MAPLE Processed Nanostructures for Antimicrobial Coatings
Abstract
:1. Introduction
2. Results and Discussions
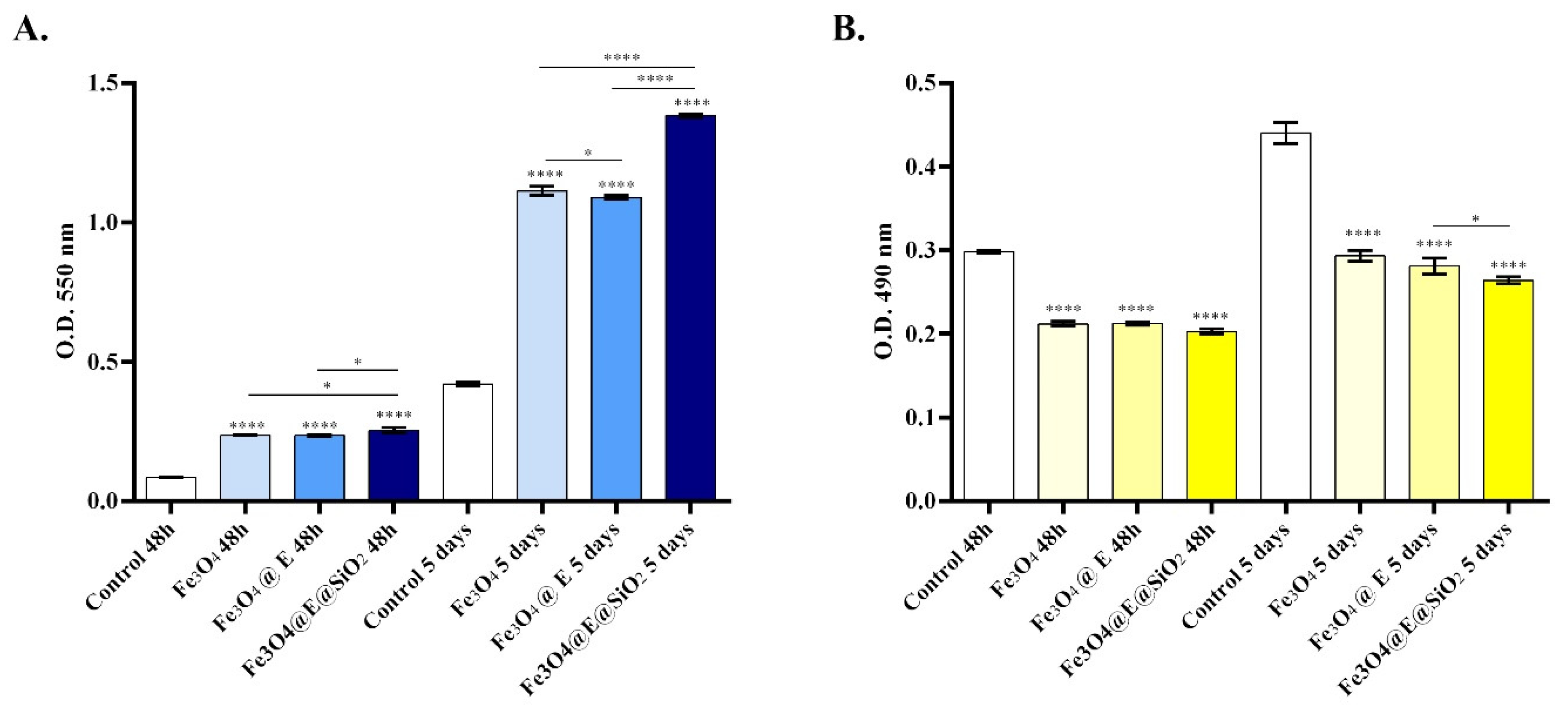
2.1. In Vitro Biocompatibility Assessment
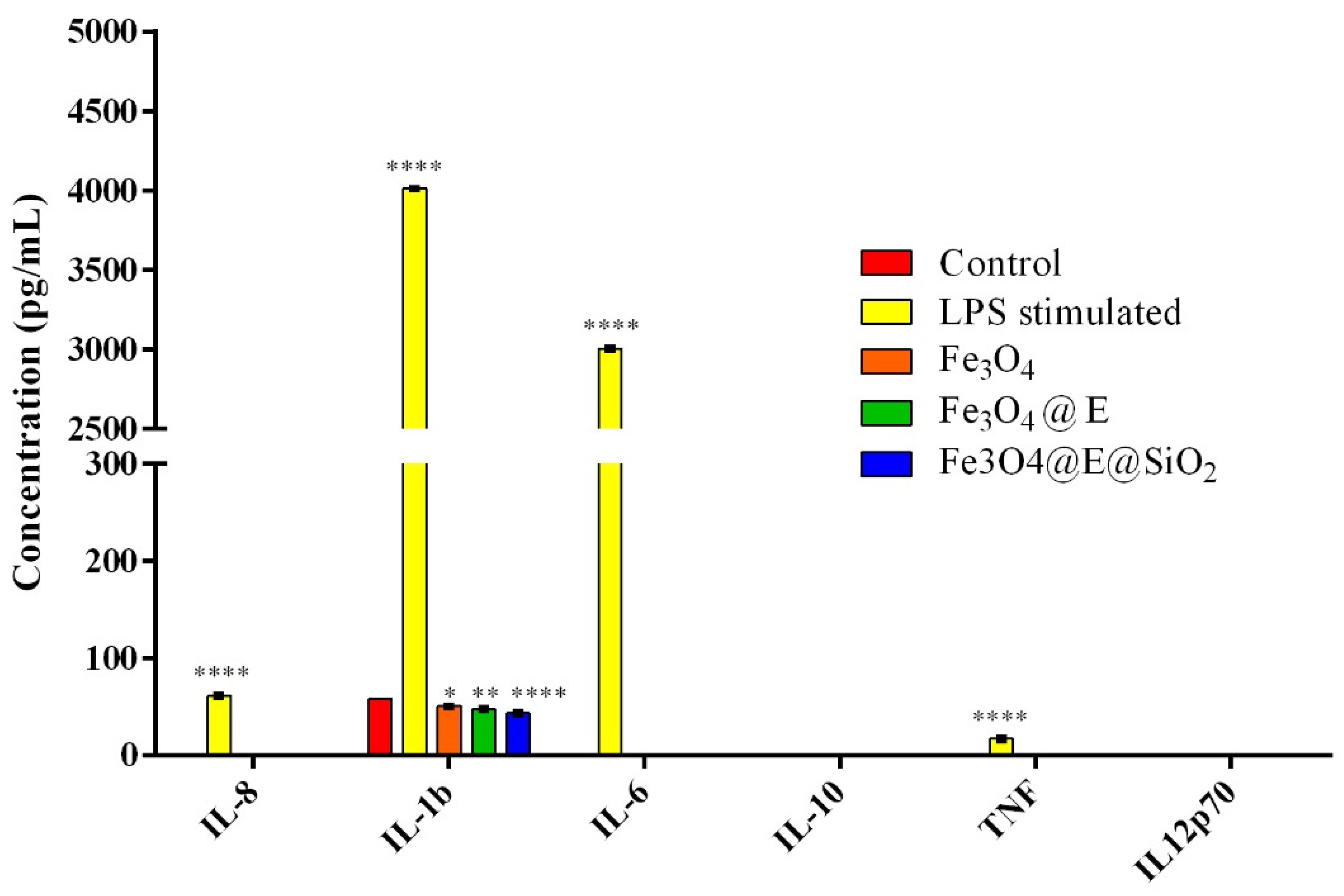
2.2. Ex Vivo Proinflammatory Potential Assessment
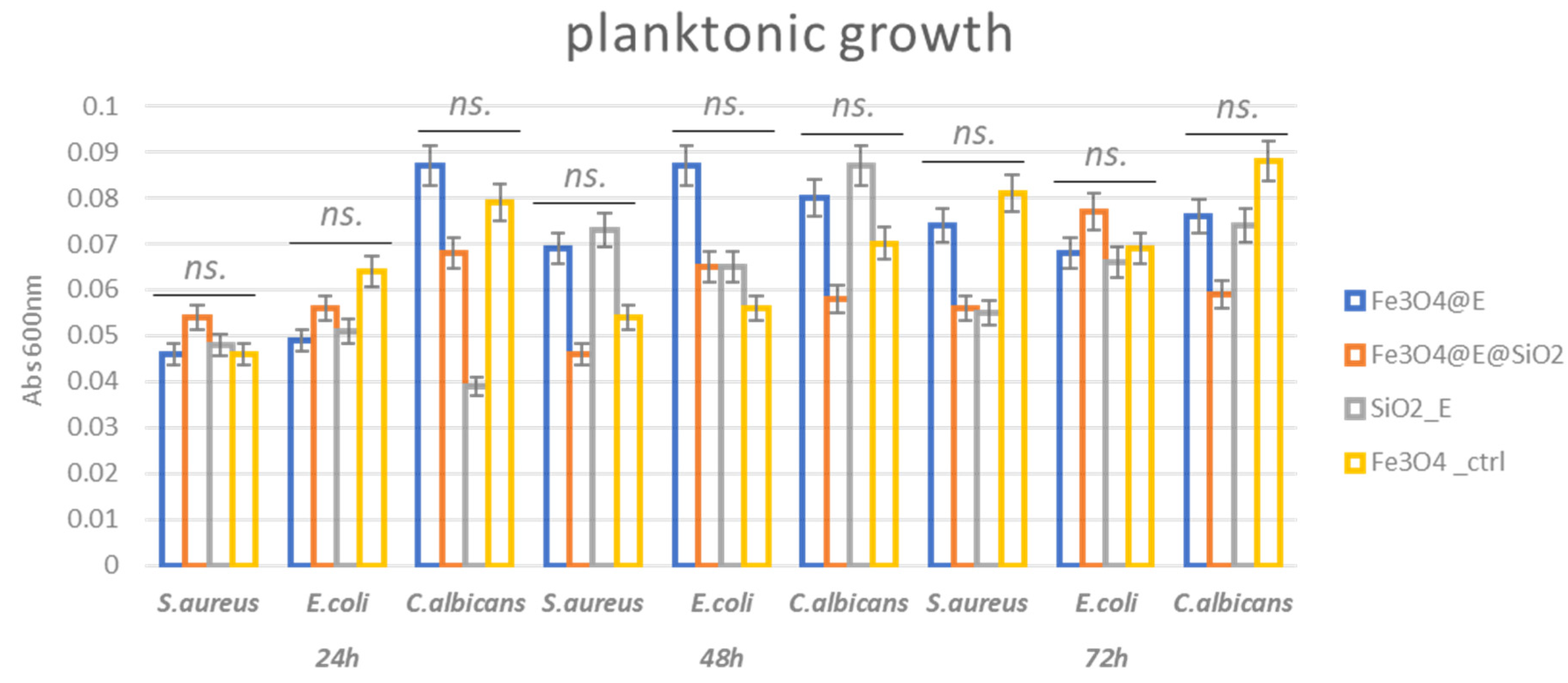
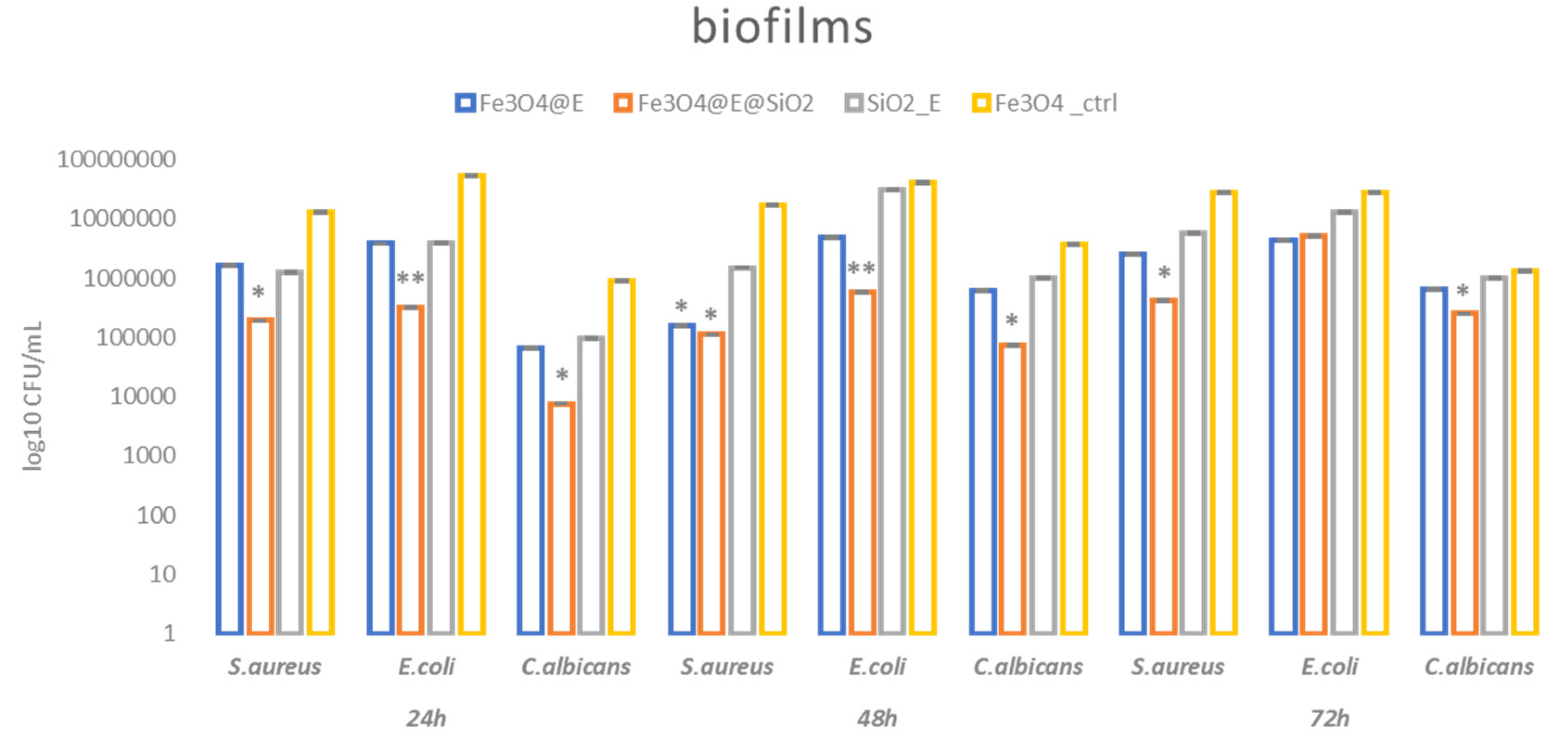
2.3. Antibacterial Efficiency
3. Materials and Methods
3.1. Materials
3.2. Synthesis Methods
3.2.1. Synthesis of Fe3O4-Based Nanosystems
3.2.2. Synthesis of Fe3O4-Based Coatings
3.3. Physicochemical Investigation
3.3.1. X-ray Diffraction (XRD)
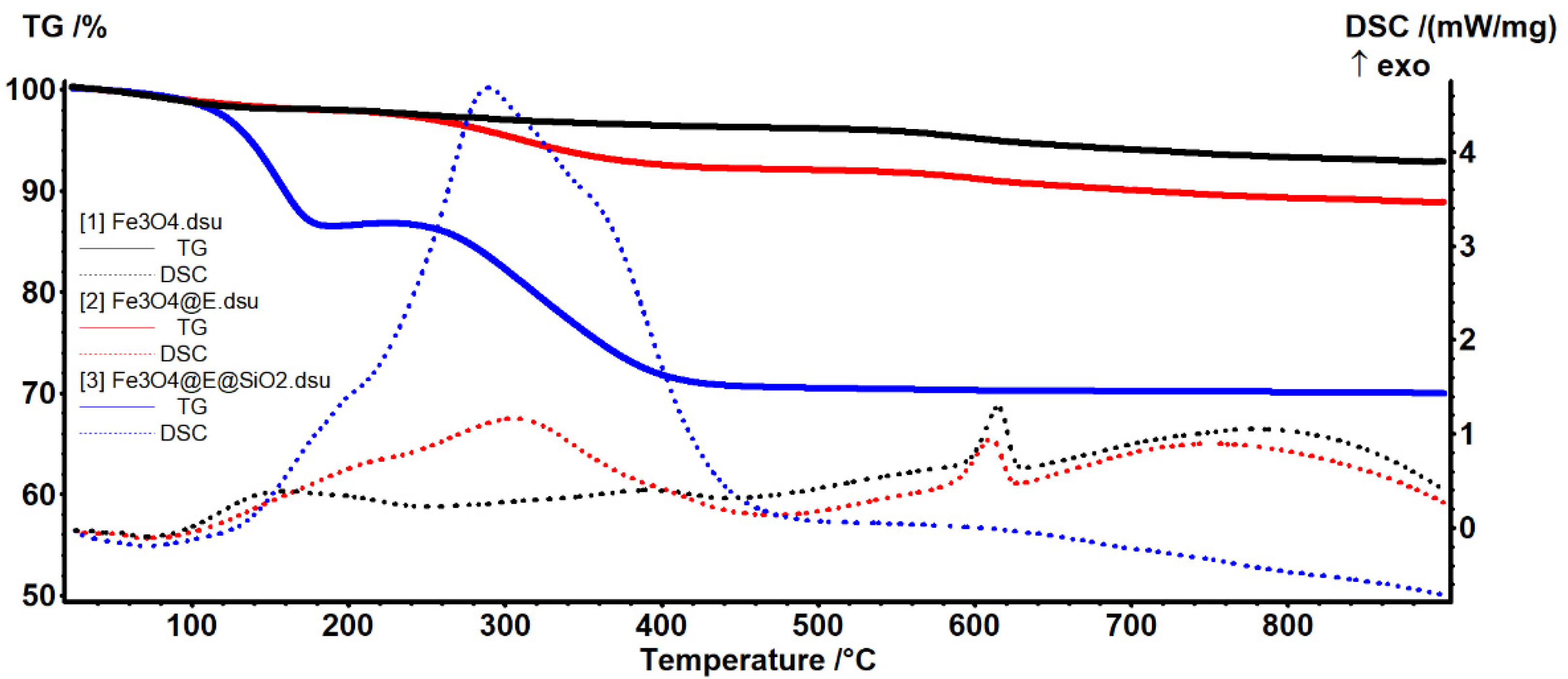
3.3.2. Thermogravimetric Analysis (TGA)
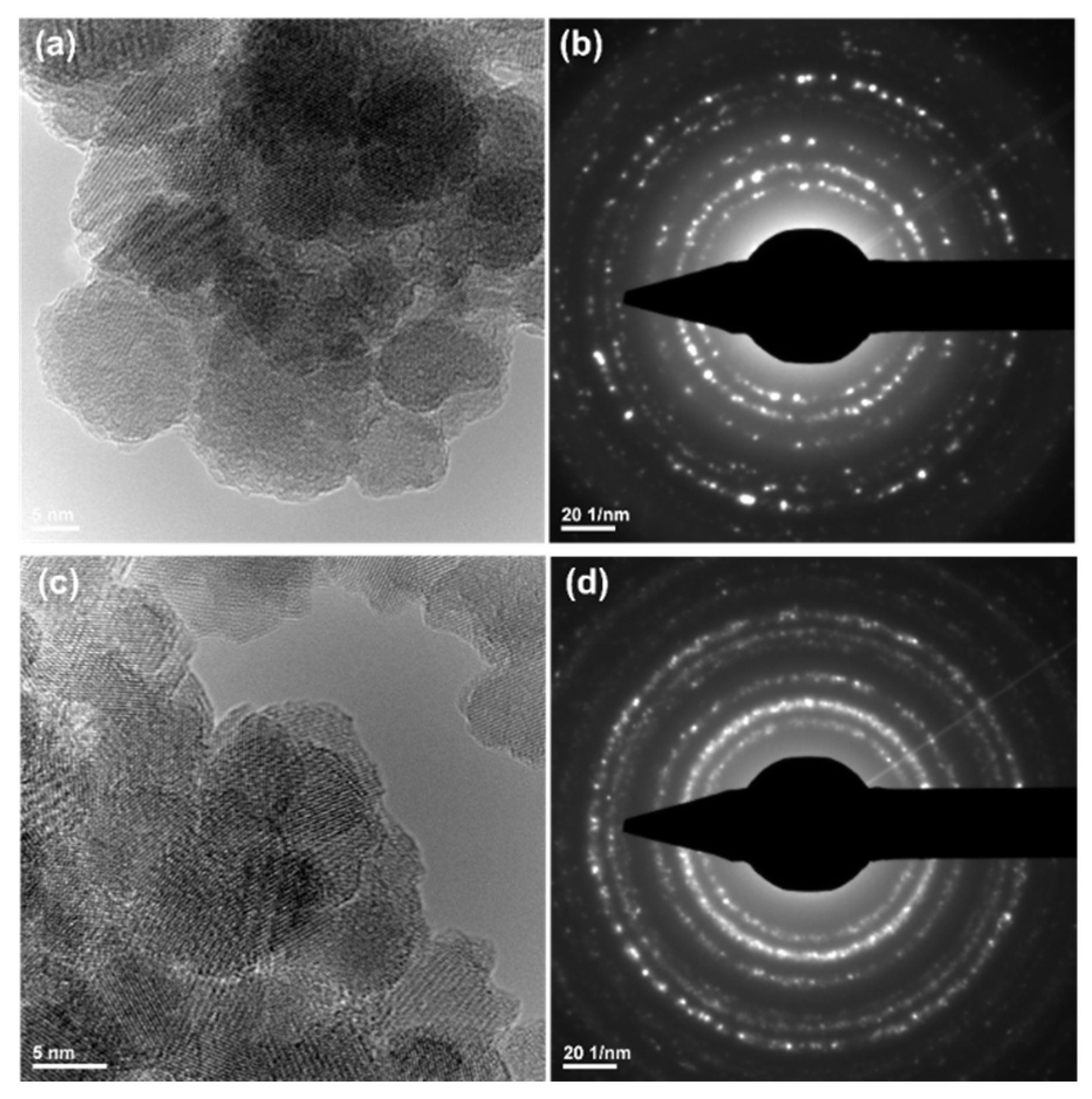
3.3.3. Transmission Electron Microscopy (TEM)
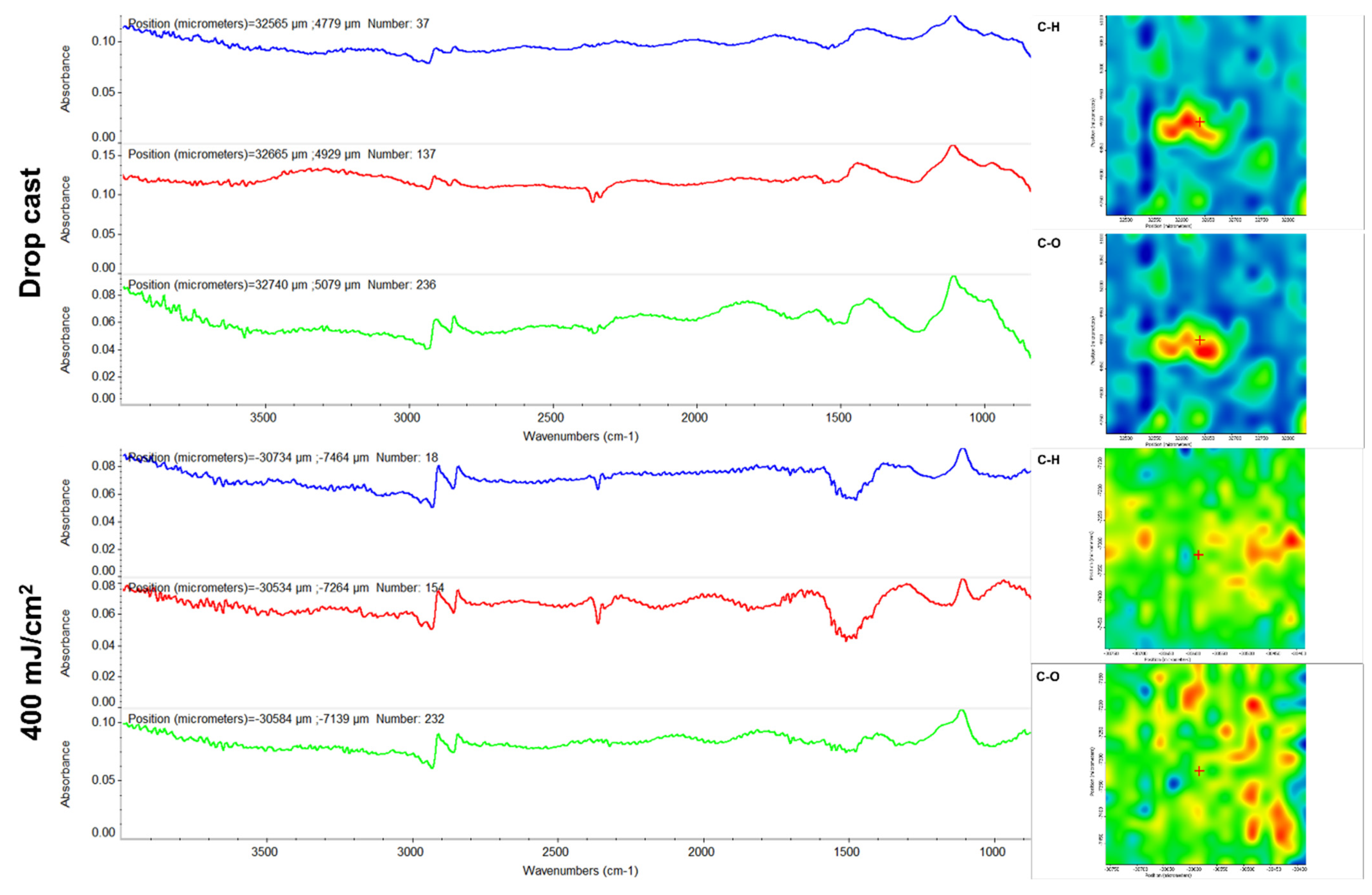
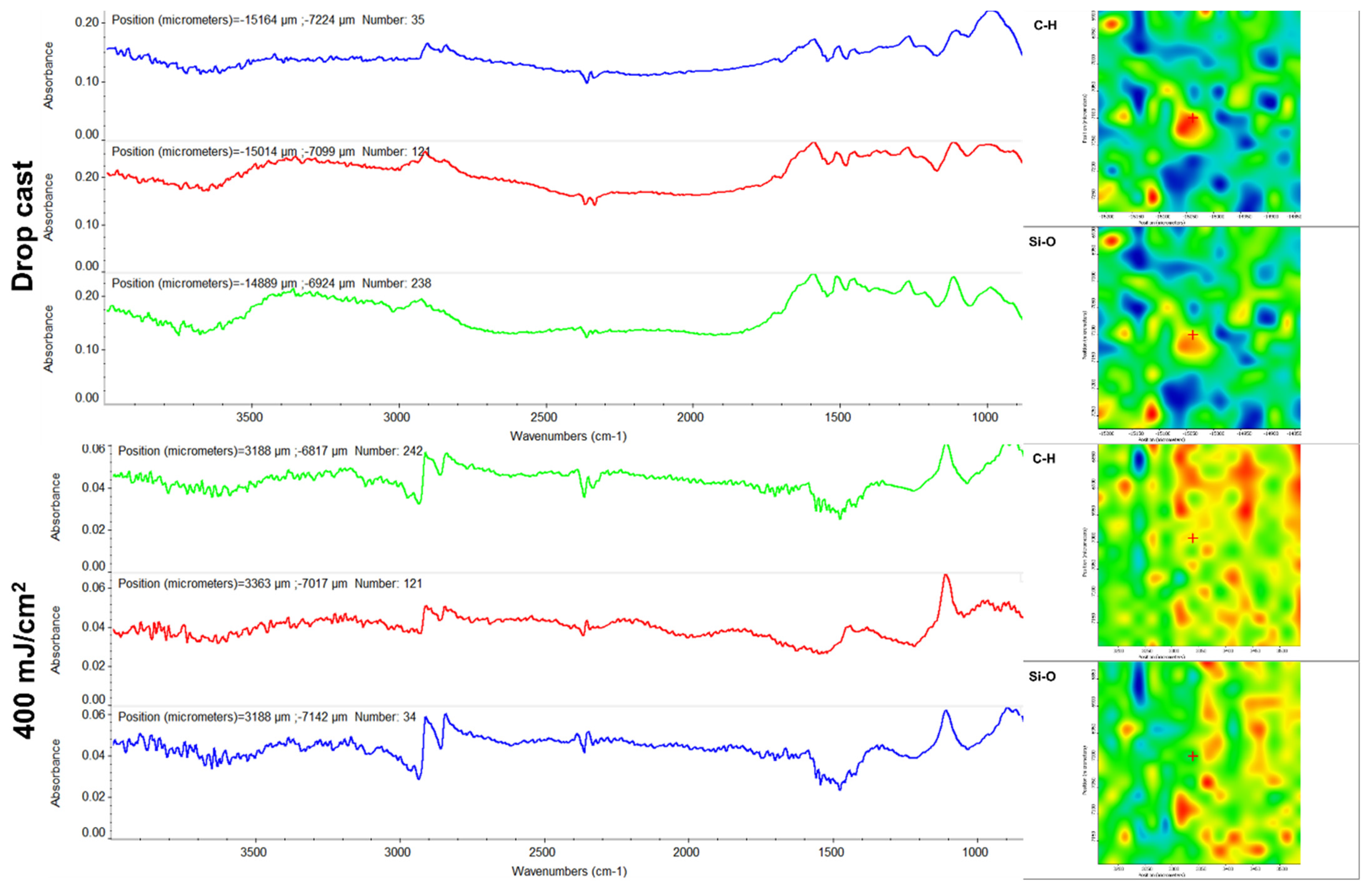
3.3.4. Infrared Microscopy (IRM)
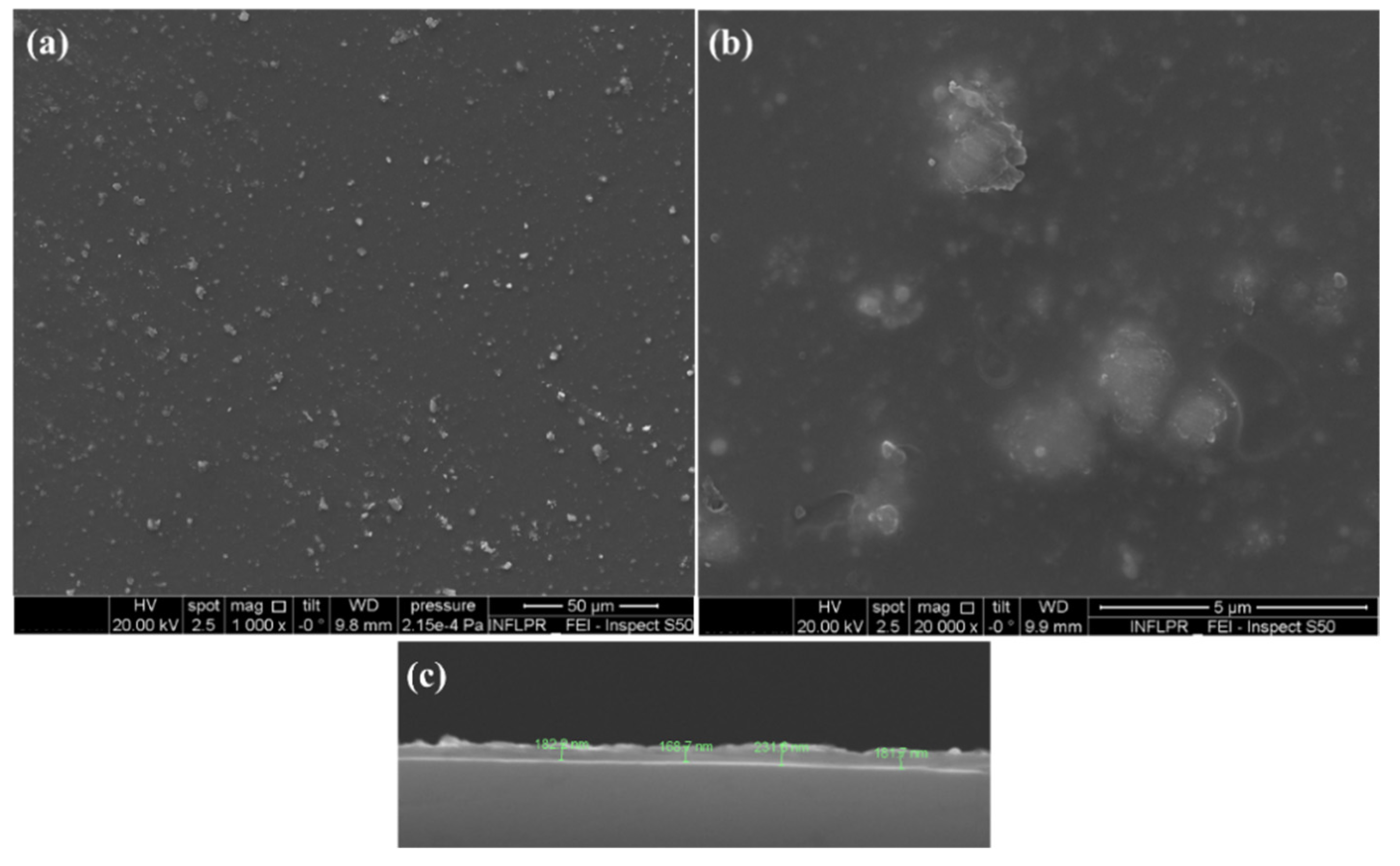
3.3.5. Scanning Electron Microscopy (SEM)
3.4. Biological Investigations
3.4.1. In Vitro Biocompatibility Screening
3.4.2. Proinflammatory Potential Assessment
3.4.3. Antimicrobial Assessment—Inhibition of Planktonic Growth
3.4.4. Antimicrobial Assessment—Modulation of Biofilm Formation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cartelle, G.M.; Holban, A.M. Advances in Nanotechnology as an Alternative against Superbugs. JSM Chem. 2014, 2, 1011. [Google Scholar]
- Lazar, V. Quorum sensing in biofilms—How to destroy the bacterial citadels or their cohesion/power? Anaerobe 2011, 17, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, M.; Aggarwal, A.; Fatima, K.H. Carbapenem-resistant bacteria on hand-held and hands-free electronic devices of healthcare workers and non-healthcare workers in Delhi, India. Infect. Prev. Pract. 2021, 3, 100162. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Koirala, N.; Kato, H.; Singh, T.; Karri, K.; Thakur, K. First Documented Case of Percutaneous Endoscopic Gastrostomy (PEG) Tube-Associated Bacterial Peritonitis due to Achromobacter Species with Literature Review. Case Rep. Gastrointest. Med. 2020, 2020, 4397930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Epps, J.S.; Younger, J.G. Implantable Device-Related Infection. Shock 2016, 46, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [Green Version]
- Dailymail. Available online: http://www.dailymail.co.uk/news/article-2477273/Weve-reached-end-antibiotics-Top-CDC-expert-declares-miracle-drugs-saved-millions-match-superbugs-people-overmedicated-themselves.html (accessed on 10 October 2022).
- Taft, D.H.; Salinero, L.K.; Vongbhavit, K.; Kalanetra, K.M.; Masarweh, C.; Yu, A.; Underwood, M.A.; Mills, D.A. Bacterial colonization and antimicrobial resistance genes in neonatal enteral feeding tubes. FEMS Microbiol. Ecol. 2019, 95, fiz039. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.A.; Magalhães, M.; Desorcy-Scherer, K.; Torrez Lamberti, M.; Lorca, G.L.; Neu, J. Neonatal Feeding Tube Colonization and the Potential Effect on Infant Health: A Review. Front. Nutr. 2022, 9, 775014. [Google Scholar] [CrossRef]
- Lu, M.; Dai, T.; Murray, C.K.; Wu, M.X. Bactericidal Property of Oregano Oil Against Multidrug-Resistant Clinical Isolates. Front. Microbiol. 2018, 9, 2329. [Google Scholar] [CrossRef] [Green Version]
- Kumari, R.; Mishra, R.C.; Sheoran, R.; Yadav, J.P. Fractionation of Antimicrobial Compounds from Acacia nilotica Twig Extract Against Oral Pathogens. Biointerface Res. Appl. Chem. 2020, 10, 7097–7105. [Google Scholar] [CrossRef]
- Lapinska, B.; Szram, A.; Zarzycka, B.; Grzegorczyk, J.; Hardan, L.; Sokolowski, J.; Lukomska-Szymanska, M. An In Vitro Study on the Antimicrobial Properties of Essential Oil Modified Resin Composite against Oral Pathogens. Materials 2020, 13, 4383. [Google Scholar] [CrossRef]
- Ghanbariasad, A.; Amoozegar, F.; Rahmani, M.; Zarenezhad, E.; Osanloo, M. Impregnated Nanofibrous Mat with Nanogel of Citrus sinensis Essential Oil as a New Type of Dressing in Cutaneous Leishmaniasis. Biointerface Res. Appl. Chem. 2021, 11, 11066–11076. [Google Scholar] [CrossRef]
- Radünz, M.; da Trindade, M.L.M.; Mota Camargo, T.; Radünz, A.L.; Dellinghausen Borges, C.; Avila Gandra, E.; Helbig, E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2019, 276, 180–186. [Google Scholar] [CrossRef]
- Abdullah, M.L.; Hafez, M.M.; Al-Hoshani, A.; Al-Shabanah, O. Anti-metastatic and anti-proliferative activity of eugenol against triple negative and HER2 positive breast cancer cells. BMC Complement. Altern. Med. 2018, 18, 321. [Google Scholar] [CrossRef] [Green Version]
- Petrocelli, G.; Farabegoli, F.; Valerii, M.C.; Giovannini, C.; Sardo, A.; Spisni, E. Molecules present in plant essential oils for prevention and Treatment of Colorectal Cancer (CRC). Molecules 2021, 26, 885. [Google Scholar] [CrossRef]
- de Araújo Lopes, A.; da Fonseca, F.N.; Rocha, T.M.; de Freitas, L.B.; Araújo EV, O.; Wong DV, T.; Pereira Lima Júnior, R.C.; Almeida Moreira Leal, L.K. Eugenol as a Promising Molecule for the Treatment of Dermatitis: Antioxidant and Anti-inflammatory Activities and Its Nanoformulation. Oxid. Med. Cell. Longev. 2018, 2018, 8194849. [Google Scholar] [CrossRef] [Green Version]
- Osman, M.A.; Mahmoud, G.I.; Elgammal, M.H.; Hasan, R.S. Bisphenol A Hormonal Disrupture and Preventive Effect of Rose Water and Clove Oil. Biointerface Res. Appl. Chem. 2021, 11, 8780–8803. [Google Scholar] [CrossRef]
- Magalhães, C.B.; Casquilho, N.V.; Machado, M.N.; Riva, D.R.; Travassos, L.H.; Leal-Cardoso, J.H.; Fortunato, R.S.; Faffe, D.S.; Zin, W.A. The anti-inflammatory and anti-oxidative actions of eugenol improve lipopolysaccharide-induced lung injury. Resp. Physiol. Neurobiol. 2019, 259, 30–36. [Google Scholar] [CrossRef]
- Anjum, N.F.; Shanmugarajan, D.; Shivaraju, V.K.; Faizan, S.; Naishima, N.L.; Prashantha Kumar, B.R.; Javid, S.; Purohit, M.H. Novel derivatives of eugenol as potent anti-inflammatory agents via PPARγ agonism: Rational design, synthesis, analysis, PPARγ protein binding assay and computational studies. RSC Adv. 2022, 12, 16966–16978. [Google Scholar] [CrossRef]
- Perugini Biasi-Garbin, R.; Saori Otaguiri, E.; Morey, A.T.; Fernandes da Silva, M.; Belotto Morguette, A.E.; Armando Contreras Lancheros, C.; Kian, D.; Perugini, M.R.; Nakazato, G.; Durán, N.; et al. Effect of Eugenol against Streptococcus agalactiae and Synergistic Interaction with Biologically Produced Silver Nanoparticles. Evid. Based Complement. Altern. Med. 2015, 2015, 861497. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, F.F.M.; Monte, F.J.Q.; de Lemos, T.L.G.; Garcia do Nascimento, P.G.; de Medeiros Costa, A.K.; de Paiva, L.M.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018, 12, 34–42. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Kong, L.-C.; Liu, J.; Ma, H.-X. Synergistic effect of eugenol with Colistin against clinical isolated Colistin-resistant Escherichia coli strains. Antimicrob. Resist. Infect. Control 2018, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Jafri, H.; Banerjee, G.; Khan, M.S.A.; Ahmad, I.; Abulreesh, H.H.; Althubiani, A.S. Synergistic interaction of eugenol and antimicrobial drugs in eradication of single and mixed biofilms of Candida albicans and Streptococcus mutans. AMB Express 2020, 10, 185. [Google Scholar] [CrossRef]
- Qian, W.; Sun, Z.; Wang, T.; Yang, M.; Liu, M.; Zhang, J.; Li, Y. Antimicrobial activity of eugenol against carbapenem-resistant Klebsiella pneumoniae and its effect on biofilms. Microb. Pathog. 2019, 139, 103924. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Gędas, A.; Simões, M. The Effects of Eugenol, Trans-Cinnamaldehyde, Citronellol, and Terpineol on Escherichia coli Biofilm Control as Assessed by Culture-Dependent and -Independent Methods. Molecules 2020, 25, 2641. [Google Scholar] [CrossRef]
- Liakos, I.; Grumezescu, A.M.; Holban, A.M. Magnetite nanostructures as novel strategies for anti-infectious therapy. Molecules 2014, 19, 12710–12726. [Google Scholar] [CrossRef]
- Mihai, A.D.; Chircov, C.; Grumezescu, A.M.; Holban, A.M. Magnetite Nanoparticles and Essential Oils Systems for Advanced Antibacterial Therapies. Int. J. Mol. Sci. 2020, 21, 7355. [Google Scholar] [CrossRef]
- Fleitas Martínez, O.; Cardoso, M.H.; Ribeiro, S.M.; Franco, O.L. Recent Advances in Anti-virulence Therapeutic Strategies With a Focus on Dismantling Bacterial Membrane Microdomains, Toxin Neutralization, Quorum-Sensing Interference and Biofilm Inhibition. Front. Cell. Infect. Microbiol. 2019, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Grumezescu, V.; Holban, A.M.; Iordache, F.; Socol, G.; Mogoşanu, G.D.; Grumezescu, A.M.; Ficai, A.; Vasile, B.Ş.; Truşcă, R.; Chifiriuc, M.C.; et al. MAPLE fabricated magnetite@eugenol and (3-hidroxybutyric acid-co-3-hidroxyvaleric acid)—polyvinyl alcohol microspheres coated surfaces with anti-microbial properties. Appl. Surf. Sci. 2014, 306, 16–22. [Google Scholar] [CrossRef]
- Balaure, P.C.; Popa, R.A.; Grumezescu, A.M.; Voicu, G.; Rădulescu, M.; Mogoantă, L.; Bălşeanu, T.A.; Mogoşanu, G.D.; Chifiriuc, M.C.; Bleotu, C.; et al. Biocompatible hybrid silica nanobiocomposites for the efficient delivery of anti-staphylococcal drugs. Int. J. Pharm. 2016, 510, 532–542. [Google Scholar] [CrossRef]
- Guzun, A.S.; Stroescu, M.; Jinga, S.I.; Voicu, G.; Grumezescu, A.M.; Holban, A.M. Plackett-Burman experimental design for bioactive bacterial cellulose-silica composites synthesis with antimicrobial adherence effect. Mater. Sci. Eng. C 2014, 42, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Favela-Camacho, S.E.; Samaniego-Benítez, E.J.; Godínez-García, A.; Avilés-Arellano, L.M.; Pérez-Robles, J.F. How to decrease the agglomeration of magnetite nanoparticles and increase their stability using surface properties. Colloids Surf. A. Physicochem. Eng. Asp. 2019, 574, 29–35. [Google Scholar] [CrossRef]
- Yuan, K.; Lee, S.S.; Cha, W.; Ulvestad, A.; Kim, H.; Abdilla, B.; Sturchio, N.C.; Fenter, P. Oxidation induced strain and defects in magnetite crystals. Nat. Commun. 2019, 10, 703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, R.C.; Andronescu, E.; Vasile, B.S. Recent Advances in Magnetite Nanoparticle Functionalization for Nanomedicine. Nanomaterials 2019, 9, 1791. [Google Scholar] [CrossRef] [Green Version]
- Rasouli, E.; Basirun, W.J.; Rezayi, M.; Shameli, K.; Nourmohammadi, E.; Khandanlou, R.; Izadiyan, Z.; Sarkarizi, H.K. Ultrasmall superparamagnetic Fe3O4 nanoparticles: Honey-based green and facile synthesis and in vitro viability assay. Int. J. Nanomed. 2018, 13, 6903–6911. [Google Scholar] [CrossRef] [Green Version]
- Temelie, M.; Popescu, R.C.; Cocioaba, D.; Vasile, B.S.; Savu, D. Biocompatibility study of magnetite nanoparticle synthesized using a green method. Rom. J. Phys. 2018, 63, 703–716. [Google Scholar]
- Gherasim, O.; Popescu, R.C.; Grumezescu, V.; Mogoșanu, G.D.; Mogoantă, L.; Iordache, F.; Holban, A.M.; Vasile, B.Ș.; Bîrcă, A.C.; Oprea, O.-C.; et al. MAPLE Coatings Embedded with Essential Oil-Conjugated Magnetite for Anti-Biofilm Applications. Materials 2021, 14, 1612. [Google Scholar] [CrossRef]
- Popescu, R.C.; Vasile, B.Ş.; Savu, D.I.; Mogoşanu, G.D.; Bejenaru, L.E.; Andronescu, E.; Grumezescu, A.M.; Mogoantă, L. Influence of Polymer Shell Molecular Weight on Functionalized Iron Oxide Nanoparticles Morphology and In Vivo Biodistribution. Pharmaceutics 2022, 14, 1877. [Google Scholar] [CrossRef]
- Li, Y.; Jiangn, R.; Liu, T.; Lv, H.; Zhang, X. Single-microemulsion-based solvothermal synthesis of magnetite microflowers. Ceram. Int. 2014, 40, 4791–4795. [Google Scholar] [CrossRef]
- Cursaru, L.M.; Piticescu, R.M.; Dragut, D.V.; Morel, R.; Thébault, C.; Carrière, M.; Joisten, H.; Dieny, B. One-Step Soft Chemical Synthesis of Magnetite Nanoparticles under Inert Gas Atmosphere. Magnetic Properties and In Vitro Study. Nanomaterials 2020, 10, 1500. [Google Scholar] [CrossRef]
- Bruckmann, F.S.; Pimentel, A.C.; Viana, A.R.; Salles, T.R.; Krause, L.M.F.; Mortari, S.R.; Rhoden, C.R.B. Synthesis, characterization and cytotoxicity evaluation of magnetic nanosilica in L929 cell line. Discip. Sci. Sér. Ciên. Nat. Tecnol. 2020, 21, 1–14. [Google Scholar] [CrossRef]
- El-Desouky, M.G.; Hassan, N.; Shahat, A.; El-Didamony, A.; El-Bindary, A.A. Synthesis and Characterization of Porous Magnetite Nanosphere Iron Oxide as a Novel Adsorbent of Anionic Dyes Removal from Aqueous Solution. Biointerface Res. Appl. Chem. 2021, 11, 13377–13401. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Niculescu, A.-G.; Slave, Ș.; Bîrcă, A.C.; Dorcioman, G.; Grumezescu, V.; Holban, A.M.; Oprea, O.-C.; Vasile, B.Ș.; Grumezescu, A.M.; et al. Anti-Biofilm Coatings Based on Chitosan and Lysozyme Functionalized Magnetite Nanoparticles. Antibiotics 2021, 10, 1269. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.B.; Rayyif, S.M.I.; Curutiu, C.; Birca, A.C.; Oprea, O.-C.; Grumezescu, A.M.; Ditu, L.-M.; Gheorghe, I.; Chifiriuc, M.C.; Mihaescu, G.; et al. Eugenol-Functionalized Magnetite Nanoparticles Modulate Virulence and Persistence in Pseudomonas aeruginosa Clinical Strains. Molecules 2021, 26, 2189. [Google Scholar] [CrossRef]
- González-Rivera, J.; Duce, C.; Falconieri, D.; Ferrari, C.; Ghezzi, L.; Piras, A.; Tine, M.R. Coaxial microwave assisted hydrodistillation of essential oils from five different herbs (lavender, rosemary, sage, fennel seeds and clove buds): Chemical composition and thermal analysis. Innov. Food Sci. Emerg. Technol. 2016, 33, 308–318. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Cabedo, L.; Lagaron, J.M.; Torres-Giner, S. Development of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Monolayers Containing Eugenol and Their Application in Multilayer Antimicrobial Food Packaging. Front. Nutr. 2020, 7, 140. [Google Scholar] [CrossRef]
- Alexandrovskaya, Y.M.; Pavley, Y.R.; Grigoriev, Y.V.; Grebenev, V.V.; Shatalova, T.B.; Obrezkova, M.V. Thermal behavior of magnetite nanoparticles with various coatings in the range 30–1000 °C. Thermochim. Acta 2022, 708, 179120. [Google Scholar] [CrossRef]
- Dolete, G.; Chircov, C.; Motelica, L.; Ficai, D.; Oprea, O.-C.; Gheorghe, M.; Ficai, A.; Andronescu, E. Magneto-Mechanically Triggered Thick Films for Drug Delivery Micropumps. Nanomaterials 2022, 12, 3598. [Google Scholar] [CrossRef]
- Moreira Ficanha, A.M.; Antunes, A.; Demaman Oro, C.E.; Dallago, R.M.; Mignoni, M.L. Immobilization of Candida antarctica B (CALB) in Silica Aerogel: Morphological Characteristics and Stability. Biointerface Res. Appl. Chem. 2020, 10, 6744–6756. [Google Scholar] [CrossRef]
- Hoseinzadeh, H.; Hayati, B.; Ghaheh, F.S.; Seifpanahi-Shabani, K.; Mahmoodi, N.M. Development of room temperature synthesized and functionalized metal-organic framework/graphene oxide composite and pollutant adsorption ability. Mater. Res. Bull. 2021, 142, 111408. [Google Scholar] [CrossRef]
- Caciandone, M.; Niculescu, A.-G.; Grumezescu, V.; Bîrcă, A.C.; Ghica, I.C.; Vasile, B.Ș.; Oprea, O.; Nica, I.C.; Stan, M.S.; Holban, A.M.; et al. Magnetite Nanoparticles Functionalized with Therapeutic Agents for Enhanced ENT Antimicrobial Properties. Antibiotics 2022, 11, 623. [Google Scholar] [CrossRef] [PubMed]
- Palimi, M.J.; Rostami, M.; Mahdavian, M.; Ramezanzadeh, B. Studying the effects of surface modification of Cr2O3 nanoparticles by 3-aminopropyltrimethoxysilane (APTMS) on its corrosion inhibitive performance. J. Sol.-Gel. Sci. Technol. 2015, 73, 141–153. [Google Scholar] [CrossRef]
- Maleky, S.; Asadipour, A.; Nasiri, A.; Luque, R.; Faraji, M. Tetracycline Adsorption from Aqueous Media by Magnetically Separable Fe3O4@Methylcellulose/APTMS: Isotherm, Kinetic and Thermodynamic Studies. J. Polym. Environ. 2022, 30, 3351–3367. [Google Scholar] [CrossRef]
- Chircov, C.; Matei, M.-F.; Neacșu, I.A.; Vasile, B.S.; Oprea, O.-C.; Croitoru, A.-M.; Trușcă, R.-D.; Andronescu, E.; Sorescu, I.; Bărbuceanu, F. Iron Oxide–Silica Core–Shell Nanoparticles Functionalized with Essential Oils for Antimicrobial Therapies. Antibiotics 2021, 10, 1138. [Google Scholar] [CrossRef]
- Naini, N.; Kalal, H.S.; Almasian, M.R.; Niknafs, D.; Taghiof, M.; Hoveidi, H. Phosphine-functionalized Fe3O4/SiO2/composites as efficient magnetic nanoadsorbents for the removal of palladium ions from aqueous solution: Kinetic, thermodynamic and isotherm studies. Mater. Chem. Phys. 2022, 287, 126242. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, Y.; He, B.; Zeng, X.; Lai, K.; Gu, Z. Effect of sodium oleate as a buffer on the synthesis of superparamagnetic magnetite colloids. J. Colloid Interface Sci. 2010, 347, 1–7. [Google Scholar] [CrossRef]
- Chircov, C.; Bîrcă, A.C.; Vasile, B.S.; Oprea, O.-C.; Huang, K.-S.; Grumezescu, A.M. Microfluidic Synthesis of -NH2- and -COOH-Functionalized Magnetite Nanoparticles. Nanomaterials 2022, 12, 3160. [Google Scholar] [CrossRef]
- Hongfang, G.; Hui, Y.; Chuang, W. Controllable preparation and mechanism of nano-silver mediated by the microemulsion system of the clove oil. Results Phys. 2017, 7, 3130–3136. [Google Scholar] [CrossRef]
- Getnet, T.G.; Kayama, M.E.; Rangel, E.C.; Cruz, N.C. Thin Film Deposition by Atmospheric Pressure Dielectric Barrier Discharges Containing Eugenol: Discharge and Coating Characterizations. Polymers 2020, 12, 2692. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Hassanein, W.S.; Alkabaa, A.S.; Ceylan, Z. Electrospun eugenol-loaded gelatin nanofibers as bioactive packaging materials to preserve quality characteristics of beef. Food Packag. Shelf Life 2022, 34, 100968. [Google Scholar] [CrossRef]
- Nuchuchua, O.; Saesoo, S.; Sramala, I.; Puttipipatkhachorn, S.; Soottitantawat, A.; Ruktanonchai, U. Physicochemical investigation and molecular modeling of cyclodextrin complexation mechanism with eugenol. Food Res. Int. 2009, 42, 1178–1185. [Google Scholar] [CrossRef]
- Yang, Y.; Song, L.X. Study on the inclusion compounds of eugenol with α-, β-, γ- and heptakis (2,6-di-O-methyl)-β-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 27–33. [Google Scholar] [CrossRef]
- Nagaraju, P.G.; Sengupta, P.; Priyadarshini, C.G.P.; Rao, P.J. Nanoencapsulation of clove oil and study of physico-chemical properties, cytotoxic, haemolytic and antioxidant activities. J. Food Process Eng. 2021, 44, e13645. [Google Scholar] [CrossRef]
- Taufiq, A.; Nikmah, A.; Hidayat, A.; Sunaryono, S.; Mufti, N.; Hidayat, N.; Susanto, H. Synthesis of magnetite/silica nanocomposites from natural sand to create a drug delivery vehicle. Helyon 2020, 6, e03784. [Google Scholar] [CrossRef]
- Demin, A.M.; Vakhrushev, A.V.; Valova, M.S.; Korolyova, M.A.; Uimin, M.A.; Minin, A.S.; Pozdina, V.A.; Byzov, I.V.; Tumashov, A.A.; Chistyakov, K.A.; et al. Effect of the Silica–Magnetite Nanocomposite Coating Functionalization on the Doxorubicin Sorption/Desorption. Pharmaceutics 2022, 14, 2271. [Google Scholar] [CrossRef]
- Ramezani, M.; Kosak, A.; Lobnik, A.; Hadela, A. Synthesis and characterization of an antimicrobial textile by hexagon silver nanoparticles with a new capping agent via the polyol process. Text. Res. J. 2019, 89, 004051751984816. [Google Scholar] [CrossRef]
- Ganapathe, L.S.; Mohamed, M.A.; Mohamad Yunus, R.; Berhanuddin, D.D. Magnetite (Fe3O4) nanoparticles in biomedical application: From synthesis to surface functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Malvindi, M.A.; Brunetti, V.; Vecchio, G.; Galeone, A.; Cingolani, R.; Pompa, P.P. SiO2 nanoparticles biocompatibility and their potential for gene delivery and silencing. Nanoscale 2012, 4, 486–495. [Google Scholar] [CrossRef]
- Escobar-García, M.; Rodríguez-Contreras, K.; Ruiz-Rodríguez, S.; Pierdant-Pérez, M.; Cerda-Cristerna, B.; Pozos-Guillén, A. Eugenol toxicity in human dental pulp fibroblasts of primary teeth. J. Clin. Pediatr. Dent. 2016, 40, 312–318. [Google Scholar] [CrossRef]
- Bakhori, S.K.M.; Mahmud, S.; Mohamad, D.; Masudi, S.A.M.; Seeni, A. Cytotoxicity determination of nano-zinc oxide eugenol on human gingival fibroblast cells. Mater. Chem. Phys. 2021, 268, 124649. [Google Scholar] [CrossRef]
- Ho, Y.C.; Huang, F.M.; Chang, Y.C. Mechanisms of cytotoxicity of eugenol in human osteoblastic cells in vitro. Int. Endod. J. 2006, 39, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.C.; Srinivasan, V.; Selvaraj, S.; Mahapatra, S.K. Versatile and synergistic potential of eugenol: A review. Pharm. Anal. Acta 2015, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Dorresteijn, M.J.; Draisma, A.; van der Hoeven, J.G.; Pickkers, P. Lipopolysaccharide-stimulated whole blood cytokine production does not predict the inflammatory response in human endotoxemia. Innate Immun. 2010, 16, 248–253. [Google Scholar] [CrossRef]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxidative Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef] [PubMed]
- Giannossa, L.C.; Longano, D.; Ditaranto, N.; Nitti, M.; Paladini, F.; Pollini, M.; Rai, M.; Sannino, A.; Valentini, A.; Cioffi, N. Metal nanoantimicrobials for textile applications. Mater. Sci. 2013, 2, 307–331. [Google Scholar] [CrossRef]
- Bezerra, S.R.; Bezerra, A.H.; de Sousa Silveira, Z.; Macedo, N.S.; Rodrigues dos Santos Barbosa, C.; Feitosa Muniz, D.; dos Santos, J.F.S.; Douglas Melo Coutinho, H.; da Cunha, F.A.B. Antibacterial activity of eugenol on the IS-58 strain of Staphylococcus aureus resistant to tetracycline and toxicity in Drosophila melanogaster. Microb. Pathog. 2022, 164, 105456. [Google Scholar] [CrossRef] [PubMed]
- Aiello, R.; Cavallaro, G.; Giammona, G.; Pasqua, L.; Pierro, P.; Testa, F. Mesoporous silicate as matrix for drug delivery systems of non-steroidal antinflammatory drugs. In Studies in Surface Science and Catalysis; Aiello, R., Giordano, G., Testa, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 142, pp. 1165–1172. [Google Scholar] [CrossRef]
- Keshavarz, H.; Khavandi, A.; Alamolhoda, S.; Naimi-Jamal, M.R. Magnetite mesoporous silica nanoparticles embedded in carboxybetaine methacrylate for application in hyperthermia and drug delivery. New J. Chem. 2020, 44, 8232–8240. [Google Scholar] [CrossRef]
- Grumezescu, A.; Vasile, B.; Holban, A.J.L.A.N. Eugenol functionalized magnetite nanostructures used in anti-infectious therapy. Lett. Appl. Nanobiosci. 2013, 2, 120–123. [Google Scholar]
- El-Nekeety, A.A.; Hassan, M.E.; Hassan, R.R.; Elshafey, O.I.; Hamza, Z.K.; Abdel-Aziem, S.H.; Hassan, N.S.; Abdel-Wahhab, M.A. Nanoencapsulation of basil essential oil alleviates the oxidative stress, genotoxicity and DNA damage in rats exposed to biosynthesized iron nanoparticles. Heliyon 2021, 7, e07537. [Google Scholar] [CrossRef]
- Chifiriuc, M.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Cotar, A.I.; Grumezescu, V.; Bezirtzoglou, E.; Lazar, V.; Radulescu, R. Water dispersible magnetite nanoparticles influence the efficacy of antibiotics against planktonic and biofilm embedded enterococcus faecalis cells. Anaerobe 2013, 22, 14–19. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Cristescu, R.; Chifiriuc, M.C.; Dorcioman, G.; Socol, G.; Mihailescu, I.N.; Mihaiescu, D.E.; Ficai, A.; Vasile, O.R.; Enculescu, M.; et al. Fabrication of magnetite-based core–shell coated nanoparticles with antibacterial properties. Biofabrication 2015, 7, 015014. [Google Scholar] [CrossRef]
- Rayyif, S.M.I.; Mohammed, H.B.; Curuțiu, C.; Bîrcă, A.C.; Grumezescu, A.M.; Vasile, B.Ș.; Dițu, L.M.; Lazăr, V.; Chifiriuc, M.C.; Mihăescu, G.; et al. ZnO Nanoparticles-Modified Dressings to Inhibit Wound Pathogens. Materials 2021, 14, 3084. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hudiță, A.; Grumezescu, V.; Gherasim, O.; Grumezescu, A.M.; Dorcioman, G.; Negut, I.; Oprea, O.-C.; Vasile, B.Ș.; Gălățeanu, B.; Curuțiu, C.; et al. MAPLE Processed Nanostructures for Antimicrobial Coatings. Int. J. Mol. Sci. 2022, 23, 15355. https://doi.org/10.3390/ijms232315355
Hudiță A, Grumezescu V, Gherasim O, Grumezescu AM, Dorcioman G, Negut I, Oprea O-C, Vasile BȘ, Gălățeanu B, Curuțiu C, et al. MAPLE Processed Nanostructures for Antimicrobial Coatings. International Journal of Molecular Sciences. 2022; 23(23):15355. https://doi.org/10.3390/ijms232315355
Chicago/Turabian StyleHudiță, Ariana, Valentina Grumezescu, Oana Gherasim, Alexandru Mihai Grumezescu, Gabriela Dorcioman, Irina Negut, Ovidiu-Cristian Oprea, Bogdan Ștefan Vasile, Bianca Gălățeanu, Carmen Curuțiu, and et al. 2022. "MAPLE Processed Nanostructures for Antimicrobial Coatings" International Journal of Molecular Sciences 23, no. 23: 15355. https://doi.org/10.3390/ijms232315355








