Fluid Shear Stress Regulates Osteogenic Differentiation via AnnexinA6-Mediated Autophagy in MC3T3-E1 Cells
Abstract
1. Introduction
2. Results
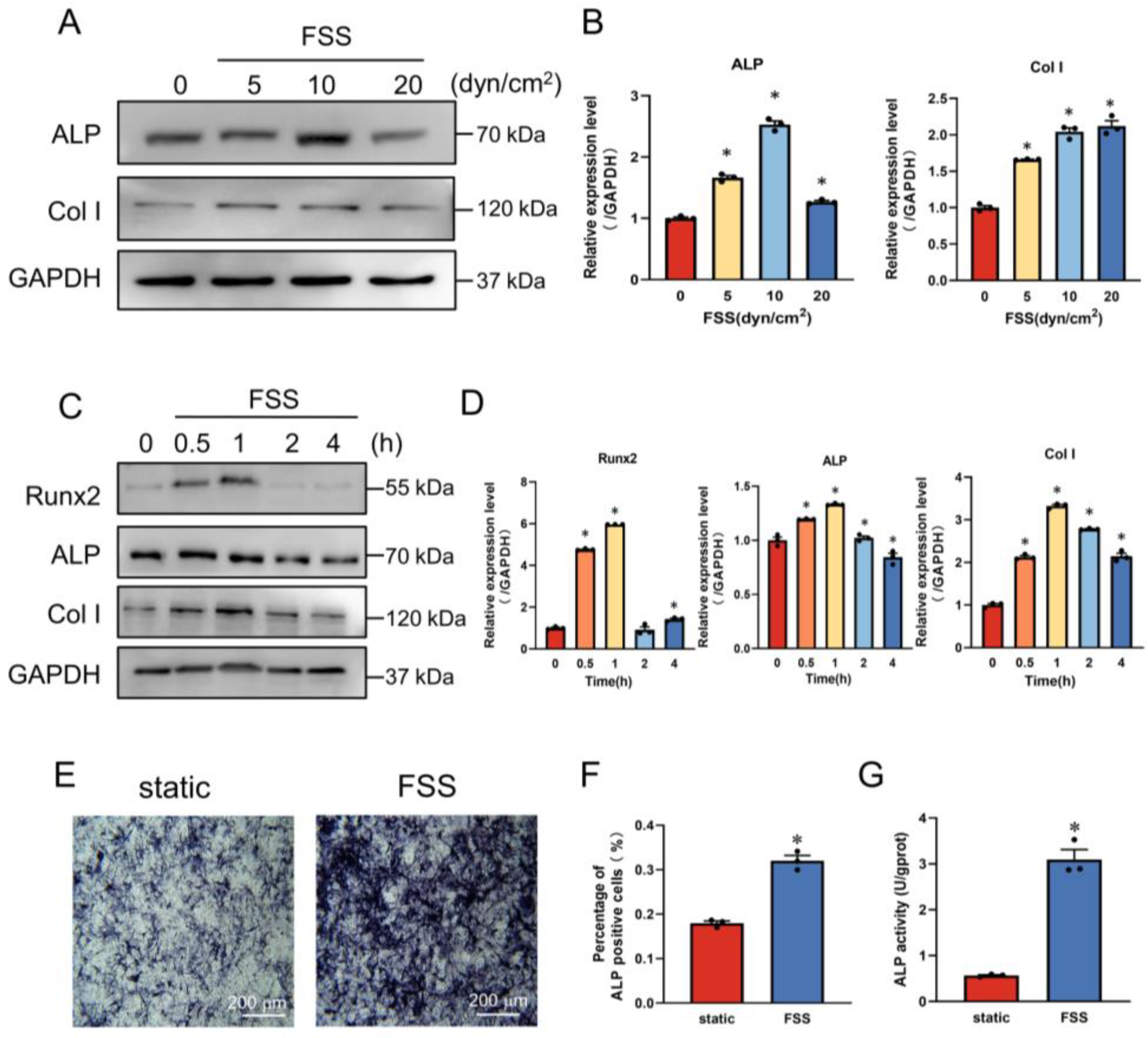
2.1. FSS Induces Osteogenic Differentiation
2.2. FSS Promotes the Expression of AnxA6 in MC3T3-E1 Cells
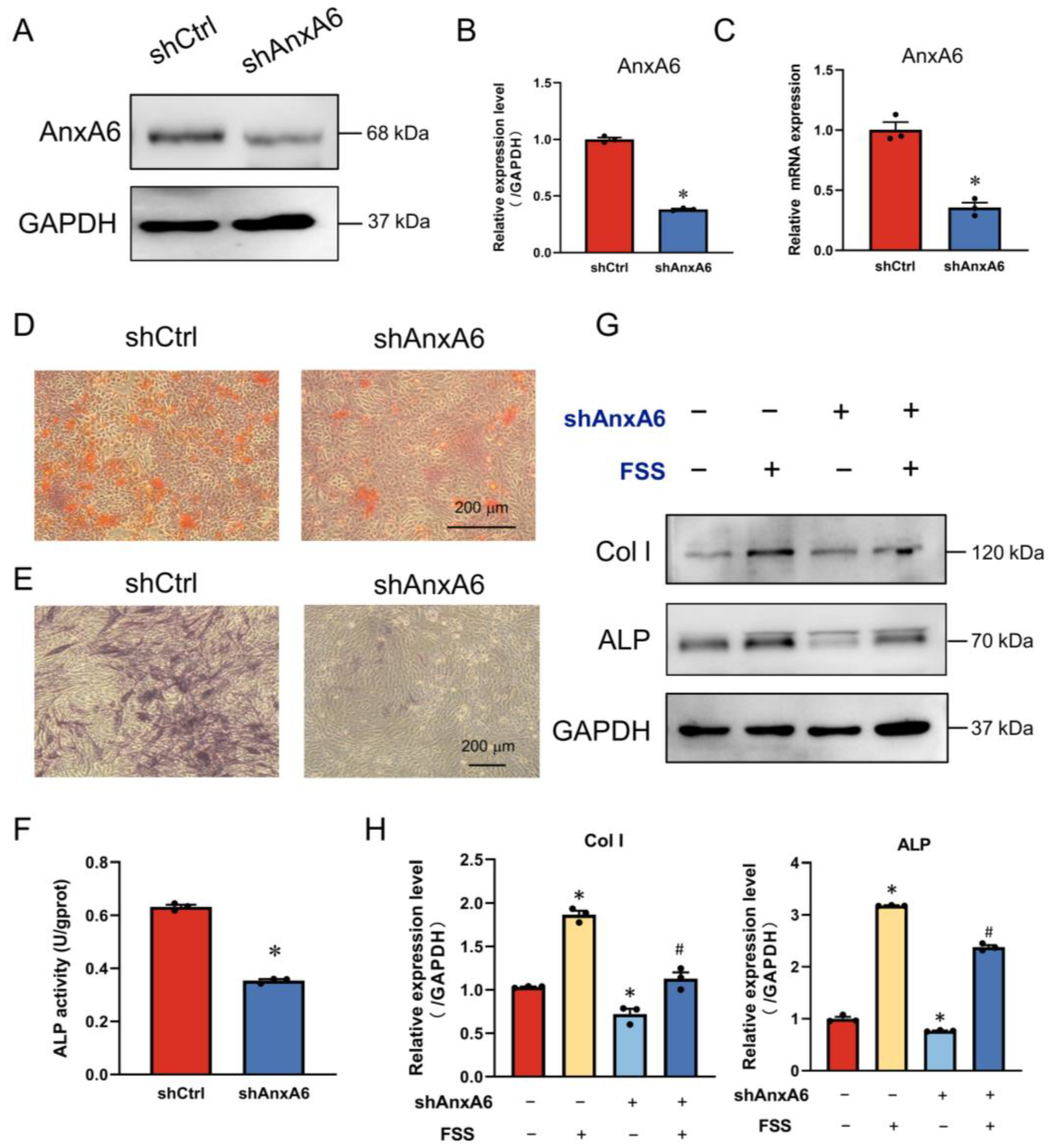
2.3. AnxA6 Involves in FSS-Induced Osteogenic Differentiation
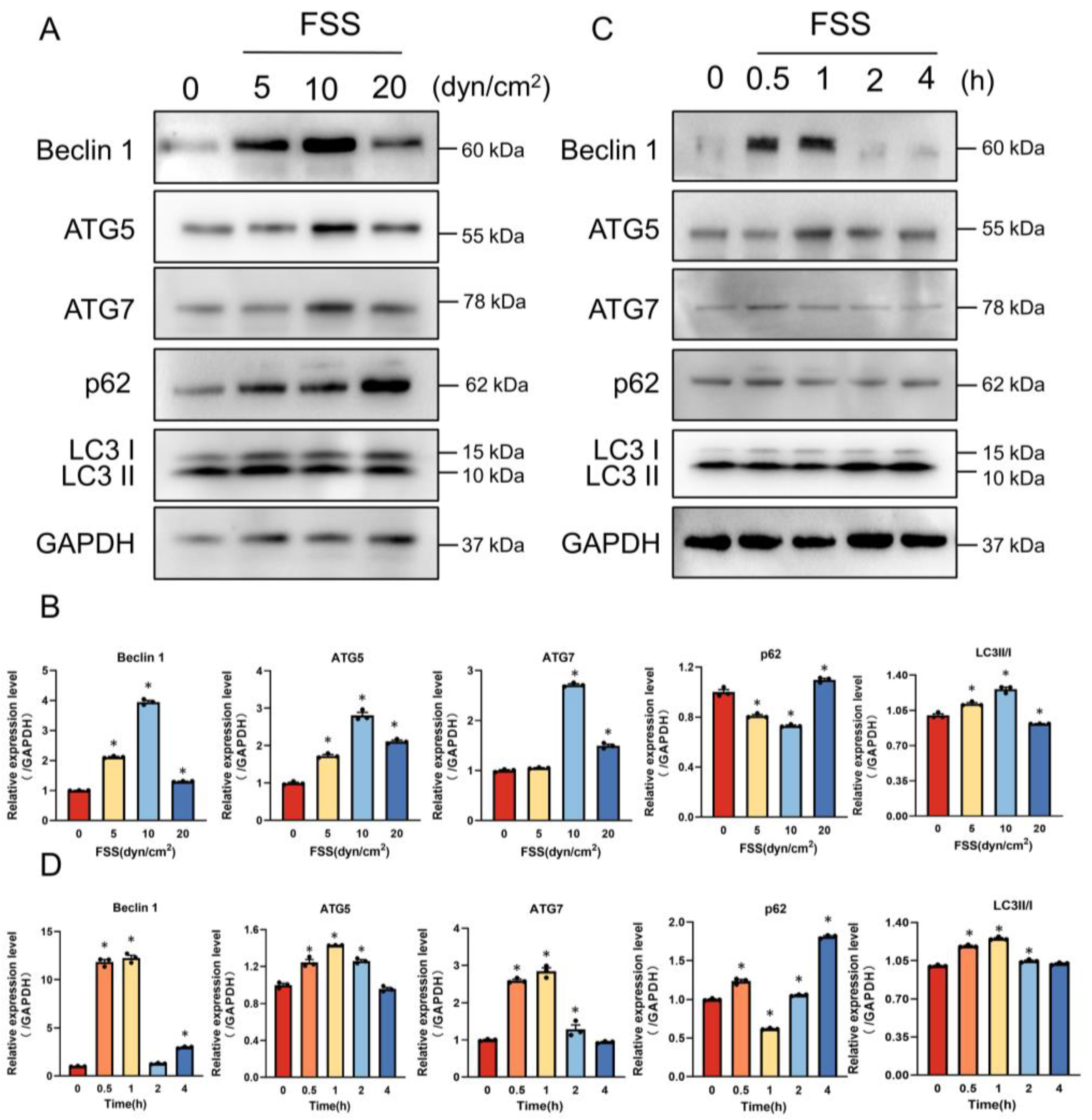
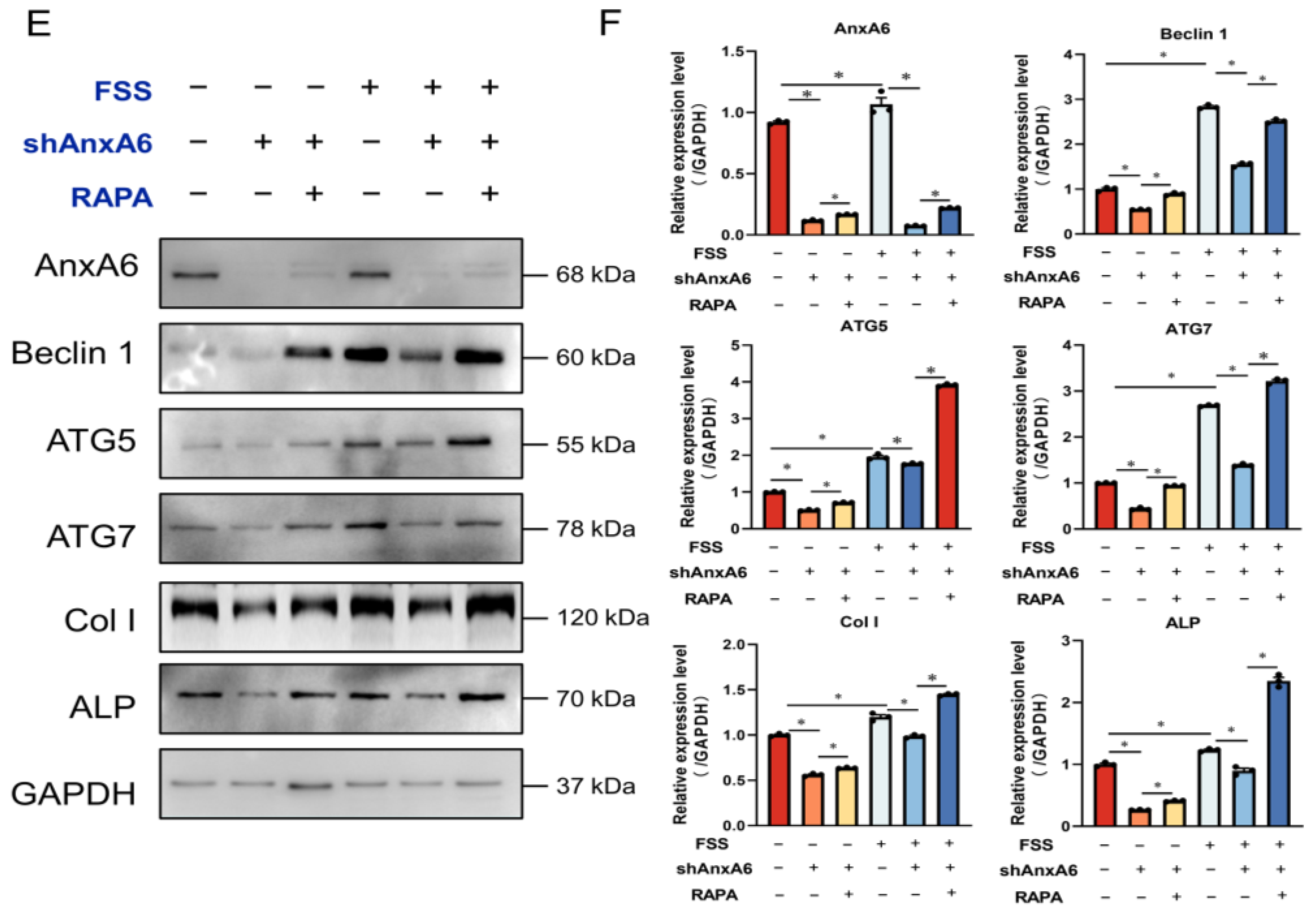
2.4. AnxA6 Knockdown Impairs Autophagy under FSS Condition
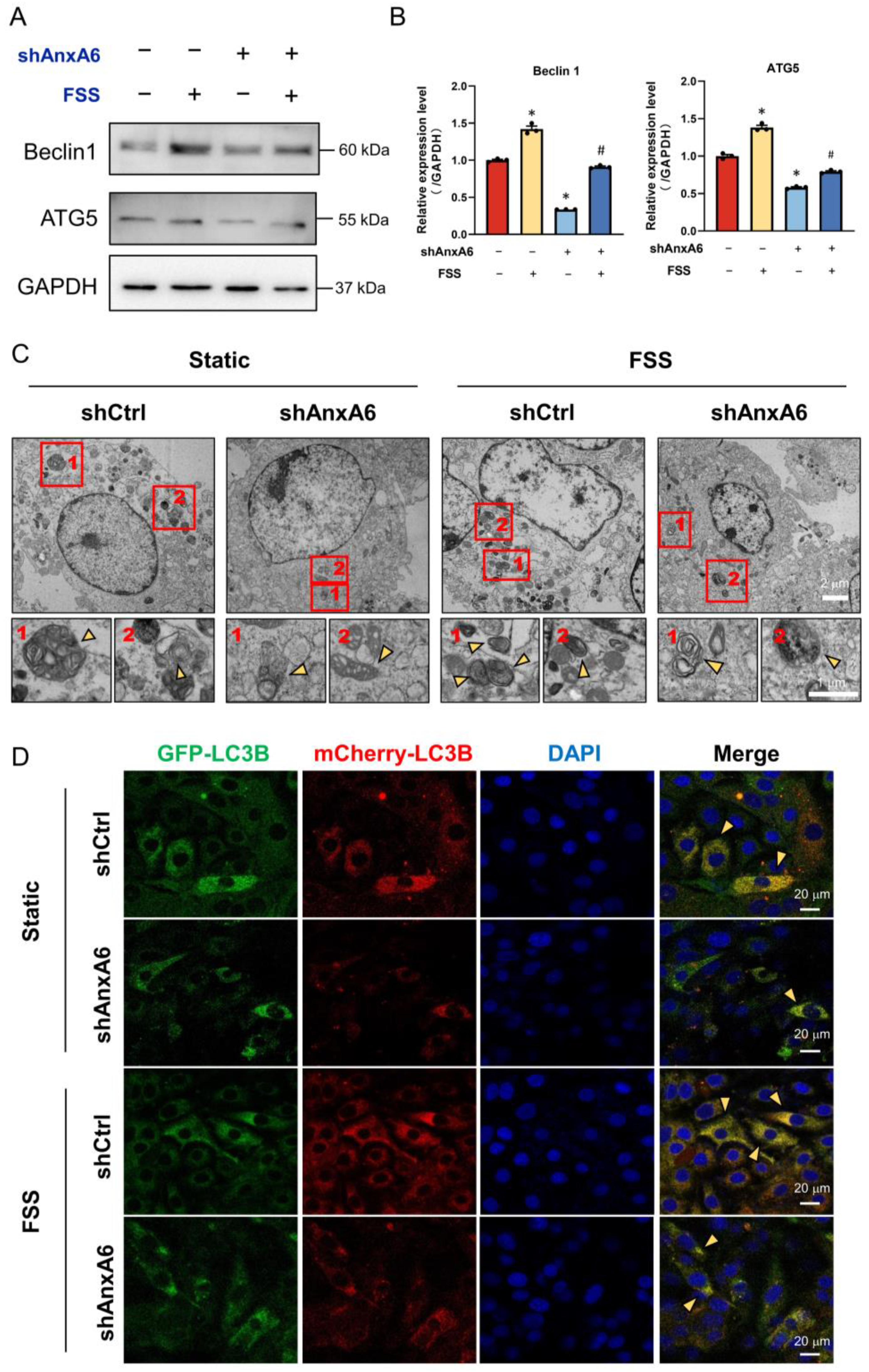
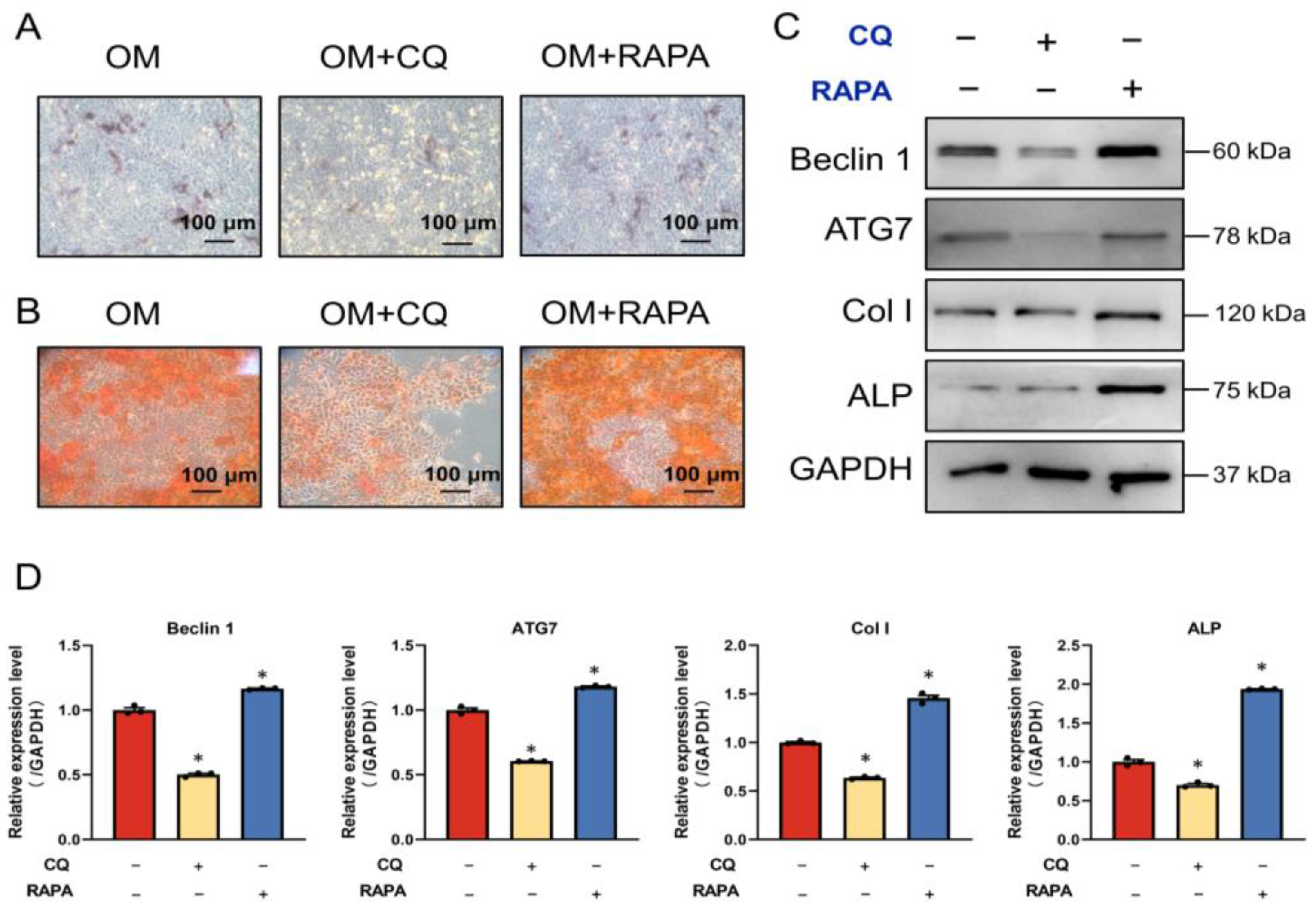
2.5. FSS Promotes Osteogenic Differentiation by Activating AnxA6-Mediated Autophagy
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents and Antibodies
4.3. Plasmid and Transfection
4.4. FSS Loading
4.5. Transmission Electron Microscope (TEM)
4.6. Alizarin Red S Staining
4.7. Alkaline Phosphatase (ALP) Staining
4.8. Alkaline Phosphatase Activity Assay
4.9. Western Blot Analysis
4.10. Quantitative Real-Time PCR (qRT-PCR)
4.11. Immunofluorescence Staining
4.12. AdPlus-mCherry-GFP-LC3B Infection
4.13. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Papachroni, K.K.; Karatzas, D.N.; Papavassiliou, K.A.; Basdra, E.K.; Papavassiliou, A.G. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol. Med. 2009, 15, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The mechanosensitive Piezo1 channel is required for bone formation. eLife 2019, 8, e47454. [Google Scholar] [CrossRef] [PubMed]
- Sikavitsas, V.I.; Temenoff, J.S.; Mikos, A.G. Biomaterials and bone mechanotransduction. Biomaterials 2001, 22, 2581–2593. [Google Scholar] [CrossRef]
- He, Y.B.; Liu, S.Y.; Deng, S.Y.; Kuang, L.P.; Xu, S.Y.; Li, Z.; Xu, L.; Liu, W.; Ni, G.X. Mechanical Stretch Promotes the Osteogenic Differentiation of Bone Mesenchymal Stem Cells Induced by Erythropoietin. Stem Cells Int. 2019, 2019, 1839627. [Google Scholar] [CrossRef]
- Wittkowske, C.; Reilly, G.C.; Lacroix, D.; Perrault, C.M. In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Front. Bioeng. Biotechnol. 2016, 4, 87. [Google Scholar] [CrossRef]
- Ma, C.; Du, T.; Niu, X.; Fan, Y. Biomechanics and mechanobiology of the bone matrix. Bone Res. 2022, 10, 59. [Google Scholar] [CrossRef]
- Thompson, W.R.; Rubin, C.T.; Rubin, J. Mechanical regulation of signaling pathways in bone. Gene 2012, 503, 179–193. [Google Scholar] [CrossRef]
- Kapur, S.; Baylink, D.J.; Lau, K.H.W. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone 2003, 32, 241–251. [Google Scholar] [CrossRef]
- Wang, X.W.; He, J.W.; Wang, H.; Zhao, D.C.; Geng, B.; Wang, S.H.; An, J.D.; Wang, C.F.; Han, H.; Xia, Y.Y. Fluid shear stress regulates osteoblast proliferation and apoptosis via the lncRNA TUG1/miR-34a/FGFR1 axis. J. Cell. Mol. Med. 2021, 25, 8734–8747. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.Y.; Wang, X.M.; Gao, X.H.; Tong, J.; Zhang, J.B. The calcium transient characteristics induced by fluid shear stress affect the osteoblast proliferation. Exp. Cell Res. 2018, 362, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J.H. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. eLife 2019, 8, e49631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Zhou, T.; Chen, Z.P.; Yan, M.; Li, B.C.; Lv, H.Z.; Wang, C.J.; Xiang, S.; Shi, L.; Zhu, Y.; et al. Coupling of Integrin alpha 5 to Annexin A2 by Flow Drives Endothelial Activation. Circ. Res. 2020, 127, 1074–1090. [Google Scholar] [CrossRef] [PubMed]
- Donahue, T.L.H.; Genetos, D.C.; Jacobs, C.R.; Donahue, H.J.; Yellowley, C.E. Annexin V disruption impairs mechanically induced calcium signaling in osteoblastic cells. Bone 2004, 35, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Grewal, T.; Enrich, C.; Rentero, C.; Buechler, C. Annexins in Adipose Tissue: Novel Players in Obesity. Int. J. Mol. Sci. 2019, 20, 3449. [Google Scholar] [CrossRef]
- Balcerzak, M.; Malinowska, A.; Thouverey, C.; Sekrecka, A.; Dadlez, M.; Buchet, R.; Pikula, S. Proteome analysis of matrix vesicles isolated from femurs of chicken embryo. Proteomics 2008, 8, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.F.; Ju, R.; Wang, Y.J. Roles of Annexin A protein family in autophagy regulation and therapy. Biomed. Pharmacother. 2020, 130, 110591. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Yoon, H.Y.; Yun, S.I.; Woo, E.R.; Song, N.K.; Kim, H.G.; Jeong, S.Y.; Chung, Y.S. In Vitro and In Vivo Inhibition of Glucocorticoid-induced Osteoporosis by the Hexane Extract of Poncirus trifoliata. Phytother. Res. 2011, 25, 1000–1010. [Google Scholar] [CrossRef]
- Li, T.T.; Yu, H.C.; Zhang, D.M.; Feng, T.; Miao, M.C.; Li, J.W.; Liu, X.H. Matrix Vesicles as a Therapeutic Target for Vascular Calcification. Front. Cell Dev. Biol. 2022, 10, 825622. [Google Scholar] [CrossRef]
- Li, Y.Z.; Wang, Y.Y.; Huang, L.; Zhao, Y.Y.; Chen, L.H.; Zhang, C. Annexin A protein family in atherosclerosis. Clin. Chim. Acta 2022, 531, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Su, G.Y.; Feng, T.; Pei, T.; Yang, F.; Sun, D.L.; Yu, H.C.; Wang, X.L.; Gao, W.B.; He, J.; Shen, Y.; et al. Autophagy modulates FSS-induced epithelial-mesenchymal transition in hepatocellular carcinoma cells. Mol. Carcinog. 2021, 60, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.P.; Su, G.Y.; Gao, W.B.; He, J.; Shen, Y.; Zeng, Y.; Liu, X.H. Fluid shear stress induces cell migration and invasion via activating autophagy in HepG2 cells. Cell Adhes. Migr. 2019, 13, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhou, C.C.; Li, J.T.; Liu, R.K.; Shi, B.; Yuan, Q.; Zou, S.J. Autophagy in bone homeostasis and the onset of osteoporosis. Bone Res. 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Zeng, Q.Z.; Wu, B.M.; Lu, J.L.; Tong, K.L.; Lin, J.B.; Liu, Q.Y.; Xu, L.P.; Yang, J.; Liu, X.H.; et al. TRIM21-regulated Annexin A2 plasma membrane trafficking facilitates osteosarcoma cell differentiation through the TFEB-mediated autophagy. Cell Death Dis. 2021, 12, 21. [Google Scholar] [CrossRef]
- Enrich, C.; Rentero, C.; Grewal, T. Annexin A6 in the liver: From the endocytic compartment to cellular physiology. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 933–946. [Google Scholar] [CrossRef]
- Trojani, M.C.; Santucci-Darmanin, S.; Breuil, V.; Carle, G.F.; Pierrefite-Carle, V. Autophagy and bone diseases. Jt. Bone Spine 2022, 89, 105301. [Google Scholar] [CrossRef]
- Minashima, T.; Small, W.; Moss, S.E.; Kirsch, T. Intracellular modulation of signaling pathways by annexin A6 regulates terminal differentiation of chondrocytes. J. Biol. Chem. 2012, 287, 14803–14815. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Guadalupe-Grau, A.; Fuentes, T.; Guerra, B.; Calbet, J.A.L. Exercise and Bone Mass in Adults. Sports Med. 2009, 39, 439–468. [Google Scholar] [CrossRef]
- Gong, X.; Yang, W.; Wang, L.; Duncan, R.L.; Pan, J. Prostaglandin E2 modulates F-actin stress fiber in FSS-stimulated MC3T3-E1 cells in a PKA-dependent manner. Acta Biochim. Biophys. Sin. 2014, 46, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.V.; Genoud, K.J.; Early, J.O.; O’Brien, F.J.; Kennedy, O.D. Impact of Fluid Flow Shear Stress on Osteoblast Differentiation and Cross-Talk with Articular Chondrocytes. Int. J. Mol. Sci. 2022, 23, 9505. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Tang, Y.; Li, Z.; Li, J.; Yu, C.; Yang, C.; Liu, L.; Wang, Y.; Liu, Y. miR-24-3p Dominates the Proliferation and Differentiation of Chicken Intramuscular Preadipocytes by Blocking ANXA6 Expression. Genes 2022, 13, 635. [Google Scholar] [CrossRef]
- Jose, J.; Hoque, M.; Engel, J.; Beevi, S.S.; Wahba, M.; Georgieva, M.I.; Murphy, K.J.; Hughes, W.E.; Cochran, B.J.; Lu, A.; et al. Annexin A6 and NPC1 regulate LDL-inducible cell migration and distribution of focal adhesions. Sci. Rep. 2022, 12, 596. [Google Scholar] [CrossRef]
- Solito, E.; Mulla, A.; Morris, J.F.; Christian, H.C.; Flower, R.J.; Buckingham, J.C. Dexamethasone induces rapid serine-phosphorylation and membrane translocation of annexin 1 in a human folliculostellate cell line via a novel nongenomic mechanism involving the glucocorticoid receptor, protein kinase c, phosphatidylinositol 3-kinase, and mitogen-activated protein kinase. Endocrinology 2003, 144, 1164–1174. [Google Scholar] [CrossRef]
- Solito, E.; Nuti, S.; Parente, L. Dexamethasone-Induced Translocation of Lipocortin (Annexin)1 to the Cell-Membrane of U-937 Cells. Br. J. Pharmacol. 1994, 112, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Kapustin, A.N.; Shanahan, C.M. Calcium Regulation of Vascular Smooth Muscle Cell-Derived Matrix Vesicles. Trends Cardiovasc. Med. 2012, 22, 133–137. [Google Scholar] [CrossRef]
- Pfander, D.; Swoboda, B.; Kirsch, T. Expression of early and late differentiation markers (proliferating cell nuclear antigen, syndecan-3, annexin VI, and alkaline phosphatase) by human osteoarthritic chondrocytes. Am. J. Pathol. 2001, 159, 1777–1783. [Google Scholar] [CrossRef]
- Veschi, E.A.; Bolean, M.; Strzelecka-Kiliszek, A.; Bandorowicz-Pikula, J.; Pikula, S.; Granjon, T.; Mebarek, S.; Magne, D.; Ramos, A.P.; Rosato, N.; et al. Localization of Annexin A6 in Matrix Vesicles During Physiological Mineralization. Int. J. Mol. Sci. 2020, 21, 1367. [Google Scholar] [CrossRef]
- Zhou, T.F.; Gao, B.; Fan, Y.; Liu, Y.C.; Feng, S.H.; Cong, Q.; Zhang, X.L.; Zhou, Y.X.; Yadav, P.S.; Lin, J.C.; et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ss-satenin. eLife 2020, 9, e52779. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 2020, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Tu, J.; Wang, W.; Li, Z.; Li, Y.; Yu, X.; Zhang, Z. Effects of Mechanical Stress Stimulation on Function and Expression Mechanism of Osteoblasts. Front. Bioeng. Biotechnol. 2022, 10, 830722. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.; Tokumaru, Y.; Mukhopadhyay, S.; Yan, L.; Matsuyama, R.; Endo, I.; Takabe, K. Annexin A1 Expression Is Associated with Epithelial-Mesenchymal Transition (EMT), Cell Proliferation, Prognosis, and Drug Response in Pancreatic Cancer. Cells 2021, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Ewing, M.M.; de Vries, M.R.; Nordzell, M.; Pettersson, K.; de Boer, H.C.; van Zonneveld, A.J.; Frostegard, J.; Jukema, J.W.; Quax, P.H. Annexin A5 therapy attenuates vascular inflammation and remodeling and improves endothelial function in mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Puerto, M.C.; Verhagen, L.P.; Braat, A.K.; Lam, E.W.F.; Coffer, P.J.; Lorenowicz, M.J. Activation of autophagy by FOXO3 regulates redox homeostasis during osteogenic differentiation. Autophagy 2016, 12, 1804–1816. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tao, J.; Yao, Y.Y.; Yang, P.; Wang, J.H.; Yu, M.L.; Hou, J.H.; Zhang, Y.; Gui, L. Resveratrol induces proliferation in preosteoblast cell MC3T3-E1 via GATA-1 activating autophagy. Acta Biochim. Biophys. Sin. 2021, 53, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.G.; Yu, Y.F.; Zheng, Q.; Zhang, W.; Wang, C.D.; Zhao, X.Y.; Tong, W.X.; Wang, H.; Liu, P.; Zhang, X.L. Autophagy protects end plate chondrocytes from intermittent cyclic mechanical tension induced calcification. Bone 2014, 66, 232–239. [Google Scholar] [CrossRef]
- Liu, F.; Fang, F.; Yuan, H.B.; Yang, D.Y.; Chen, Y.Q.; Williams, L.; Goldstein, S.A.; Krebsbach, P.H.; Guan, J.L. Suppression of Autophagy by FIP200 Deletion Leads to Osteopenia in Mice Through the Inhibition of Osteoblast Terminal Differentiation. J. Bone Miner. Res. 2013, 28, 2414–2430. [Google Scholar] [CrossRef]
- Luo, D.; Ren, H.; Li, T.; Lian, K.; Lin, D. Rapamycin reduces severity of senile osteoporosis by activating osteocyte autophagy. Osteoporos. Int. 2016, 27, 1093–1101. [Google Scholar] [CrossRef]
- Zhang, B.B.; Hou, R.T.; Zou, Z.; Luo, T.T.; Zhang, Y.; Wang, L.Y.; Wang, B. Mechanically induced autophagy is associated with ATP metabolism and cellular viability in osteocytes in vitro. Redox Biol. 2018, 14, 492–498. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhang, Y.Y.; Feng, T.; Su, G.Y.; He, J.; Gao, W.B.; Shen, Y.; Liu, X.H. Fluid Shear Stress Promotes Autophagy in Hepatocellular Carcinoma Cells. Int. J. Biol. Sci. 2018, 14, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yan, Z.P.; Ma, L.J.; Liu, X.H. Research Progress of Cell Autophagy Induced by Mechanical Stress. Prog. Biochem. Biophys. 2019, 46, 555–564. [Google Scholar] [CrossRef]
- Chen, Q.P.; Zheng, W.; Zhu, L.; Yao, D.; Wang, C.; Song, Y.M.; Hu, S.L.; Liu, H.X.; Bai, Y.; Pan, Y.; et al. ANXA6 Contributes to Radioresistance by Promoting Autophagy via Inhibiting the PI3K/AKT/mTOR Signaling Pathway in Nasopharyngeal Carcinoma. Front. Cell Dev. Biol. 2020, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shu, Y.H.; Xu, M.T.; Jiang, J.K.; Wang, L.M.; Wang, J.G.; Huang, D.S.; Zhang, J.B. ANXA6 suppresses the tumorigenesis of cervical cancer through autophagy induction. Clin. Transl. Med. 2020, 10, e208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Z.; Shi, G.X.; Zheng, X.F.; Jiang, S.D.; Jiang, L.S. Autophagy activation facilitates mechanical stimulation-promoted osteoblast differentiation and ameliorates hindlimb unloading-induced bone loss. Biochem. Biophys. Res. Commun. 2018, 498, 667–673. [Google Scholar] [CrossRef]
- Wang, L.; Yan, J.; Hu, X.; Zhu, X.; Hu, S.; Qian, J.; Zhang, F.; Liu, M. Effect of nanoscale bioactive glass with radial spherical particles on osteogenic differentiation of rat bone marrow mesenchymal stem cells. Mater. Med. 2020, 31, 29. [Google Scholar] [CrossRef]
- Kim, B.S.; Kang, H.J.; Park, J.Y.; Lee, J. Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem cells. Exp. Mol. Med. 2015, 47, e128. [Google Scholar] [CrossRef]


| Category | Antibody | Isotype | Manufacturer | Cat. No | Dilution |
|---|---|---|---|---|---|
| Autophagic markers | Beclin1 | Rabbit pAb | Santa Cruz | sc-11427 | 1:100 |
| ATG7 | Mouse mAb | Proteintech | 67341-1-Ig | 1:1000 | |
| P62(SQSTM1) | Rabbit pAb | HuaBio | R1309-8 | 1:1000 | |
| ATG5 | Rabbit mAb | HuaBio | ET1611-38 | 1:1000 | |
| LC3B | Rabbit mAb | Abcam | Ab192890 | 1:1000 | |
| Osteoblastic differentiation marker | ALP | Rabbit pAb | HuaBio | ET1601-21 | 1:500 |
| Col I | Rabbit pAb | Proteintech | 14695-1-AP | 1:1000 | |
| Runx2 | Rabbit mAb | CST | #12556 | 1:1000 | |
| Annexin | AnxA6 | Rabbit pAb | Proteintech | 12542-1-AP | 1:500 for IF 1:1000 for WB |
| GAPDH | Rabbit mAb | SAB | 21337 | 1:1000 |
| Name | Forward | Reverse |
|---|---|---|
| GAPDH | 5′-AACATCAAATGGGGTGAGGCC-3′ | 5′-GTTGTCATGGATGACCTGGC-3′ |
| AnxA6 | 5′-CTTCGGCAGTGACAAGGAGT-3′ | 5′-TGGCGTCACAATAGGCAAGT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, T.; Su, G.; Yang, J.; Gao, W.; Yang, X.; Zhang, Y.; Ren, J.; Shen, Y.; Liu, X. Fluid Shear Stress Regulates Osteogenic Differentiation via AnnexinA6-Mediated Autophagy in MC3T3-E1 Cells. Int. J. Mol. Sci. 2022, 23, 15702. https://doi.org/10.3390/ijms232415702
Pei T, Su G, Yang J, Gao W, Yang X, Zhang Y, Ren J, Shen Y, Liu X. Fluid Shear Stress Regulates Osteogenic Differentiation via AnnexinA6-Mediated Autophagy in MC3T3-E1 Cells. International Journal of Molecular Sciences. 2022; 23(24):15702. https://doi.org/10.3390/ijms232415702
Chicago/Turabian StylePei, Tong, Guanyue Su, Jie Yang, Wenbo Gao, Xinrui Yang, Yaojia Zhang, Jie Ren, Yang Shen, and Xiaoheng Liu. 2022. "Fluid Shear Stress Regulates Osteogenic Differentiation via AnnexinA6-Mediated Autophagy in MC3T3-E1 Cells" International Journal of Molecular Sciences 23, no. 24: 15702. https://doi.org/10.3390/ijms232415702
APA StylePei, T., Su, G., Yang, J., Gao, W., Yang, X., Zhang, Y., Ren, J., Shen, Y., & Liu, X. (2022). Fluid Shear Stress Regulates Osteogenic Differentiation via AnnexinA6-Mediated Autophagy in MC3T3-E1 Cells. International Journal of Molecular Sciences, 23(24), 15702. https://doi.org/10.3390/ijms232415702






