Senolytic Therapy: A Potential Approach for the Elimination of Oncogene-Induced Senescent HPV-Positive Cells
Abstract
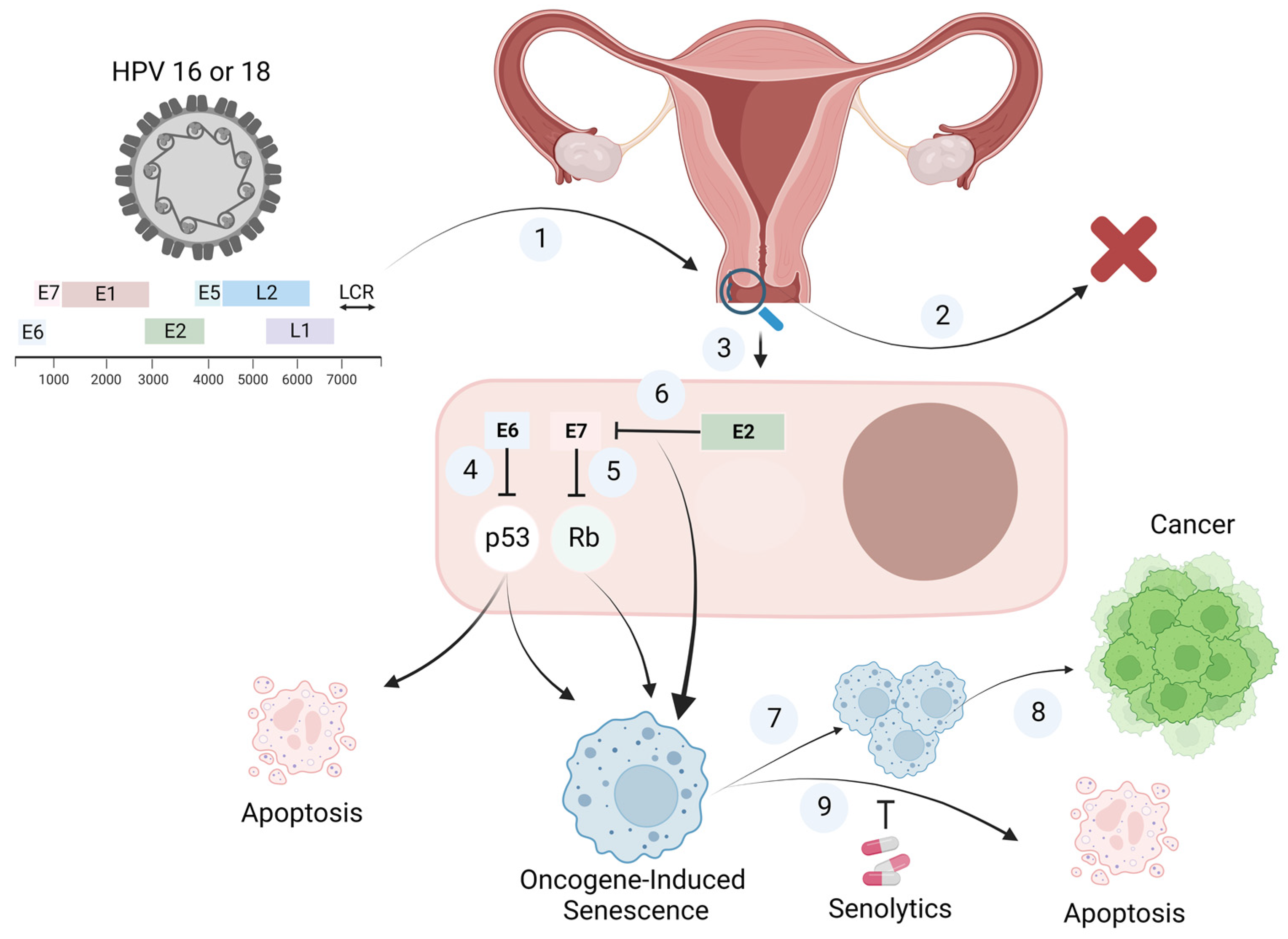
1. Introduction
2. Oncogene-Induced Senescence
2.1. Hallmarks of Senescence
2.1.1. Growth Arrest
2.1.2. Morphological and Macromolecular Changes
2.1.3. DNA Damage
2.1.4. Mitochondrial Dysfunction
2.1.5. Epigenetic Changes
2.1.6. Resistance to Apoptosis
2.1.7. The SASP
2.2. Evidence for Oncogene-Induced Senescence (OIS)
| Oncogene/Tumor Suppressor Gene | Alteration | Function | Model | Premalignant/Malignant Lesion | Reference |
|---|---|---|---|---|---|
| c-mos | Overexpression | Serine/threonine kinase | Human fibroblasts | Lung cancer | [92] |
| PTEN | Loss of function | Tumor suppressor gene | Murine embryonic fibroblast | Prostate cancer | [129] |
| Ras | Activation | Regulation of signal transduction | Murine embryonic fibroblast | Pancreas, colon, and lung cancers | [60] |
| Raf | Activation | Ras signaling | Human diploid fibroblast | Lung adenomas | [141] |
| Akt | Activation | Akt signaling | Murine embryonic fibroblasts Endothelial cells | - | [142] |
| E2F1 | Overexpression | Promotes G1 to S-phase | Human diploid fibroblasts | Pituitary gland hyperplasia | [143] |
| Cyclin E | Overexpression | Activation of cyclin dependent kinase-2 | - | Breast cancer | [92] |
| E7 | Overexpression | Inactivation of Rb | HCA2 human fibroblasts | - | [18] |
3. Human Papilloma Virus (HPV)-Induced Senescence
4. Should Senolytics Be Considered for the Elimination of HPV-Infected Senescent Cells?
Author Contributions
Funding
Conflicts of Interest
References
- Hayflick, L.; Moorhead, P.S. The Serial Cultivation of Human Diploid Cell Strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp. Cell Res. 1965, 636, 614–636. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, N.E.; Sherr, C.J. Forging a Signature of in Vivo Senescence. Nat. Rev. Cancer 2015, 15, 397–408. [Google Scholar] [CrossRef]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres Shorten during Ageing of Human Fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef]
- Poele, R.H.; Okorokov, A.L.; Jardine, L.; Cummings, J.; Joel, S.P.; te Poele, R.H.; Okorokov, A.L.; Jardine, L.; Cummings, J.; Joel, S.P. DNA Damage Is Able to Induce Senescence in Tumor Cells In Vitro and In Vivo. Cancer Res. 2002, 62, 1876–1883. [Google Scholar]
- Braig, M.; Schmitt, C.A. Oncogene-Induced Senescence: Putting the Brakes on Tumor Development. Cancer Res. 2006, 66, 2881–2884. [Google Scholar] [CrossRef]
- Campisi, J. Cellular Senescence as a Tumor-Suppressor Mechanism. Trends Cell Biol. 2001, 11, 27–31. [Google Scholar] [CrossRef]
- Collado, M.; Gil, J.; Efeyan, A.; Guerra, C.; Schuhmacher, A.J.; Barradas, M.; Benguría, A.; Zaballos, A.; Flores, J.M.; Barbacid, M.; et al. Tumour Biology: Senescence in Premalignant Tumours. Nature 2005, 436, 642. [Google Scholar] [CrossRef]
- Michaloglou, C.; Vredeveld, L.C.W.; Soengas, M.S.; Denoyelle, C.; Kuilman, T.; Van Der Horst, C.M.A.M.; Majoor, D.M.; Shay, J.W.; Mooi, W.J.; Peeper, D.S. BRAFE600-Associated Senescence-like Cell Cycle Arrest of Human Naevi. Nature 2005, 436, 720–724. [Google Scholar] [CrossRef]
- Courtois-Cox, S.; Jones, S.L.; Cichowski, K. Many Roads Lead to Oncogene-Induced Senescence. Oncogene 2008, 27, 2801–2809. [Google Scholar] [CrossRef] [PubMed]
- Kolodkin-Gal, D.; Roitman, L.; Ovadya, Y.; Azazmeh, N.; Assouline, B.; Schlesinger, Y.; Kalifa, R.; Horwitz, S.; Khalatnik, Y.; Hochner-Ger, A.; et al. Senolytic Elimination of Cox2-Expressing Senescent Cells Inhibits the Growth of Premalignant Pancreatic Lesions. Gut 2022, 71, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Fumagalli, M.; Cicalese, A.; Piccinin, S.; Gasparini, P.; Luise, C.; Schurra, C.; Garré, M.; Giovanni Nuciforo, P.; Bensimon, A.; et al. Oncogene-Induced Senescence Is a DNA Damage Response Triggered by DNA Hyper-Replication. Nature 2006, 444, 638–642. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; de Jong, T.V.; Melov, S.; Guryev, V.; Campisi, J.; Demaria, M. Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr. Biol. 2017, 27, 2652–2660. [Google Scholar] [CrossRef]
- Faridi, R.; Zahra, A.; Khan, K.; Idrees, M. Oncogenic Potential of Human Papillomavirus (HPV) and Its Relation with Cervical Cancer. Virol. J. 2011, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Hoppe-seyler, K.; Bossler, F.; Braun, J.A.; Herrmann, A.L.; Hoppe-seyler, F. The HPV E6 / E7 Oncogenes: Key Factors for Viral Carcinogenesis and Therapeutic Targets. Trends Microbiol. 2018, 26, 158–168. [Google Scholar] [CrossRef]
- Burkhart, D.L.; Sage, J. Cellular Mechanisms of Tumour Suppression by the Retinoblastoma Gene. Nat. Rev. Cancer 2008, 8, 671–682. [Google Scholar] [CrossRef]
- Rodier, F.; Muñoz, D.P.; Teachenor, R.; Chu, V.; Le, O.; Bhaumik, D.; Coppé, J.P.; Campeau, E.; Beauséjour, C.M.; Kim, S.H.; et al. DNA-SCARS: Distinct Nuclear Structures That Sustain Damage-Induced Senescence Growth Arrest and Inflammatory Cytokine Secretion. J. Cell Sci. 2011, 124, 68–81. [Google Scholar] [CrossRef]
- Tomaić, V. Functional Roles of E6 and E7 Oncoproteins in HPV-Induced Malignancies at Diverse Anatomical Sites. Cancers 2016, 8, 95. [Google Scholar] [CrossRef]
- Fischer, M.; Uxa, S.; Stanko, C.; Magin, T.M.; Engeland, K. Human Papilloma Virus E7 Oncoprotein Abrogates the P53-P21-DREAM Pathway. Sci. Rep. 2017, 7, 2603. [Google Scholar] [CrossRef]
- Saleh, T.; Carpenter, V.J. Potential Use of Senolytics for Pharmacological Targeting of Precancerous Lesions. Mol. Pharmacol. 2021, 100, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I. Oncogene-Induced Senescence and Tumour Control in Complex Biological Systems. Cell Death Differ. 2018, 25, 1005–1006. [Google Scholar] [CrossRef]
- Zhang, R.; Poustovoitov, M.V.; Ye, X.; Santos, H.A.; Chen, W.; Daganzo, S.M.; Erzberger, J.P.; Serebriiskii, I.G.; Canutescu, A.A.; Dunbrack, R.L.; et al. Formation of MacroH2A-Containing Senescence-Associated Heterochromatin Foci and Senescence Driven by ASF1a and HIRA. Dev. Cell 2005, 8, 19–30. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskensi, M.; Rubelj, I.; Pereira-Smith, O.; et al. A Biomarker That Identifies Senescent Human Cells in Culture and in Aging Skin In Vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Kurz, D.J.D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D.J.D. Senescence-Associated (Beta)-Galactosidase Reflects an Increase in Lysosomal Mass during Replicative Ageing of Human Endothelial Cells. J. Cell Sci. 2000, 113, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Kucheryavenko, O.; Wordsworth, J.; von Zglinicki, T. The Senescent Bystander Effect Is Caused by ROS-Activated NF-ΚB Signalling. Mech. Ageing Dev. 2018, 170, 30–36. [Google Scholar] [CrossRef]
- Rodier, F.; Coppé, J.; Patil, C.K.; Hoeijmakers, W.A.M.; Muñoz, D.P.; Raza, S.R.; Freund, A.; Campeau, E.; Davalos, A.R.; Campisi, J. Persistent DNA Damage Signalling Triggers Senescence- Associated Inflammatory Cytokine Secretion. Nat. Cell Biol. 2009, 11, 973–979. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.Y.; Campisi, J.; Coppe, J.-P.; et al. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the P53 Tumor Suppressor. Aging Cell 2008, 6, 2853–2868. [Google Scholar] [CrossRef]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A Guide to Assessing Cellular Senescence In Vitro and In Vivo. FEBS J. 2020, 288, 56–80. [Google Scholar] [CrossRef] [PubMed]
- Yosef, R.; Pilpel, N.; Papismadov, N.; Gal, H.; Ovadya, Y.; Vadai, E.; Miller, S.; Porat, Z.; Ben-Dor, S.; Krizhanovsky, V. P21 Maintains Senescent Cell Viability under Persistent DNA Damage Response by Restraining JNK and Caspase Signaling. EMBO J. 2017, 36, 2280–2295. [Google Scholar] [CrossRef] [PubMed]
- Beausejour, C.M.; Krtolica, A.; Galimi, F.; Narita, M.; Lowe, S.W.; Yaswen, P.; Campisi, J. Reversal of Human Cellular Senescence: Roles of the P53 and P16 Pathways. Eur. Mol. Biol. Organ. J. 2003, 22, 4212–4222. [Google Scholar] [CrossRef] [PubMed]
- Lessard, F.; Igelmann, S.; Trahan, C.; Huot, G.; Saint-Germain, E.; Mignacca, L.; Del Toro, N.; Lopes-Paciencia, S.; Le Calvé, B.; Montero, M.; et al. Senescence-Associated Ribosome Biogenesis Defects Contributes to Cell Cycle Arrest through the Rb Pathway. Nat. Cell Biol. 2018, 20, 789–799. [Google Scholar] [CrossRef]
- Nishimura, K.; Kumazawa, T.; Kuroda, T.; Katagiri, N.; Tsuchiya, M.; Goto, N.; Furumai, R.; Murayama, A.; Yanagisawa, J.; Kimura, K. Perturbation of Ribosome Biogenesis Drives Cells into Senescence through 5S RNP-Mediated P53 Activation. Cell Rep. 2015, 10, 1310–1323. [Google Scholar] [CrossRef]
- Sikora, E.; Mosieniak, G.; Alicja Sliwinska, M. Morphological and Functional Characteristic of Senescent Cancer Cells. Curr. Drug Targets 2016, 17, 377–387. [Google Scholar] [CrossRef]
- Karlseder, J.; Smogorzewska, A.; De Lange, T. Senescence Induced by Altered Telomere State, Not Telomere Loss. Science 2002, 295, 2446–2449. [Google Scholar] [CrossRef]
- Höhn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; König, J.; Grune, T.; Castro, J.P. Happily (n)Ever after: Aging in the Context of Oxidative Stress, Proteostasis Loss and Cellular Senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef]
- Georgakopoulou, E.A.; Tsimaratou, K.; Evangelou, K.; Fernandez-Marcos, P.J.; Zoumpourlis, V.; Trougakos, I.P.; Kletsas, D.; Bartek, J.; Serrano, M.; Gorgoulis, V.G. Specific Lipofuscin Staining as a Novel Biomarker to Detect Replicative and Stress-Induced Senescence. A Method Applicable in Cryo-Preserved and Archival Tissues. Aging 2013, 5, 37–50. [Google Scholar] [CrossRef]
- Sitte, N.; Merker, K.; Grune, T.; Von Zglinicki, T. Lipofuscin Accumulation in Proliferating Fibroblasts in Vitro: An Indicator of Oxidative Stress. Exp. Gerontol. 2001, 36, 475–486. [Google Scholar] [CrossRef]
- Kaplon, J.; Zheng, L.; Meissl, K.; Chaneton, B.; Selivanov, V.A.; MacKay, G.; Van Der Burg, S.H.; Verdegaal, E.M.E.; Cascante, M.; Shlomi, T.; et al. A Key Role for Mitochondrial Gatekeeper Pyruvate Dehydrogenase in Oncogene-Induced Senescence. Nature 2013, 498, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Darzynkiewicz, Z. Biomarkers of Cell Senescence Assessed by Imaging Cytometry. Methods Mol. Biol. 2013, 965, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Adams, P.D. Heterochromatin and Its Relationship to Cell Senescence and Cancer Therapy. Cell Cycle 2007, 6, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A Proteomic Atlas of Senescence-Associated Secretomes for Aging Biomarker Development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef]
- Ortiz-Montero, P.; Londoño-Vallejo, A.; Vernot, J.P. Senescence-Associated IL-6 and IL-8 Cytokines Induce a Self- and Cross-Reinforced Senescence/Inflammatory Milieu Strengthening Tumorigenic Capabilities in the MCF-7 Breast Cancer Cell Line. Cell Commun. Signal. 2017, 15, 17. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; Deursen, J.M. Van Cellular Senescence in Aging and Age-Related Disease: From Mechanisms to Therapy. Nat. Med. 2016, 21, 1424–1435. [Google Scholar] [CrossRef]
- Chen, Q.M.; Bartholomew, J.C.; Campisi, J.; Acosta, M.; Reagan, J.D.; Ames, B.N. Molecular Analysis of H2O2-Induced Senescent-like Growth Arrest in Normal Human Fibroblasts: P53 and Rb Control G1 Arrest but Not Cell Replication. Biochem. J. 1998, 332, 43–50. [Google Scholar] [CrossRef]
- Alcorta, D.A.; Xiong, Y.; Phelps, D.; Hannon, G.; Beach, D.; Barrett, J.C. Involvement of the Cyclin-Dependent Kinase Inhibitor P16 (INK4a) in Replicative Senescence of Normal Human Fibroblasts. Proc. Natl. Acad. Sci. USA 1996, 93, 13742–13747. [Google Scholar] [CrossRef]
- Stein, G.H.; Drullinger, L.F.; Soulard, A.; Dulić, V. Differential Roles for Cyclin-Dependent Kinase Inhibitors P21 and P16 in the Mechanisms of Senescence and Differentiation in Human Fibroblasts. Mol. Cell. Biol. 1999, 19, 2109–2117. [Google Scholar] [CrossRef]
- Riley, T.; Sontag, E.; Chen, P.; Levine, A. Transcriptional Control of Human P53-Regulated Genes. Nat. Rev. Mol. Cell Biol. 2008, 9, 402–412. [Google Scholar] [CrossRef]
- Sullivan, K.D.; Galbraith, M.D.; Andrysik, Z.; Espinosa, J.M. Mechanisms of Transcriptional Regulation by P53. Cell Death Differ. 2018, 25, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Caspari, T. Checkpoints: How to Activate P53. Curr. Biol. 2000, 10, R315–R317. [Google Scholar] [CrossRef] [PubMed]
- Goh, A.M.; Coffill, C.R.; Lane, D.P. The Role of Mutant P53 in Human Cancer. J. Pathol. 2011, 223, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Ausserlechner, M.J.; Obexer, P.; Geley, S.; Kofler, R. G1 Arrest by P16INK4A Uncouples Growth from Cell Cycle Progression in Leukemia Cells with Deregulated Cyclin E and C-Myc Expression. Leukemia 2005, 19, 1051–1057. [Google Scholar] [CrossRef][Green Version]
- Al-Khalaf, H.H.; Aboussekhra, A. P16 Controls P53 Protein Expression through Mir-Dependent Destabilization of MDM2. Mol. Cancer Res. 2018, 16, 1299–1308. [Google Scholar] [CrossRef]
- Shay, J.W.; Pereira-Smith, O.M.; Wright, W.E. A Role for Both RB and P53 in the Regulation of Human Cellular Senescence. Exp. Cell Res. 1991, 196, 33–39. [Google Scholar] [CrossRef]
- Benson, E.K.; Mungamuri, S.K.; Attie, O.; Kracikova, M.; Sachidanandam, R.; Manfredi, J.J.; Aaronson, S.A. P53-Dependent Gene Repression through P21 Is Mediated by Recruitment of E2F4 Repression Complexes. Oncogene 2014, 33, 3959–3969. [Google Scholar] [CrossRef]
- Pantoja, C.; Serrano, M. Murine Fibroblasts Lacking P21 Undergo Senescence and Are Resistant to Transformation by Oncogenic Ras. Oncogene 1999, 18, 4974–4982. [Google Scholar] [CrossRef]
- Lin, A.W.; Barradas, M.; Stone, J.C.; Aelst, L.V.; Serrano, M.; Lowe, S.W. Premature Senescence Involving P53 and P16 Is Activated in Response to Constitutive MEK/MAPK Mitogenic Signaling. Genes Dev. 1998, 12, 3008–3019. [Google Scholar] [CrossRef]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic Ras Provokes Premature Cell Senescence Associated with Accumulation of P53 and P16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef]
- Liu, J.Y.; Souroullas, G.P.; Diekman, B.O.; Krishnamurthy, J.; Hall, B.M.; Sorrentino, J.A.; Parker, J.S.; Sessions, G.A.; Gudkov, A.V.; Sharpless, N.E. Cells Exhibiting Strong P16 INK4a Promoter Activation in Vivo Display Features of Senescence. Proc. Natl. Acad. Sci. USA 2019, 116, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Romagosa, C.; Simonetti, S.; López-Vicente, L.; Mazo, A.; Lleonart, M.E.; Castellvi, J.; Ramon y Cajal, S. P16Ink4a Overexpression in Cancer: A Tumor Suppressor Gene Associated with Senescence and High-Grade Tumors. Oncogene 2011, 30, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Liggett, W.H.; Sidransky, D. Role of the P16 Tumor Suppressor Gene in Cancer. J. Clin. Oncol. 1998, 16, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Choi, B.Y.; Lee, M.H.; Bode, A.M.; Dong, Z. Implications of Genetic and Epigenetic Alterations of CDKN2A (P16INK4a) in Cancer. EBioMedicine 2016, 8, 30–39. [Google Scholar] [CrossRef]
- Garbuglia, A.R. Human Papillomavirus in Head and Neck Cancer. Cancers 2014, 6, 1705–1726. [Google Scholar] [CrossRef]
- Parry, D.; Bates, S.; Mann1, D.J.; Peters, G. Lack of Cyclin D-Cdk Complexes in Rb-Negative Cells Correlates with High Levels of P16INK4IMTS1 Tumour Suppressor Gene Product. EMBO J. 1995, 14, 503–511. [Google Scholar] [CrossRef]
- Seshadri, T.; Campisi, J. Growth-Factor-Inducible Gene Expression in Senescent Human Fibroblasts. Exp. Gerontol. 1989, 24, 515–522. [Google Scholar] [CrossRef]
- Saleh, T.; Bloukh, S.; Carpenter, V.J.; Alwohoush, E.; Bakeer, J.; Darwish, S.; Azab, B.; Gewirtz, D.A. Therapy-Induced Senescence: An “Old” Friend Becomes the Enemy. Cancers 2020, 12, 822. [Google Scholar] [CrossRef]
- Roberson, R.S.; Kussick, S.J.; Vallieres, E.; Chen, S.Y.J.; Wu, D.Y. Escape from Therapy-Induced Accelerated Cellular Senescence in P53-Null Lung Cancer Cells and in Human Lung Cancers. Cancer Res. 2005, 65, 2795–2803. [Google Scholar] [CrossRef]
- Saleh, T.; Tyutyunyk-Massey, L.; Murray, G.F.; Alotaibi, M.R.; Kawale, A.S.; Elsayed, Z.; Henderson, S.C.; Yakovlev, V.; Elmore, L.W.; Toor, A.; et al. Tumor Cell Escape from Therapy-Induced Senescence. Biochem. Pharmacol. 2019, 162, 202–212. [Google Scholar] [CrossRef]
- Carpenter, V.; Saleh, T.; Min Lee, S.; Murray, G.; Reed, J.; Souers, A.; Faber, A.C.; Harada, H.; Gewirtz, D.A. Androgen-Deprivation Induced Senescence in Prostate Cancer Cells Is Permissive for the Development of Castration-Resistance but Susceptible to Senolytic Therapy. Biochem. Pharmacol. 2021, 193, 114765. [Google Scholar] [CrossRef] [PubMed]
- Ahmadinejad, F.; Bos, T.; Hu, B.; Britt, E.; Koblinski, J.; Souers, A.J.; Leverson, J.D.; Faber, A.C.; Gewirtz, D.A.; Harada, H. Senolytic-Mediated Elimination of Head and Neck Tumor Cells Induced Into Senescence by Cisplatin. Mol. Pharmacol. 2022, 101, 168–180. [Google Scholar] [CrossRef]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Däbritz, J.H.M.; Zhao, Z.; Yu, Y.; Dörr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-Associated Reprogramming Promotes Cancer Stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Dirac, A.M.G.; Bernards, R. Reversal of Senescence in Mouse Fibroblasts through Lentiviral Suppression of P53. J. Biol. Chem. 2003, 278, 11731–11734. [Google Scholar] [CrossRef] [PubMed]
- Carrire, C.; Gore, A.J.; Norris, A.M.; Gunn, J.R.; Young, A.L.; Longnecker, D.S.; Korc, M. Deletion of Rb Accelerates Pancreatic Carcinogenesis by Oncogenic Kras and Impairs Senescence in Premalignant Lesions. Gastroenterology 2011, 141, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Seoane, M.; Iglesias, P.; Gonzalez, T.; Dominguez, F.; Fraga, M.; Aliste, C.; Forteza, J.; Costoya, J.A. Retinoblastoma Loss Modulates DNA Damage Response Favoring Tumor Progression. PLoS ONE 2008, 3, e3632. [Google Scholar] [CrossRef]
- Majumder, P.K.; Grisanzio, C.; O’Connell, F.; Barry, M.; Brito, J.M.; Xu, Q.; Guney, I.; Berger, R.; Herman, P.; Bikoff, R.; et al. A Prostatic Intraepithelial Neoplasia-Dependent P27 Kip1 Checkpoint Induces Senescence and Inhibits Cell Proliferation and Cancer Progression. Cancer Cell 2008, 14, 146–155. [Google Scholar] [CrossRef]
- Funayama, R.; Ishikawa, F. Cellular Senescence and Chromatin Structure. Chromosoma 2007, 116, 431–440. [Google Scholar] [CrossRef]
- Zhao, H.; Halicka, H.D.; Traganos, F.; Jorgensen, E.; Darzynkiewicz, Z. New Biomarkers Probing Depth of Cell Senescence Assessed by Laser Scanning Cytometry. Cytom. Part A 2010, 77, 999–1007. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to Detect Senescence-Associated Beta-Galactosidase (SA-Betagal) Activity, a Biomarker of Senescent Cells in Culture and In Vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef]
- Evangelou, K.; Lougiakis, N.; Rizou, S.V.; Kotsinas, A.; Kletsas, D.; Muñoz-Espín, D.; Kastrinakis, N.G.; Pouli, N.; Marakos, P.; Townsend, P.; et al. Robust, Universal Biomarker Assay to Detect Senescent Cells in Biological Specimens. Aging Cell 2017, 16, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Jung, T.; Grune, T.; Höhn, A. Accumulation of Modified Proteins and Aggregate Formation in Aging. Exp. Gerontol. 2014, 57, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Ogrodnik, M.; Miwa, S.; Tchkonia, T.; Tiniakos, D.; Wilson, C.L.; Lahat, A.; Day, C.P.; Burt, A.; Palmer, A.; Anstee, Q.M.; et al. Cellular Senescence Drives Age-Dependent Hepatic Steatosis. Nat. Commun. 2017, 8, 15691. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Senescence and Immortalization: Role of Telomeres and Telomerase. Carcinogenesis 2005, 26, 867–874. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. How Telomeres Solve the End-Protection Problem. Science 2009, 326, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Herbig, U.; Jobling, W.A.; Chen, B.P.C.; Chen, D.J.; Sedivy, J.M. Telomere Shortening Triggers Senescence of Human Cells through a Pathway Involving ATM, P53, and P21CIP1, but Not P16INK4a. Mol. Cell 2004, 14, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Rossiello, F.; Clerici, M.; Barozzi, S.; Cittaro, D.; Kaplunov, J.M.; Bucci, G.; Dobreva, M.; Matti, V.; Beausejour, C.M.; et al. Telomeric DNA Damage Is Irreparable and Causes Persistent DNA-Damage-Response Activation. Nat. Cell Biol. 2012, 14, 355–365. [Google Scholar] [CrossRef]
- d’Adda di Fagagna, F.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA Damage Checkpoint Response in Telomere-Initiated Senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef]
- Bartkova, J.; Hořejší, Z.; Koed, K.; Krämer, A.; Tort, F.; Zieger, K.; Guldberg, P.; Sehested, M.; Nesland, J.M.; Lukas, C.; et al. DNA Damage Response as a Candidate Anti-Cancer Barrier in Early Human Tumorigenesis. Nature 2005, 434, 864–870. [Google Scholar] [CrossRef]
- Halazonetis, T.D.; Gorgoulis, V.G.; Bartek, J. An Oncogene-Induced DNA Damage Model for Cancer Development. Science 2008, 319, 1352–1355. [Google Scholar] [CrossRef]
- Malumbres, M.; Pérez De Castro, I.; Hernández, M.I.; Jiménez, M.; Corral, T.; Pellicer, A. Cellular Response to Oncogenic Ras Involves Induction of the Cdk4 and Cdk6 Inhibitor P15INK4b. Mol. Cell. Biol. 2000, 20, 2915–2925. [Google Scholar] [CrossRef] [PubMed]
- Bartkova, J.; Rezaei, N.; Liontos, M.; Karakaidos, P.; Kletsas, D.; Issaeva, N.; Vassiliou, L.V.F.; Kolettas, E.; Niforou, K.; Zoumpourlis, V.C.; et al. Oncogene-Induced Senescence Is Part of the Tumorigenesis Barrier Imposed by DNA Damage Checkpoints. Nature 2006, 444, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial Dysfunction in Cell Senescence and Aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef]
- Moiseeva, O.; Bourdeau, V.; Roux, A.; Deschênes-Simard, X.; Ferbeyre, G. Mitochondrial Dysfunction Contributes to Oncogene-Induced Senescence. Mol. Cell. Biol. 2009, 29, 4495–4507. [Google Scholar] [CrossRef]
- Korolchuk, V.I.; Miwa, S.; Carroll, B.; von Zglinicki, T. Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine 2017, 21, 7–13. [Google Scholar] [CrossRef]
- Nacarelli, T.; Lau, L.; Fukumoto, T.; Zundell, J.; Fatkhutdinov, N.; Wu, S.; Aird, K.M.; Iwasaki, O.; Kossenkov, A.V.; Schultz, D.; et al. NAD+ Metabolism Governs the Proinflammatory Senescence-Associated Secretome. Nat. Cell Biol. 2019, 21, 397–407. [Google Scholar] [CrossRef]
- Wiel, C.; Lallet-Daher, H.; Gitenay, D.; Gras, B.; Le Calvé, B.; Augert, A.; Ferrand, M.; Prevarskaya, N.; Simonnet, H.; Vindrieux, D.; et al. Endoplasmic Reticulum Calcium Release through ITPR2 Channels Leads to Mitochondrial Calcium Accumulation and Senescence. Nat. Commun. 2014, 5, 3792. [Google Scholar] [CrossRef]
- Lai, D.; Tan, C.L.; Gunaratne, J.; Quek, L.S.; Nei, W.; Thierry, F.; Bellanger, S. Localization of HPV-18 E2 at Mitochondrial Membranes Induces ROS Release and Modulates Host Cell Metabolism. PLoS ONE 2013, 8, e75625. [Google Scholar] [CrossRef]
- Zhu, H.; Blake, S.; Kusuma, F.K.; Pearson, R.B.; Kang, J.; Chan, K.T. Oncogene-Induced Senescence: From Biology to Therapy. Mech. Ageing Dev. 2020, 187, 111229. [Google Scholar] [CrossRef]
- Ye, X.; Zerlanko, B.; Zhang, R.; Somaiah, N.; Lipinski, M.; Salomoni, P.; Adams, P.D. Definition of PRB- and P53-Dependent and -Independent Steps in HIRA/ASF1a-Mediated Formation of Senescence-Associated Heterochromatin Foci. Mol. Cell. Biol. 2007, 27, 2452–2465. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Nun, S.; Heard, E.; Narita, M.; Lin, A.W.; Hearn, S.A.; Spector, D.L.; Hannon, G.J.; Lowe, S.W. Rb-Mediated Heterochromatin Formation and Silencing of E2F Target Genes during Cellular Senescence. Cell 2003, 113, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Nelyudova, A.; Aksenov, N.; Pospelov, V.; Pospelova, T. By Blocking Apoptosis, Bcl-2 in P38-Dependent Manner Promotes Cell Cycle Arrest and Accelerated Senescence after DNA Damage and Serum Withdrawal. Cell Cycle 2007, 6, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Yosef, R.; Pilpel, N.; Tokarsky-amiel, R.; Biran, A.; Ovadya, Y.; Cohen, S.; Vadai, E.; Dassa, L.; Shahar, E.; Condiotti, R.; et al. Directed Elimination of Senescent Cells by Inhibition of BCL-W and BCL-XL. Nat. Commun. 2016, 7, 11190. [Google Scholar] [CrossRef]
- Sasaki, M.; Kumazaki, T.; Takano, H.; Nishiyama, M.; Mitsui, Y. Senescent Cells Are Resistant to Death despite Low Bcl-2 Level. Mech. Ageing Dev. 2001, 122, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Troiani, M.; Colucci, M.; D’Ambrosio, M.; Guccini, I.; Pasquini, E.; Varesi, A.; Valdata, A.; Mosole, S.; Revandkar, A.; Attanasio, G.; et al. Single-Cell Transcriptomics Identifies Mcl-1 as a Target for Senolytic Therapy in Cancer. Nat. Commun. 2022, 13, 2177. [Google Scholar] [CrossRef]
- Marcotte, R.; Lacelle, C.; Wang, E. Senescent Fibroblasts Resist Apoptosis by Downregulating Caspase-3. Mech. Ageing Dev. 2004, 125, 777–783. [Google Scholar] [CrossRef]
- Bourgeois, B.; Madl, T. Regulation of Cellular Senescence via the FOXO4-P53 Axis. FEBS Lett. 2018, 592, 2083–2097. [Google Scholar] [CrossRef]
- De Keizer, P.L.J.; Packer, L.M.; Szypowska, A.A.; Riedl-Polderman, P.E.; Van Den Broek, N.J.F.; De Bruin, A.; Dansen, T.B.; Marais, R.; Brenkman, A.B.; Burgering, B.M.T. Activation of Forkhead Box O Transcription Factors by Oncogenic BRAF Promotes P21cip1-Dependent Senescence. Cancer Res. 2010, 70, 8526–8536. [Google Scholar] [CrossRef] [PubMed]
- Baar, M.P.; Brandt, R.M.C.; Putavet, D.A.; Klein, J.D.D.; Derks, K.W.J.; Bourgeois, B.R.M.; Stryeck, S.; Rijksen, Y.; van Willigenburg, H.; Feijtel, D.A.; et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 2017, 169, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Le, H.H.; Cinaroglu, S.S.; Manalo, E.C.; Ors, A.; Gomes, M.M.; Duan Sahbaz, B.; Bonic, K.; Origel Marmolejo, C.A.; Quentel, A.; Plaut, J.S.; et al. Molecular Modelling of the FOXO4-TP53 Interaction to Design Senolytic Peptides for the Elimination of Senescent Cancer Cells. EBioMedicine 2021, 73, 103646. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.R.; Coppe, J.P.; Campisi, J.; Desprez, P.Y. Senescent Cells as a Source of Inflammatory Factors for Tumor Progression. Cancer Metastasis Rev. 2010, 29, 273–283. [Google Scholar] [CrossRef]
- Pereira, B.I.; Devine, O.P.; Vukmanovic-Stejic, M.; Chambers, E.S.; Subramanian, P.; Patel, N.; Virasami, A.; Sebire, N.J.; Kinsler, V.; Valdovinos, A.; et al. Senescent Cells Evade Immune Clearance via HLA-E-Mediated NK and CD8+ T Cell Inhibition. Nat. Commun. 2019, 10, 2387. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, D.P.; Yannone, S.M.; Daemen, A.; Sun, Y.; Vakar-Lopez, F.; Kawahara, M.; Freund, A.M.; Rodier, F.; Wu, J.D.; Desprez, P.-Y.; et al. Targetable Mechanisms Driving Immunoevasion of Persistent Senescent Cells Link Chemotherapy-Resistant Cancer to Aging. JCI Insight 2019, 4, e124716. [Google Scholar] [CrossRef] [PubMed]
- Barakat, D.J.; Zhang, J.; Barberi, T.; Denmeade, S.R.; Friedman, A.D. CCAAT/Enhancer Binding Protein β Controls Androgen-Deprivation-Induced Senescence in Prostate Cancer Cells. Oncogene 2015, 34, 5912–5922. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.; Scuoppo, C.; Wang, X.; Fang, X.; Balgley, B.; Bolden, J.E.; Premsrirut, P.; Luo, W.; Chicas, A.; Lee, C.S.; et al. Control of the Senescence-Associated Secretory Phenotype by NF- k B Promotes Senescence and Enhances Chemosensitivity. Genes Dev. 2011, 25, 2125–2136. [Google Scholar] [CrossRef]
- Loo, T.M.; Miyata, K.; Tanaka, Y.; Takahashi, A. Cellular Senescence and Senescence-Associated Secretory Phenotype via the CGAS-STING Signaling Pathway in Cancer. Cancer Sci. 2020, 111, 304–311. [Google Scholar] [CrossRef]
- Xue, W.; Zender, L.; Miething, C.; Dickins, R.A.; Hernando, E.; Krizhanovsky, V.; Cordon-cardo, C.; Lowe, S.W. Senescence and Tumour Clearance Is Triggered by P53 Restoration in Murine Liver Carcinomas. Nature 2007, 445, 656–660. [Google Scholar] [CrossRef]
- Acosta, J.C.; Loghlen, A.O.; Banito, A.; Guijarro, M.V.; Augert, A.; Raguz, S.; Fumagalli, M.; Costa, M.D.; Brown, C.; Popov, N.; et al. Chemokine Signaling via the CXCR2 Receptor Reinforces Senescence. Cell 2008, 133, 1006–1018. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.W.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-Induced Senescence Relayed by an Interleukin-Dependent Inflammatory Network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef]
- Ancrile, B.; Lim, K.H.; Counter, C.M. Oncogenic Ras-Induced Secretion of IL6 Is Required for Tumorigenesis. Genes Dev. 2007, 21, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Sparmann, A.; Bar-Sagi, D. Ras-Induced Interleukin-8 Expression Plays a Critical Role in Tumor Growth and Angiogenesis. Cancer Cell 2004, 6, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.; Lasitschka, F.; Andrulis, M.; et al. A Complex Secretory Program Orchestrated by the Inflammasome Controls Paracrine Senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hornsby, P.J. Senescent Human Fibroblasts Increase the Early Growth of Xenograft Tumors via Matrix Metalloproteinase Secretion. Cancer Res. 2007, 67, 3117–3127. [Google Scholar] [CrossRef]
- Liu, X.L.; Ding, J.; Meng, L.H. Oncogene-Induced Senescence: A Double Edged Sword in Cancer. Acta Pharmacol. Sin. 2018, 39, 1553–1558. [Google Scholar] [CrossRef]
- Sarkisian, C.J.; Keister, B.A.; Stairs, D.B.; Boxer, R.B.; Moody, S.E.; Chodosh, L.A. Dose-Dependent Oncogene-Induced Senescence in Vivo and Its Evasion during Mammary Tumorigenesis. Nat. Cell Biol. 2007, 9, 493–505. [Google Scholar] [CrossRef]
- Maldonado, J.L.; Timmerman, L.; Fridlyand, J.; Bastian, B.C. Mechanisms of Cell-Cycle Arrest in Spitz Nevi with Constitutive Activation of the MAP-Kinase Pathway. Am. J. Pathol. 2004, 164, 1783–1787. [Google Scholar] [CrossRef]
- Astle, M.V.; Hannan, K.M.; Ng, P.Y.; Lee, R.S.; George, A.J.; Hsu, A.K.; Haupt, Y.; Hannan, R.D.; Pearson, R.B. AKT Induces Senescence in Human Cells via MTORC1 and P53 in the Absence of DNA Damage: Implications for Targeting MTOR during Malignancy. Oncogene 2012, 31, 1949–1962. [Google Scholar] [CrossRef]
- Chen, Z.; Trotman, L.C.; Shaffer, D.; Lin, H.K.; Dotan, Z.A.; Niki, M.; Koutcher, J.A.; Scher, H.I.; Ludwig, T.; Gerald, W.; et al. Crucial Role of P53-Dependent Cellular Senescence in Suppression of Pten-Deficient Tumorigenesis. Nature 2005, 436, 725–730. [Google Scholar] [CrossRef]
- Natarajan, E.; Saeb, M.; Crum, C.P.; Woo, S.B.; McKee, P.H.; Rheinwald, J.G. Co-Expression of P16INK4A and Laminin 5 Γ2 by Microinvasive and Superficial Squamous Cell Carcinomas in Vivo and by Migrating Wound and Senescent Keratinocytes in Culture. Am. J. Pathol. 2003, 163, 477–491. [Google Scholar] [CrossRef]
- Tateishi, K.; Ohta, M.; Kanai, F.; Guleng, B.; Tanaka, Y.; Asaoka, Y.; Tada, M.; Seto, M.; Jazag, A.; Lianjie, L.; et al. Dysregulated Expression of Stem Cell Factor Bmi1 in Precancerous Lesions of the Gastrointestinal Tract. Clin. Cancer Res. 2006, 12, 6960–6966. [Google Scholar] [CrossRef]
- Miyasaka, Y.; Nagai, E.; Ohuchida, K.; Fujita, H.; Nakata, K.; Hayashi, A.; Mizumoto, K.; Tsuneyoshi, M.; Tanaka, M. Senescence in Intraductal Papillary Mucinous Neoplasm of the Pancreas. Hum. Pathol. 2011, 42, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Gray-Schopfer, V.C.; Cheong, S.C.; Chong, H.; Chow, J.; Moss, T.; Abdel-Malek, Z.A.; Marais, R.; Wynford-Thomas, D.; Bennett, D.C. Cellular Senescence in Naevi and Immortalisation in Melanoma: A Role for P16? Br. J. Cancer 2006, 95, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Bascones-Martínez, A.; López-Durán, M.; Cano-Sánchez, J.; Sánchez-Verde, L.; Díez-Rodríguez, A.; Aguirre-Echebarría, P.; Alvarez-Fernández, E.; González-Moles, M.A.; Bascones-Ilundain, J.; Muzio, L.L.; et al. Differences in the Expression of Five Senescence Markers in Oral Cancer, Oral Leukoplakia and Control Samples in Humans. Oncol. Lett. 2012, 3, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.M.; Yip, Y.L.; Lo, K.W.; Deng, W.; To, K.F.; Hau, P.M.; Lau, V.M.Y.; Takada, K.; Lui, V.W.Y.; Lung, M.L.; et al. Cyclin D1 Overexpression Supports Stable EBV Infection in Nasopharyngeal Epithelial Cells. Proc. Natl. Acad. Sci. USA 2012, 109, E3473–E3482. [Google Scholar] [CrossRef]
- Song, P.; An, J.; Zou, M.-H. Immune Clearance of Senescent Cells to Combat Ageing and Chronic Diseases. Cells 2020, 9, 671. [Google Scholar] [CrossRef]
- Kale, A.; Sharma, A.; Stolzing, A.; Stolzing, A.; Desprez, P.Y.; Desprez, P.Y.; Campisi, J.; Campisi, J. Role of Immune Cells in the Removal of Deleterious Senescent Cells. Immun. Ageing 2020, 17, 16. [Google Scholar] [CrossRef]
- Kang, T.W.; Yevsa, T.; Woller, N.; Hoenicke, L.; Wuestefeld, T.; Dauch, D.; Hohmeyer, A.; Gereke, M.; Rudalska, R.; Potapova, A.; et al. Senescence Surveillance of Pre-Malignant Hepatocytes Limits Liver Cancer Development. Nature 2011, 479, 547–551. [Google Scholar] [CrossRef]
- Greenman, C.; Stephens, P.; Smith, R.; Dalgliesh, G.L.; Hunter, C.; Bignell, G.; Davies, H.; Teague, J.; Butler, A.; Stevens, C.; et al. Patterns of Somatic Mutation in Human Cancer Genomes. Nature 2007, 446, 153–158. [Google Scholar] [CrossRef]
- Tort, F.; Bartkova, J.; Sehested, M.; Ørntoft, T.; Lukas, J.; Bartek, J. Retinoblastoma Pathway Defects Show Differential Ability to Activate the Constitutive DNA Damage Response in Human Tumorigenesis. Cancer Res. 2006, 66, 10258–10263. [Google Scholar] [CrossRef]
- Dankort, D.; Filenova, E.; Collado, M.; Serrano, M.; Jones, K.; McMahon, M. A New Mouse Model to Explore the Initiation, Progression, and Therapy of BRAFV600E-Induced Lung Tumors. Genes Dev. 2007, 21, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, H.; Minamino, T.; Tateno, K.; Kunieda, T.; Toko, H.; Komuro, I. Akt Negatively Regulates the in Vitro Lifespan of Human Endothelial Cells via a P53/P21-Dependent Pathway. EMBO J. 2004, 23, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Itahana, K.; Acosta, M.; Campisi, J. Regulation of a Senescence Checkpoint Response by the E2F1 Transcription Factor and P14ARF Tumor Suppressor. Mol. Cell. Biol. 2000, 20, 273–285. [Google Scholar] [CrossRef]
- Schosserer, M.; Grillari, J.; Breitenbach, M. The Dual Role of Cellular Senescence in Developing Tumors and Their Response to Cancer Therapy. Front. Oncol. 2017, 7, 278. [Google Scholar] [CrossRef]
- Alimirah, F.; Pulido, T.; Valdovinos, A.; Alptekin, S.; Chang, E.; Jones, E.; Diaz, D.A.; Flores, J.; Velarde, M.C.; Demaria, M.; et al. Cellular Senescence Promotes Skin Carcinogenesis through P38MAPK and P44/42MAPK Signaling. Cancer Res. 2020, 80, 3606–3619. [Google Scholar] [CrossRef] [PubMed]
- Cahu, J.; Bustany, S.; Sola, B. Senescence-Associated Secretory Phenotype Favors the Emergence of Cancer Stem-like Cells. Cell Death Dis. 2012, 3, e446–e448. [Google Scholar] [CrossRef] [PubMed]
- Laberge, R.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-edell, K.A.; Liu, S.; et al. MTOR Regulates the Pro-Tumorigenic Senescence-Associated Secretory Phenotype by Promoting IL1A Translation. Nat. Cell Biol. 2015, 17, 1049–1061. [Google Scholar] [CrossRef]
- Eggert, T.; Wolter, K.; Ji, J.; Ma, C.; Yevsa, T.; Klotz, S.; Medina-Echeverz, J.; Longerich, T.; Forgues, M.; Reisinger, F.; et al. Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression. Cancer Cell 2016, 30, 533–547. [Google Scholar] [CrossRef]
- Bent, E.H.; Gilbert, L.A.; Hemann, M.T. A Senescence Secretory Switch Mediated by PI3K/AKT/MTOR Activation Controls Chemoprotective Endothelial Secretory Responses. Genes Dev. 2016, 30, 1811–1821. [Google Scholar] [CrossRef]
- Hubackova, S.; Krejcikova, K.; Bartek, J.; Hodny, Z. IL1- and TGFβ-Nox4 Signaling, Oxidative Stress and DNA Damage Response Are Shared Features of Replicative, Oncogene-Induced, and Drug-Induced Paracrine ‘Bystander Senescence’. Aging 2012, 4, 932–951. [Google Scholar] [CrossRef]
- Shannon, C.L.; Klausner, J.D. The Growing Epidemic of Sexually Transmitted Infections in Adolescents: A Neglected Population. Curr. Opin. Pediatr. 2019, 30, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; Tully, S.; Franceschi, S. Carcinogenicity of Human Papillomavirus (HPV) Types in HIV-Positive Women: A Meta-Analysis from HPV Infection to Cervical Cancer. Clin. Infect. Dis. 2017, 64, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide Burden of Cancer Attributable to HPV by Site, Country and HPV Type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Wang, J.; Tang, D.; Wang, K.; Wang, J.; Zhang, Z.; Chen, Y.; Zhang, X.; Ma, C. HPV Genotype Prevalence and Distribution during 2009-2018 in Xinjiang, China: Baseline Surveys Prior to Mass HPV Vaccination. BMC Womens Health 2019, 19, 90. [Google Scholar] [CrossRef]
- Delany-Moretlwe, S.; Kelley, K.F.; James, S.; Scorgie, F.; Subedar, H.; Dlamini, N.R.; Pillay, Y.; Naidoo, N.; Chikandiwa, A.; Rees, H. Human Papillomavirus Vaccine Introduction in South Africa: Implementation Lessons from an Evaluation of the National School-Based Vaccination Campaign. Glob. Health Sci. Pract. 2018, 6, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Trus, B.L.; Roden, R.B.S.; Greenstone, H.L.; Vrhel, M.; Schiller, J.T.; Booy, F.P. Novel Structural Features of Bovine Papillomavirus Capsid Revealed by a Three-Dimensional Reconstruction to 9 A Resolution. Nat. Struct. Biol. 1997, 4, 413–420. [Google Scholar] [CrossRef] [PubMed]
- DiGiuseppe, S.; Bienkowska-Haba, M.; Guion, L.G.M.; Keiffer, T.R.; Sapp, M. Human Papillomavirus Major Capsid Protein L1 Remains Associated with the Incoming Viral Genome throughout the Entry Process. J. Virol. 2017, 91, e00537-17. [Google Scholar] [CrossRef] [PubMed]
- Godi, A.; Bissett, S.L.; Masloh, S.; Fleury, M.; Li, S.; Zhao, Q.; Xia, N.; Cocuzza, C.E.; Beddows, S. Impact of Naturally Occurring Variation in the Human Papillomavirus 52 Capsid Proteins on Recognition by Type-Specific Neutralising Antibodies. J. Gen. Virol. 2019, 100, 237–245. [Google Scholar] [CrossRef]
- Pouyanfard, S.; Spagnoli, G.; Bulli, L.; Balz, K.; Yang, F.; Odenwald, C.; Seitz, H.; Mariz, F.C.; Bolchi, A.; Ottonello, S.; et al. Minor Capsid Protein L2 Polytope Induces Broad Protection against Oncogenic and Mucosal Human Papillomaviruses. J. Virol. 2018, 92, e01930-17. [Google Scholar] [CrossRef]
- Castro-Muñoz, L.J.; Manzo-Merino, J.; Muñoz-Bello, J.O.; Olmedo-Nieva, L.; Cedro-Tanda, A.; Alfaro-Ruiz, L.A.; Hidalgo-Miranda, A.; Madrid-Marina, V.; Lizano, M. The Human Papillomavirus (HPV) E1 Protein Regulates the Expression of Cellular Genes Involved in Immune Response. Sci. Rep. 2019, 9, 13620. [Google Scholar] [CrossRef]
- Graham, S.V. Human Papillomavirus E2 Protein: Linking Replication, Transcription, and RNA Processing. J. Virol. 2016, 90, 8384–8388. [Google Scholar] [CrossRef]
- Chojnacki, M.; Melendy, T. The HPV E2 Transcriptional Transactivation Protein Stimulates Cellular DNA Polymerase Epsilon. Viruses 2018, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Knight, G.L.; Pugh, A.G.; Yates, E.; Bell, I.; Wilson, R.; Moody, C.A.; Laimins, L.A.; Roberts, S. A Cyclin-Binding Motif in Human Papillomavirus Type 18 (HPV18) E1^E4 Is Necessary for Association with CDK-Cyclin Complexes and G2/M Cell Cycle Arrest of Keratinocytes, but Is Not Required for Differentiation-Dependent Viral Genome Amplification or L1 Cap. Virology 2011, 412, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Gaykalova, D.A.; Guo, T.; Favorov, A.V.; Fertig, E.J.; Tamayo, P.; Callejas-Valera, J.L.; Allevato, M.; Gilardi, M.; Santos, J.; et al. HPV E2, E4, E5 Drive Alternative Carcinogenic Pathways in HPV Positive Cancers. Oncogene 2020, 39, 6327–6339. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.T.; Han, A.; Fife, K.H.; Brown, D.R. The Human Papillomavirus Type 11 E1E4 Protein Is Phosphorylated in Genital Epithelium. Virology 2000, 268, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Ilahi, N.E.; Bhatti, A. Impact of HPV E5 on Viral Life Cycle via EGFR Signaling. Microb. Pathog. 2020, 139, 103923. [Google Scholar] [CrossRef]
- Baz-Martínez, M.; Da Silva-Álvarez, S.; Rodríguez, E.; Guerra, J.; El Motiam, A.; Vidal, A.; Garciá-Caballero, T.; González-Barcia, M.; Sánchez, L.; Munõz-Fontela, C.; et al. Cell Senescence Is an Antiviral Defense Mechanism. Sci. Rep. 2016, 6, 37007. [Google Scholar] [CrossRef]
- Kim, J.A.; Seong, R.K.; Shin, O.S. Enhanced Viral Replication by Cellular Replicative Senescence. Immune Netw. 2016, 16, 286–295. [Google Scholar] [CrossRef]
- Velasco, J.A.; Stevens, C.W.; Esteban, J.M.; Ruthsatz, M.K.J.; Ramsamooj, P.; Dritschilo, A.; Notario, V. Modulation of Proliferation and Tumorigenic Potential of Cervical-Carcinoma Cells by the Expression of Sense and Antisense P53. Int. J. Oncol. 1995, 7, 883–888. [Google Scholar] [CrossRef]
- Holt, S.; Gollahon, L.; Willingham, T.; Barbosa, M.; Shay, J. P53 Levels in Human Mammary Epithelial Cells Expressing Wild-Type and Mutant Human Papillomavirus Type 16 (HPV-16) E6 Proteins. Int. J. Oncol. 1996, 8, 263–270. [Google Scholar] [CrossRef]
- Litaker, J.R.; Pan, J.; Cheung, Y.C.; Zhang, D.K.; Liu, Y.; Wong, S.C.H.; Wan, T.S.K.; Tsao, S.W. Expression Profile of Senescence-Associated Beta-Galactosidase and Activation of Telomerase in Human Ovarian Surface Epithelial Cells Undergoing Immortalization. Int. J. Oncol. 1998, 13, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Filatov, L.; Golubovskaya, V.; Hurt, J.C.; Byrd, L.L.; Phillips, J.M.; Kaufmann, W.K. Chromosomal Instability Is Correlated with Telomere Erosion and Inactivation of G2 Checkpoint Function in Human Fibroblasts Expressing Human Papillomavirus Type 16 E6 Oncoprotein. Oncogene 1998, 16, 1825–1838. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Helt, A.-M.; Funk, J.O.; Galloway, D.A. Inactivation of Both the Retinoblastoma Tumor Suppressor and P21 by the Human Papillomavirus Type 16 E7 Oncoprotein Is Necessary to Inhibit Cell Cycle Arrest in Human Epithelial Cells. J. Virol. 2002, 76, 10559–10568. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.I. Papillomavirus E2 Induces Senescence in HPV-Positive Cells via PRB- and P21CIP-Dependent Pathways. EMBO J. 2000, 19, 5762–5771. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.I.; Aronow, B.J.; Wise, T.M.; Williams, S.S.; Couget, J.A.; Howley, P.M. Transcriptome Signature of Irreversible Senescence in Human Papillomavirus-Positive Cervical Cancer Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 7093–7098. [Google Scholar] [CrossRef] [PubMed]
- Thierry, F.; Howley, P.M. Functional Analysis of E2-Mediated Repression of the HPV18 P105 Promoter. New Biol. 1991, 3, 90–100. [Google Scholar]
- Goodwin, E.C.; DiMaio, D. Induced Senescence in HeLa Cervical Carcinoma Cells Containing Elevated Telomerase Activity and Extended Telomeres. Cell Growth Differ. 2001, 12, 525–534. [Google Scholar]
- Lee, C.J.; Suh, E.J.; Kang, H.T.; Im, J.S.; Um, S.J.; Park, J.S.; Hwang, E.S. Induction of Senescence-like State and Suppression of Telomerase Activity through Inhibition of HPV E6/E7 Gene Expression in Cells Immortalized by HPV16 DNA. Exp. Cell Res. 2002, 277, 173–182. [Google Scholar] [CrossRef]
- DeFilippis, R.A.; Goodwin, E.C.; Wu, L.; DiMaio, D. Endogenous Human Papillomavirus E6 and E7 Proteins Differentially Regulate Proliferation, Senescence, and Apoptosis in HeLa Cervical Carcinoma Cells. J. Virol. 2003, 77, 1551–1563. [Google Scholar] [CrossRef]
- Gu, W.; Putral, L.; Hengst, K.; Minto, K.; Saunders, N.A.; Leggatt, G.; McMillan, N.A.J. Inhibition of Cervical Cancer Cell Growth in Vitro and in Vivo with Lentiviral-Vector Delivered Short Hairpin RNA Targeting Human Papillomavirus E6 and E7 Oncogenes. Cancer Gene Ther. 2006, 13, 1023–1032. [Google Scholar] [CrossRef]
- Celegato, M.; Messa, L.; Goracci, L.; Mercorelli, B.; Bertagnin, C.; Spyrakis, F.; Suarez, I.; Cousido-Siah, A.; Travé, G.; Banks, L.; et al. A Novel Small-Molecule Inhibitor of the Human Papillomavirus E6-P53 Interaction That Reactivates P53 Function and Blocks Cancer Cells Growth. Cancer Lett. 2020, 470, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.A.; Herrmann, A.L.; Blase, J.I.; Frensemeier, K.; Bulkescher, J.; Scheffner, M.; Galy, B.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Effects of the Antifungal Agent Ciclopirox in HPV-Positive Cancer Cells: Repression of Viral E6/E7 Oncogene Expression and Induction of Senescence and Apoptosis. Int. J. Cancer 2020, 146, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Inturi, R.; Jemth, P. CRISPR/Cas9-Based Inactivation of Human Papillomavirus Oncogenes E6 or E7 Induces Senescence in Cervical Cancer Cells. Virology 2021, 562, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Itahana, K.; Zou, Y.; Itahana, Y.; Martinez, J.-L.; Beausejour, C.; Jacobs, J.J.L.; van Lohuizen, M.; Band, V.; Campisi, J.; Dimri, G.P. Control of the Replicative Life Span of Human Fibroblasts by P16 and the Polycomb Protein Bmi-1. Mol. Cell. Biol. 2003, 23, 389–401. [Google Scholar] [CrossRef]
- Ren, C.; Cheng, X.; Lu, B.; Yang, G. Activation of Interleukin-6/Signal Transducer and Activator of Transcription 3 by Human Papillomavirus Early Proteins 6 Induces Fibroblast Senescence to Promote Cervical Tumourigenesis through Autocrine and Paracrine Pathways in Tumour Microenvironment. Eur. J. Cancer 2013, 49, 3889–3899. [Google Scholar] [CrossRef]
- Von Keyserling, H.; Kühn, W.; Schneider, A.; Bergmann, T.; Kaufmann, A.M. P16INK4a and P14ARF MRNA Expression in Pap Smears Is Age-Related. Mod. Pathol. 2012, 25, 465–470. [Google Scholar] [CrossRef][Green Version]
- Feng, W.; Xiao, J.; Zhang, Z.; Rosen, D.G.; Brown, R.E.; Liu, J.; Duan, X. Senescence and Apoptosis in Carcinogenesis of Cervical Squamous Carcinoma. Mod. Pathol. 2007, 20, 961–966. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Hara, S.P.O.; et al. The Achilles’ Heel of Senescent Cells: From Transcriptome to Senolytic Drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- van Deursen, J.M. Senolytic Therapies for Healthy Longevity. Science 2019, 364, 636–637. [Google Scholar] [CrossRef]
- Roos, C.M.; Zhang, B.; Palmer, A.K.; Ogrodnik, M.B.; Pirtskhalava, T.; Thalji, N.M.; Hagler, M.; Jurk, D.; Smith, L.A.; Casaclang-Verzosa, G.; et al. Chronic Senolytic Treatment Alleviates Established Vasomotor Dysfunction in Aged or Atherosclerotic Mice. Aging Cell 2016, 15, 973–977. [Google Scholar] [CrossRef]
- Jeon, O.H.; Kim, C.; Laberge, R.-M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local Clearance of Senescent Cells Attenuates the Development of Post-Traumatic Osteoarthritis and Creates a pro-Regenerative Environment. Nat. Med. 2017, 23, 712–781. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Recalde, U.; Lorenzo-Gómez, I.; Blanco, F.J.; Loza, M.I.; Grassi, D.; Shirinsky, V.; Shirinsky, I.; Lotz, M.; Robbins, P.D.; Domínguez, E.; et al. Fibrates as Drugs with Senolytic and Autophagic Activity for Osteoarthritis Therapy. EBioMedicine 2019, 45, 588–605. [Google Scholar] [CrossRef] [PubMed]
- Bussian, T.J.; Aziz, A.; Meyer, C.F.; Swenson, B.L.; van Deursen, J.M.; Baker, D.J. Clearance of Senescent Glial Cells Prevents Tau-Dependent Pathology and Cognitive Decline. Nature 2018, 562, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Sen, J.M.; Gorospe, M.; et al. Senolytic Therapy Alleviates Aβ-Associated Oligodendrocyte Progenitor Cell Senescence and Cognitive Deficits in an Alzheimer’s Disease Model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Acklin, S.; Zhang, M.; Du, W.; Zhao, X.; Plotkin, M.; Chang, J.; Campisi, J.; Zhou, D.; Xia, F. Depletion of Senescent-like Neuronal Cells Alleviates Cisplatin-Induced Peripheral Neuropathy in Mice. Sci. Rep. 2020, 10, 14170. [Google Scholar] [CrossRef]
- Pan, J.; Li, D.; Xu, Y.; Zhang, J.; Wang, Y.; Chen, M.; Lin, S.; Huang, L.; Chung, E.J.; Citrin, D.E.; et al. Inhibition of Bcl-2/Xl With ABT-263 Selectively Kills Senescent Type II Pneumocytes and Reverses Persistent Pulmonary Fibrosis Induced by Ionizing Radiation in Mice. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Xu, M.; Zhu, Y.; Pirtskhalava, T.; Weivoda, M.M.; Hachfeld, C.M.; Prata, L.G.; van Dijk, T.H.; Verkade, E.; Casaclang-Verzosa, G.; et al. Targeting Senescent Cells Alleviates Obesity-Induced Metabolic Dysfunction. Aging Cell 2019, 18, e12950. [Google Scholar] [CrossRef]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular Senescence and Senolytics: The Path to the Clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in Idiopathic Pulmonary Fibrosis: Results from a First-in-Human, Open-Label, Pilot Study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef]
- Hickson, L.T.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Corrigendum to “Senolytics Decrease Senescent Cells in Humans: Preliminary Report from a Clinical Trial of Dasatinib plus Quercetin in Individuals with Diabetic Kidney Disease” EBioMedicine 47 (2019) 446-456. EBioMedicine 2020, 52, 102595. [Google Scholar] [CrossRef]
- Short, S.; Fielder, E.; Miwa, S.; von Zglinicki, T. Senolytics and Senostatics as Adjuvant Tumour Therapy. EBioMedicine 2019, 41, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Kahlem, P.; Dörken, B.; Schmitt, C.A.; Kahlem, P.; Dörken, B.; Schmitt, C.A. Cellular Senescence in Cancer Treatment: Friend or Foe ? J. Clin. Investig. 2004, 113, 169–174. [Google Scholar] [CrossRef]
- Saleh, T.; Carpenter, V.; Tyutyunyk-Massey, L.; Murray, G.; Leverson, J.; Souers, A.; Alotaibi, M.; Faber, A.; Reed, J.; Harada, H.; et al. Clearance of Therapy-induced Senescent Tumor Cells by the Senolytic ABT-263 via Interference with BCL-XL-BAX Interaction. Mol. Oncol. 2020, 14, 2504–2519. [Google Scholar] [CrossRef] [PubMed]
- Shahbandi, A.; Rao, S.G.; Anderson, A.Y.; Frey, W.D.; Olayiwola, J.O.; Ungerleider, N.A.; Jackson, J.G. BH3 Mimetics Selectively Eliminate Chemotherapy-Induced Senescent Cells and Improve Response in TP53 Wild-Type Breast Cancer. Cell Death Differ. 2020, 27, 3097–3116. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting Senescence for the Treatment of Cancer. Nat. Rev. Cancer 2022, 22, 340–355. [Google Scholar] [CrossRef]
- Fleury, H.; Malaquin, N.; Tu, V.; Gilbert, S.; Martinez, A.; Olivier, M.A.; Sauriol, A.; Communal, L.; Leclerc-Desaulniers, K.; Carmona, E.; et al. Exploiting Interconnected Synthetic Lethal Interactions between PARP Inhibition and Cancer Cell Reversible Senescence. Nat. Commun. 2019, 10, 2556. [Google Scholar] [CrossRef]
- Demaria, M.; Leary, M.N.O.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017, 7, 165–177. [Google Scholar] [CrossRef]
- Carpenter, V.J.; Saleh, T.; Gewirtz, D.A. Senolytics for Cancer Therapy: Is All That Glitters Really Gold? Cancers 2021, 13, 723. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.; Demaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W.; et al. Clearance of Senescent Cells by ABT263 Rejuvenates Aged Hematopoietic Stem Cells in Mice. Nat. Med. 2016, 22, 78–83. [Google Scholar] [CrossRef]
- Selt, F.; Hohloch, J.; Hielscher, T.; Sahm, F.; Capper, D.; Korshunov, A.; Usta, D.; Brabetz, S.; Ridinger, J.; Ecker, J.; et al. Establishment and Application of a Novel Patient-Derived KIAA1549: BRAF-Driven Pediatric Pilocytic Astrocytoma Model for Preclinical Drug Testing. Oncotarget 2017, 8, 11460–11479. [Google Scholar] [CrossRef]
- Buhl, J.L.; Selt, F.; Hielscher, T.; Guiho, R.; Ecker, J.; Sahm, F.; Ridinger, J.; Riehl, D.; Usta, D.; Ismer, B.; et al. The Senescence-Associated Secretory Phenotype Mediates Oncogene-Induced Senescence in Pediatric Pilocytic Astrocytoma. Clin. Cancer Res. 2019, 25, 1851–1866. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, J.; Liu, X.; Zhang, X.; Zhang, S.; Zhang, X.; Zhou, D.; Zheng, G. Discovery of Piperlongumine as a Potential Novel Lead for the Development of Senolytic Agents. Aging 2016, 8, 2915–2926. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Herranz, N.; Sun, B.; Wagner, V.; Gallage, S.; Guiho, R.; Wolter, K.; Pombo, J.; Irvine, E.E.; Innes, A.J.; et al. Cardiac Glycosides Are Broad-Spectrum Senolytics. Nat. Metab. 2019, 1, 1074–1088. [Google Scholar] [CrossRef] [PubMed]
- Mario Gonzalez-Meljem, J.; Haston, S.; Carreno, G.; Apps, J.R.; Pozzi, S.; Stache, C.; Kaushal, G.; Virasami, A.; Panousopoulos, L.; Neda Mousavy-Gharavy, S.; et al. Stem Cell Senescence Drives Age-Attenuated Induction of Pituitary Tumours in Mouse Models of Paediatric Craniopharyngioma. Nat. Commun. 2017, 8, 1819. [Google Scholar] [CrossRef] [PubMed]
- L’Hôte, V.; Courbeyrette, R.; Pinna, G.; Cintrat, J.-C.; Pavec, G.L.; Delaunay-Moisan, A.; Thuret, J.-Y.; Moisan, A.D.; Mann, C. Ouabain and Chloroquine Trigger Senolysis of BRAF-V600E-Induced Senescent Cells by Targeting Autophagy. Aging Cell 2021, 20, e13447. [Google Scholar] [CrossRef]
- Denholm, M.; Rintoul, R.C.; Muñoz-Espín, D. SARS-CoV-2-Induced Senescence as a Potential Therapeutic Target. Eur. Respir. J. 2022, 60, 2201101. [Google Scholar] [CrossRef]
- Camell, C.D.; Yousefzadeh, M.J.; Zhu, Y.; Langhi Prata, L.G.P.; Huggins, M.A.; Pierson, M.; Zhang, L.; O’Kelly, R.D.; Pirtskhalava, T.; Xun, P.; et al. Senolytics Reduce Coronavirus-Related Mortality in Old Mice. Science 2021, 373, eabe4832. [Google Scholar] [CrossRef]
- Lee, S.; Yu, Y.; Trimpert, J.; Benthani, F.; Mairhofer, M.; Richter-Pechanska, P.; Wyler, E.; Belenki, D.; Kaltenbrunner, S.; Pammer, M.; et al. Virus-Induced Senescence Is a Driver and Therapeutic Target in COVID-19. Nature 2021, 599, 283–289. [Google Scholar] [CrossRef]
- Pham, A.M.; Ortiz, L.E.; Lukacher, A.E.; Kwun, H.J. Cellular Senescence Preserves Viral Genome Maintenance. bioRxiv 2022. [Google Scholar] [CrossRef]
- Seoane, R.; Vidal, S.; Bouzaher, Y.H.; El Motiam, A.; Rivas, C. The Interaction of Viruses with the Cellular Senescence Response. Biology 2020, 9, 455. [Google Scholar] [CrossRef]
- Giannakoulis, V.G.; Dubovan, P.; Papoutsi, E.; Kataki, A.; Koskinas, J. Senescence in HBV-, HCV- and NAFLD- Mediated Hepatocellular Carcinoma and Senotherapeutics: Current Evidence and Future Perspective. Cancers 2021, 13, 4732. [Google Scholar] [CrossRef] [PubMed]
- Szaniawski, M.A.; Spivak, A.M. Senotherapeutics and HIV-1 Persistence. Curr. HIV/AIDS Rep. 2020, 17, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Hoppe-Seyler, K.; Herrmann, A.L.; Däschle, A.; Kuhn, B.J.; Strobel, T.D.; Lohrey, C.; Bulkescher, J.; Krijgsveld, J.; Hoppe-Seyler, F. Effects of Metformin on the Virus/Host Cell Crosstalk in Human Papillomavirus-positive Cancer Cells. Int. J. Cancer 2021, 149, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
| Hallmark | Description | Reference |
|---|---|---|
| Growth arrest | Upregulation of p21Cip1 | [32] |
| Upregulation of p16INK4a | [33] | |
| Downregulation of ribosomal biogenesis | [34,35] | |
| Morphological changes | Large and flattened appearance | [36] |
| Suborganellar damage | Telomere dysfunction | [37] |
| Persistent activation of the DNA damage repair response (DDR) | [28] | |
| DNA-SCARSs | [18] | |
| Proteosomal activity | [22] | |
| Accumulation of reactive oxygen species (ROS) | [38] | |
| Enhanced lysosomal biogenesis (SA-β-galactosidase) | [26] | |
| Accumulation of protein aggregates (lipofuscin) | [39,40] | |
| Mitochondrial dysfunction | [41] | |
| Epigenetic changes; SAHF | Histone edits (H3K9Me3, HP-1, γH2AX) | [42,43] |
| The SASP | Growth factors | [44] |
| Chemokines | [29] | |
| Cytokines | [45] | |
| Angiogenic factors | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, T.; Khasawneh, A.I.; Himsawi, N.; Abu-Raideh, J.; Ejeilat, V.; Elshazly, A.M.; Gewirtz, D.A. Senolytic Therapy: A Potential Approach for the Elimination of Oncogene-Induced Senescent HPV-Positive Cells. Int. J. Mol. Sci. 2022, 23, 15512. https://doi.org/10.3390/ijms232415512
Saleh T, Khasawneh AI, Himsawi N, Abu-Raideh J, Ejeilat V, Elshazly AM, Gewirtz DA. Senolytic Therapy: A Potential Approach for the Elimination of Oncogene-Induced Senescent HPV-Positive Cells. International Journal of Molecular Sciences. 2022; 23(24):15512. https://doi.org/10.3390/ijms232415512
Chicago/Turabian StyleSaleh, Tareq, Ashraf I. Khasawneh, Nisreen Himsawi, Jumana Abu-Raideh, Vera Ejeilat, Ahmed M. Elshazly, and David A. Gewirtz. 2022. "Senolytic Therapy: A Potential Approach for the Elimination of Oncogene-Induced Senescent HPV-Positive Cells" International Journal of Molecular Sciences 23, no. 24: 15512. https://doi.org/10.3390/ijms232415512
APA StyleSaleh, T., Khasawneh, A. I., Himsawi, N., Abu-Raideh, J., Ejeilat, V., Elshazly, A. M., & Gewirtz, D. A. (2022). Senolytic Therapy: A Potential Approach for the Elimination of Oncogene-Induced Senescent HPV-Positive Cells. International Journal of Molecular Sciences, 23(24), 15512. https://doi.org/10.3390/ijms232415512







