Analysis and Functional Verification of PlPM19L Gene Associated with Drought-Resistance in Paeonia lactiflora Pall.
Abstract
1. Introduction
2. Result
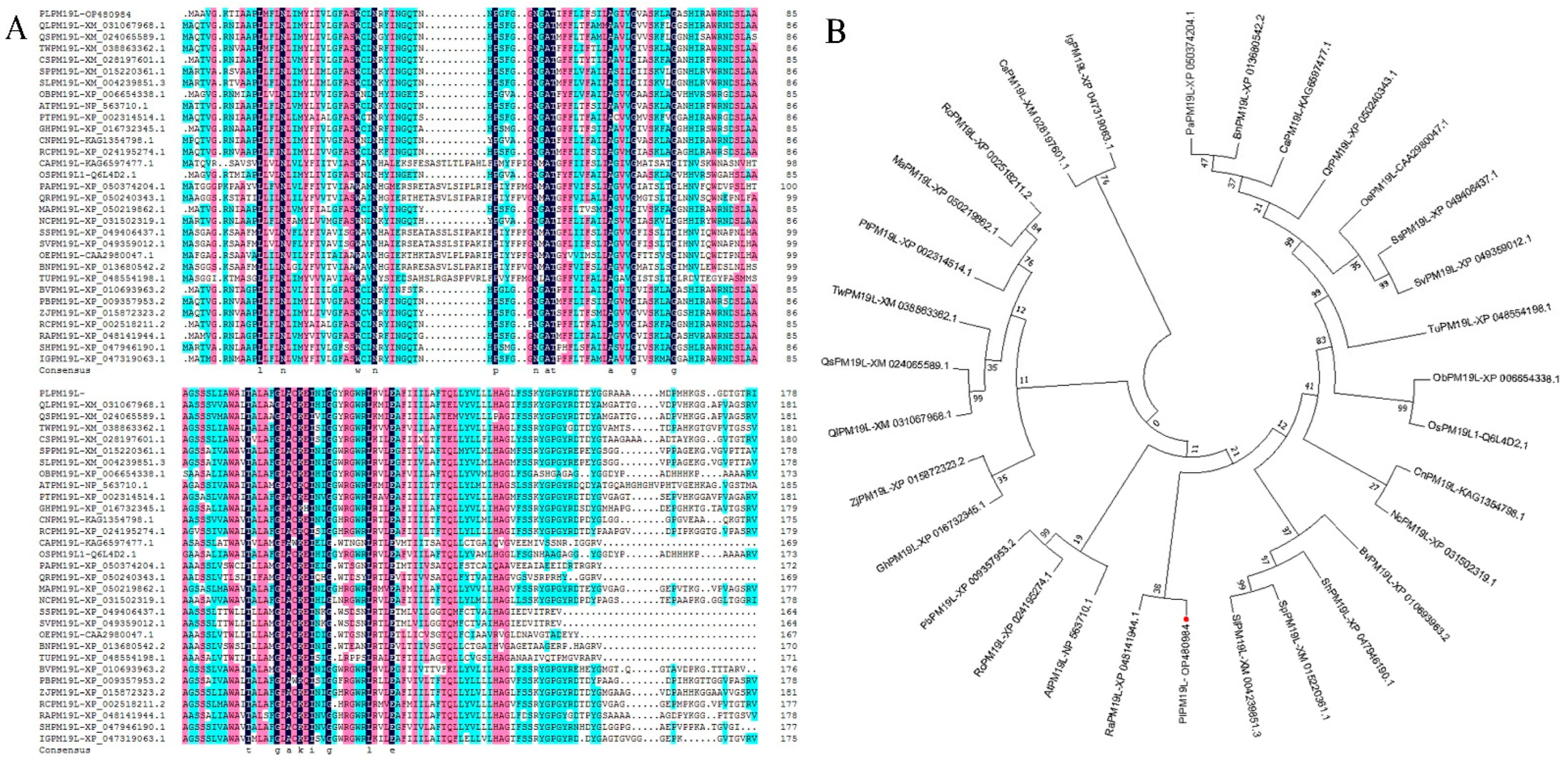
2.1. Gene Clone and Sequence Analysis of PlPM19L from P. lactiflora
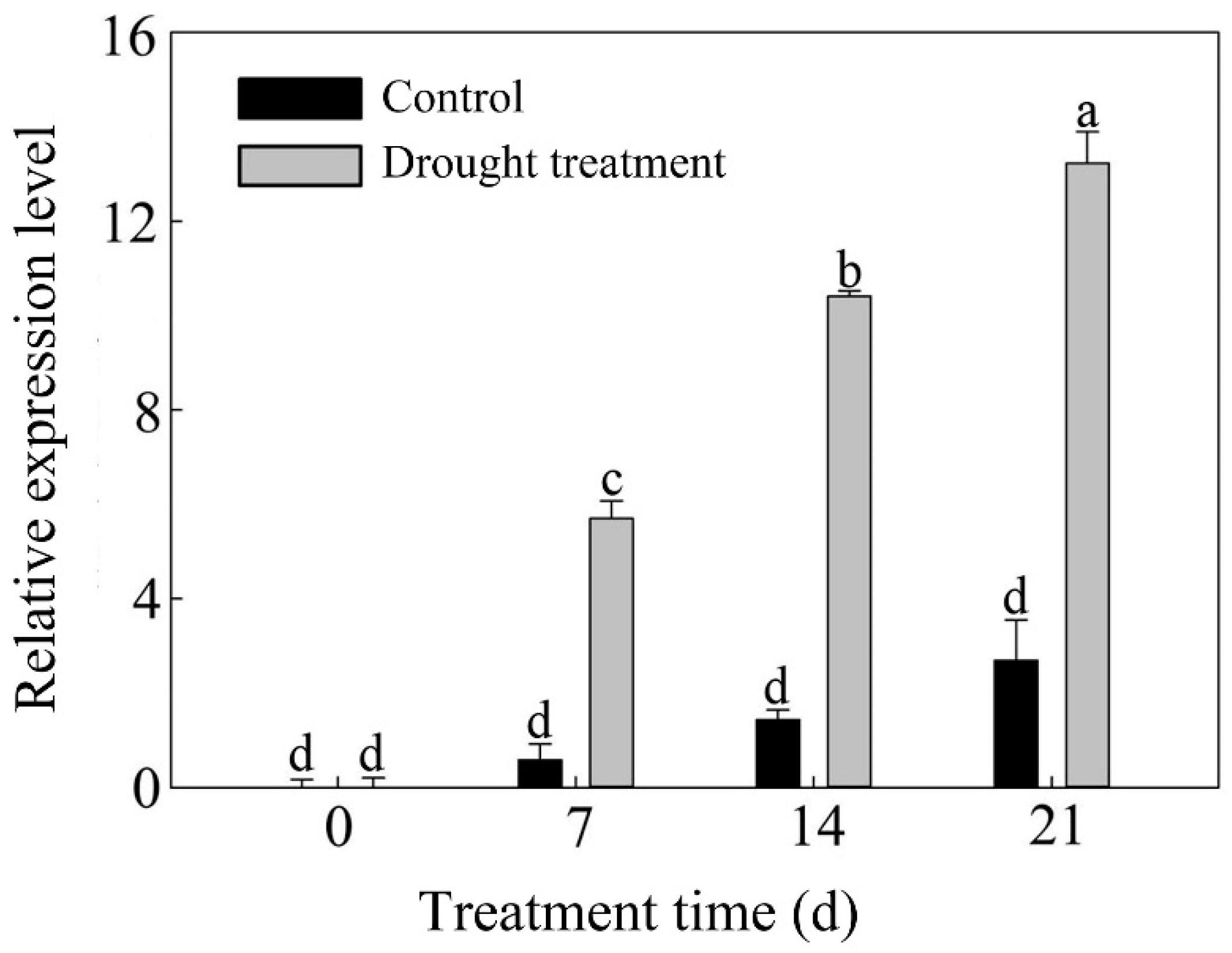
2.2. Expression Level Analysis of PlPM19L in P. lactiflora
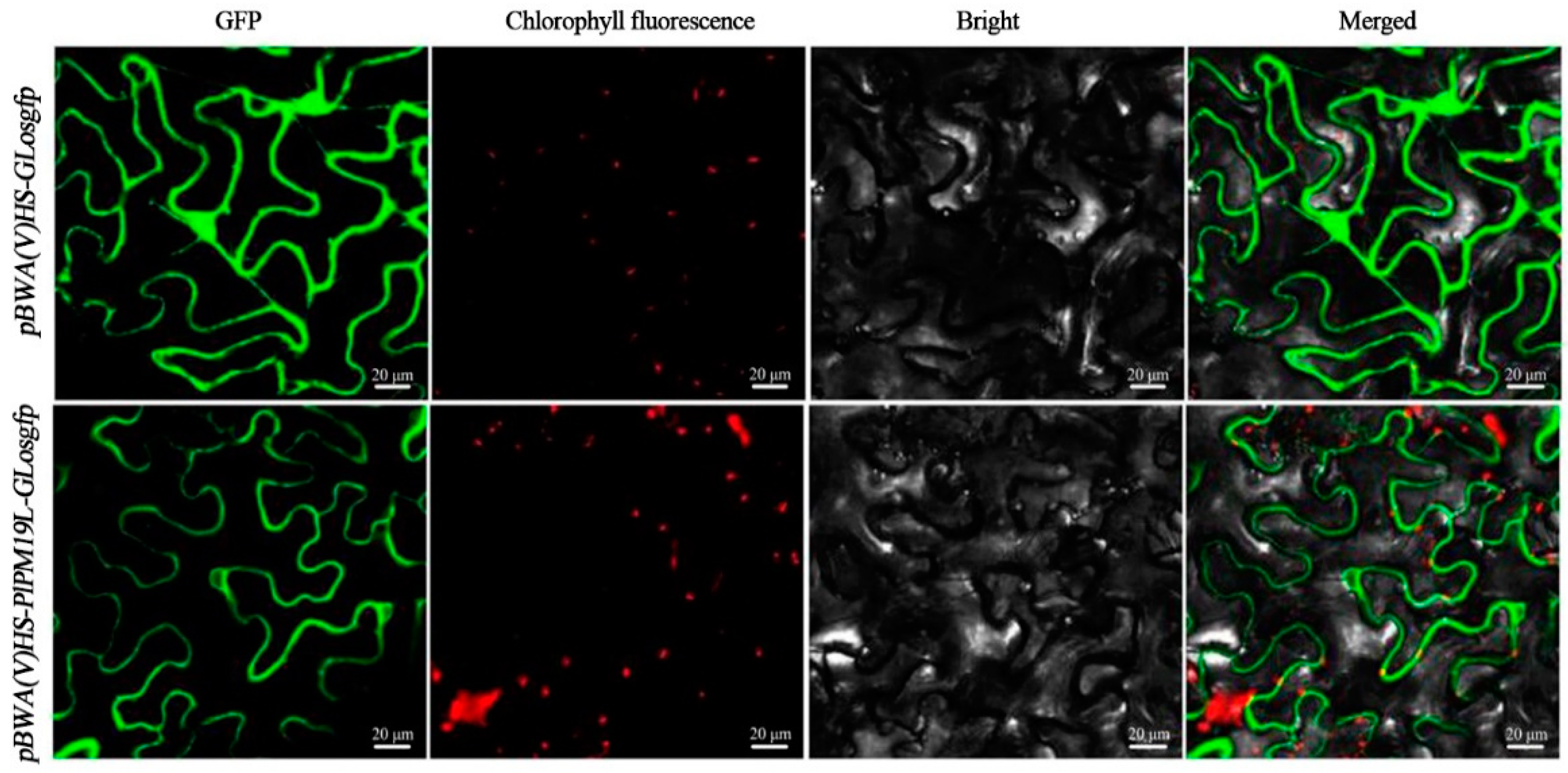
2.3. Subcellular Localization of the PlPM19L Protein
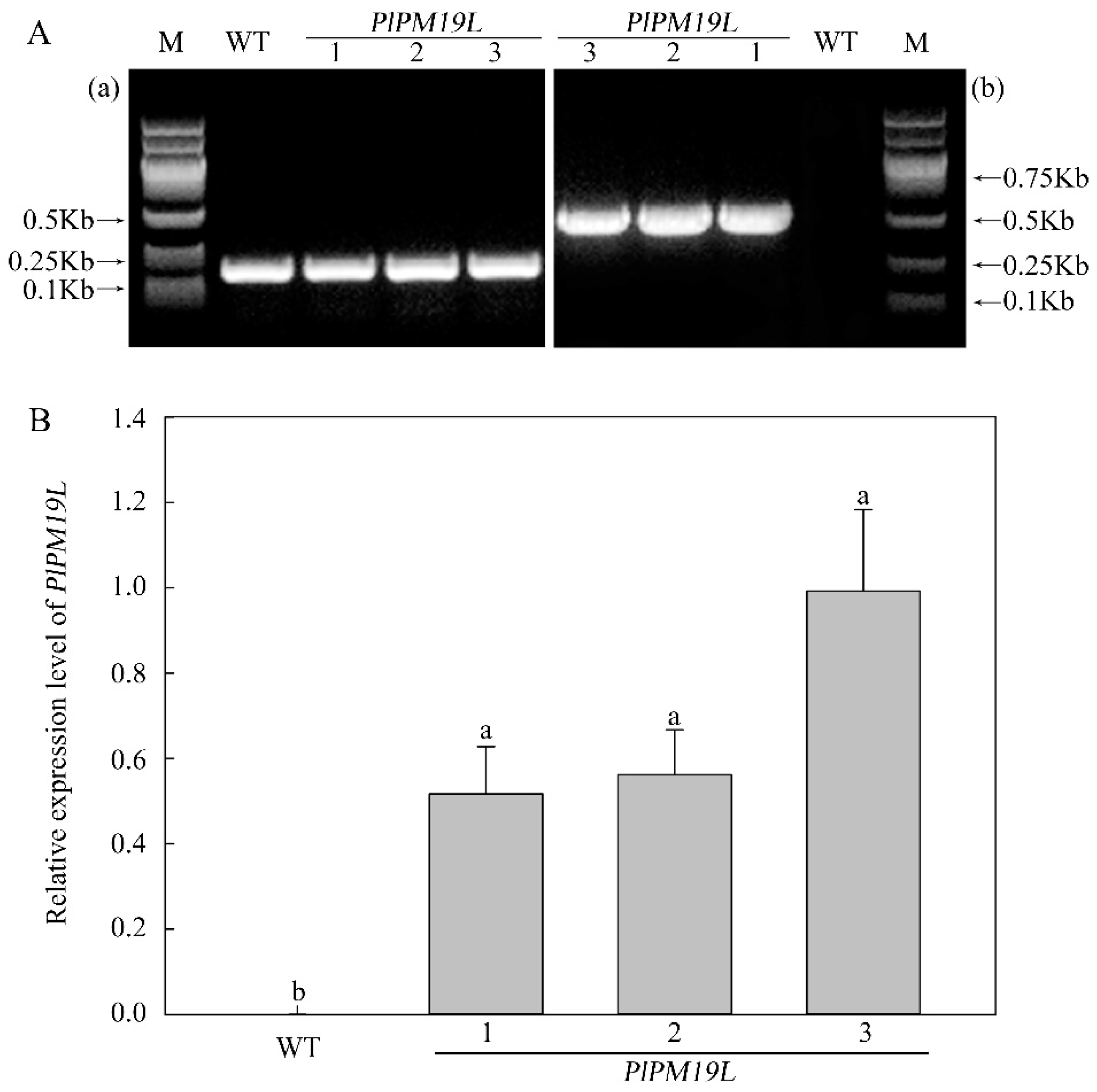
2.4. Identification of Transgenic PlPM19L in N. tabacum
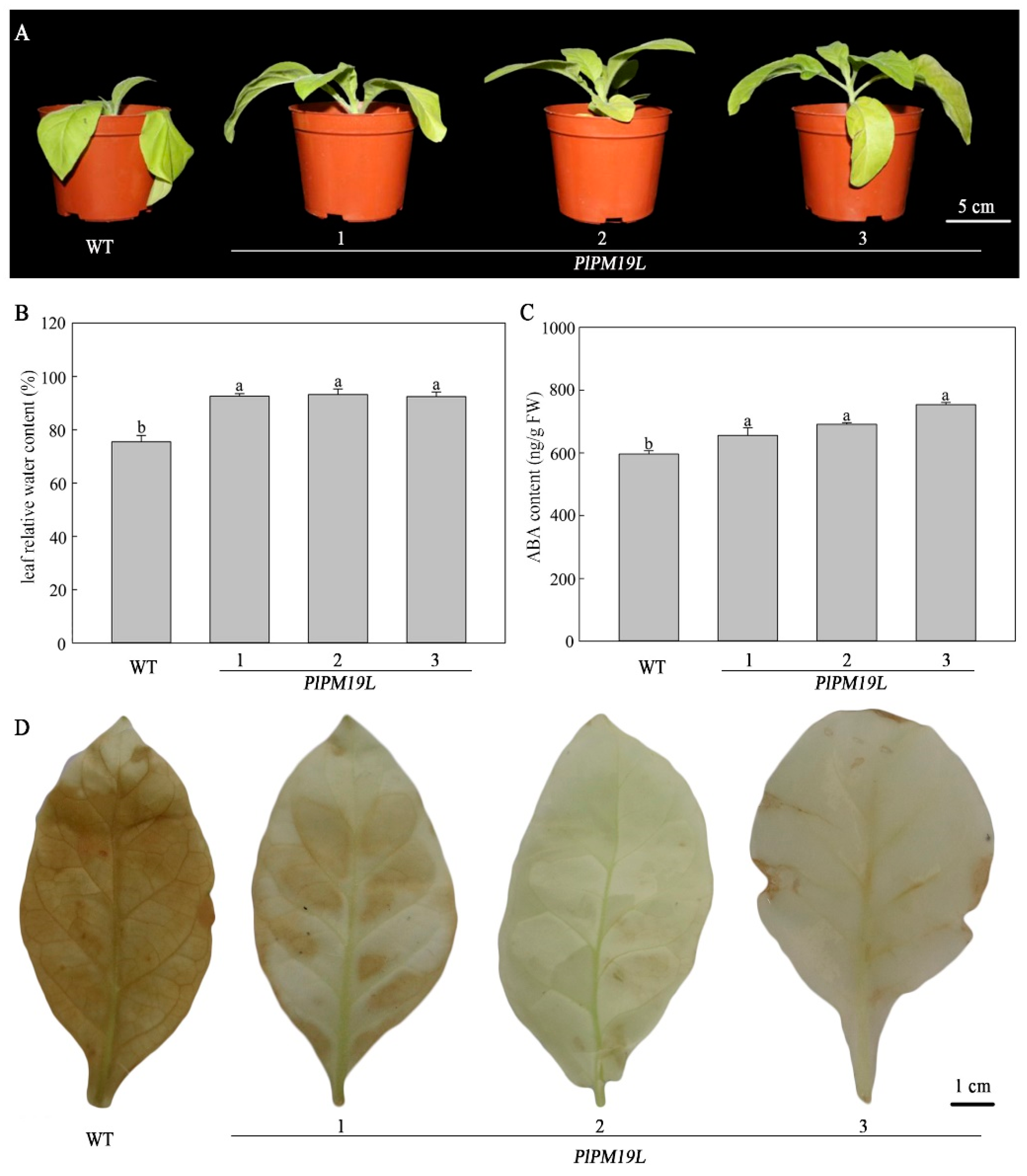
2.5. Effects of Drought Stress on the Phenotype of Transgenic PlPM19L in N. tabacum
2.6. Effects of Drought Stress on the Physiological Indices of Transgenic PlPM19L in N. tabacum
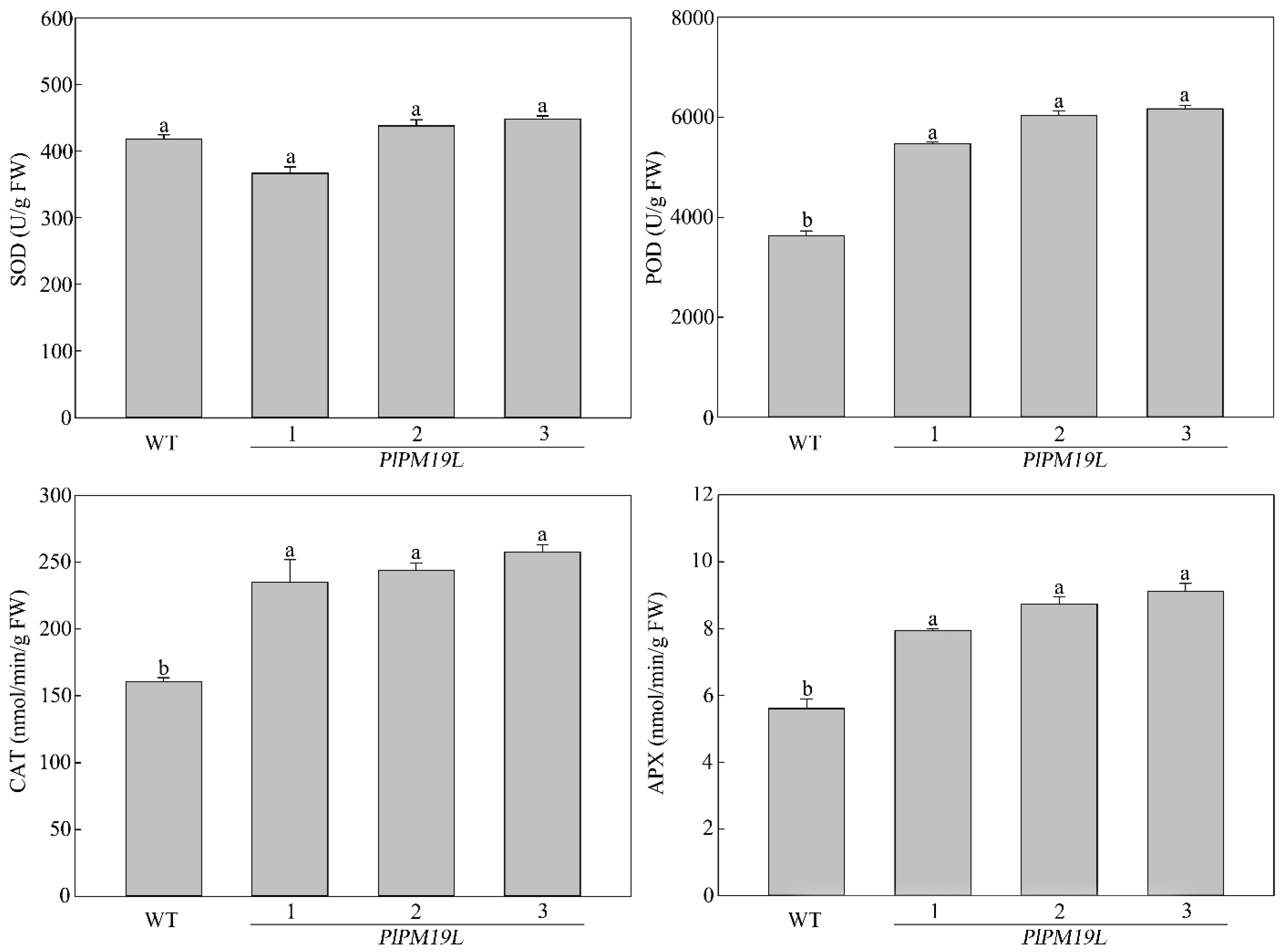
2.7. Effects of Drought Stress on the Antioxidant Enzyme Activity of Transgenic PlPM19L in N. tabacum
2.8. Effects of Drought Stress on the Photosynthetic Characteristics of Transgenic PlPM19L in N. tabacum
2.9. Effects of Drought Stress on the Chlorophyll Fluorescence Parameters of Transgenic PlPM19L in N. tabacum
3. Discussion
3.1. Structural Characteristics of PlPM19L and Its Expression Level in P. lactiflora
3.2. Phenotype and ABA Content of PlPM19L Transgenic N. tabacum
3.3. Photosynthesis of PlPM19L Transgenic N. tabacum
4. Material and Method
4.1. Plant Materials and Treatment
4.2. Gene Cloning and Sequence Analysis
4.3. Gene Expression Analysis Using Quantitative Real-Time PCR (qRT-PCR)
4.4. Construction of Expression Vector
4.5. Subcellular Localization
4.6. Overexpression of PlPM19L in the N. tabacum
4.7. Measurement of Relative Water Content
4.8. Measurement of ABA Content
4.9. Accumulation Level of H2O2
4.10. Measurement of Antioxidant Enzyme Activities
4.11. Measurement of Photosynthetic Characteristics
4.12. Measurement of Chlorophyll Fluorescence Parameters
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qing, K.J. Illustration of One Hundred Ornamental Flowers Bonsai—The Herbaceous Peony; China Forestry Press: Beijing, China, 2004; pp. 2–5. (In Chinese) [Google Scholar]
- Zhao, D.Q.; Luan, Y.T.; Xia, X.; Shi, W.B.; Tang, Y.H.; Tao, J. Lignin provides mechanical support to herbaceous peony (Paeonia lactiflora Pall.) stems. Hortic. Res. 2020, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.S.; Tang, Y.H.; Sun, J.; Zhao, D.Q.; Zhang, K.L.; Tao, J. Identification of genes asscociated with the biosynthesis of unsaurated fatty acid and oil accumulation in herbaceous peony ‘Hangshao’ (Paeonia lactiflora ‘Hangshao’) seeds based on transcriptome analysis. BMC Genom. 2021, 22, 94. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, H.X.; Tao, J. Effects of harvest stage, storage, and preservation technology on postharvest ornamtal value of cut peony (Paeonia lactilfora) flowers. Agronomy 2022, 12, 230. [Google Scholar] [CrossRef]
- Kamenetsky, R.; Dole, J. Herbaceous peony (Paeonia): Genetics, physiology and cut flower production. Floric. Ornam. Biotechnol. 2012, 6, 62–77. [Google Scholar]
- Zhang, W.T.; Yang, X.J.; Tang, D.Q. Study on plant hardiness and heat zones in China. Chin. Landsc. Archit. 2011, 27, 63–69. (In Chinese) [Google Scholar]
- Zhang, J.P.; Zhang, D.; Wei, J.F.; Shi, X.H.; Ding, H.Q.; Qiu, S.; Guo, J.; Li, D.Q.; Zhu, K.Y.; Horvath, D.P.; et al. Annual growth cycle observation, hybridization and forcing culture for improving the ornamental application of Paeonia lactiflora Pall. in the low-latitude regions. PLoS ONE 2019, 14, e0218164. [Google Scholar] [CrossRef]
- Li, T.T.; Wang, R.; Zhao, D.Q.; Tao, J. Effect of drought stress on physiological responses and gene expression changes in herbaceous peony (Paeonia lactiflora Pall.). Plant Signal. Behav. 2020, 15, 1746034. [Google Scholar] [CrossRef]
- Huang, J.P.; Ma, J.R.; Guan, X.D.; Li, Y.; He, Y.L. Progress in semi-arid climate change studies in China. Adv. Atmos. Sci. 2019, 36, 922–937. [Google Scholar] [CrossRef]
- Qin, Y.W.; Liu, J.Y.; Shi, W.J.; Tao, F.L.; Yan, H.M. Saptial-temporal changes of cropland and climate potential productivity in northern China during 1990–2010. Food Secur. 2013, 5, 499–512. [Google Scholar] [CrossRef]
- Soma, F.; Takahshi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Celluar phophorylation signaling and gene expression in drought stress responses: ABA-dependent and ABA-independent regulatory system. Plants 2021, 10, 756. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Luan, Y.T.; Shi, W.B.; Zhang, X.Y.; Meng, J.S.; Tao, J. A Paeonia ostia caffeoyl-CoA O-methyltransferase confers drought stress tolerance by promoting lignin synthesis and ROS scavenging. Plant Sci. 2021, 303, 110765. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Maruyama, K.; Mogami, J.; Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Four Arabidopsis AREB/ABF transciption factors function predominantly in gene expressioin downstrean of SnRK2 kinases in abscisic signalling in response to osmotic stress. Plant Cell Environ. 2015, 38, 35–49. [Google Scholar] [CrossRef]
- Kidokoro, S.; Watanabe, K.; Ohori, T.; Moriwaki, T.; Maruyama, K.; Mizoi, J.; Myint, N.; Htwe, P.S.; Fujita, Y.; Sekita, S.; et al. Soybean DREB1/CBF-type transcription factors function in heat and drought as well as cold stress-responsive gene expression. Plant J. 2015, 81, 505–518. [Google Scholar] [CrossRef]
- Chen, S.J.; Xu, K.; Kong, D.Y.; Wu, L.Y.; Chen, Q.; Ma, X.S.; Li, T.F.; Xie, Q.; Liu, H.Y.; Luo, L.J. Ubiquitin ligase OsRINGzf1 regulates drought resistance by controlling the turnover of OsPIP2;1. Plant Biotechnol. J. 2022, 20, 1743–1755. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetic research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Kuromori, T.; Sato, H.; Shinozaki, K. Regulatory gene networks in drought stress responses and resistance in Plants. Adv. Exp. Med. Biol. 2018, 1081, 189–214. [Google Scholar] [PubMed]
- Kar, R.K. Plant responses to water stress: Role of reactive oxygen species. Plant Signal. Behav. 2011, 6, 1741–1745. [Google Scholar] [CrossRef] [PubMed]
- Postinglion, A.E.; Muday, G.K. The role of ROS homeostasis in ABA-induced guard cell signaling. Front. Plant Sci. 2020, 11, 968. [Google Scholar] [CrossRef]
- Chen, H.; Lan, H.; Huang, P.; Zhang, Y.; Yuan, X.; Huang, X.; Huang, J.; Zhang, H. Characterization of OsPM19L1 encoding an AWPM-19-like family protein that is dramatically induced by osmotic stress in rice. Genet. Mol. Res. 2015, 14, 11994–12005. [Google Scholar] [CrossRef]
- Barrero, J.M.; Dorr, M.M.; Talbot, M.J.; Ishikawa, S.; Umezawa, T.; White, R.G.; Gubler, F. A role for PM19-Like 1 in seed dormancy in Arabidopsis. Seed Sci. Res. 2019, 29, 184–196. [Google Scholar] [CrossRef]
- Koike, M.; Takezawa, D.; Arakawa, K.; Yoshida, S. Accumulation of 19-kDa plasma membrane polypeptide during induction of freezing tolerance in wheat suspension-cultured cells by abscisic acid. Plant Cell Physiol. 1997, 38, 707–716. [Google Scholar] [CrossRef]
- Ranford, J.C.; Bryce, J.H.; Morris, P.C. PM19, a barley (Hordeum vulgare L.) gene encoding a putative plasma membrane protein, is expressed during embryo development and dormancy. J. Exp. Bot. 2002, 53, 147–148. [Google Scholar] [PubMed]
- Zhao, D.Q.; Tao, J.; Han, C.X.; Ge, J.T. Actin as an alternative internal control gene for gene expression analysis in herbaceous peony (Paeonia lactiflora Pall.). Afr. J. Agr. Res. 2012, 7, 2153–2159. [Google Scholar]
- Weaver, J.; Goklany, S.; Rizvi, N.; Cram, E.J.; Lee-Parsons, C.W. Optimizing the transient Fast Agro-mediated Seedling Transformation (FAST) method in Catharanthus roseus seedling. Plant Cell Rep. 2014, 33, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Rerksiri, W.; Zhang, X.; Xiong, H.; Chen, X. Expression and promoter analysis of six heat stress-inducible genes in rice. Sci. World J. 2013, 2013, 397401. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2006, 58, 221–227. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Ostrowska, A. Drought-stress induced physiological and molecular changes in plants. Int. J. Mol. Sci. 2022, 23, 4698. [Google Scholar] [CrossRef]
- Yao, L.Y.; Cheng, X.; Gu, Z.Y.; Huang, W.; Li, S.; Wang, L.B.; Wang, Y.F.; Xu, P.; Ma, H.; Ge, X.C. The AWPM-19 family protein OsPM1 mediates abscisic acid influx and drought response in rice. Plant Cell 2018, 30, 1258–1276. [Google Scholar] [CrossRef]
- Seo, M.; Marion-Poll, A. Abscisic acid metabolism and transport. Adv. Bot. Res. 2019, 92, 1–49. [Google Scholar]
- Wang, Q.; Zhao, R.; Chen, Q.H.; da Silva, J.A.T.; Chen, L.Q.; Yu, X.N. Physiological and biochemical responses of two herbaceous peony cultivars to drought stress. HortScience 2018, 54, 492–498. [Google Scholar] [CrossRef]
- Nguyen, T.T.Q.; Trinh, L.T.H.; Pham, H.B.V.; Le, T.V.; Phung, T.K.H.; Lee, S.K.; Cheong, J.J. Evaluation of proline, soluble sugar and ABA content in soybean Glycine max (L.) under drought stress memory. AIMS Bioeng. 2020, 7, 114–123. [Google Scholar] [CrossRef]
- Spollen, W.; LeNoble, M.; Samuels, T.; Bernstein, N.; Sharp, R. Abscisic acid accumulation maintains maize primary root at low water potentials by restricting ethylene production. Plant Physiol. 2000, 12, 967–976. [Google Scholar] [CrossRef]
- Cenzano, A.M.; Reginato, M.; Varela, M.C.; Luna, M.V. Abscisic acid and its metabolites are involved in drought tolerance in four native species of Patagonian semiarid shrublands (Argentina). Aust. J. Bot. 2018, 66, 589–600. [Google Scholar] [CrossRef]
- Uenyayar, S.; Keles, Y.; Cekic, F.O. The antioxidative response of two tomato species with different drought tolerances as a results of drought and cadmium stress combinations. Plant Soil Environ. 2005, 51, 57–64. [Google Scholar] [CrossRef]
- Zhao, J.; Lang, Y.; Zhang, S.Y.; Zhao, Q.K.; Zhang, C.Y.; Xia, J.B. Photosynthetic characteristics and chlorophyll a fluorescence transient in Lonicera japonica under drought stress. Acta Physilogogiae Plant. 2019, 41, 219. [Google Scholar] [CrossRef]
- Meng, J.S.; Li, M.; Hao, Z.J.; Zhao, D.Q.; Tao, J. Paclobutrazol can enhance the themar-taolerant on herbaceous peony (Paeonia lactiflora). Russ. J. Plant Physiol. 2022, 69, 57. [Google Scholar] [CrossRef]
- Simeneh, A.T. Photosynthesis limiting stresses under climate change scenarios and role of chlorophyll fluorescence: A review article. Cogent Food Agric. 2020, 6, 1785136. [Google Scholar]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understandings new applications. J. Exp. Bot. 2013, 13, 3983–3998. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Z.H.; Yao, B.Q.; Ma, Z.; Huang, X.T.; Zhou, B.R.; Xu, M.H.; Guo, J.; Zhou, H.K. Effects of drought and heat on the productivity and photosynthetic characteristics of alpine meadow plants on the Qinghai-Tibetan Plateau. J. Mt. Sci. 2021, 18, 2079–2093. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, P.; Tian, J.; Li, J. Study on Photosyntheic and chlorophyll fluorescence characteristics of transgenic tobacco with AcCBF1 gene. Xinjiang Agric. Sci. 2019, 56, 267–277. (In Chinese) [Google Scholar]
- Çiçek, N.; Pekcan, V.; Arslan, Ö.; Erdal, Ş.Ç.; Nalçaiyi, A.S.B.; Çil, A.N.; Şahin, V.; Kaya, Y.; Ekmekçi, Y. Assessing drought tolerance in field-grown sunflower hybrids by chlorophyll fluorescence kinetics. Braz. J. Bot. 2019, 42, 249–260. [Google Scholar] [CrossRef]
- Killi, D.; Raschi, A.; Bussotti, F. Lipid peroxidation and chlorophyll fluorescence of photosystem II performance during drought and heat stress in associated with the antioxidant capacities of C3 sunflower and C4 maize varieties. Int. J. Mol. Sci. 2020, 21, 4846. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Zhang, X.Y.; Wang, R.; Liu, D.; Sun, J.; Tao, J. Herbaceous peony tryptophan decarboxylase confers drought and salt stresses tolerance. Environ. Exp. Bot. 2019, 162, 345–356. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic tress. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Sunilkumar, G.; Vijayachandra, K.; Veluthambi, K. Preincubation of cut tobacco leaf explants promotes Agrobacterium-mediated transformation by increasing vir gene induction. Plant Sci. 1999, 141, 51–58. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, J.; Guo, J.; Li, T.; Chen, Z.; Li, M.; Zhao, D.; Tao, J. Analysis and Functional Verification of PlPM19L Gene Associated with Drought-Resistance in Paeonia lactiflora Pall. Int. J. Mol. Sci. 2022, 23, 15695. https://doi.org/10.3390/ijms232415695
Meng J, Guo J, Li T, Chen Z, Li M, Zhao D, Tao J. Analysis and Functional Verification of PlPM19L Gene Associated with Drought-Resistance in Paeonia lactiflora Pall. International Journal of Molecular Sciences. 2022; 23(24):15695. https://doi.org/10.3390/ijms232415695
Chicago/Turabian StyleMeng, Jiasong, Jinhui Guo, Tingting Li, Zijie Chen, Miao Li, Daqiu Zhao, and Jun Tao. 2022. "Analysis and Functional Verification of PlPM19L Gene Associated with Drought-Resistance in Paeonia lactiflora Pall." International Journal of Molecular Sciences 23, no. 24: 15695. https://doi.org/10.3390/ijms232415695
APA StyleMeng, J., Guo, J., Li, T., Chen, Z., Li, M., Zhao, D., & Tao, J. (2022). Analysis and Functional Verification of PlPM19L Gene Associated with Drought-Resistance in Paeonia lactiflora Pall. International Journal of Molecular Sciences, 23(24), 15695. https://doi.org/10.3390/ijms232415695





