Complex Formation between Cytochrome c and a Tetra-alanino-calix[4]arene
Abstract
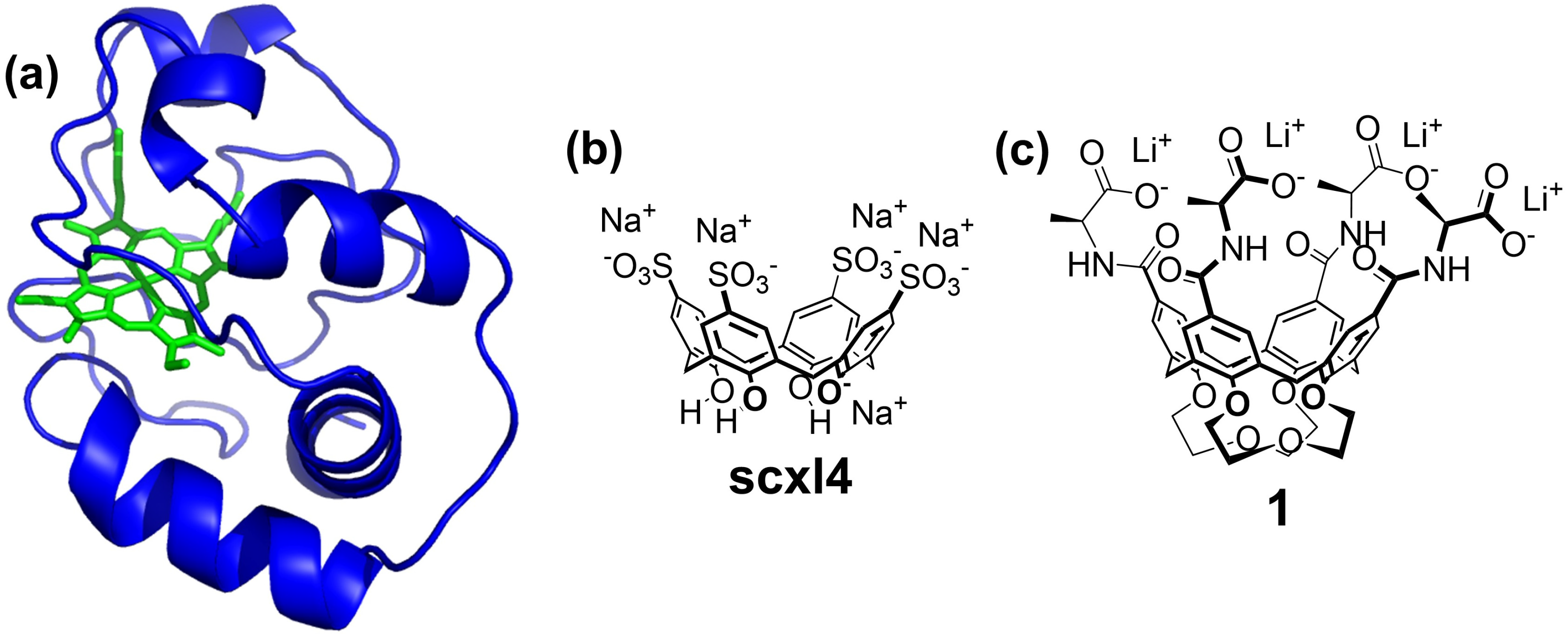
1. Introduction
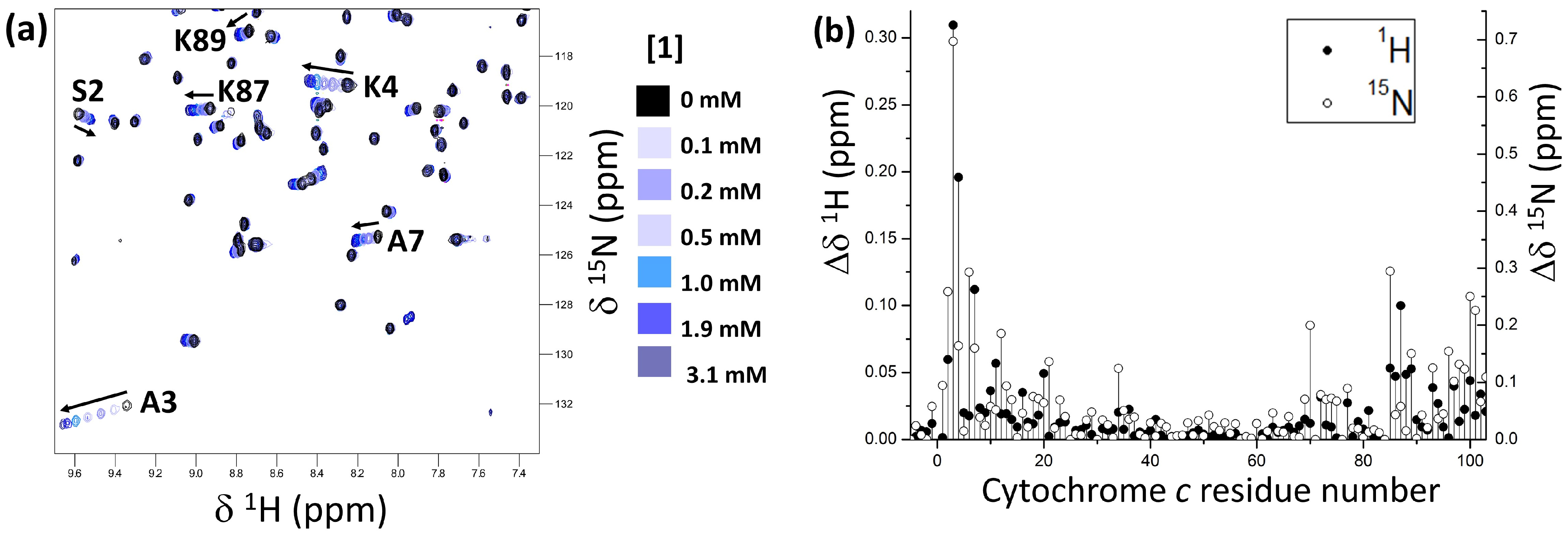
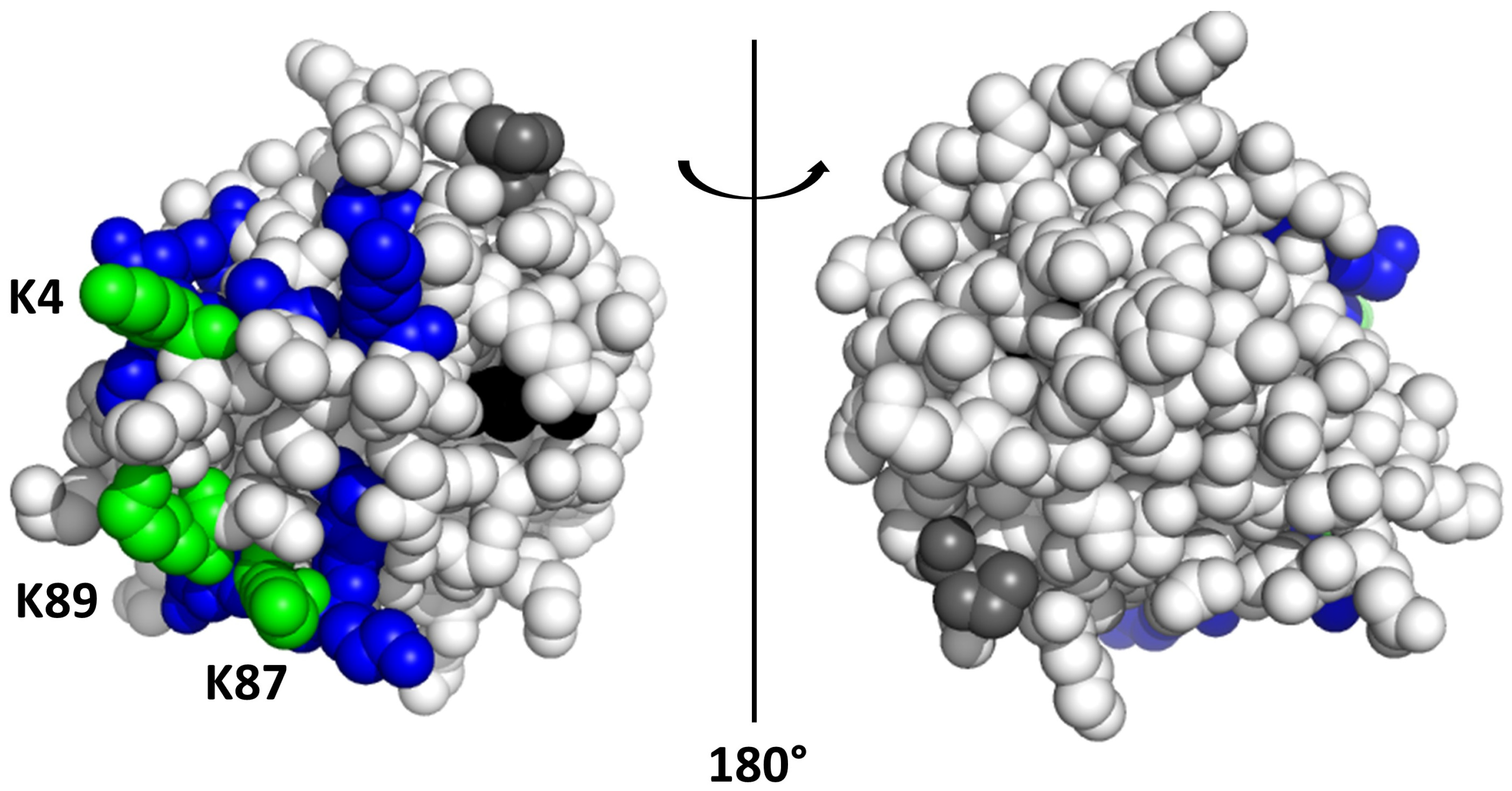
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. NMR Titrations
3.3. ITC Titrations
3.4. Cocrystallization Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, X.; Zhao, Y. Biomedical Applications of Supramolecular Systems Based on Host-Guest Interactions. Chem. Rev. 2015, 115, 7794–7839. [Google Scholar] [CrossRef] [PubMed]
- Hof, F. Host-Guest Chemistry That Directly Targets Lysine Methylation: Synthetic Host Molecules as Alternatives to Bio-Reagents. Chem. Commun. 2016, 52, 10093–10108. [Google Scholar] [CrossRef] [PubMed]
- Baldini, L.; Casnati, A.; Sansone, F. Multivalent and Multifunctional Calixarenes in Bionanotechnology. Eur. J. Org. Chem. 2020, 5056–5069. [Google Scholar] [CrossRef]
- Pan, Y.C.; Hu, X.Y.; Guo, D.S. Biomedical Applications of Calixarenes: State of the Art and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 2768–2794. [Google Scholar] [CrossRef]
- Pinalli, R.; Pedrini, A.; Dalcanale, E. Biochemical Sensing with Macrocyclic Receptors. Chem. Soc. Rev. 2018, 47, 7006–7026. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, A.; Singh, H.; Suating, P.; Kim, H.S.; Sunwoo, K.; Shim, I.; Gibb, B.C.; Kim, J.S. Revisiting Fluorescent Calixarenes: From Molecular Sensors to Smart Materials. Chem. Rev. 2019, 119, 9657–9721. [Google Scholar] [CrossRef]
- Yu, G.; Jie, K.; Huang, F. Supramolecular Amphiphiles Based on Host-Guest Molecular Recognition Motifs. Chem. Rev. 2015, 115, 7240–7303. [Google Scholar] [CrossRef]
- Crowley, P.B. Protein–Calixarene Complexation: From Recognition to Assembly. Acc. Chem. Res. 2022, 55, 2019–2032. [Google Scholar] [CrossRef]
- Hamuro, Y.; Calama, M.C.; Park, H.S.; Hamilton, A.D. A Calixarene with Four Peptide Loops: An Antibody Mimic for Recognition of Protein Surfaces. Angew. Chem. (Int. Ed. Engl.) 1997, 36, 2680–2683. [Google Scholar] [CrossRef]
- Sebti, S.M.; Hamilton, A.D. Design of Growth Factor Antagonists with Antiangiogenic and Antitumor Properties. Oncogene 2000, 19, 6566–6573. [Google Scholar] [CrossRef]
- Cecioni, S.; Lalor, R.; Blanchard, B.; Praly, J.P.; Imberty, A.; Matthews, S.E.; Vidal, S. Achieving High Affinity towards a Bacterial Lectin through Multivalent Topological Isomers of Calix[4]arene Glycoconjugates. Chem.—Eur. J. 2009, 15, 13232–13240. [Google Scholar] [CrossRef]
- André, S.; Sansone, F.; Kaltner, H.; Casnati, A.; Kopitz, J.; Gabius, H.J.; Ungaro, R. Calix[n]Arene-Based Glycoclusters: Bioactivity of Thiourea- Linked Galactose/Lactose Moieties as Inhibitors of Binding of Medically Relevant Lectins to a Glycoprotein and Cell- Surface Glycoconjugates and Selectivity among Human Adhesion/Growth-Regulatory. ChemBioChem 2008, 9, 1649–1661. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Grandjean, C.; Gautier, F.M.; Bernardi, S.; Sansone, F.; Gabius, H.J.; Ungaro, R. Combining Carbohydrate Substitutions at Bioinspired Positions with Multivalent Presentation towards Optimising Lectin Inhibitors: Case Study with Calixarenes. Chem. Commun. 2011, 47, 6126–6128. [Google Scholar] [CrossRef]
- Garcia-Hartjes, J.; Bernardi, S.; Weijers, C.A.G.M.; Wennekes, T.; Gilbert, M.; Sansone, F.; Casnati, A.; Zuilhof, H. Picomolar Inhibition of Cholera Toxin by a Pentavalent Ganglioside GM1os-Calix[5]Arene. Org. Biomol. Chem. 2013, 11, 4340–4349. [Google Scholar] [CrossRef]
- Martos, V.; Bell, S.C.; Santos, E.; Isacoff, E.Y.; Trauner, D.; De Mendoza, J. Calix[4]arene-Based Conical-Shaped Ligands for Voltage-Dependent Potassium Channels. Proc. Natl. Acad. Sci. USA 2009, 106, 10482–10486. [Google Scholar] [CrossRef]
- Gordo, S.; Martos, V.; Santos, E.; Menéndez, M.; Bo, C.; Giralt, E.; De Mendoza, J. Stability and Structural Recovery of the Tetramerization Domain of P53-R337H Mutant Induced by a Designed Templating Ligand. Proc. Natl. Acad. Sci. USA 2008, 105, 16426–16431. [Google Scholar] [CrossRef]
- McGovern, R.E.; Fernandes, H.; Khan, A.R.; Power, N.P.; Crowley, P.B. Protein Camouflage in Cytochrome c–Calixarene Complexes. Nat. Chem. 2012, 4, 527–533. [Google Scholar] [CrossRef]
- Doolan, A.M.; Rennie, M.L.; Crowley, P.B. Protein Recognition by Functionalized Sulfonatocalix[4]arenes. Chem.—Eur. J. 2018, 24, 984–991. [Google Scholar] [CrossRef]
- Mummidivarapu, V.V.S.; Rennie, M.L.; Doolan, A.M.; Crowley, P.B. Noncovalent PEGylation via Sulfonatocalix[4]arene—A Crystallographic Proof. Bioconjug. Chem. 2018, 29, 3999–4003. [Google Scholar] [CrossRef]
- Rennie, M.L.; Fox, G.C.; Pérez, J.; Crowley, P.B. Auto-Regulated Protein Assembly on a Supramolecular Scaffold. Angew. Chem. Int. Ed. 2018, 57, 13764–13769. [Google Scholar] [CrossRef]
- Engilberge, S.; Rennie, M.L.; Dumont, E.; Crowley, P.B. Tuning Protein Frameworks via Auxiliary Supramolecular Interactions. ACS Nano 2019, 13, 10343–10350. [Google Scholar] [CrossRef] [PubMed]
- Alex, J.M.; Brancatelli, G.; Volpi, S.; Bonaccorso, C.; Casnati, A.; Geremia, S.; Crowley, P.B. Probing the Determinants of Porosity in Protein Frameworks: Co-Crystals of Cytochrome c and an Octa-Anionic Calix[4]arene. Org. Biomol. Chem. 2020, 18, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Mockler, N.M.; Ramberg, K.O.; Guagnini, F.; Raston, C.L.; Crowley, P.B. Noncovalent Protein-Pseudorotaxane Assembly Incorporating an Extended Arm Calix[8]Arene with α-Helical Recognition Properties. Cryst. Growth Des. 2021, 21, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
- Ramberg, K.O.; Engilberge, S.; Skorek, T.; Crowley, P.B. Facile Fabrication of Protein-Macrocycle Frameworks. J. Am. Chem. Soc. 2021, 143, 1896–1907. [Google Scholar] [CrossRef] [PubMed]
- Mockler, N.M.; Engilberge, S.; Rennie, M.L.; Raston, C.L.; Crowley, P.B. Protein-Macrocycle Framework Engineering: Supramolecular Copolymerisation with Two Disparate Calixarenes. Supramol. Chem. 2021, 33, 122–128. [Google Scholar] [CrossRef]
- Rennie, M.L.; Doolan, A.M.; Raston, C.L.; Crowley, P.B. Protein Dimerization on a Phosphonated Calix[6]Arene Disc. Angew. Chem. Int. Ed. 2017, 56, 5517–5521. [Google Scholar] [CrossRef]
- Alex, J.M.; Rennie, M.L.; Volpi, S.; Sansone, F.; Casnati, A.; Crowley, P.B. Phosphonated Calixarene as a “Molecular Glue” for Protein Crystallization. Cryst. Growth Des. 2018, 18, 2467–2473. [Google Scholar] [CrossRef]
- Sansone, F.; Barboso, S.; Casnati, A.; Sciotto, D.; Ungaro, R. A New Chiral Rigid Cone Water Soluble Peptidocalix[4]arene and Its Inclusion Complexes with α-Amino Acids and Aromatic Ammonium Cations. Tetrahedron Lett. 1999, 40, 4741–4744. [Google Scholar] [CrossRef]
- Fielding, L. NMR Methods for the Determination of Protein-Ligand Dissociation Constants. Prog. Nucl. Magn. Reson. Spectrosc. 2007, 51, 219–242. [Google Scholar] [CrossRef]
- Crowley, P.B.; Chow, E.; Papkovskaia, T. Protein Interactions in the Escherichia Coli Cytosol: An Impediment to In-Cell NMR Spectroscopy. ChemBioChem 2011, 12, 1043–1048. [Google Scholar] [CrossRef]
- Volkov, A.N.; Vanwetswinkel, S.; de Water, K.; van Nuland, N.A.J. Redox-Dependent Conformational Changes in Eukaryotic Cytochromes Revealed by Paramagnetic NMR Spectroscopy. J. Biomol. NMR 2012, 52, 245–256. [Google Scholar] [CrossRef]
- Studier, W.F. Protein Production by Auto-Induction in High-Density Shaking Cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef]
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. NMRPipe: A Multidimensional Spectral Processing System Based on UNIX Pipes. J. Biomol. NMR 1995, 6, 277–293. [Google Scholar] [CrossRef]
- Thordarson, P. Determining Association Constants from Titration Experiments in Supramolecular Chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]
- Hibbert, D.B.; Thordarson, P. The Death of the Job Plot, Transparency, Open Science and Online Tools, Uncertainty Estimation Methods and Other Developments in Supramolecular Chemistry Data Analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef]
- Rennie, M.L.; Crowley, P.B. A Thermodynamic Model of Auto-Regulated Protein Assembly by a Supramolecular Scaffold. ChemPhysChem 2019, 20, 1011–1017. [Google Scholar] [CrossRef]
- Krauss, I.R.; Merlino, A.; Vergara, A.; Sica, F. An Overview of Biological Macromolecule Crystallization. Int. J. Mol. Sci. 2013, 14, 11643–11691. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpi, S.; Doolan, A.; Baldini, L.; Casnati, A.; Crowley, P.B.; Sansone, F. Complex Formation between Cytochrome c and a Tetra-alanino-calix[4]arene. Int. J. Mol. Sci. 2022, 23, 15391. https://doi.org/10.3390/ijms232315391
Volpi S, Doolan A, Baldini L, Casnati A, Crowley PB, Sansone F. Complex Formation between Cytochrome c and a Tetra-alanino-calix[4]arene. International Journal of Molecular Sciences. 2022; 23(23):15391. https://doi.org/10.3390/ijms232315391
Chicago/Turabian StyleVolpi, Stefano, Aishling Doolan, Laura Baldini, Alessandro Casnati, Peter B. Crowley, and Francesco Sansone. 2022. "Complex Formation between Cytochrome c and a Tetra-alanino-calix[4]arene" International Journal of Molecular Sciences 23, no. 23: 15391. https://doi.org/10.3390/ijms232315391
APA StyleVolpi, S., Doolan, A., Baldini, L., Casnati, A., Crowley, P. B., & Sansone, F. (2022). Complex Formation between Cytochrome c and a Tetra-alanino-calix[4]arene. International Journal of Molecular Sciences, 23(23), 15391. https://doi.org/10.3390/ijms232315391





