Circ_0011129 Encapsulated by the Small Extracellular Vesicles Derived from Human Stem Cells Ameliorate Skin Photoaging
Abstract
:1. Introduction
2. Results
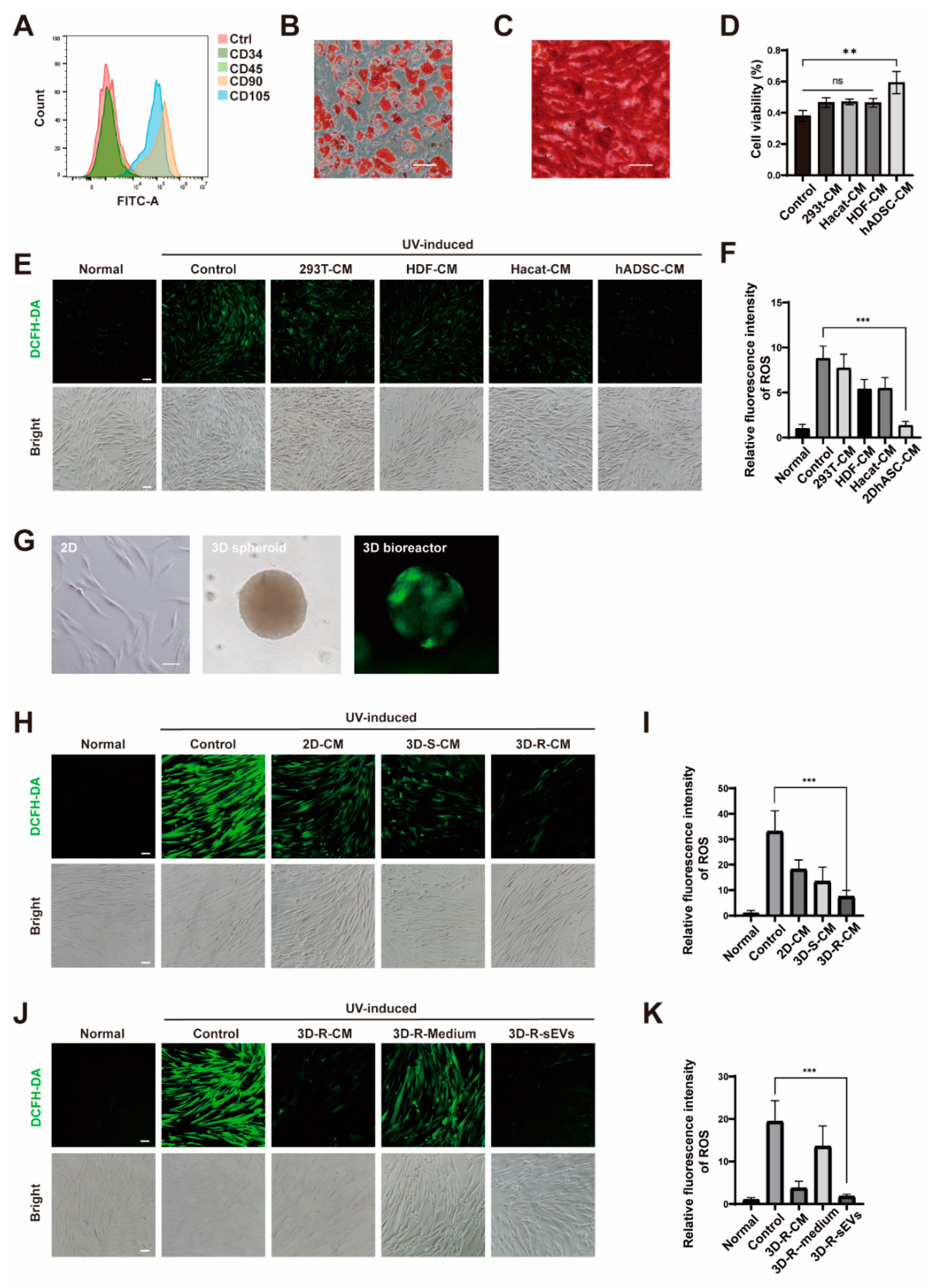
2.1. Cell-Conditioned Media from Human Adipose-Derived Stem Cells in 3D Bioreactor Culture Exibits the Best Anti-Photoaging Effect
2.2. hADSCs-sEVs in 3D Bioreactor Culture Had an Optimal Anti-Photoaging Function In Vitro
2.3. Preparation and Characterization of sEVs from hADSCs Overexpressing circ_0011129 in 3D Bioreactor Culture
2.4. Preventive Effects of 3D-circ-sEVs on Photoaging In Vitro
2.5. Preventive Effects of 3D-circ-sEVs on Oxidative Stress Damage In Vitro
3. Discussion
4. Materials and Methods
4.1. Isolation, Characterization, and Differentiation of ADSCs
4.2. Cell Lines and Cell Culture
4.3. Plasmids and Stable Cell Lines
4.4. Small Extracellular Vesicles Isolation and Characterization
4.5. UVA Irradiation of HDFs
4.6. Cell Viability Assay
4.7. Detection of Cellular ROS by Fluorescence Microscopy
4.8. Western Blotting
4.9. RNA Isolation and qPCR Analysis
4.10. Stability Determination of circ_0011129
4.11. β-Galactosidase Staining
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flament, F.; Bazin, R.; Laquieze, S.; Rubert, V.; Simonpietri, E.; Piot, B. Effect of the sun on visible clinical signs of aging in Caucasian skin. Clin. Cosmet. Investig. Dermatol. 2013, 6, 221–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittie, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Rodriguez, S.; Folgueras, A.R.; Lopez-Otin, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys Acta Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.F.; Hou, W.; Zheng, Y.; Liu, C.; Gong, Z.J.; Lu, C.; Lai, W.; Maibach, H.I. Ultraviolet A-Induced Cathepsin K Expression Is Mediated via MAPK/AP-1 Pathway in Human Dermal Fibroblasts. PLoS ONE 2014, 9, e102732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Fisher, G.J.; Voorhees, J.J. Photoaging and topical tretinoin: Therapy, pathogenesis, and prevention. Arch. Dermatol. 1997, 133, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Sjerobabski Masnec, I.; Poduje, S. Photoaging. Coll Antropol. 2008, 32 (Suppl. S2), 177–180. [Google Scholar] [PubMed]
- Infante, V.H.P.; Bagatin, E.; Maia Campos, P. Skin photoaging in young men: A clinical study by skin imaging techniques. Int. J. Cosmet Sci. 2021, 43, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P. Sunscreens. Protection against skin cancers and photoaging. Hautarzt 2003, 54, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.W.; Haylett, A.K.; Ling, T.C.; Rhodes, L.E. Comparison of Demographic and Photobiological Features of Chronic Actinic Dermatitis in Patients with Lighter vs Darker Skin Types. JAMA Dermatol 2017, 153, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, J.E.; Choi, Y.J.; Jin, Y.J.; Roh, Y.J.; Seol, A.Y.; Song, H.J.; Park, S.H.; Uddin, M.S.; Lee, S.W.; et al. Antioxidative Role of Hygrophila erecta (Brum. F.) Hochr. on UV-Induced Photoaging of Dermal Fibroblasts and Melanoma Cells. Antioxidants 2022, 11, 1317. [Google Scholar] [CrossRef]
- Ke, Y.; Wang, X.J. TGFbeta Signaling in Photoaging and UV-Induced Skin Cancer. J. Invest. Dermatol. 2021, 141, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Obi, P.; Chen, Y.G. The design and synthesis of circular RNAs. Methods 2021, 196, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Zhu, J.; Hui, B.; Jia, C.; Yan, X.; Jiang, T.; Wang, X. Circ-HSP90A expedites cell growth, stemness, and immune evasion in non-small cell lung cancer by regulating STAT3 signaling and PD-1/PD-L1 checkpoint. Cancer Immunol. Immunother. 2022. [Google Scholar] [CrossRef]
- Khorsandi, K.; Esfahani, H.; Abrahamse, H. Characteristics of circRNA and its approach as diagnostic tool in melanoma. Expert Rev. Mol. Diagn 2021, 21, 1079–1094. [Google Scholar] [CrossRef]
- Peng, Y.; Song, X.; Zheng, Y.; Cheng, H.; Lai, W. circCOL3A1-859267 regulates type I collagen expression by sponging miR-29c in human dermal fibroblasts. Eur. J. Dermatol. 2018, 28, 613–620. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, Y.; Wang, X.; Shen, J.; An, W. Advances in Circular RNA and Its Applications. Int. J. Med. Sci. 2022, 19, 975–985. [Google Scholar] [CrossRef]
- Moller, A.; Lobb, R.J. The evolving translational potential of small extracellular vesicles in cancer. Nat. Rev. Cancer 2020, 20, 697–709. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, B.; Chu, H.; Cheng, P.; Li, H.; Huang, K.; He, X.; Xu, W. Assembly and in vitro assessment of a powerful combination: Aptamer-modified exosomes combined with gold nanorods for effective photothermal therapy. Nanotechnology 2020, 31, 485101. [Google Scholar] [CrossRef]
- Bernardi, S.; Balbi, C. Extracellular Vesicles: From Biomarkers to Therapeutic Tools. Biology 2020, 9, 258. [Google Scholar] [CrossRef]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, T.; Zhang, S.Y.; Chen, P.; Cao, Z.; Lian, W.Q.; Guo, J.Y.; Kang, Y. Adipose-derived stem cell-conditioned medium protects fibroblasts at different senescent degrees from UVB irradiation damages. Mol. Cell Biochem. 2020, 463, 67–78. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Cores, J.; Huang, K.; Su, T.; Dinh, P.U.; Cheng, K. Needle-Free Injection of Exosomes Derived from Human Dermal Fibroblast Spheroids Ameliorates Skin Photoaging. ACS Nano 2019, 13, 11273–11282. [Google Scholar] [CrossRef]
- Lin, M.B.; Zheng, Y.; Li, Q.; Liu, Y.F.; Xu, Q.F.; Li, Y.Y.; Lai, W. Circular RNA expression profiles significantly altered in UVA-irradiated human dermal fibroblasts. Exp. Ther Med. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, Y.; Mori, M.; Yoshimura, K. Adipose-Derived Stem Cell Conditioned Medium and Wound Healing: A Systematic Review. Tissue Eng. Part. B Rev. 2022, 28, 830–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, J.; Shi, P.; Su, D.; Cheng, X.; Yi, W.; Yan, J.; Chen, H.; Cheng, F. Small Extracellular Vesicles Derived from MSCs Have Immunomodulatory Effects to Enhance Delivery of ASO-210 for Psoriasis Treatment. Front. Cell Dev. Biol. 2022, 10, 842813. [Google Scholar] [CrossRef]
- Zheng, Y.; Lai, W.; Wan, M.; Maibach, H.I. Expression of cathepsins in human skin photoaging. Skin Pharmacol. Physiol. 2011, 24, 10–21. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Luo, L.; Wu, Z.; Wang, Y.; Li, H. Regulating the production and biological function of small extracellular vesicles: Current strategies, applications and prospects. J. Nanobiotechnol. 2021, 19, 422. [Google Scholar] [CrossRef]
- Abhange, K.; Makler, A.; Wen, Y.; Ramnauth, N.; Mao, W.; Asghar, W.; Wan, Y. Small extracellular vesicles in cancer. Bioact. Mater. 2021, 6, 3705–3743. [Google Scholar] [CrossRef] [PubMed]





| Gene | Forward Primer Sequence 5′→3′ | Reverse Primer Sequence 5′→3′ |
|---|---|---|
| H-GAPDH | AGAAGGCTGGGGCTCATTTG | GCAGGAGGCATTGCTGATGAT |
| H-MMP1 | AAAATTACACGCCAGATTTGCC | GGTGTGACATTACTCCAGAGTTG |
| H-MMP3 | AGTCTTCCAATCCTACTGTTGCT | TCCCCGTCACCTCCAATCC |
| H-COL1A1 | GAGGGCCAAGACGAAGACATC | CAGATCACGTCATCGCACAAC |
| H-COL3A1 | GGAGCTGGCTACTTCTCGC | GGGAACATCCTCCTTCAACAG |
| H-Eastin | GGAGCTGGCTACTTCTCGC | GGGAACATCCTCCTTCAACAG |
| H-Cathepsin K | ACACCCACTGGGAGCTATG | GACAGGGGTACTTTGAGTCCA |
| H-p53 | CAGCACATGACGGAGGTTGT | TCATCCAAATACTCCACACGC |
| H-p21 | TGTCCGTCAGAACCCATGC | AAAGTCGAAGTTCCATCGCTC |
| H-p16 | GATCCAGGTGGGTAGAAGGTC | GATCCAGGTGGGTAGAAGGTC |
| H-HO-1 | AAGACTGCGTTCCTGCTCAAC | AAAGCCCTACAGCAACTGTCG |
| H-NQO1 | GAAGAGCACTGATCGTACTGGC | GGATACTGAAAGTTCGCAGGG |
| H-GPX1 | CAGTCGGTGTATGCCTTCTCG | GAGGGACGCCACATTCTCG |
| H-CAT | TGGAGCTGGTAACCCAGTAGG | CCTTTGCCTTGGAGTATTTGGTA |
| H-SOD2 | GCTCCGGTTTTGGGGTATCTG | GCGTTGATGTGAGGTTCCAG |
| H-linear_0011129 | TTCCGGGCCCAGGTC | CATCACCACTGCCAGGTT |
| H-circ_0011129 | GAGGACTTCACTCGGAGAGG | CACCACTGCCAGGTTGTCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, M.; Yao, A.; Xie, Y.; Lin, J.; Sharifullah, F.; Hong, Y.; Chen, H.; Cheng, F.; Lai, W. Circ_0011129 Encapsulated by the Small Extracellular Vesicles Derived from Human Stem Cells Ameliorate Skin Photoaging. Int. J. Mol. Sci. 2022, 23, 15390. https://doi.org/10.3390/ijms232315390
Zhang Y, Zhang M, Yao A, Xie Y, Lin J, Sharifullah F, Hong Y, Chen H, Cheng F, Lai W. Circ_0011129 Encapsulated by the Small Extracellular Vesicles Derived from Human Stem Cells Ameliorate Skin Photoaging. International Journal of Molecular Sciences. 2022; 23(23):15390. https://doi.org/10.3390/ijms232315390
Chicago/Turabian StyleZhang, Yu, Manqi Zhang, Amin Yao, Yalin Xie, Jingxiong Lin, Farooqi Sharifullah, Yixin Hong, Hongbo Chen, Fang Cheng, and Wei Lai. 2022. "Circ_0011129 Encapsulated by the Small Extracellular Vesicles Derived from Human Stem Cells Ameliorate Skin Photoaging" International Journal of Molecular Sciences 23, no. 23: 15390. https://doi.org/10.3390/ijms232315390
APA StyleZhang, Y., Zhang, M., Yao, A., Xie, Y., Lin, J., Sharifullah, F., Hong, Y., Chen, H., Cheng, F., & Lai, W. (2022). Circ_0011129 Encapsulated by the Small Extracellular Vesicles Derived from Human Stem Cells Ameliorate Skin Photoaging. International Journal of Molecular Sciences, 23(23), 15390. https://doi.org/10.3390/ijms232315390







