Immune Ablation and Stem Cell Rescue in Two Pediatric Patients with Progressive Severe Chronic Graft-Versus-Host Disease
Abstract
1. Introduction
2. Results
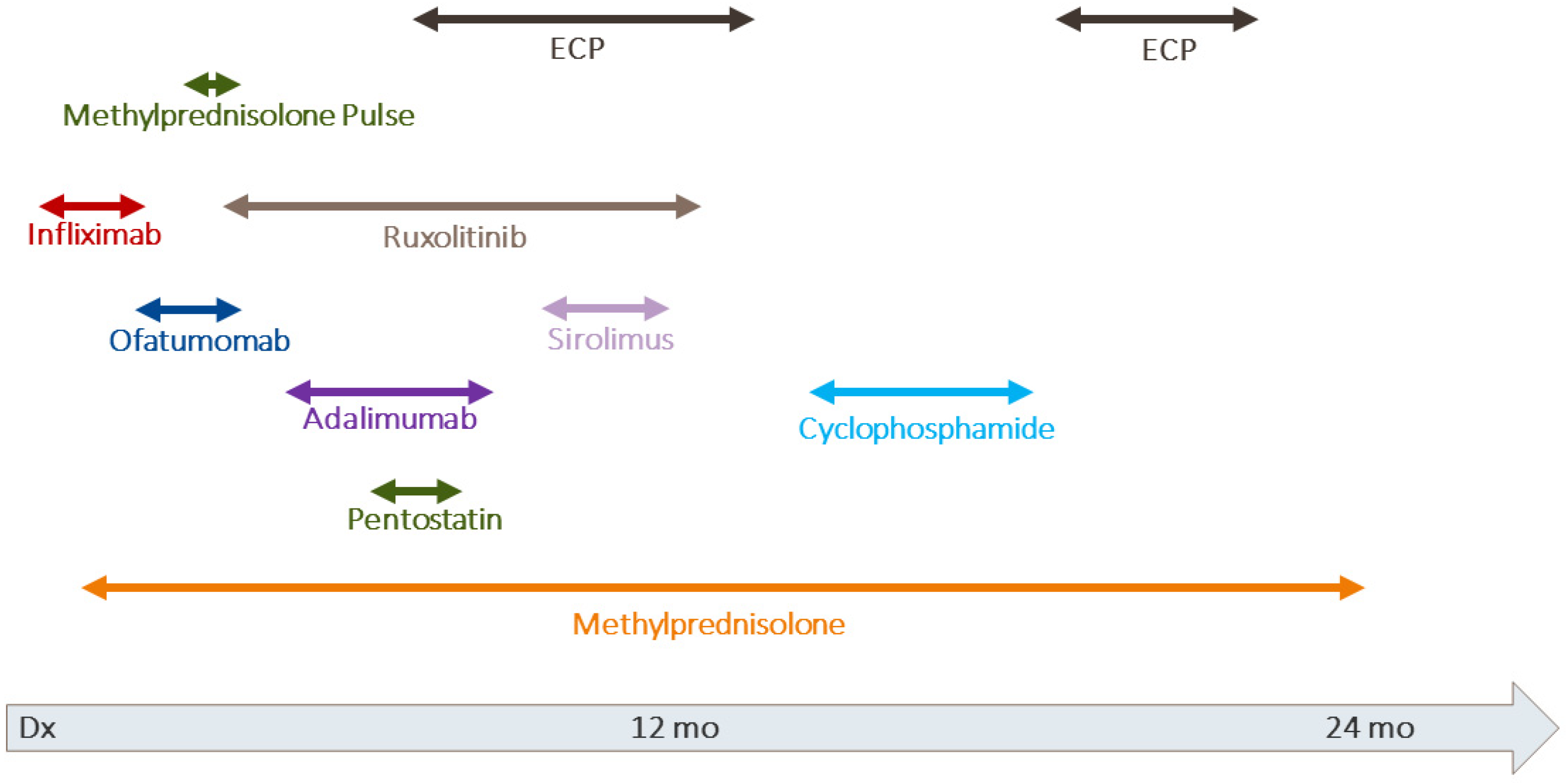
2.1. Case 1
2.2. Case 2
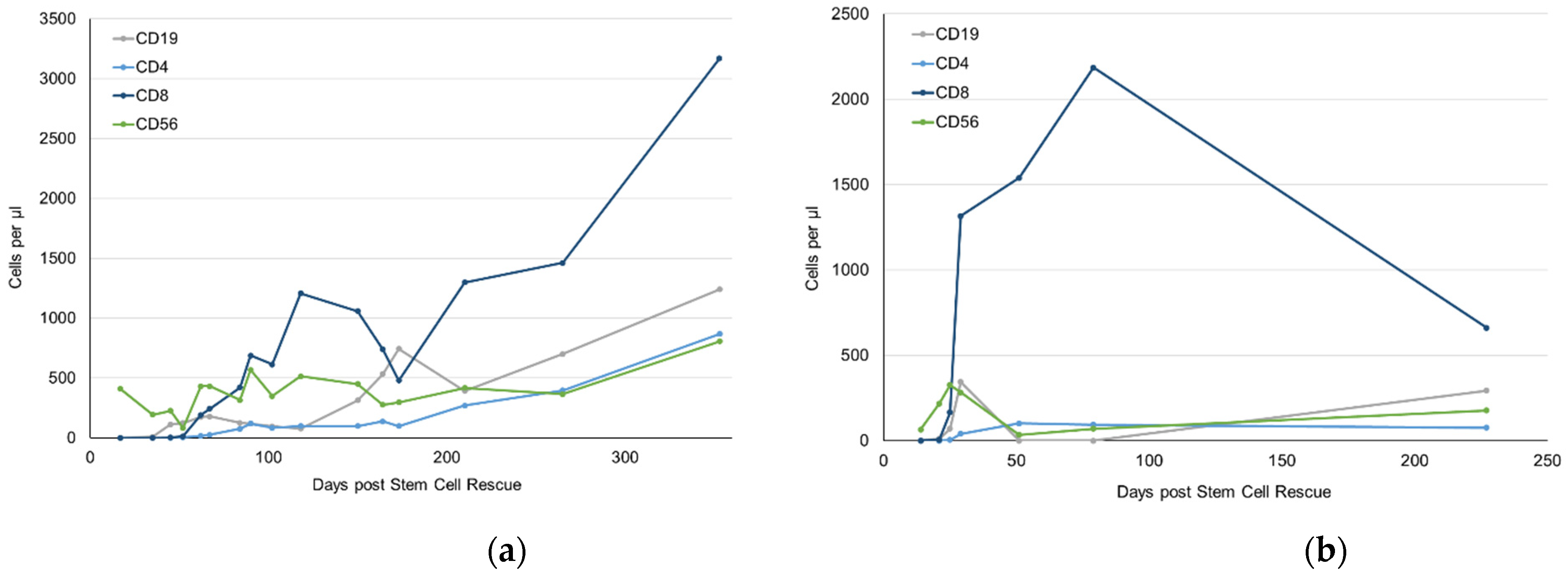
2.3. Immune Reconstitution after Immunoablation
3. Discussion
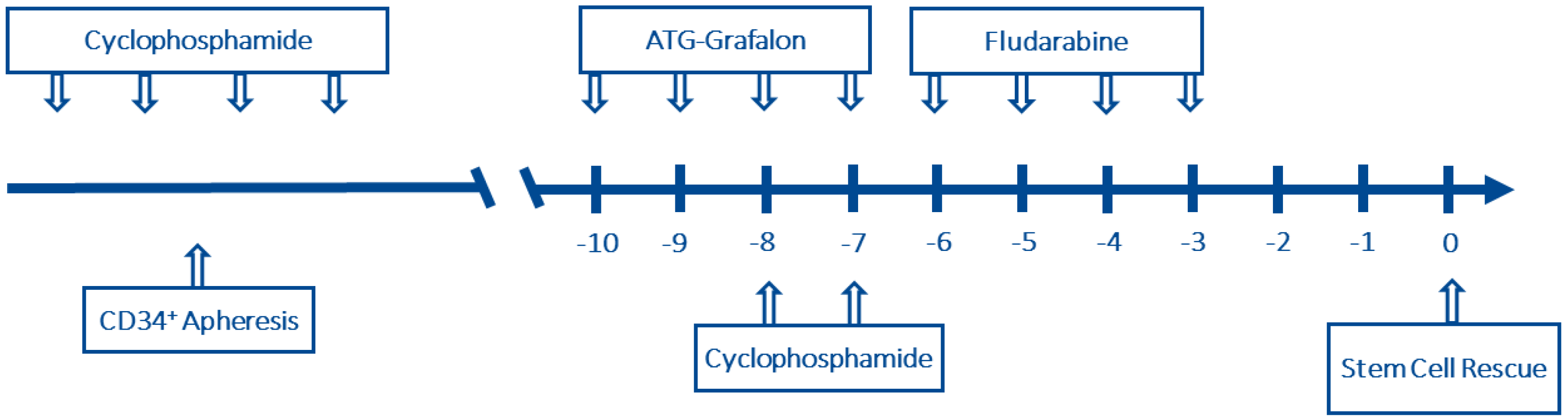
4. Materials and Methods
Vβ-Spectratyping
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cuvelier, G.D.E.; Ng, B.; Abdossamadi, S.; Nemecek, E.R.; Melton, A.; Kitko, C.L.; Lewis, V.A.; Schechter, T.; Jacobsohn, D.A.; Harris, A.C.; et al. A diagnostic classifier for pediatric chronic graft-versus-host disease: Results of the ABLE/PBMTC 1202 study. Blood Adv. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Klein, J.P.; Barrett, A.J.; Ringden, O.; Antin, J.H.; Cahn, J.Y.; Carabasi, M.H.; Gale, R.P.; Giralt, S.; Hale, G.A.; et al. Severity of chronic graft-versus-host disease: Association with treatment-related mortality and relapse. Blood 2002, 100, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Dalle, J.H.; Locatelli, F.; Poetschger, U.; Sedlacek, P.; Buechner, J.; Shaw, P.J.; Staciuk, R.; Ifversen, M.; Pichler, H.; et al. Total Body Irradiation or Chemotherapy Conditioning in Childhood ALL: A Multinational, Randomized, Noninferiority Phase III Study. J. Clin. Oncol. 2021, 39, 295–307. [Google Scholar] [CrossRef]
- Cooke, K.R.; Luznik, L.; Sarantopoulos, S.; Hakim, F.T.; Jagasia, M.; Fowler, D.H.; van den Brink, M.R.M.; Hansen, J.A.; Parkman, R.; Miklos, D.B.; et al. The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2017, 23, 211–234. [Google Scholar] [CrossRef]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant. 2015, 21, 389–401.e381. [Google Scholar] [CrossRef]
- Perez-Simon, J.A.; Encinas, C.; Silva, F.; Arcos, M.J.; Diez-Campelo, M.; Sanchez-Guijo, F.M.; Colado, E.; Martin, J.; Vazquez, L.; Del Canizo, C.; et al. Prognostic factors of chronic graft-versus-host disease following allogeneic peripheral blood stem cell transplantation: The national institutes health scale plus the type of onset can predict survival rates and the duration of immunosuppressive therapy. Biol. Blood Marrow Transplant. 2008, 14, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Sobkowiak-Sobierajska, A.; Lindemans, C.; Sykora, T.; Wachowiak, J.; Dalle, J.H.; Bonig, H.; Gennery, A.; Lawitschka, A. Management of Chronic Graft-vs.-Host Disease in Children and Adolescents With ALL: Present Status and Model for a Personalised Management Plan. Front. Pediatr. 2022, 10, 808103. [Google Scholar] [CrossRef] [PubMed]
- Doring, M.; Kluba, T.; Cabanillas Stanchi, K.M.; Kahle, P.; Lenglinger, K.; Tsiflikas, I.; Treuner, C.; Vaegler, M.; Mezger, M.; Erbacher, A.; et al. Longtime Outcome After Intraosseous Application of Autologous Mesenchymal Stromal Cells in Pediatric Patients and Young Adults with Avascular Necrosis After Steroid or Chemotherapy. Stem Cells Dev. 2020, 29, 811–822. [Google Scholar] [CrossRef]
- Zeiser, R.; Burchert, A.; Lengerke, C.; Verbeek, M.; Maas-Bauer, K.; Metzelder, S.K.; Spoerl, S.; Ditschkowski, M.; Ecsedi, M.; Sockel, K.; et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: A multicenter survey. Leukemia 2015, 29, 2062–2068. [Google Scholar] [CrossRef]
- Bertolotto, A.; Martire, S.; Mirabile, L.; Capobianco, M.; De Gobbi, M.; Cilloni, D. Autologous Hematopoietic Stem Cell Transplantation (AHSCT): Standard of Care for Relapsing-Remitting Multiple Sclerosis Patients. Neurol. Ther. 2020, 9, 197–203. [Google Scholar] [CrossRef]
- Holzer, U.; van Royen-Kerkhof, A.; van der Torre, P.; Kuemmerle-Deschner, J.; Well, C.; Handgretinger, R.; Mueller, I.; Wulffraat, N. Successful autologous stem cell transplantation in two patients with juvenile dermatomyositis. Scand. J. Rheumatol. 2010, 39, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Keyes-Elstein, L.; Brittain, E.; Pinckney, A.; Goldmuntz, E.A.; Sullivan, K.M. Safety and efficacy of HSCT for systemic sclerosis across clinical trials. Nat. Rev. Rheumatol. 2020, 16, 661. [Google Scholar] [CrossRef]
- Saidu, N.E.B.; Bonini, C.; Dickinson, A.; Grce, M.; Inngjerdingen, M.; Koehl, U.; Toubert, A.; Zeiser, R.; Galimberti, S. New Approaches for the Treatment of Chronic Graft-Versus-Host Disease: Current Status and Future Directions. Front. Immunol. 2020, 11, 578314. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.P.; Blazar, B.R.; Hill, G.R. Cytokine mediators of chronic graft-versus-host disease. J. Clin. Invest. 2017, 127, 2452–2463. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat. Med. 2007, 13, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Warnatz, K.; Rosshart, S.; Sagar; Tanriver, Y. GVHD, IBD, and primary immunodeficiencies: The gut as a target of immunopathology resulting from impaired immunity. Eur. J. Immunol. 2022, 52, 1406–1418. [Google Scholar] [CrossRef]
- Arat, M.; Ilhan, O.; Iayan, E.A.a.; Çelebi, H.; Koç, H.; Akan, H. Treatment of extensive chronic sclerodermatous graft-versus-host disease with high-dose immunosuppressive therapy and CD34+ autologous stem cell rescue. Blood 2001, 98, 892. [Google Scholar] [CrossRef]
- Cencioni, M.T.; Genchi, A.; Brittain, G.; de Silva, T.I.; Sharrack, B.; Snowden, J.A.; Alexander, T.; Greco, R.; Muraro, P.A. Immune Reconstitution Following Autologous Hematopoietic Stem Cell Transplantation for Multiple Sclerosis: A Review on Behalf of the EBMT Autoimmune Diseases Working Party. Front. Immunol. 2021, 12, 813957. [Google Scholar] [CrossRef]
- Kawashima-Vasconcelos, M.Y.; Santana-Goncalves, M.; Zanin-Silva, D.C.; Malmegrim, K.C.R.; Oliveira, M.C. Reconstitution of the immune system and clinical correlates after stem cell transplantation for systemic sclerosis. Front. Immunol. 2022, 13, 941011. [Google Scholar] [CrossRef]
- Krenger, W.; Hollander, G.A. The thymus in GVHD pathophysiology. Best Pract. Res. Clin. Haematol. 2008, 21, 119–128. [Google Scholar] [CrossRef]
- Pannetier, C.; Cochet, M.; Darche, S.; Casrouge, A.; Zoller, M.; Kourilsky, P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc. Natl. Acad. Sci. USA 1993, 90, 4319–4323. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.J.; Langerak, A.W.; Bruggemann, M.; Evans, P.A.; Hummel, M.; Lavender, F.L.; Delabesse, E.; Davi, F.; Schuuring, E.; Garcia-Sanz, R.; et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003, 17, 2257–2317. [Google Scholar] [CrossRef] [PubMed]



| Primer Forward | Modifi-Cation | Sequence | Annealing Temperature |
|---|---|---|---|
| Vβ 2 | 6-Fam | AAC TAT GTT TTG GTA TCG TCA | 56 °C |
| Vβ 4 | 6-Fam | CAC GAT GTT CTG GTA CCG TCA GCA | 56 °C |
| Vβ 5/1 | 6-Fam | CAG TGT GTC CTG GTA CCA ACA G | 58 °C |
| Vβ 6a/11 | 6-Fam | AAC CCT TTA TTG GTA CCG ACA | 56 °C |
| Vβ 8a | 6-Fam | CTC CCG TTT TCT GGT ACA GAC AGA C | 56 °C |
| Reverse | |||
| Jß 1.1 | CTT ACC TAC AAC TGT GAA TCT GGT G | 56 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kloehn, J.; Kruchen, A.; Schütze, K.; Wustrau, K.; Schrum, J.; Müller, I. Immune Ablation and Stem Cell Rescue in Two Pediatric Patients with Progressive Severe Chronic Graft-Versus-Host Disease. Int. J. Mol. Sci. 2022, 23, 15403. https://doi.org/10.3390/ijms232315403
Kloehn J, Kruchen A, Schütze K, Wustrau K, Schrum J, Müller I. Immune Ablation and Stem Cell Rescue in Two Pediatric Patients with Progressive Severe Chronic Graft-Versus-Host Disease. International Journal of Molecular Sciences. 2022; 23(23):15403. https://doi.org/10.3390/ijms232315403
Chicago/Turabian StyleKloehn, Jaspar, Anne Kruchen, Kerstin Schütze, Katharina Wustrau, Johanna Schrum, and Ingo Müller. 2022. "Immune Ablation and Stem Cell Rescue in Two Pediatric Patients with Progressive Severe Chronic Graft-Versus-Host Disease" International Journal of Molecular Sciences 23, no. 23: 15403. https://doi.org/10.3390/ijms232315403
APA StyleKloehn, J., Kruchen, A., Schütze, K., Wustrau, K., Schrum, J., & Müller, I. (2022). Immune Ablation and Stem Cell Rescue in Two Pediatric Patients with Progressive Severe Chronic Graft-Versus-Host Disease. International Journal of Molecular Sciences, 23(23), 15403. https://doi.org/10.3390/ijms232315403






