Cancer-Specific miRNAs Extracted from Tissue-Exudative Extracellular Vesicles in Ovarian Clear Cell Carcinoma
Abstract
1. Introduction
2. Results
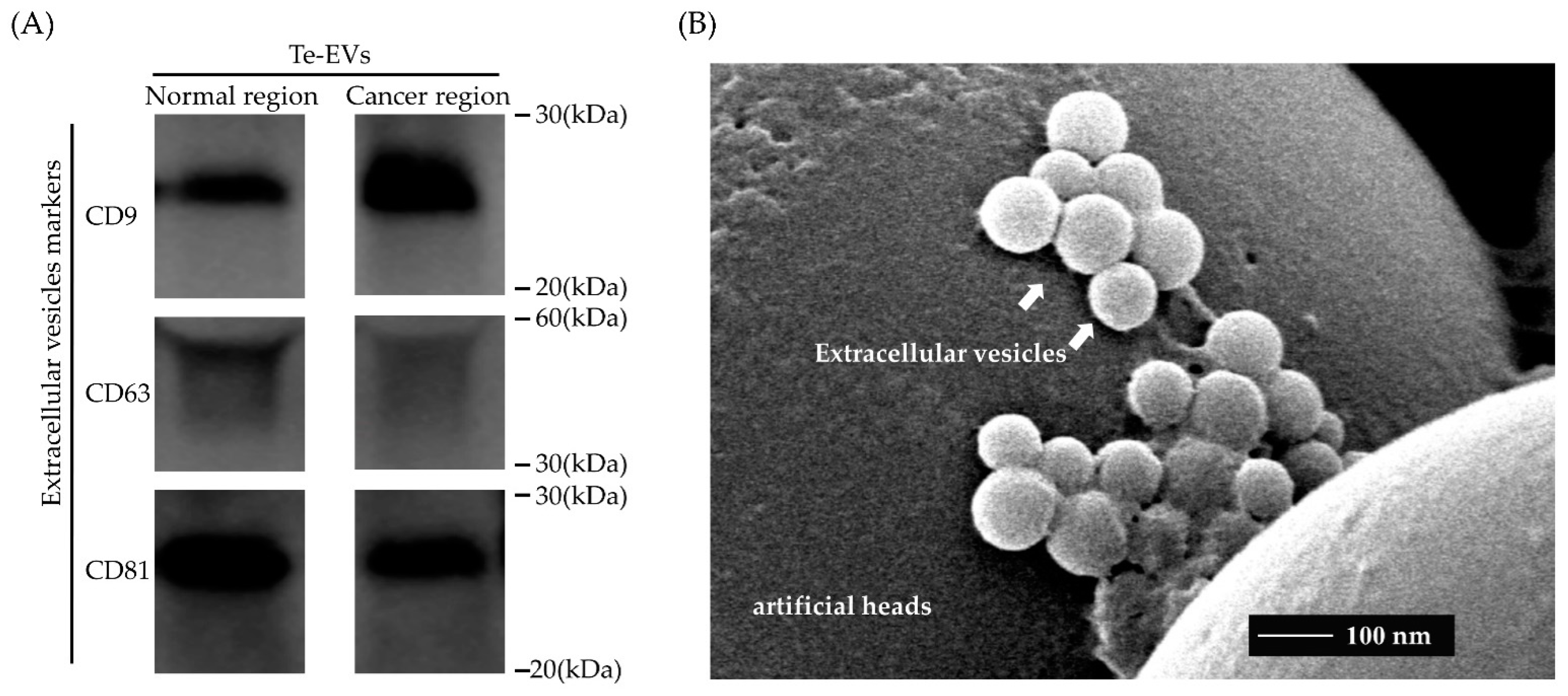
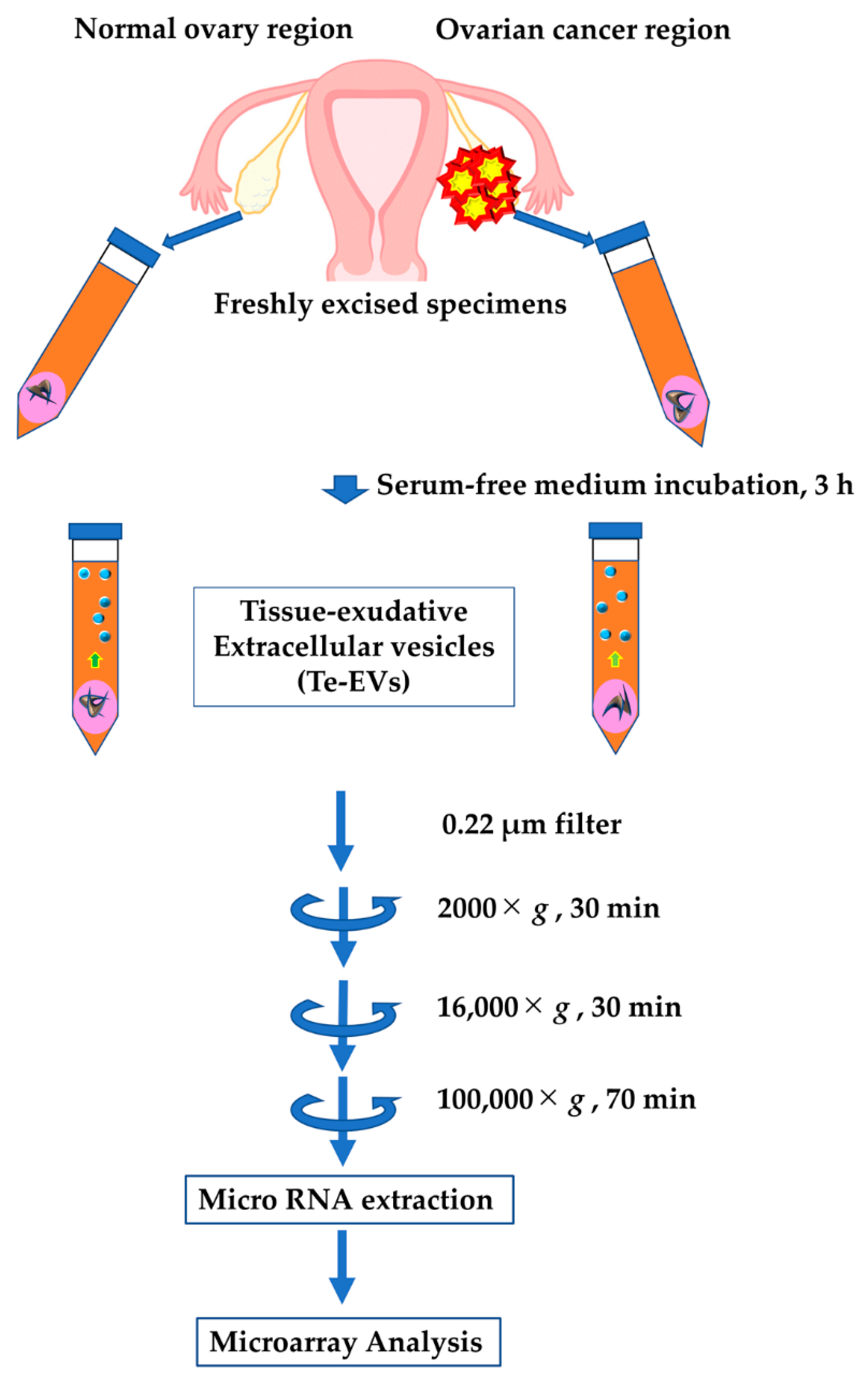
2.1. Isolation of Extracellular Vesicles from Cultured Patient-Derived Tissue
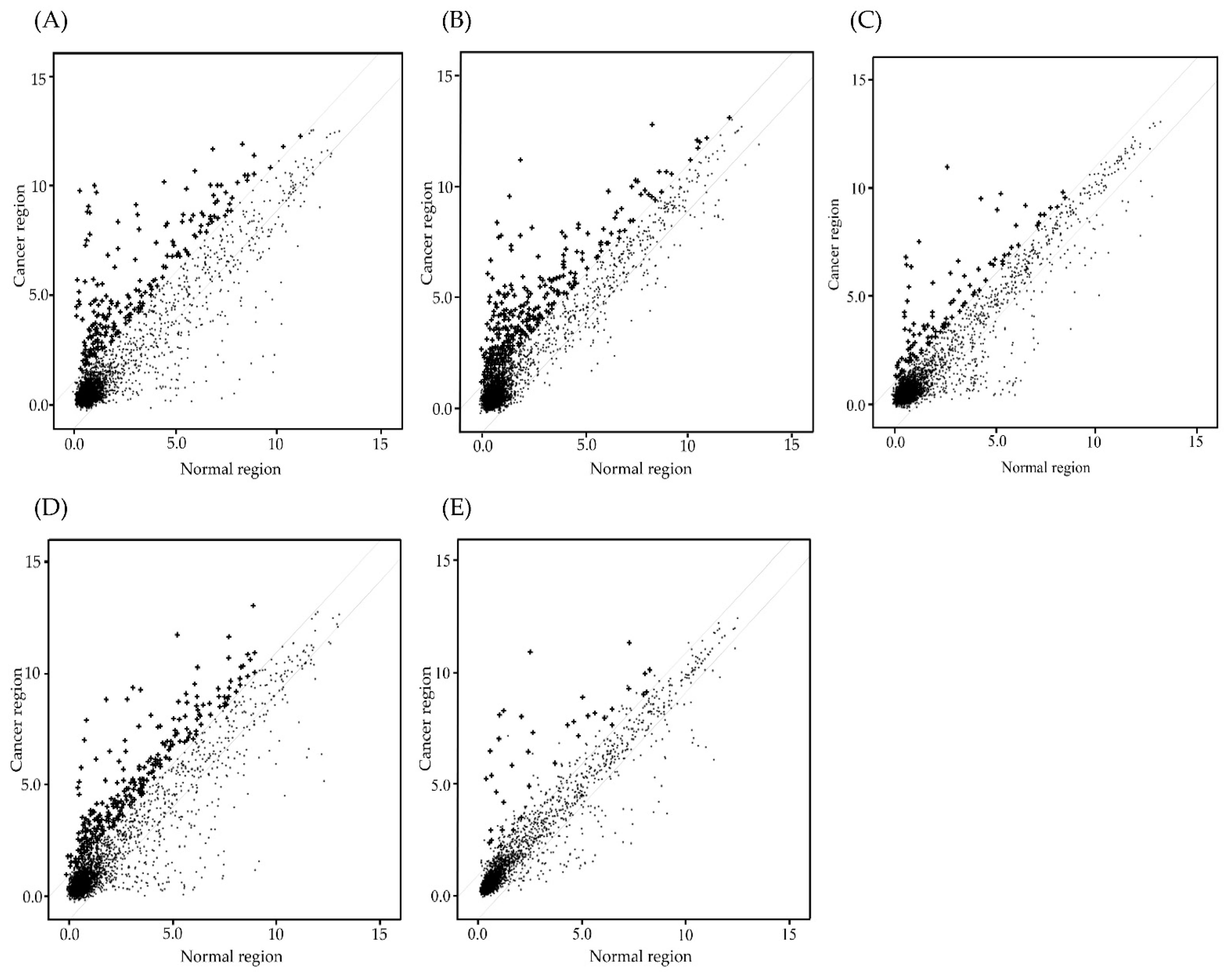
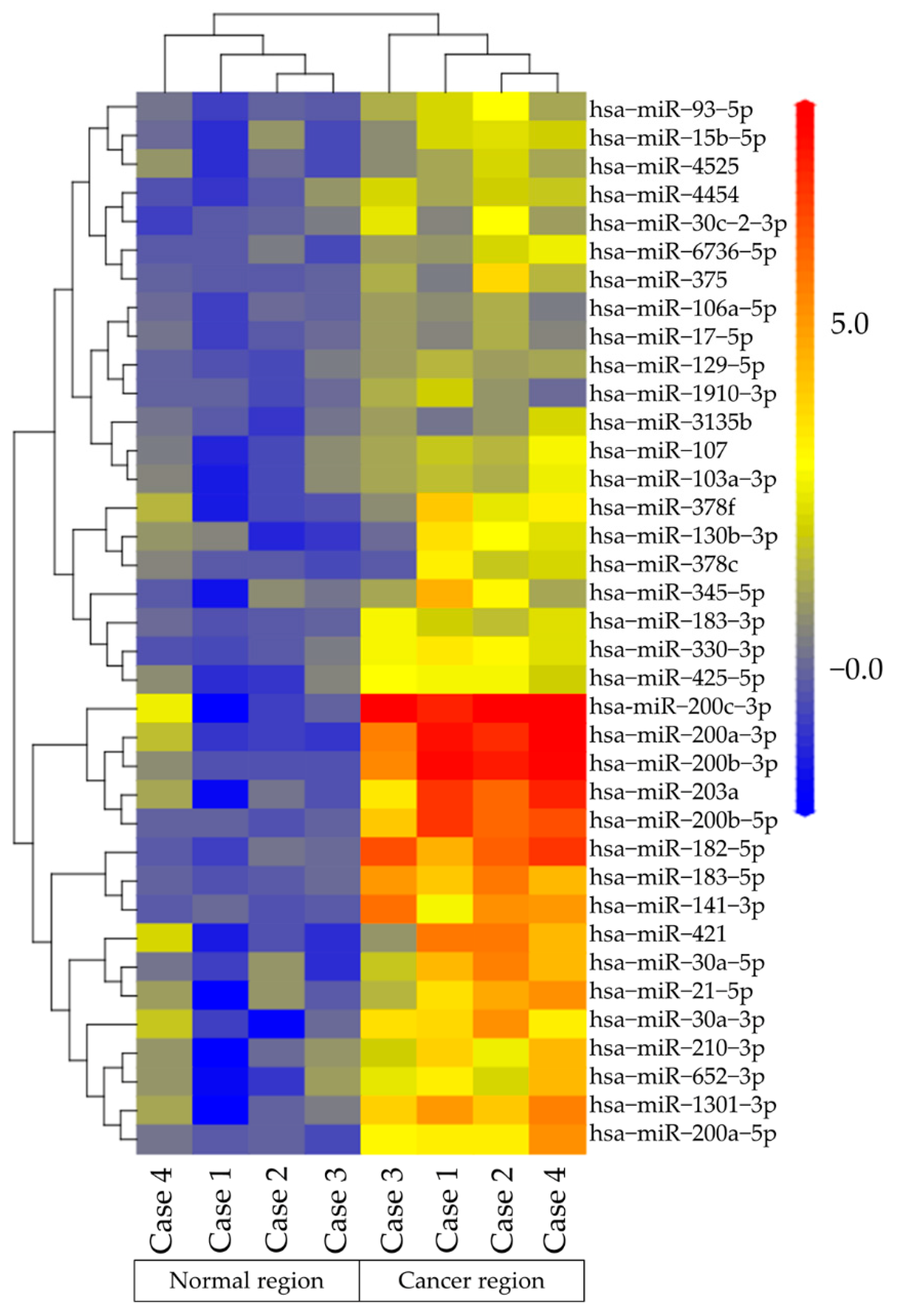
2.2. MiRNA Microarray Analysis of Te-EVs
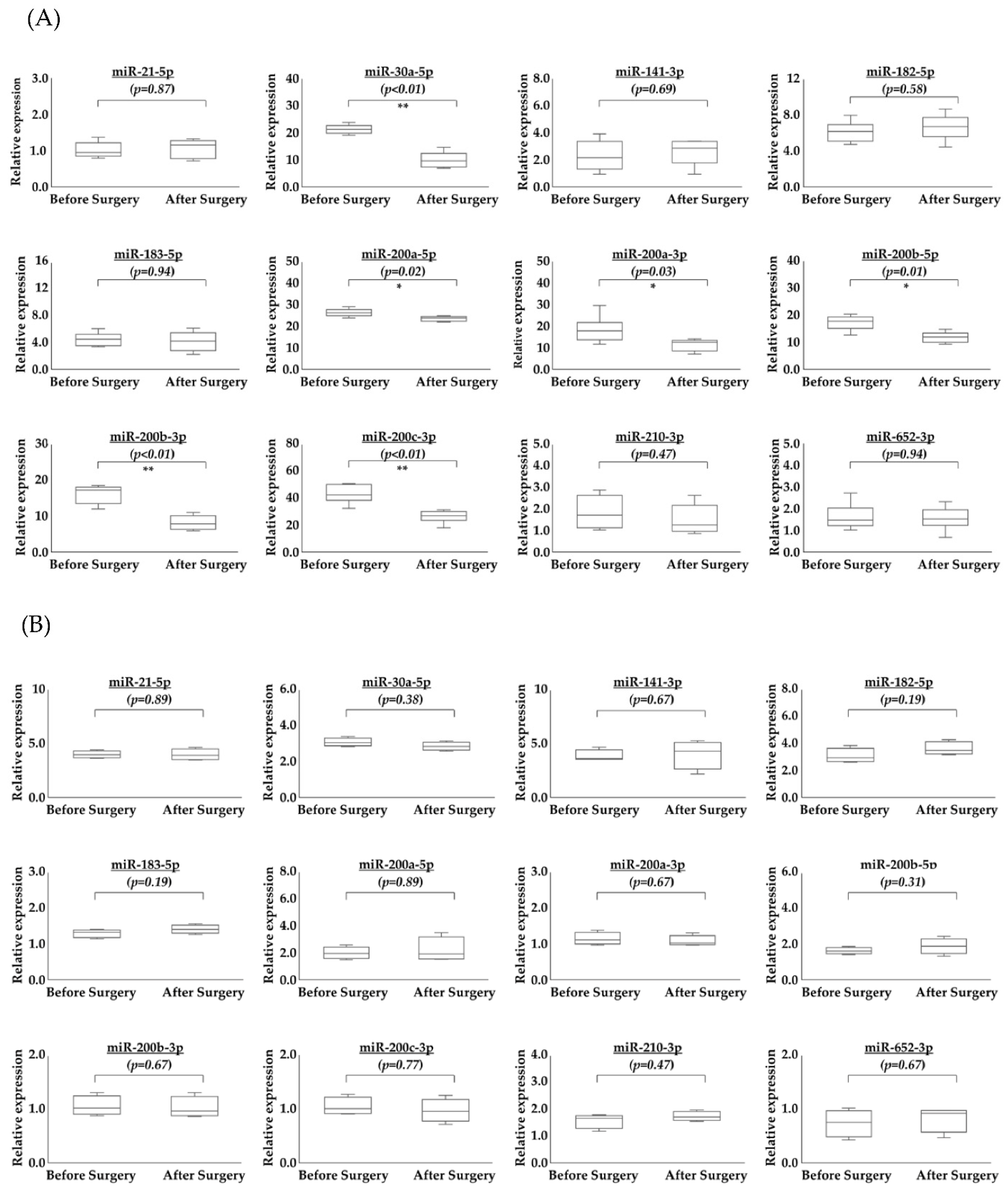
2.3. Comparison of Serum miRNAs in EVs before and after Surgery
3. Discussion
4. Materials and Methods
4.1. Patients and Tissue Samples
4.2. Purification of Extracellular Vesicles
4.3. Western Blot Analysis
4.4. Transmission Electron Microscopy
4.5. Microarray Analysis of miRNA
4.6. Extraction of EV miRNAs
4.7. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR) of miRNAs from Serum EVs before and after Surgery
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today (accessed on 19 November 2022).
- Huttenhain, R.; Soste, M.; Selevsek, N.; Rost, H.; Sethi, A.; Carapito, C.; Farrah, T.; Deutsch, E.W.; Kusebauch, U.; Moritz, R.L.; et al. Reproducible quantification of cancer-associated proteins in body fluids using targeted proteomics. Sci. Transl. Med. 2012, 4, 142ra94. [Google Scholar] [CrossRef] [PubMed]
- Nolen, B.M.; Lokshin, A.E. Protein biomarkers of ovarian cancer: The forest and the trees. Future Oncol. 2012, 8, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers 2020, 12, 3730. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. ARID1A Mutations in Endometriosis-Associated Ovarian Carcinomas. N. Engl. J. Med. 2010, 363, 1532–1543. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Henderson, J.T.; Webber, E.M.; Sawaya, G.F. Screening for Ovarian Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 319, 595–606. [Google Scholar] [CrossRef]
- Samborski, A.; Miller, M.C.; Blackman, A.; MacLaughlan-David, S.; Jackson, A.; Lambert-Messerlian, G.; Rowswell-Turner, R.; Moore, R.G. HE4 and CA125 serum biomarker monitoring in women with epithelial ovarian cancer. Tumor Biol. 2022, 44, 205–213. [Google Scholar] [CrossRef]
- Niu, L.; Song, X.; Wang, N.; Xue, L.; Song, X.; Xie, L. Tumor-derived exosomal proteins as diagnostic biomarkers in non-small cell lung cancer. Cancer Sci. 2019, 110, 433–442. [Google Scholar] [CrossRef]
- Heitzer, E.; Ulz, P.; Geigl, J.B. Circulating Tumor DNA as a Liquid Biopsy for Cancer. Clin. Chem. 2015, 61, 112–123. [Google Scholar] [CrossRef]
- Pessoa, L.S.; Heringer, M.; Ferrer, V.P. ctDNA as a cancer biomarker: A broad overview. Crit. Rev. Oncol. Hematol. 2020, 155, 103109. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.L.; Pectasides, E.; Hanna, G.J.; Parsons, H.A.; Choudhury, A.D.; Oxnard, G.R. Circulating tumor DNA in advanced solid tumors: Clinical relevance and future directions. CA Cancer J. Clin. 2021, 71, 176–190. [Google Scholar] [CrossRef]
- Testa, A.; Venturelli, E.; Brizzi, M.F. Extracellular Vesicles: New Tools for Early Diagnosis of Breast and Genitourinary Cancers. Int. J. Mol. Sci. 2021, 22, 8430. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xu, J.; Xu, Q.; Zhang, B.; Lam, E.W.F.; Sun, Y. Extracellular vesicles in the tumor microenvironment: Therapeutic resistance, clinical biomarkers, and targeting strategies. Med. Res. Rev. 2017, 37, 1318–1349. [Google Scholar] [CrossRef]
- Bevacqua, E.; Ammirato, S.; Cione, E.; Curcio, R.; Dolce, V.; Tucci, P. The potential of microRNAs as non-invasive prostate cancer biomarkers: A systematic literature review based on a machine learning approach. Cancers 2022, 14, 5418. [Google Scholar] [CrossRef]
- Dansero, L.; Ricceri, F.; De Marco, L.; Fiano, V.; Nesi, G.; Padroni, L.; Milani, L.; Caini, S.; Masala, G.; Agnoli, C.; et al. Investigating the role of circulating miRNAs as biomarkers in colorectal cancer: An epidemiological systematic review. Biomedicines 2022, 10, 2224. [Google Scholar] [CrossRef]
- Nascimento, N.P.G.; Gally, T.B.; Borges, G.F.; Campos, L.C.G.; Kaneto, C.M. Systematic review of circulating MICRORNAS as biomarkers of cervical carcinogenesis. BMC Cancer 2022, 22, 862. [Google Scholar] [CrossRef]
- Nguyen, T.H.N.; Nguyen, T.T.N.; Nguyen, T.T.M.; Nguyen, L.H.M.; Huynh, L.H.; Phan, H.N.; Nguyen, H.T. Panels of circulating microRNAs as potential diagnostic biomarkers for breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2022, 196, 1–15. [Google Scholar] [CrossRef]
- Sehovic, E.; Urru, S.; Chiorino, G.; Doebler, P. Meta-analysis of diagnostic cell-free circulating microRNAs for breast cancer detection. BMC Cancer 2022, 22, 634. [Google Scholar] [CrossRef]
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Müller, V.; Milde-Langosch, K.; Trillsch, F.; Pantel, K.; Schwarzenbach, H. Diagnostic and prognostic relevance of circulating exosomal miR-373, miR-200a, miR-200b and miR-200c in patients with epithelial ovarian cancer. Oncotarget 2016, 7, 16923–16935. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Stevic, I.; Müller, V.; Ni, Q.; Oliveira-Ferrer, L.; Pantel, K.; Schwarzenbach, H. Exosomal microRNAs as tumor markers in epithelial ovarian cancer. Mol. Oncol. 2018, 12, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Wyman, S.K.; Parkin, R.K.; Mitchell, P.S.; Fritz, B.R.; O’Briant, K.; Godwin, A.K.; Urban, N.; Drescher, C.W.; Knudsen, B.S.; Tewari, M. Repertoire of microRNAs in Epithelial Ovarian Cancer as Determined by Next Generation Sequencing of Small RNA cDNA Libraries. PLoS ONE 2009, 4, e5311. [Google Scholar] [CrossRef] [PubMed]
- Yanaihara, N.; Noguchi, Y.; Saito, M.; Takenaka, M.; Takakura, S.; Yamada, K.; Okamoto, A. MicroRNA Gene Expression Signature Driven by miR-9 Overexpression in Ovarian Clear Cell Carcinoma. PLoS ONE 2016, 11, e0162584. [Google Scholar] [CrossRef]
- Horie, K.; Nanashima, N.; Yokoyama, Y.; Yoshioka, H.; Watanabe, J. Exosomal MicroRNA as Biomarkers for Diagnosing or Monitoring the Progression of Ovarian Clear Cell Carcinoma: A Pilot Study. Molecules 2022, 27, 3953. [Google Scholar] [CrossRef]
- Jenike, A.E.; Halushka, M.K. miR-21: A non-specific biomarker of all maladies. Biomark. Res. 2021, 9, 18. [Google Scholar] [CrossRef]
- Haider, B.A.; Baras, A.S.; McCall, M.N.; Hertel, J.A.; Cornish, T.C.; Halushka, M.K. A Critical Evaluation of microRNA Biomarkers in Non-Neoplastic Disease. PLoS ONE 2014, 9, e89565. [Google Scholar] [CrossRef]
- Muralidhar, G.; Barbolina, M. The miR-200 Family: Versatile Players in Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2015, 16, 16833–16847. [Google Scholar] [CrossRef]
- Choi, P.W.; Ng, S.W. The Functions of MicroRNA-200 Family in Ovarian Cancer: Beyond Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2017, 18, 1207. [Google Scholar] [CrossRef]
- Sulaiman, S.A.; Ab Mutalib, N.S.; Jamal, R. miR-200c Regulation of Metastases in Ovarian Cancer: Potential Role in Epithelial and Mesenchymal Transition. Front. Pharmacol. 2016, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Yin, J.J.; Abou-Kheir, W.; Hynes, P.G.; Casey, O.M.; Fang, L.; Yi, M.; Stephens, R.M.; Seng, V.; Sheppard-Tillman, H.; et al. MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene 2013, 32, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Burk, U.; Schubert, J.; Wellner, U.; Schmalhofer, O.; Vincan, E.; Spaderna, S.; Brabletz, T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008, 9, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Koutsaki, M.; Libra, M.; Spandidos, D.A.; Zaravinos, A. The miR-200 family in ovarian cancer. Oncotarget 2017, 8, 66629–66640. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, Y.; Cai, J.; Cui, K.; Li, R.X.; Wang, H.; Shang, X.; Wei, D. MiR-30a-5p Suppresses Tumor Metastasis of Human Colorectal Cancer by Targeting ITGB3. Cell. Physiol. Biochem. 2016, 39, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lai, X.; Zhang, W.; Zhu, H.; Zhang, S.; Wu, W.; Wang, S.; Tang, M.; Deng, Z.; Tan, J. MiR-30a-5p frequently downregulated in prostate cancer inhibits cell proliferation via targeting PCLAF. Artif. Cells Nanomed. Biotechnol. 2019, 47, 278–289. [Google Scholar] [CrossRef]
- Ye, Y.Y.; Mei, J.W.; Xiang, S.S.; Li, H.F.; Ma, Q.; Song, X.L.; Wang, Z.; Zhang, Y.C.; Liu, Y.C.; Jin, Y.P.; et al. MicroRNA-30a-5p inhibits gallbladder cancer cell proliferation, migration and metastasis by targeting E2F7. Cell Death Dis. 2018, 9, 410. [Google Scholar] [CrossRef]
- Liu, J.; Wu, X.; Liu, H.; Liang, Y.; Gao, X.; Cai, Z.; Wang, W.; Zhang, H. Expression of microRNA-30a-5p in drug-resistant and drug-sensitive ovarian cancer cell lines. Oncol. Lett. 2016, 12, 2065–2070. [Google Scholar] [CrossRef]
| Case | Age (Years) | BMI (kg/m2) | FIGO Stage | Intraperitoneal Cytology | Follow-Up (Months) | Recurrence | Menopause | Other Disease | Medication |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | 20.1 | IA | Negative | 29 | No | Yes | No | No |
| 2 | 54 | 20.2 | IA | Negative | 26 | No | Yes | No | No |
| 3 | 64 | 21.4 | IC1 | Negative | 29 | No | Yes | No | No |
| 4 | 65 | 26.8 | IIIA | Positive | 27 | Yes | Yes | No | No |
| 5 | 66 | 22.3 | IIIB | Positive | 33 | Yes | Yes | No | No |
| 6 | 70 | 20.1 | IA | Negative | 32 | No | Yes | HT | Ca blocker |
| Case | Age (Years) | BMI (kg/m2) | Intraperitoneal Cytology | Follow-Up (Months) | Recurrence | Menopause | Other Disease | Medication |
|---|---|---|---|---|---|---|---|---|
| 1 | 54 | 24.4 | Negative | 26 | No | Yes | No | No |
| 2 | 57 | 25.3 | Negative | 39 | No | Yes | No | No |
| 3 | 57 | 20.2 | Negative | 25 | No | Yes | No | No |
| 4 | 58 | 26.1 | Negative | 25 | No | Yes | No | No |
| Case | Age (Years) | BMI (kg/m2) | FIGO Stage | Tumor Origin | Intraperitoneal Cytology | Follow-Up (Months) | Recurrence | Menopause | Other Disease | Medication |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | 17.9 | IC1 | Right ovary | Negative | 45 | No | No | No | No |
| 2 | 67 | 17.5 | IC1 | Right ovary | Negative | 43 | No | Yes | HT | Ca blocker |
| 3 | 55 | 21.8 | IIIB | Left ovary | Negative | 26 | No | Yes | No | No |
| 4 | 45 | 16.7 | IC2 | Right ovary | Negative | 40 | Yes | No | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruoka, H.; Tanaka, T.; Murakami, H.; Tsuchihashi, H.; Toji, A.; Nunode, M.; Daimon, A.; Miyamoto, S.; Nishie, R.; Ueda, S.; et al. Cancer-Specific miRNAs Extracted from Tissue-Exudative Extracellular Vesicles in Ovarian Clear Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 15715. https://doi.org/10.3390/ijms232415715
Maruoka H, Tanaka T, Murakami H, Tsuchihashi H, Toji A, Nunode M, Daimon A, Miyamoto S, Nishie R, Ueda S, et al. Cancer-Specific miRNAs Extracted from Tissue-Exudative Extracellular Vesicles in Ovarian Clear Cell Carcinoma. International Journal of Molecular Sciences. 2022; 23(24):15715. https://doi.org/10.3390/ijms232415715
Chicago/Turabian StyleMaruoka, Hiroshi, Tomohito Tanaka, Hikaru Murakami, Hiromitsu Tsuchihashi, Akihiko Toji, Misa Nunode, Atsushi Daimon, Shunsuke Miyamoto, Ruri Nishie, Shoko Ueda, and et al. 2022. "Cancer-Specific miRNAs Extracted from Tissue-Exudative Extracellular Vesicles in Ovarian Clear Cell Carcinoma" International Journal of Molecular Sciences 23, no. 24: 15715. https://doi.org/10.3390/ijms232415715
APA StyleMaruoka, H., Tanaka, T., Murakami, H., Tsuchihashi, H., Toji, A., Nunode, M., Daimon, A., Miyamoto, S., Nishie, R., Ueda, S., Hashida, S., Terada, S., Konishi, H., Kogata, Y., Taniguchi, K., Komura, K., & Ohmichi, M. (2022). Cancer-Specific miRNAs Extracted from Tissue-Exudative Extracellular Vesicles in Ovarian Clear Cell Carcinoma. International Journal of Molecular Sciences, 23(24), 15715. https://doi.org/10.3390/ijms232415715





