Locus Coeruleus Neurons’ Firing Pattern Is Regulated by ERG Voltage-Gated K+ Channels
Abstract
:1. Introduction
2. Results
2.1. Immunohistochemical Localization of ERG Channels within Murine LC Nuclei
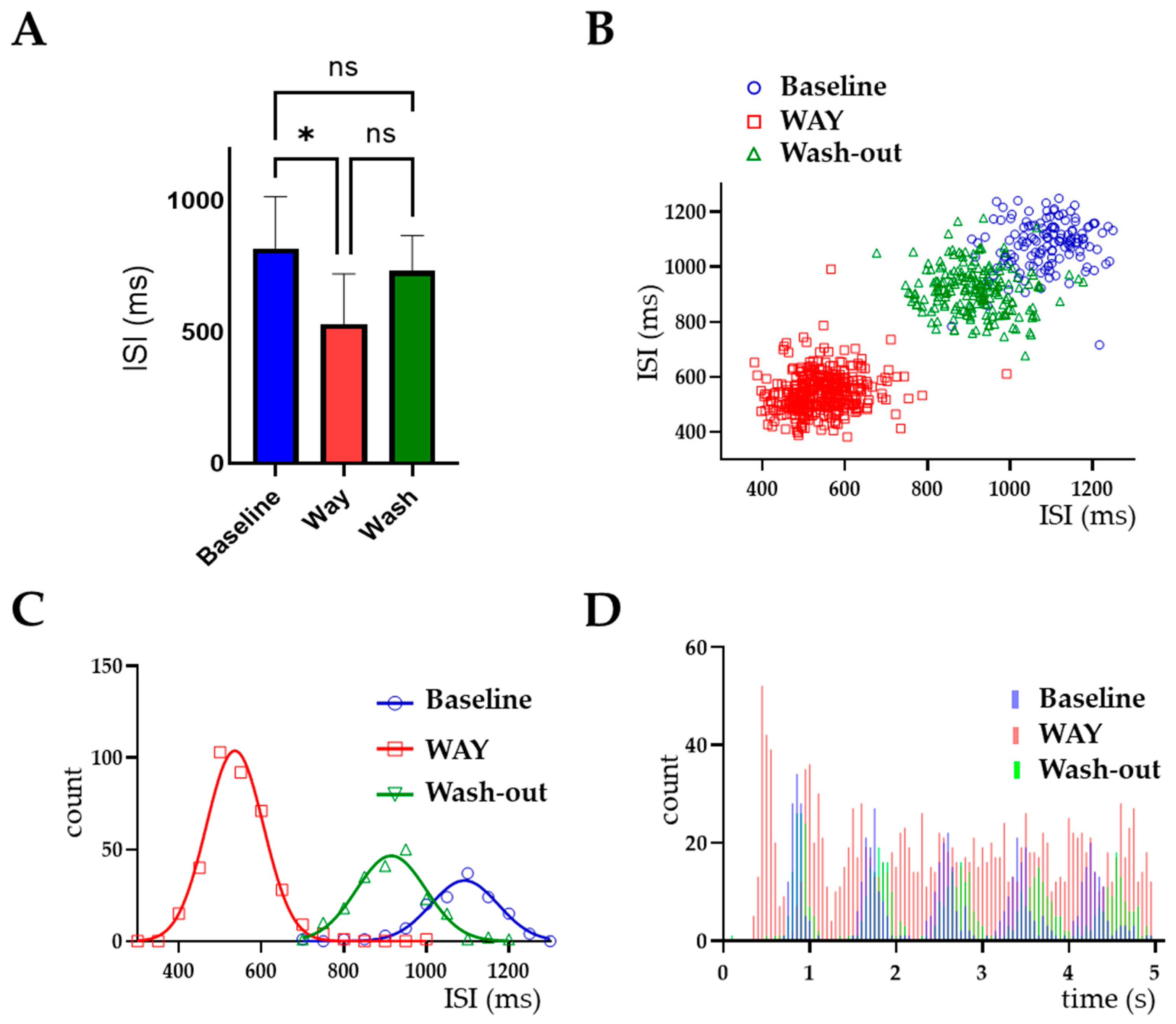
2.2. ERG Channels Regulate the Spontaneous Activity of LC Neurons
3. Discussion
4. Materials and Methods
4.1. Immunohistochemistry
4.2. Tight-Seal, Whole-Cell Recordings
4.3. ERG Channels’ Blockage
4.4. Data Analysis and Statistical Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Egroo, M.; Koshmanova, E.; Vandewalle, G.; Jacobs, H.I.L. Importance of the locus coeruleus-norepinephrine system in sleep-wake regulation: Implications for aging and Alzheimer’s disease. Sleep Med. Rev. 2022, 62, 101592. [Google Scholar] [CrossRef] [PubMed]
- Borodovitsyna, O.; Tkaczynski, J.A.; Corbett, C.M.; Loweth, J.A.; Chandler, D.J. Ageand Sex-Dependent Changes in Locus Coeruleus Physiology and Anxiety-Like Behavior Following Acute Stressor Exposure. Front. Behav. Neurosci. 2022, 16, 808590. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.J.; Mather, M.; Werkle-Bergner, M. Noradrenergic modulation of rhythmic neural activity shapes selective attention. Trends Cogn. Sci. 2022, 26, 38–52. [Google Scholar] [CrossRef]
- Llorca-Torralba, M.; Camarena-Delgado, C.; Suárez-Pereira, I.; Bravo, L.; Mariscal, P.; Garcia-Partida, J.A.; López-Martín, C.; Wei, H.; Pertovaara, A.; Mico, J.A.; et al. Pain and depression comorbidity causes asymmetric plasticity in the locus coeruleus neurons. Brain 2022, 145, 154–167. [Google Scholar] [CrossRef] [PubMed]
- James, T.; Kula, B.; Choi, S.; Khan, S.S.; Bekar, L.K.; Smith, N.A. Locus coeruleus in memory formation and Alzheimer’s disease. Eur. J. Neurosci. 2021, 54, 6948–6959. [Google Scholar] [CrossRef]
- Ross, J.A.; Van Bockstaele, E.J. The Locus Coeruleus- Norepinephrine System in Stress and Arousal: Unraveling Historical, Current, and Future Perspectives. Front. Psychiatry 2021, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, A.; Tan, B.Z.; Ycu, E.A.; Cuevas, J.S.; Koivumaa, J.; Junyent, F.; Kremer, E.; Witten, I.B.; Deisseroth, K.; Johansen, J.P. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci. 2017, 20, 1602–1611. [Google Scholar] [CrossRef]
- D’Adamo, M.C.; Shang, L.; Imbrici, P.; Brown, S.D.; Pessia, M.; Tucker, S.J. Genetic inactivation of Kcnj16 identifies Kir5.1 as an im-portant determinant of neuronal PCO2/pH sensitivity. J. Biol. Chem. 2011, 286, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Filosa, J.A.; Dean, J.B.; Putnam, R.W. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J. Physiol. 2002, 541, 493–509. [Google Scholar] [CrossRef]
- Masuko, S.; Nakajima, Y.; Nakajima, S.; Yamaguchi, K. Noradrenergic neurons from the locus ceruleus in dissociated cell culture: Culture methods, morphology, and electrophysiology. J. Neurosci. 1986, 6, 3229–3241. [Google Scholar] [CrossRef]
- Forsythe, I.D.; Linsdell, P.; Stanfield, P.R. Unitary A-currents of rat locus coeruleus neurones grown in cell culture: Rectification caused by internal Mg2+ and Na+. J. Physiol. 1992, 451, 553–583. [Google Scholar] [CrossRef] [PubMed]
- Pineda, J.; Aghajanian, G. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience 1997, 77, 723–743. [Google Scholar] [CrossRef] [PubMed]
- Sausbier, U.; Sausbier, M.; Sailer, C.A.; Arntz, C.; Knaus, H.-G.; Neuhuber, W.; Ruth, P. Ca2+-activated K+ channels of the BK-type in the mouse brain. Histochem. Cell Biol. 2005, 125, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Filosa, J.A.; Putnam, R.W. Multiple targets of chemosensitive signaling in locus coeruleus neurons: Role of K+ and Ca2+ channels. Am. J. Physiol. Cell Physiol. 2003, 284, C145–C155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imber, A.N.; Putnam, R.W. Postnatal development, and activation of L-type Ca2+ currents in locus coeruleus neurons: Implica-tions for a role for Ca2+ in central chemosensitivity. J. Appl. Physiol. 2012, 112, 1715–1726. [Google Scholar] [CrossRef] [Green Version]
- Bauer, C.K.; Schwarz, J.R. Ether-à-go-go K+ channels: Effective modulators of neuronal excitability. J. Physiol. 2018, 596, 769–783. [Google Scholar] [CrossRef] [Green Version]
- Chiesa, N.; Rosati, B.; Arcangeli, A.; Olivotto, M.; Wanke, E. A novel role for HERG K+ channels: Spike-frequency adaptation. J. Physiol. 1997, 501, 313–318. [Google Scholar] [CrossRef]
- Sacco, T.; Bruno, A.; Wanke, E.; Tempia, F. Functional Roles of an ERG Current Isolated in Cerebellar Purkinje Neurons. J. Neurophysiol. 2003, 90, 1817–1828. [Google Scholar] [CrossRef]
- Guasti, L.; Cilia, E.; Crociani, O.; Hofmann, G.; Polvani, S.; Becchetti, A.; Wanke, E.; Tempia, F.; Arcangeli, A. Expression pattern of the ether-a-go-go-related (ERG) family proteins in the adult mouse central nervous system: Evidence for coassembly of dif-ferent subunits. J. Comp. Neurol. 2005, 491, 157–174. [Google Scholar] [CrossRef]
- Pessia, M.; Servettini, I.; Panichi, R.; Guasti, L.; Grassi, S.; Arcangeli, A.; Wanke, E.; Pettorossi, V.E. ERG voltage-gated K+ channels regulate excitability and discharge dynamics of the medial vestibular nucleus neurones. J. Physiol. 2008, 586, 4877–4890. [Google Scholar] [CrossRef]
- Huang, C.-S.; Wang, G.-H.; Tai, C.-H.; Hu, C.-C.; Yang, Y.-C. Antiarrhythmics cure brain arrhythmia: The imperativeness of subthalamic ERG K + channels in parkinsonian discharges. Sci. Adv. 2017, 3, e1602272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanguinetti, M.C.; Tristani-Firouzi, M. hERG potassium channels and cardiac arrhythmia. Nature 2006, 440, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, J.I.; Perry, M.D.; Perrin, M.J.; Mann, S.A.; Ke, Y.; Hill, A.P. hERG K+ channels: Structure, function, and clinical significance. Physiol. Rev. 2012, 92, 1393–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlan, F.; Taccola, G.; Grandolfo, M.; Guasti, L.; Arcangeli, A.; Nistri, A.; Ballerini, L.; Guasti, L. ERG Conductance Expression Modulates the Excitability of Ventral Horn GABAergic Interneurons That Control Rhythmic Oscillations in the Developing Mouse Spinal Cord. J. Neurosci. 2007, 27, 919–928. [Google Scholar] [CrossRef] [Green Version]
- Hagendorf, S.; Fluegge, D.; Engelhardt, C.; Spehr, M. Homeostatic Control of Sensory Output in Basal Vomeronasal Neurons: Activity-Dependent Expression of Ether-à-Go-Go-Related Gene Potassium Channels. J. Neurosci. 2009, 29, 206–221. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Tucker, K.R.; Putzier, I.; Huertas, M.A.; Horn, J.P.; Canavier, C.C.; Levitan, E.S.; Shepard, P.D. Functional characterization of ether-à-go-go-related gene potassium channels in midbrain dopamine neurons—Implications for a role in depolarization block. Eur. J. Neurosci. 2012, 36, 2906–2916. [Google Scholar] [CrossRef] [Green Version]
- Saganich, M.J.; Machado, E.; Rudy, B. Differential Expression of Genes Encoding Subthreshold-Operating Voltage-Gated K+ Channels in Brain. J. Neurosci. 2001, 21, 4609–4624. [Google Scholar] [CrossRef] [Green Version]
- Papa, M.; Boscia, F.; Canitano, A.; Castaldo, P.; Sellitti, S.; Annunziato, L.; Taglialatela, M. Expression pattern of the ether-a-gogo-related (ERG) k+ channel-encoding genes ERG1, ERG2, and ERG3 in the adult rat central nervous system. J. Comp. Neurol. 2003, 466, 119–135. [Google Scholar] [CrossRef]
- Spinelli, W.; Moubarak, I.F.; Parsons, R.W.; Colatsky, T.J. Cellular electrophysiology of WAY-123,398, a new class III antiarrhythmic agent: Specificity of IK block and lack of reverse use dependence in cat ventricular myocytes. Cardiovasc. Res. 1993, 27, 1580–1591. [Google Scholar] [CrossRef]
- Faravelli, L.; Arcangeli, A.; Olivotto, M.; Wanke, E. A HERG-like K+ channel in rat F-11 DRG cell line: Pharmacological identification and biophysical characterization. J. Physiol. 1996, 496, 13–23. [Google Scholar] [CrossRef]
- Williams, J.T.; North, A.R. Opiate-receptor interactions on single locus coeruleus neurones. Mol. Pharmacol. 1984, 26, 489–497. [Google Scholar] [PubMed]
- Cui, E.D.; Strowbridge, B.W. Modulation of Ether-à-Go-Go Related Gene (ERG) Current Governs Intrinsic Persistent Activity in Rodent Neocortical Pyramidal Cells. J. Neurosci. 2017, 38, 423–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noriega, N.C.; Garyfallou, V.T.; Kohama, S.G.; Urbanski, H.F. Glutamate receptor subunit expression in the rhesus macaque locus coeruleus. Brain Res. 2007, 1173, 53–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cockerill, S.L.; Tobin, A.B.; Torrecilla, I.; Willars, G.B.; Standen, N.B.; Mitcheson, J. Modulation of hERG potassium currents in HEK-293 cells by protein kinase C. Evidence for direct phosphorylation of pore forming subunits. J. Physiol. 2007, 581, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Hirdes, W.; Horowitz, L.F.; Hille, B. Muscarinic modulation of erg potassium current. J. Physiol. 2004, 559, 67–84. [Google Scholar] [CrossRef]
- Egan, T.; North, R. Acetylcholine acts on m2-muscarinic receptors to excite rat locus coeruleus neurones. J. Cereb. Blood Flow Metab. 1985, 85, 733–735. [Google Scholar] [CrossRef] [Green Version]
- Freedman, S.B.; Beer, M.S.; Harley, E.A. Muscarinic M1, M2 receptor binding. relationship with functional efficacy. Eur. J. Pharmacol. 1988, 156, 133. [Google Scholar] [CrossRef]
- Niculescu, D.; Hirdes, W.; Hornig, S.; Pongs, O.; Schwarz, J.R. Erg Potassium Currents of Neonatal Mouse Purkinje Cells Exhibit Fast Gating Kinetics and Are Inhibited by mGluR1 Activation. J. Neurosci. 2013, 33, 16729–16740. [Google Scholar] [CrossRef] [Green Version]
- Carter, M.; Yizhar, O.; Chikahisa, S.; Nguyen, H.; Adamantidis, A.; Nishino, S.; Deisseroth, K.; De Lecea, L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 2010, 13, 1526–1533. [Google Scholar] [CrossRef] [Green Version]
- Poe, G.R.; Foote, S.; Eschenko, O.; Johansen, J.P.; Bouret, S.; Aston-Jones, G.; Harley, C.W.; Manahan-Vaughan, D.; Weinshenker, D.; Valentino, R.; et al. Locus coeruleus: A new look at the blue spot. Nat. Rev. Neurosci. 2020, 21, 644–659. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Chow, C.C.; Van Bockstaele, E.J.; Williams, J.T. Frequency-dependent synchrony in locus ceruleus: Role of electrotonic coupling. Proc. Natl. Acad. Sci. USA 2002, 99, 4032–4036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCall, J.G.; Al-Hasani, R.; Siuda, E.R.; Hong, D.Y.; Norris, A.J.; Ford, C.P.; Bruchas, M.R. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron 2015, 87, 605–620. [Google Scholar] [CrossRef] [Green Version]
- Aston-Jones, G.; Cohen, J.D. An Integrative Theory of Locus Coeruleus-Norepinephrine Function: Adaptive Gain and Optimal Performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howells, F.M.; Stein, D.; Russell, V.A. Synergistic tonic and phasic activity of the locus coeruleus norepinephrine (LC-NE) arousal system is required for optimal attentional performance. Metab. Brain Dis. 2012, 27, 267–274. [Google Scholar] [CrossRef]
- Janitzky, K. Impaired Phasic Discharge of Locus Coeruleus Neurons Based on Persistent High Tonic Discharge—A New Hypothesis with Potential Implications for Neurodegenerative Diseases. Front. Neurol. 2020, 11, 371. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Soohoo, S.; Tiwari, P.B.; Piszczek, G.; Brelidze, T.I. Chlorpromazine binding to the PAS domains uncovers the effect of ligand modulation on EAG channel activity. J. Biol. Chem. 2020, 295, 4114–4123. [Google Scholar] [CrossRef] [PubMed]
- Clos, M.; Bunzeck, N.; Sommer, T. Dopamine is a double-edged sword: Dopaminergic modulation enhances memory retrieval performance but impairs metacognition. Neuropsychopharmacology 2019, 44, 555–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Piñol, R.A.; Byrne, P.; Mendelowitz, D. Optogenetic stimulation of locus ceruleus neurons augments inhibitory transmission to parasympathetic cardiac vagal neurons via activation of brainstem α1 and β1 receptors. J. Neurosci. 2014, 34, 6182–6189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.N.; Hofman, N.; Haglund, C.M.; Cascino, G.D.; Wilde, A.; Ackerman, M.J. Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology 2008, 72, 224–231. [Google Scholar] [CrossRef] [Green Version]
- Omichi, C.; Momose, Y.; Kitahara, S. Congenital long QT syndrome presenting with a history of epilepsy: Misdiagnosis or rela-tionship between channelopathies of the heart and brain? Epilepsia 2009, 51, 289–292. [Google Scholar] [CrossRef]
- Kuo, P.H.; Chuang, L.C.; Liu, J.R.; Liu, C.M.; Huang, M.C.; Lin, S.K.; Sunny Sun, H.; Hsieh, M.H.; Hung, H.; Lu, R.B. Identification of novel loci for bipolar I disorder in a multi-stage genome-wide association study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 51, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.A.; Markx, S.; Georgi, B.; Paul, S.M.; Jinks, R.N.; Hoshi, T.; McDonald, A.; First, M.B.; Liu, W.; Benkert, A.R.; et al. A population-based study of KCNH7 p.Arg394His and bipolar spectrum disorder. Hum. Mol. Genet. 2014, 23, 6395–6406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäki-Marttunen, V.; Andreassen, O.A.; Espeseth, T. The role of norepinephrine in the pathophysiology of schizophrenia. Neurosci. Biobehav. Rev. 2020, 118, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Huffaker, S.J.; Chen, J.; Nicodemus, K.K.; Sambataro, F.; Yang, F.; Mattay, V.; Lipska, B.K.; Hyde, T.M.; Song, J.; Rujescu, D.; et al. A primate-specific, brain isoform of KCNH2 affects cortical physiology, cognition, neuronal repolarization and risk of schizophrenia. Nat. Med. 2009, 15, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Atalar, F.; Acuner, T.T.; Cine, N.; Oncu, F.; Yesilbursa, D.; Ozbek, U.; Turkcan, S. Two four-marker haplotypes on 7q36.1 region indicate that the potassium channel gene HERG1 (KCNH2, Kv11.1) is related to schizophrenia: A case control study. Behav. Brain Funct. 2010, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Apud, J.A.; Zhang, F.; Decot, H.; Bigos, K.L.; Weinberger, D.R. Genetic Variation in KCNH2 Associated with Expression in the Brain of a Unique hERG Isoform Modulates Treatment Response in Patients with Schizophrenia. Am. J. Psychiatry 2012, 169, 725–734. [Google Scholar] [CrossRef]
- Hashimoto, R.; Ohi, K.; Yasuda, Y.; Fukumoto, M.; Yamamori, H.; Kamino, K.; Morihara, T.; Iwase, M.; Kazui, H.; Takeda, M. The KCNH2 gene is associated with neurocognition and the risk of schizophrenia. World J. Biol. Psychiatry 2013, 14, 114–120. [Google Scholar] [CrossRef]
- Matschke, L.A.; Komadowski, M.A.; Stöhr, A.; Lee, B.; Henrich, M.T.; Griesbach, M.; Rinné, S.; Geibl, F.F.; Chiu, W.-H.; Koprich, J.B.; et al. Enhanced firing of locus coeruleus neurons and SK channel dysfunction are conserved in distinct models of prodromal Parkinson’s disease. Sci. Rep. 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Lottini, T.; Iorio, J.; Lastraioli, E.; Carraresi, L.; Duranti, C.; Sala, C.; Armenio, M.; Noci, I.; Pillozzi, S.; Arcangeli, A. Transgenic mice overexpressing the LH receptor in the female reproductive system spontaneously develop endometrial tumour masses. Sci. Rep. 2021, 11, 8847. [Google Scholar] [CrossRef]



| WAY-123,398 10 μM (n = 6 Neurons) | Wash-Out (n = 6 Neurons) | ||||
|---|---|---|---|---|---|
| Firing Freq + | ISI + | CV + | Firing Freq + | ISI + | CV + |
| 188 ± 35 * | 62 ± 10 * | 124 ± 14 | 109 ± 14 * | 102 ± 14.4 | 112 ± 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, S.; Delicata, F.; Guasti, L.; Duranti, C.; Haidar, F.M.; Arcangeli, A.; Imbrici, P.; Pessia, M.; Valentino, M.; D’Adamo, M.C. Locus Coeruleus Neurons’ Firing Pattern Is Regulated by ERG Voltage-Gated K+ Channels. Int. J. Mol. Sci. 2022, 23, 15334. https://doi.org/10.3390/ijms232315334
Hasan S, Delicata F, Guasti L, Duranti C, Haidar FM, Arcangeli A, Imbrici P, Pessia M, Valentino M, D’Adamo MC. Locus Coeruleus Neurons’ Firing Pattern Is Regulated by ERG Voltage-Gated K+ Channels. International Journal of Molecular Sciences. 2022; 23(23):15334. https://doi.org/10.3390/ijms232315334
Chicago/Turabian StyleHasan, Sonia, Francis Delicata, Leonardo Guasti, Claudia Duranti, Fatemah Mousalem Haidar, Annarosa Arcangeli, Paola Imbrici, Mauro Pessia, Mario Valentino, and Maria Cristina D’Adamo. 2022. "Locus Coeruleus Neurons’ Firing Pattern Is Regulated by ERG Voltage-Gated K+ Channels" International Journal of Molecular Sciences 23, no. 23: 15334. https://doi.org/10.3390/ijms232315334
APA StyleHasan, S., Delicata, F., Guasti, L., Duranti, C., Haidar, F. M., Arcangeli, A., Imbrici, P., Pessia, M., Valentino, M., & D’Adamo, M. C. (2022). Locus Coeruleus Neurons’ Firing Pattern Is Regulated by ERG Voltage-Gated K+ Channels. International Journal of Molecular Sciences, 23(23), 15334. https://doi.org/10.3390/ijms232315334











