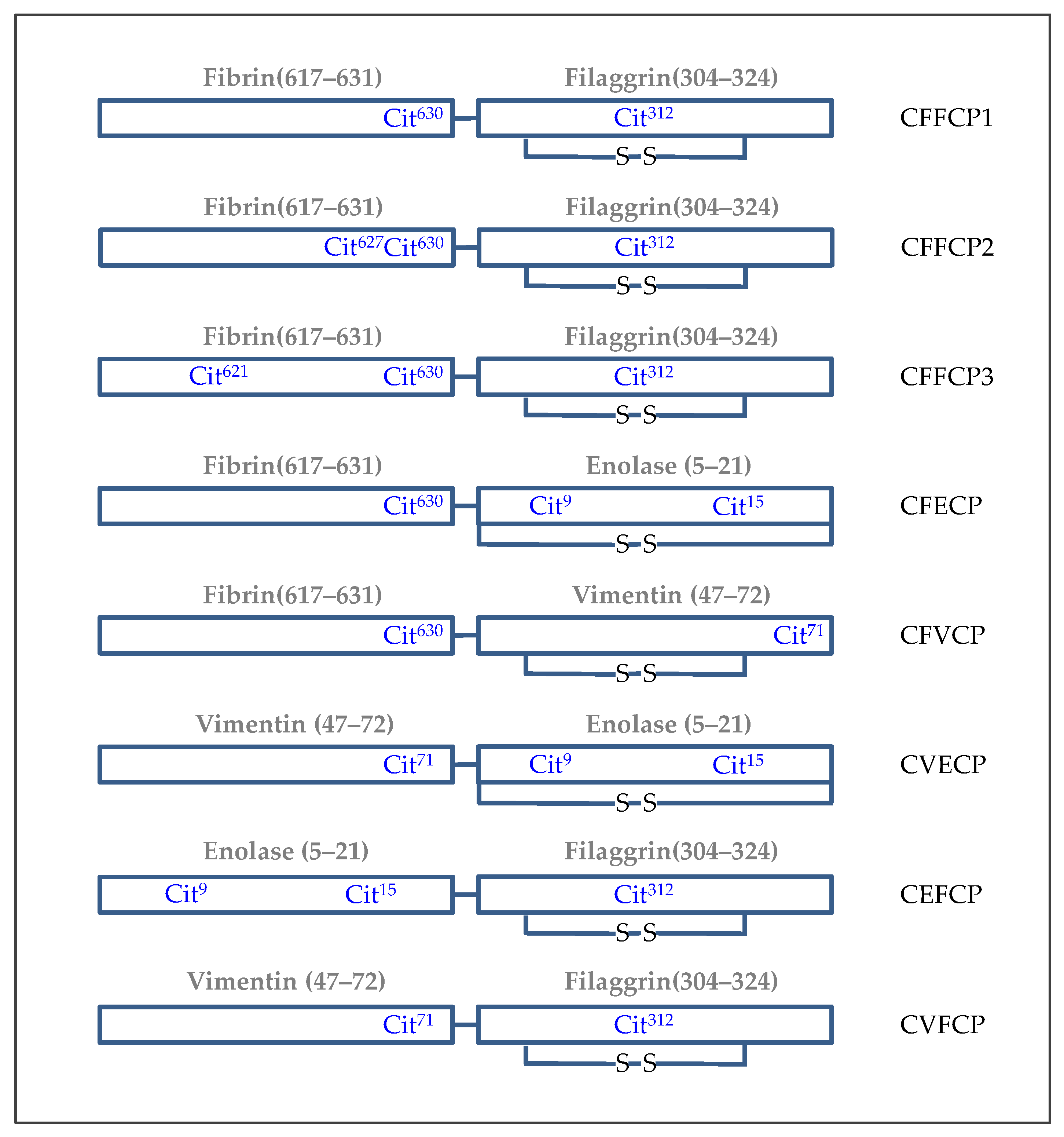
- Sanmartí, R.; Graell, E.; Perez, M.L.; Ercilla, G.; Vinas, O.; Gomez-Puerta, J.A.; Gratacos, J.; Balsa, A.; Gomara, M.J.; Larrosa, M.; et al. Diagnostic and prognostic value of antibodies against chimeric fibrin/filaggrin citrullinated synthetic peptides in rheumatoid arthritis. Arthritis Res. Ther. 2009, 11, R135. [Google Scholar] [CrossRef]
- Gomara, M.J.; Rodrıguez, J.; Bleda, M.J.; Salvador, J.P.; Sanmartí, R.; Haro, I. Comparative study of the diagnostic and prognostic value of antibodies against chimeric citrullinated synthetic peptides and CCP3/CCP3.1 assays. Clin. Chem. Lab. Med. 2018, 56, 285–293. [Google Scholar] [CrossRef]
- Hueber, W.; Kidd, B.A.; Tomooka, B.H.; Lee, B.J.; Bruce, B.; Fries, J.F.; Sønderstrup, G.; Monach, P.; Drijfhout, J.W.; van Venrooij, W.J.; et al. Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2005, 52, 2645–2655. [Google Scholar] [CrossRef]
- Wagner, C.A.; Sokolove, J.; Lahey, L.J.; Bengtsson, C.; Saevarsdottir, S.; Alfredsson, L.; Delanoy, M.; Lindstrom, T.M.; Walker, R.P.; Bromberg, R.; et al. Identification of anticitrullinated protein antibody reactivities in a subset of anti-CCP-negative rheumatoid arthritis: Association with cigarette smoking and HLA-DRB1 ‘shared epitope’ alleles. Ann. Rheum. Dis. 2015, 74, 579–586. [Google Scholar] [CrossRef]
- Rönnelid, J.; Hansson, M.; Mathsson-Alm, L.; Cornillet, M.; Reed, E.; Jakobsson, P.J.; Alfredsson, L.; Holmdahl, R.; Skriner, K.; Serre, G.; et al. Anticitrullinated protein/peptide antibody multiplexing defines an extended group of ACPA-positive rheumatoid arthritis patients with distinct genetic and environmental determinants. Ann. Rheum. Dis. 2018, 77, 203–211. [Google Scholar] [CrossRef]
- García-Moreno, C.; Gomara, M.J.; Bleda, M.J.; Sanmartí, R.; Haro, I. Development of a multiplex assay based on chimeric citrullinated peptides as proof of concept for diagnosis of rheumatoid arthritis. PLoS ONE 2019, 14, e0215927. [Google Scholar] [CrossRef] [PubMed]
- Grönwall, C.; Liljefors, L.; Bang, H.; Hensvold, A.H.; Hansson, M.; Mathsson-Alm, L.; Israelsson, L.; Joshua, V.; Svärd, A.; Stålesen, R.; et al. A Comprehensive evaluation of the relationship between different IgG and IgA anti-modified protein autoantibodies in rheumatoid arthritis. Front. Immunol. 2021, 12, 627986. [Google Scholar] [CrossRef] [PubMed]
- Sahlström, P.; Hansson, M.; Steen, J.; Amara, K.; Titcombe, P.J.; Forsström, B.; Stålesen, R.; Israelsson, L.; Piccoli, L.; Lundberg, K.; et al. Different hierarchies of anti–modified protein autoantibody reactivities in rheumatoid arthritis. Arthritis Rheum. 2020, 72, 1643–1657. [Google Scholar] [CrossRef]
- van Beers, J.B.C.; Willemze, A.; Jansen, J.J.; Engbers, G.H.M.; Salden, M.; Raats, J.; Drijfhout, J.W.; van der Helm-van Mil, A.H.M.; Toes, R.E.M.; Pruijn, G.J.M. ACPA fine-specificity profiles in early rheumatoid arthritis patients do not correlate with clinical features at baseline or with disease progression. Arthritis Res. Ther. 2013, 15, R140. [Google Scholar] [CrossRef] [PubMed]
- Hansson, M.; Mathsson, L.; Schlederer, T.; Israelsson, L.; Matsson, P.; Nogueria, L.; Jakobsson, P.J.; Lundberg, K.; Malmström, V.; Serre, G.; et al. Validation of a multiplex chip-based for the detection of autoantibodies against citrullinated peptides. Arthritis Res. Ther. 2012, 14, R201. [Google Scholar] [CrossRef] [PubMed]
- Brink, M.; Hansson, M.; Mathsson, L.; Jakobsson, P.J.; Holmdahl, R.; Hallmans, G.; Stenlund, H.; Rönnelid, J.; Klareskog, L.; Rantapää-Dahlqvist, S. Multiplex analyses of antibodies against citrullinated peptides in individuals prior to development of rheumatoid arthritis. Arthritis Rheum. 2013, 65, 899–910. [Google Scholar] [CrossRef]
- van Heemst, J.; Trouw, L.A.; Nogueira, L.; van Steenbergen, H.W.; van der Helm-van Mil, A.H.M.; Allaart, C.F.; Serre, G.; Holmdahl, R.; Huizinga, T.W.J.; Toes, R.E.M.; et al. An investigation of the added value of an ACPA multiplex assay in an early rheumatoid arthritis setting. Arthritis Res Ther. 2015, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Kissel, T.; Reijm, S.; Slot, L.M.; Cavallari, M.; Wortel, C.M.; Vergroesen, R.D.; Stoeken-Rijsbergen, G.; Kwekkeboom, J.C.; Kampstra, A.; Levarht, E.; et al. Antibodies and B cells recognizing citrullinated proteins display a broad cross-reactivity towards other post-translational modifications. Ann. Rheum. Dis. 2020, 79, 472–480. [Google Scholar] [CrossRef]
- Chandra, P.E.; Sokolove, J.; Hipp, B.G.; Lindstrom, T.M.; Elder, J.T.; Reveille, J.D.; Eberl, H.; Klause, U.; Robinson, W.H. Novel multiplex technology for diagnostic characterization of rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, R102. [Google Scholar] [CrossRef]
- Iobagiu, C.; Magyar, A.; Nogueira, L.; Cornillet, M.; Sebbag, M.; Arnaud, J.; Hudecz, F.; Serre, G. The antigen specificity of the rheumatoid arthritis-associated ACPA directed to citrullinated fibrin is very closely restricted. J. Autoimmun. 2011, 37, 263–272. [Google Scholar] [CrossRef]
- Sokolove, J.; Bromberg, R.; Deane, K.D.; Lahey, L.J.; Derber, L.A.; Chandra, P.E.; Edison, J.D.; Gilliland, W.R.; Tibshirani, R.J.; Norris, J.M.; et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS ONE 2012, 7, e35296. [Google Scholar] [CrossRef]
- Fernandes-Cerqueira, C.; Ossipova, E.; Gunasekera, S.; Hansson, M.; Mathsson, L.; Catrina, A.I.; Sommarin, Y.; Klareskog, L.; Lundberg, K.; Rönnelid, J.; et al. Targeting of anti-citrullinated protein/peptide antibodies in rheumatoid arthritis using peptides mimicking endogenously citrullinated fibrinogen antigens. Arthritis Res. Ther. 2015, 17, 155. [Google Scholar] [CrossRef] [PubMed]
- Juarez, M.; Bang, H.; Hammar, F.; Reimer, U.; Dyke, B.; Sahbudin, I.; Buckley, C.D.; Fisher, B.; Filer, A.; Raza, K. Identification of novel antiacetylated vimentin antibodies in patients with early inflammatory arthritis. Ann. Rheum. Dis. 2016, 75, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Nijjar, J.S.; Morton, F.R.; Bang, H.; Buckley, C.D.; van der Heijde, D.; Gilmour, A.; Paterson, C.; McInnes, L.B.; Porter, D.; Raza, K. The impact of autoantibodies against citrullinated, carbamylated, and acetylated peptides on radiographic progression in patients with new-onset rheumatoid arthritis: An observational cohort study. Lancet Rheumatol. 2021, 3, e284–e293. [Google Scholar] [CrossRef]
- Schwenzer, A.; Jiang, X.; Mikuls, T.R.; Payne, J.B.; Sayles, H.R.; Quirke, A.M.; Kessler, B.M.; Fischer, R.; Venables, P.J.; Lundberg, K.; et al. Identification of an immunodominant peptide from citrullinated tenascin-C as a major target for autoantibodies in rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 1876–1883. [Google Scholar] [CrossRef]
- Shi, J.; Knevel, R.; Suwannalai, P.; van der Linden, M.P.; Janssen, G.M.C.; van Veelen, P.A.; Levarht, N.E.W.; van der Helm-van Mil, A.H.M.; Cerami, A.; Huizinga, T.W.J.; et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc. Natl. Acad. Sci. USA 2011, 108, 7372–7377. [Google Scholar] [CrossRef] [PubMed]
- Studenic, P.; Alunno, A.; Sieghart, D.; Bang, H.; Aletaha, D.; Blüml, S.; Haslacher, H.; Smolen, J.S.; Gerli, R.; Steiner, G. Presence of anti-acetylated peptide antibodies (AAPA) in inflammatory arthritis and other rheumatic diseases suggests discriminative diagnostic capacity towards early rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211022533. [Google Scholar] [CrossRef]
- Jones, J.D.; Hamilton, B.J.; Rigby, W.F.C. Brief Report: Anti-carbamylated protein antibodies in rheumatoid arthritis patients are reactive with specific epitopes of the human fibrinogen beta-chain. Arthritis Rheumatol. 2017, 69, 1381–1386. [Google Scholar] [CrossRef][Green Version]
- Brevet, P.; Lattard, C.; Guillou, C.; Rottenberg, P.; Fardellone, P.; Le-Loët, X.; Lequerre, T.; Cosette, P.; Boyer, O.; Freret, M.; et al. Anti-carbamylated fibrinogen antibodies might be associated with a specific rheumatoid phenotype and include a subset recognizing in vivo epitopes of its γ chain one of which is not cross reactive with anti-citrullinated protein antibodiess. Front. Immunol. 2021, 12, 733511. [Google Scholar] [CrossRef]
- Reed, E.; Jiang, X.; Kharlamova, N.; Ytterberg, A.J.; Catrina, A.I.; Israelsson, L.; Mathsson-Alm, L.; Hansson, M.; Alfredsson, L.; Rönnelid, J.; et al. Antibodies to carbamylated alpha-enolase epitopes in rheumatoid arthritis also bind citrullinated epitopes and are largely indistinct from anti-citrullinated protein antibodies. Arthritis Res. Ther. 2016, 18, 96. [Google Scholar] [CrossRef]
- Lloyd, K.A.; Wigerblad, G.; Sahlström, P.; Garimella, M.G.; Chemin, K.; Steen, J.; Titcombe, P.J.; Marklein, B.; Zhou, D.; Stålesen, R.; et al. Differential ACPA binding to nuclear antigens reveals a PAD-independent pathway and a distinct dubset of acetylation cross-reactive autoantibodies in rheumatoid arthritis. Front. Immunol. 2019, 9, 3033. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Moreira, R.; Rodriguez-Garcia, S.C.; Cabrera-Villalba, S.; Gomara, M.J.; Salvador, G.; Ruiz-Esquide, V.; Ramirez, J.; Inciarte-Mundo, J.; Morla, R.; Garcia-Moreno, C.; et al. Anti-carbamylated protein antibody isotype pattern differs between palindromic rheumatism and rheumatoid arthritis. Ther. Adv. Musculoskel. Dis. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Verheul, M.K.; van Erp, S.J.H.; van der Woude, D.; Levarht, E.W.N.; Mallat, M.J.K.; Verspaget, H.W.; Stolk, J.; Toes, R.E.M.; van der Meulen-de Jong, A.E.; Hiemstra, P.S.; et al. Anti-carbamylated protein antibodies: A specific hallmark for rheumatoid arthritis. Comparison to conditions known for enhanced carbamylation; renal failure, smoking and chronic inflammation. Ann. Rheum. Dis. 2016, 75, 1575–1576. [Google Scholar] [CrossRef]
- Castellanos-Moreira, R.; Rodríguez-García, S.C.; Gomara, M.J.; Ruiz-Esquide, V.; Cuervo, A.; Casafont-Solé, I.; Ramírez, J.; Holgado, S.; Gómez-Puerta, J.A.; Cañete, J.D.; et al. Anti-carbamylated proteins antibody repertoire in rheumatoid arthritis: Evidence of a new autoantibody linked to interstitial lung disease. Ann. Rheum. Dis. 2020, 79, 587–594. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, C.; Gómara, M.J.; Castellanos-Moreira, R.; Sanmartí, R.; Haro, I. Peptides bearing multiple post-translational modifications as antigenic targets for severe rheumatoid arthritis patients. Int. J. Mol. Sci. 2021, 22, 13290. [Google Scholar] [CrossRef] [PubMed]


| Human Disease | Target Proteins | PTM | References |
|---|---|---|---|
| Multiple Sclerosis | Myelin proteins (MBP), GFAP, Histone H3 | Citrullination | [9] |
| Rheumatoid Arthritis Palindromic Rheumatism | Proteins of inflamed joints and synovium: Collagen type II, Fibrinogen, Fibrin, Vimentin, α-Enolase | Citrullination Carbamylation Acetylation Glycosylation | [10,11,12,13] |
| Type 1 Diabetes | Proteins modified in beta-cells and neutrophils (IAPP, IA-2, IGRP, GAD65, GRP78) | Citrullination | [14] |
| Systemic Lupus Erythematosus | Intracellular macromolecules: ribonucleoproteins and nucleosomes (U1-70K, Ro/SSA, La/SSB, SmD1, H2B, H4, …) | Phosphorylation Glycosylation Acetylation Iso-aspartylation Methylation Ubiquitination | [15,16,17] |
| Celiac disease | Adherence and tight junction proteins; transcription factor (PPARγ); serum IgG and IgA1 Wheat gliadin | Phosphorylation Ubiquitination Glycosylation Citrullination | [18,19] |
| Psoriasis/Psoriatic arthritis | Keratin; Cathelicidin LL37 | Citrullination Carbamylation | [20,21] |
| Antiphospholipid Syndrome | Apolipoprotein (β2GPI), Vimentin | Oxidation/ Sialylation Citrullination | [22] |
| Primary Biliary Cirrhosis | PDC-E2 | Acylation/ oxidation | [23] |
| Inflammatory Bowel Disease | Kinases: MAPKs and PKB/Akt; DNA-binding transcription factor; NF-κB; Histone H3 | Phosphorylation Ubiquitination Glycosylation Citrullination | [19,24] |
| Sjogren’s Syndrome | α-Enolase | Citrullination | [25] |
| Protein | PTM | Peptide Sequence | References |
|---|---|---|---|
| Filaggrin | Homocitrulline Acetyl-Lysine | HQCHQESThCitGRSRGRCGRSGS HQCHQESTKAcGRSRGRCGRSGS | [99] |
| Vimentin | Homocitrulline Acetyl-Lysine Acetyl-ornithine | GRVYAThCitSSAVR GRVYATKAcSSAVR GRVYATOrAcSSAVR | [93,104,105,108,109] |
| Vimentin | Homocitrulline Acetyl-Lysine | VYAThCitSSAVhCitLhCitSSVP VYATKAcSSAVKAcLKAcSSVP | [99] |
| Fibrinogen α | Homocitrulline Acetyl-Lysine | FLAEGGGVhCitGPRVVERH FLAEGGGVKAcGPRVVERH | [99] |
| Fibrinogen β | Homocitrulline | ARGHRPLDKhCitREEA | [93,109] |
| Fibrinogen β | Homocitrulline | AKAAATQhCitKVER (cyclic) | [93,109] |
| Fibrinogen β | Homocitrulline Acetyl-Lysine | NEEGFFSAhCitGHRPLDKK NEEGFFSAKAcGHRPLDKK | [99] |
| Fibrinogen γ | Carbamylation | QKIVNLKEKVAQLEAQCQEPCKDTVQI * WMNKCHAGHLNGVYYQGGTYSKASTPN * | [110] |
| Enolase | Homocitrulline Homocitrulline Acetyl-Lysine | KIHAhCitEIFDShCitGNPTVE (cyclic) KIHAhCitEIFDShCitGNPTV (linear) KIHAKAcEIFDSKAcGNPTV (linear) | [93,111] [99] |
| Histone 2B | Acetyl-Lysine | SAPAPKKAcGSKKAVTKAQ (cyclic) | [93,112] |
| Histone 4 | Acetyl-Lysine Acetyl-Lysine Acetyl-Lysine | SGRGKAcGGKAcGLGKAcGGAKAcRH SGRGKAcGGKGLGKGGAKRH SGRGKGGKGLGKGGAKAcRH | [93,112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haro, I.; Sanmartí, R.; Gómara, M.J. Implications of Post-Translational Modifications in Autoimmunity with Emphasis on Citrullination, Homocitrullination and Acetylation for the Pathogenesis, Diagnosis and Prognosis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 15803. https://doi.org/10.3390/ijms232415803
Haro I, Sanmartí R, Gómara MJ. Implications of Post-Translational Modifications in Autoimmunity with Emphasis on Citrullination, Homocitrullination and Acetylation for the Pathogenesis, Diagnosis and Prognosis of Rheumatoid Arthritis. International Journal of Molecular Sciences. 2022; 23(24):15803. https://doi.org/10.3390/ijms232415803
Chicago/Turabian StyleHaro, Isabel, Raimon Sanmartí, and María J. Gómara. 2022. "Implications of Post-Translational Modifications in Autoimmunity with Emphasis on Citrullination, Homocitrullination and Acetylation for the Pathogenesis, Diagnosis and Prognosis of Rheumatoid Arthritis" International Journal of Molecular Sciences 23, no. 24: 15803. https://doi.org/10.3390/ijms232415803
APA StyleHaro, I., Sanmartí, R., & Gómara, M. J. (2022). Implications of Post-Translational Modifications in Autoimmunity with Emphasis on Citrullination, Homocitrullination and Acetylation for the Pathogenesis, Diagnosis and Prognosis of Rheumatoid Arthritis. International Journal of Molecular Sciences, 23(24), 15803. https://doi.org/10.3390/ijms232415803






