Disease-Modifying Activity of Huperzine A on Alzheimer’s Disease: Evidence from Preclinical Studies on Rodent Models
Abstract
1. Introduction
2. Results
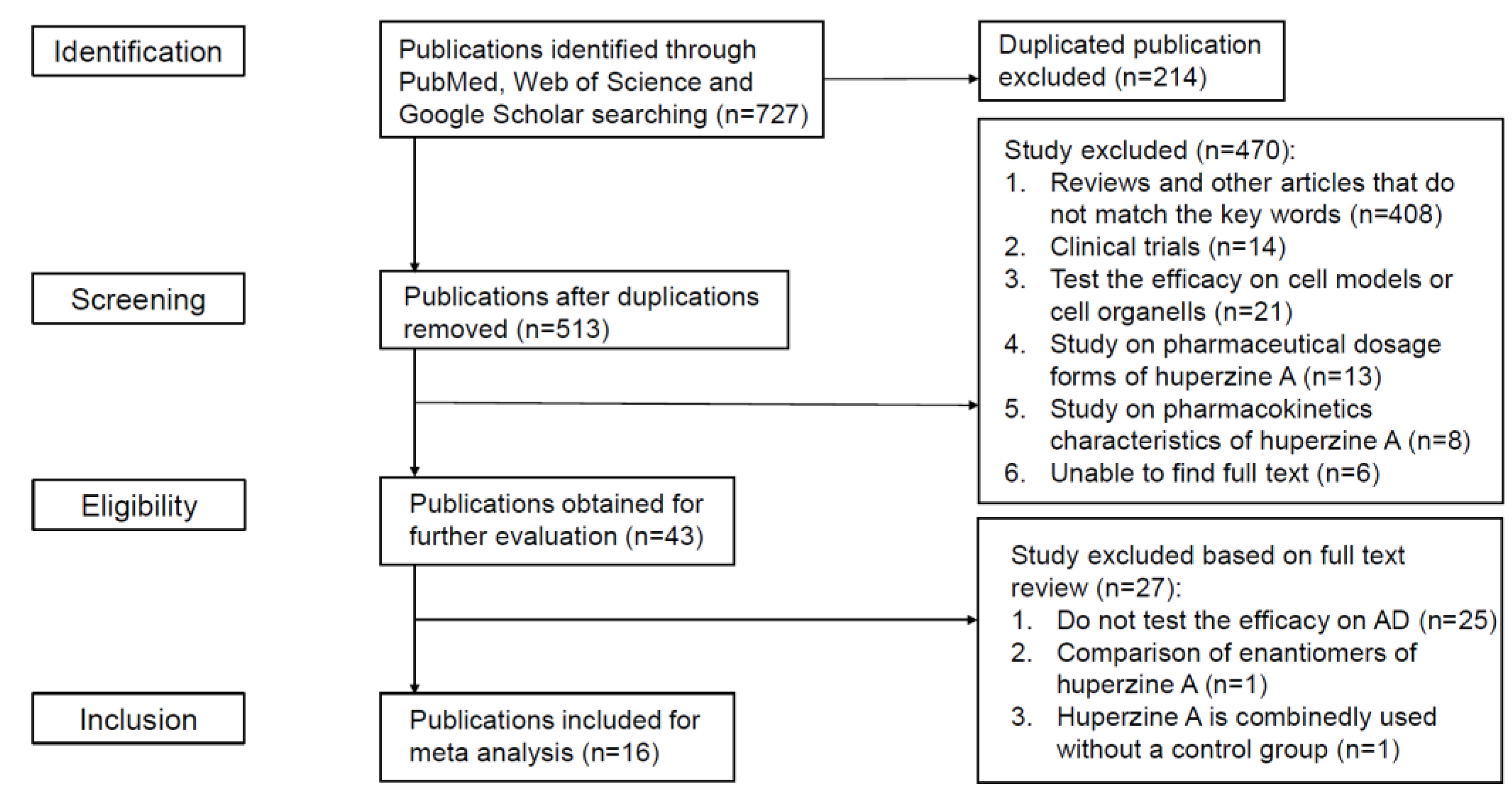
2.1. Study Selection
2.2. Study Characteristics
2.2.1. Animal Models
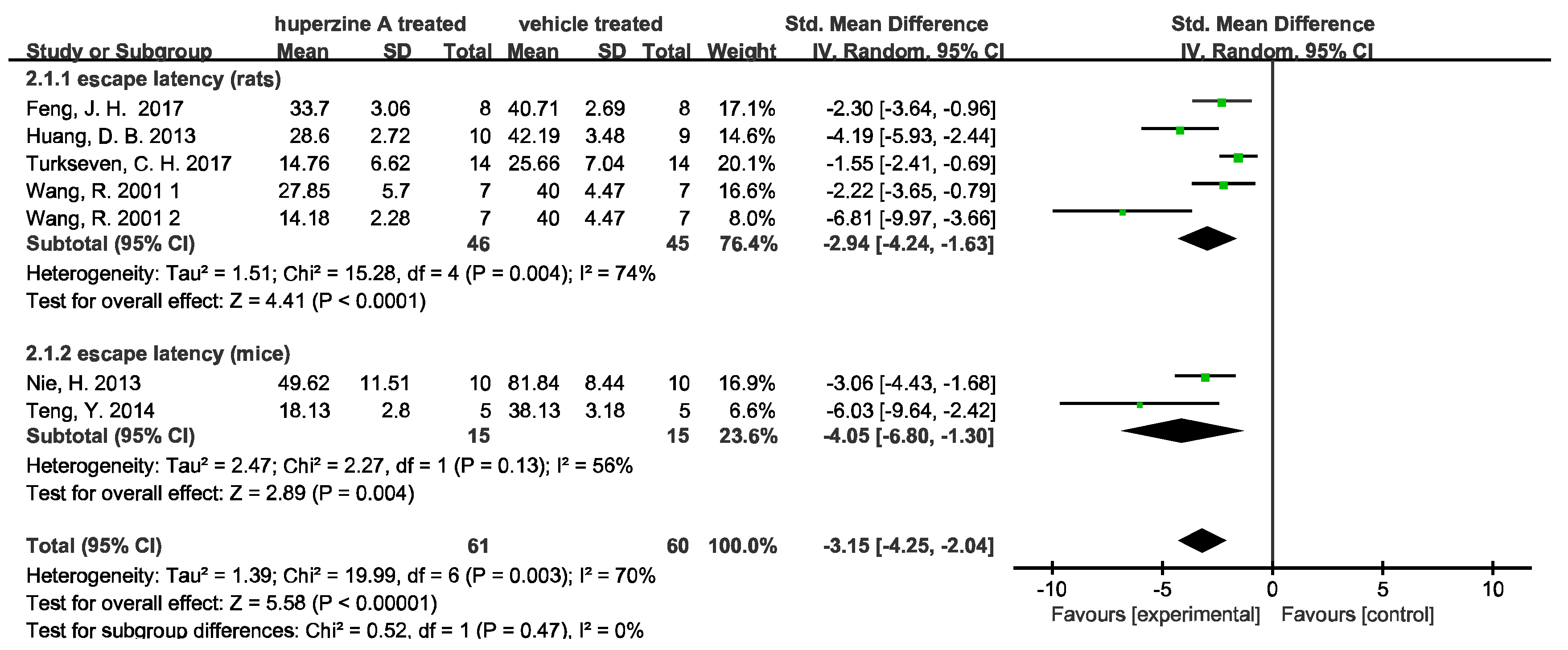
2.2.2. Behavioral Test Analysis
2.2.3. Neuroprotective Mechanisms Analysis
- (1)
- Inhibition of Aβ pathway
- (2)
- Enhancement of cholinergic activity
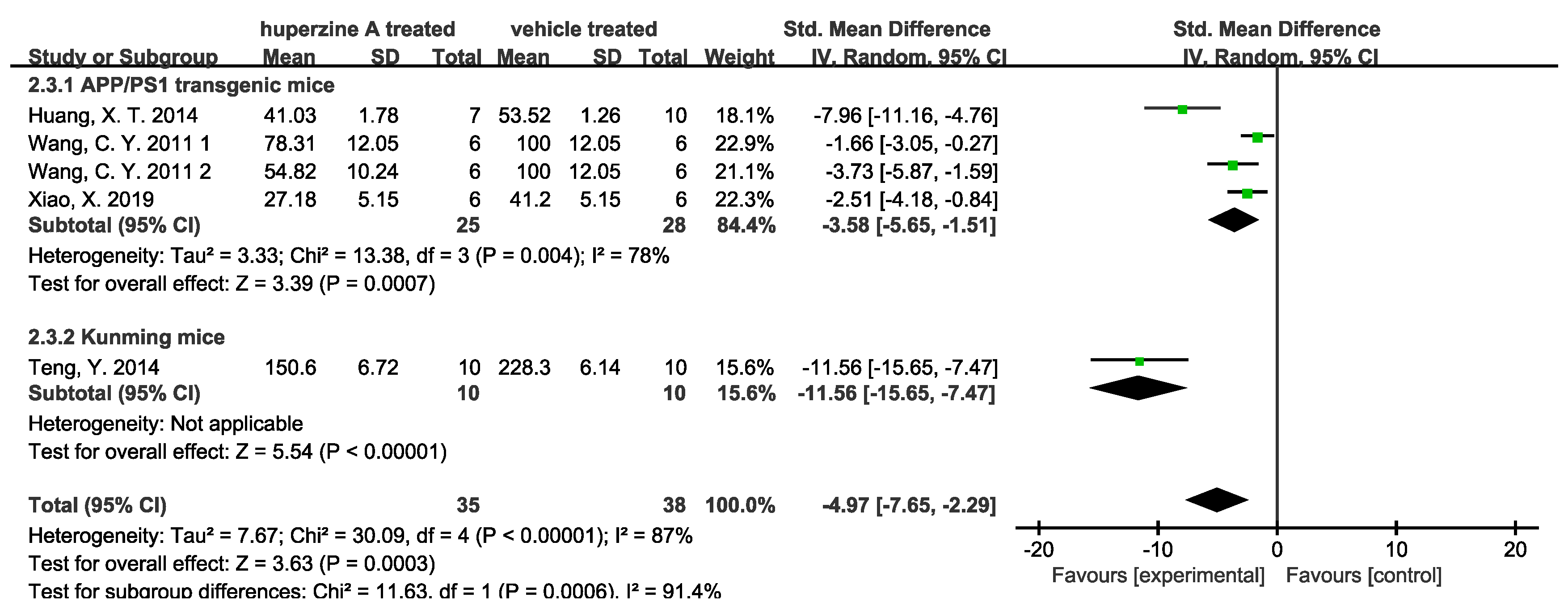
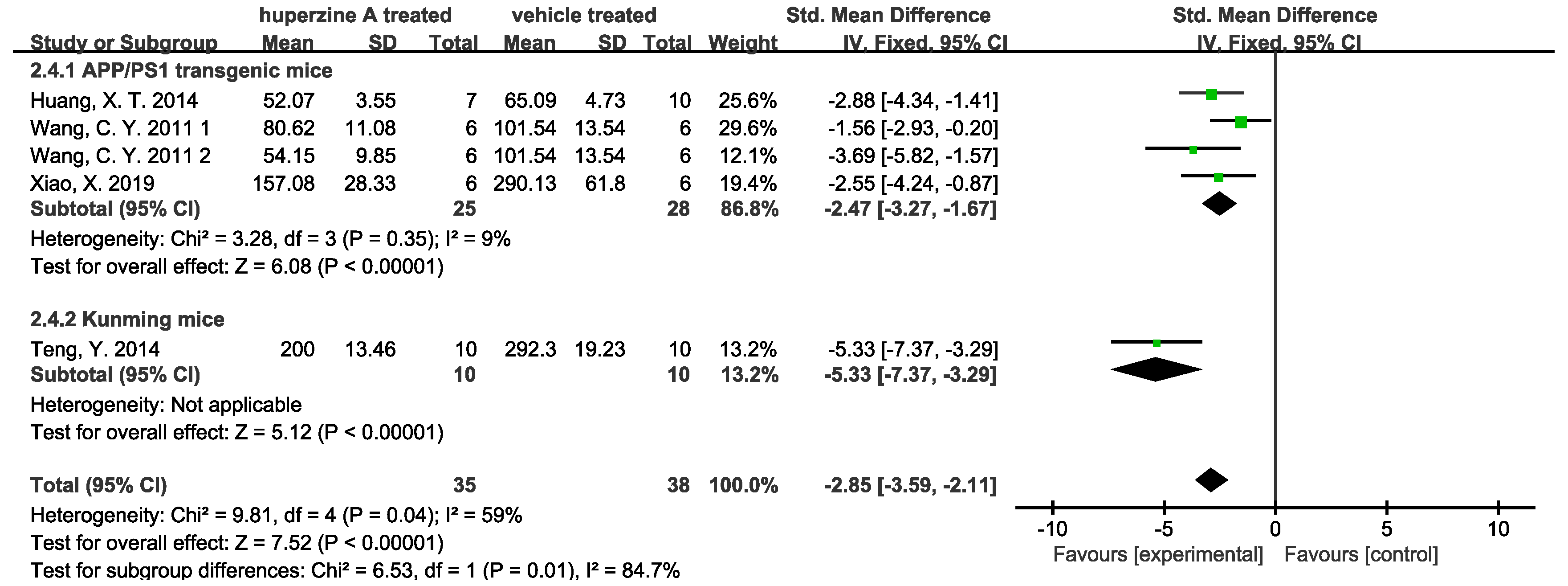
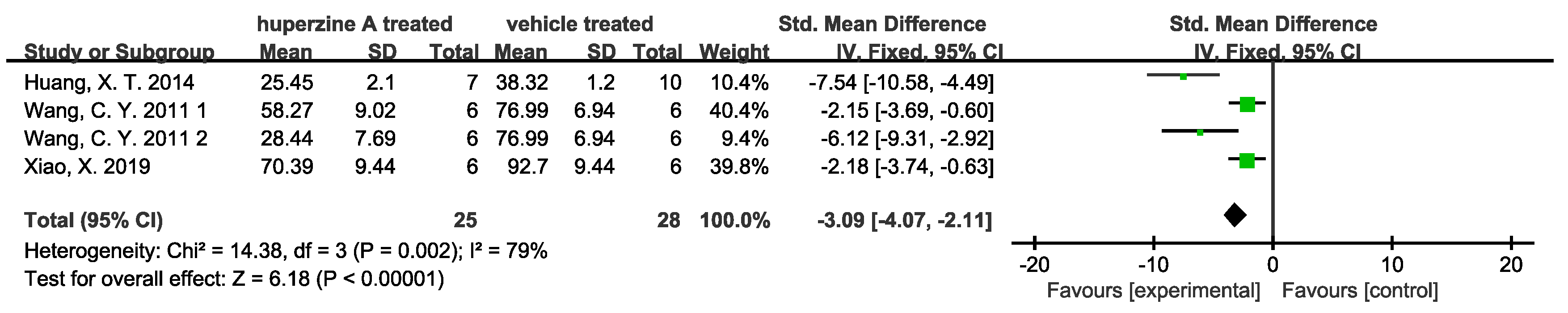


- (3)
- Other effects and mechanisms
2.3. Methodological Quality Assessment
| Study | a | b | c | d | e | f | g | h | i | j | Scores |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cheng, D. H. 1998 [23] | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 3 |
| Feng, J. H. 2017 [24] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
| Huang, D. B. 2013 [25] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
| Huang, X. T. 2014 [26] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
| Huang, Z. S. 2009 [27] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 |
| Liang, Y. Q. 2008 [28] | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 4 |
| Nie, H. 2013 [29] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
| Rispoli, V. 2013 [30] | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 4 |
| Teng, Y. 2014 [31] | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 3 |
| Turkseven, C. H. 2017 [32] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
| Wang, C. Y. 2011 [33] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 |
| Wang, R. 2001 [34] | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 3 |
| Wang, T. 1998 [35] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 6 |
| Wang, Y. 2012 [36] | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 3 |
| Xiao, X. 2019 [37] | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 3 |
| Xu, Z. W. 2006 [38] | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 4 |
3. Discussion
4. Materials and Methods
4.1. Search Strategy
4.2. Inclusion and Exclusion Criteria
- (1)
- Types of animals: laboratory animals of any breed, age, sex, or strain were included.
- (2)
- Types of involvement: the study must contain at least a control group and a Huperzine A administration group. The control group should include physiological saline or other solvent control.
- (3)
- Types of results: any results that reflected the effects of Huperzine A on Alzheimer’s disease models.
- (1)
- No access to the full text.
- (2)
- Reviews, case reports, comments, letters, and clinical trials.
- (3)
- Not testing the effect of Huperzine A on Alzheimer’s disease animal models.
4.3. Data Extraction and Quality Assessment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2017, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Kivipelto, M.; von Strauss, E. Epidemiology of Alzheimer’s disease: Occurrence, determinants, and strategies toward intervention. Dialog.-Clin. Neurosci. 2009, 11, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Onstead, L.; Randle, S.; Price, R.; Smithson, L.; Zwizinski, C.; Dickson, D.W.; Golde, T.; McGowan, E. Aβ40 Inhibits Amyloid Deposition In Vivo. J. Neurosci. 2007, 27, 627–633. [Google Scholar] [CrossRef]
- Ferreira, S.T.; Vieira, M.N.N.; De Felice, F.G. Soluble protein oligomers as emerging toxins in alzheimer’s and other amyloid diseases. IUBMB Life 2007, 59, 332–345. [Google Scholar] [CrossRef]
- Sakono, M.; Zako, T. Amyloid oligomers: Formation and toxicity of Aβ oligomers. FEBS J. 2010, 277, 1348–1358. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Valincius, G.; Heinrich, F.; Budvytyte, R.; Vanderah, D.J.; McGillivray, D.; Sokolov, Y.; Hall, J.E.; Lösche, M. Soluble Amyloid β-Oligomers Affect Dielectric Membrane Properties by Bilayer Insertion and Domain Formation: Implications for Cell Toxicity. Biophys. J. 2008, 95, 4845–4861. [Google Scholar] [CrossRef]
- Magdesian, M.H.; Carvalho, M.M.V.F.; Mendes, F.A.; Saraiva, L.M.; Juliano, M.A.; Juliano, L.; Garcia-Abreu, J.; Ferreira, S.T. Amyloid-β Binds to the Extracellular Cysteine-rich Domain of Frizzled and Inhibits Wnt/β-Catenin Signaling. J. Biol. Chem. 2008, 283, 9359–9368. [Google Scholar] [CrossRef]
- Tseng, B.P.; Green, K.N.; Chan, J.L.; Blurton-Jones, M.; LaFerla, F.M. Aβ inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiol. Aging 2008, 29, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Kosik, K.S.; Joachim, C.L.; Selkoe, D.J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1986, 83, 4044–4048. [Google Scholar] [CrossRef]
- Thies, E.; Mandelkow, E.-M. Missorting of Tau in Neurons Causes Degeneration of Synapses That Can Be Rescued by the Kinase MARK2/Par-1. J. Neurosci. 2007, 27, 2896–2907. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Eisenberg, D.S.; Crowther, R.A. Propagation of Tau Aggregates and Neurodegeneration. Annu. Rev. Neurosci. 2017, 40, 189–210. [Google Scholar] [CrossRef]
- Maphis, N.; Xu, G.; Kokiko-Cochran, O.N.; Jiang, S.; Cardona, A.; Ransohoff, R.M.; Lamb, B.T.; Bhaskar, K. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain 2015, 138, 1738–1755. [Google Scholar] [CrossRef]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-S.; Zhu, Y.-L.; Yu, C.-M.; Zhou, Y.-Z.; Han, Y.-Y.; Wu, F.-W.; Qi, B.-F. The structures of Huperzine A and B, two new alkaloids exhibiting marked anticholinesterase activity. Can. J. Chem. 1986, 64, 837–839. [Google Scholar] [CrossRef]
- Gao, X.; Tang, X.C. Huperzine a attenuates mitochondrial dysfunction in β-amyloid-treated PC12 cells by reducing oxygen free radicals accumulation and improving mitochondrial energy metabolism. J. Neurosci. Res. 2006, 83, 1048–1057. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Zhang, H.Y.; Tang, X.C. Huperzine A attenuates mitochondrial dysfunction after middle cerebral artery occlusion in rats. J. Neurosci. Res. 2008, 86, 2432–2440. [Google Scholar] [CrossRef]
- Xiao, X.Q.; Yang, J.W.; Tang, X.C. Huperzine A protects rat pheochromocytoma cells against hydrogen peroxide-induced injury. Neurosci. Lett. 1999, 275, 73–76. [Google Scholar] [CrossRef]
- Gordon, R.K.; Nigam, S.V.; Weitz, J.A.; Dave, J.R.; Doctor, B.P.; Ved, H.S. The NMDA receptor ion channel: A site for binding of Huperzine A. J. Appl. Toxicol. 2001, 21, S47–S51. [Google Scholar] [CrossRef]
- Tang, L.-L.; Wang, R.; Tang, X.-C. Huperzine A protects SHSY5Y neuroblastoma cells against oxidative stress damage via nerve growth factor production. Eur. J. Pharmacol. 2005, 519, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.H. Comparative Studies of Huperzine A, E2020, and Tacrine on Behavior and Cholinesterase Activities. Pharmacol. Biochem. Behav. 1998, 60, 377–386. [Google Scholar] [CrossRef]
- Feng, J.-H.; Cai, B.-C.; Guo, W.-F.; Wang, M.-Y.; Ma, Y.; Lu, Q.-X. Neuroprotective effects of Tongmai Yizhi Decoction against Alzheimer’s disease through attenuating cyclin-dependent kinase-5 expression. Chin. J. Integr. Med. 2016, 23, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Hu, Z.; Yu, Z. Eleutheroside B or E enhances learning and memory in experimentally aged rats. Neural Regen. Res. 2013, 8, 1103–1112. [Google Scholar] [CrossRef]
- Huang, X.-T.; Qian, Z.-M.; He, X.; Gong, Q.; Wu, K.-C.; Jiang, L.-R.; Lu, L.-N.; Zhu, Z.-J.; Zhang, H.-Y.; Yung, W.-H.; et al. Reducing iron in the brain: A novel pharmacologic mechanism of Huperzine A in the treatment of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.S.; Zhang, S.J.; Xie, H.Y.; Lin, X.; Jiang, W.Z.; Huang, R.B. Key gene and protein changes in the beta-amyloid pathway following Longyanshen poly-saccharides treatment in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2009, 4, 756–762. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Huang, X.T.; Tang, X.C. Huperzine A Reverses Cholinergic and Monoaminergic Dysfunction Induced by Bilateral Nucleus Basalis Magnocellularis Injection of β-Amyloid Peptide (1–40) in Rats. Cell. Mol. Neurobiol. 2007, 28, 87–101. [Google Scholar] [CrossRef]
- Nie, H.; Wang, Z.; Zhao, W.; Lu, J.; Zhang, C.; Lok, K.; Wang, Y.; Shen, H.; Xu, Z.; Yin, M. New nicotinic analogue ZY-1 enhances cognitive functions in a transgenic mice model of Alzheimer’s disease. Neurosci. Lett. 2013, 537, 29–34. [Google Scholar] [CrossRef]
- Rispoli, V.; Ragusa, S.; Nisticò, R.; Marra, R.; Russo, E.; Leo, A.; Felicitá, V.; Rotiroti, D. Huperzine A Restores Cortico-Hippocampal Functional Connectivity after Bilateral AMPA Lesion of the Nucleus Basalis of Meynert. J. Alzheimers Dis. 2013, 35, 833–846. [Google Scholar] [CrossRef]
- Teng, Y.; Zhang, M.-Q.; Wang, W.; Liu, L.-T.; Zhou, L.-M.; Miao, S.-K.; Wan, L.-H. Compound danshen tablet ameliorated aβ25-35-induced spatial memory impairment in mice via rescuing imbalance between cytokines and neurotrophins. BMC Complement. Altern. Med. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Turkseven, C.H.; Buyukakilli, B.; Balli, E.; Yetkin, D.; Erdal, M.E.; Yilmaz, S.G.; Sahin, L. Effects of Huperzin-A on the Beta-amyloid accumulation in the brain and skeletal muscle cells of a rat model for Alzheimer’s disease. Life Sci. 2017, 184, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Zheng, W.; Wang, T.; Xie, J.-W.; Wang, S.-L.; Zhao, B.-L.; Teng, W.-P.; Wang, Z.-Y. Huperzine A Activates Wnt/β-Catenin Signaling and Enhances the Nonamyloidogenic Pathway in an Alzheimer Transgenic Mouse Model. Neuropsychopharmacology 2011, 36, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, H.Y.; Tang, X.C. Huperzine A attenuates cognitive dysfunction and neuronal degeneration caused by β-amyloid protein-(1–40) in rat. Eur. J. Pharmacol. 2001, 421, 149–156. [Google Scholar] [CrossRef]
- Wang, T.; Tang, X.C. Reversal of scopolamine-induced deficits in radial maze performance by (−)-Huperzine A: Comparison with E2020 and tacrine. Eur. J. Pharmacol. 1998, 349, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, X.C.; Zhang, H.Y. Huperzine a alleviates synaptic deficits and modulates amyloidogenic and nonamyloidogenic pathways in APPswe/PS1dE9 transgenic mice. J. Neurosci. Res. 2011, 90, 508–517. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, Q.; Zhu, X.; Wang, Y. ABAD/17β-HSD10 reduction contributes to the protective mechanism of Huperzine a on the cerebral mitochondrial function in APP/PS1 mice. Neurobiol. Aging 2019, 81, 77–87. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, H.; Law, S.L.; So, D.D.; Han, Y.; Xue, H. Effects of a memory enhancing peptide on cognitive abilities of brain-lesioned mice: Additivity with Huperzine A and relative potency to tacrine. J. Pept. Sci. 2005, 12, 72–78. [Google Scholar] [CrossRef]
- Sandberg, K.; Schnaar, R.L.; McKinney, M.; Hanin, I.; Fisher, A.; Coyle, J.T. AF64A: An Active Site Directed Irreversible Inhibitor of Choline Acetyltransferase. J. Neurochem. 1985, 44, 439–445. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Ikonomovic, M.D.; Styren, S.D.; Beckett, L.; Wisniewski, S.; Bennett, D.A.; Cochran, E.J.; Kordower, J.H.; Mufson, E.J. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann. Neurol. 2002, 51, 145–155. [Google Scholar] [CrossRef]
- Wang, R.; Yan, H.; Tang, X.-C. Progress in studies of Huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine1. Acta Pharmacol. Sin. 2006, 27, 1–26. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Rafii, M.S.; Walsh, S.; Little, J.T.; Behan, K.; Reynolds, B.; Ward, C.; Jin, S.; Thomas, R.; Aisen, P.S. For the Alzheimer’s Disease Cooperative Study A phase II trial of Huperzine A in mild to moderate Alzheimer disease. Neurology 2011, 76, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, Y.; Tian, J.; Liu, J.-P. Huperzine A for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. PLOS ONE 2013, 8, e74916. [Google Scholar] [CrossRef] [PubMed]
- Desilets, A.R.; Gickas, J.J.; Dunican, K.C. Role of Huperzine a in the Treatment of Alzheimer’s Disease. Ann. Pharmacother. 2009, 43, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Ghassab-Abdollahi, N.; Mobasseri, K.; Ahmadabad, A.D.; Nadrian, H.; Mirghafourvand, M. The effects of Huperzine A on dementia and mild cognitive impairment: An overview of systematic reviews. Phytotherapy Res. 2021, 35, 4971–4987. [Google Scholar] [CrossRef]
- Silva, D.F.; Esteves, A.R.; Oliveira, C.; Cardoso, S.M. Mitochondria: The Common Upstream Driver of Amyloid-β and Tau Pathology in Alzheimers Disease. Curr. Alzheimer Res. 2011, 8, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ye, C.-Y.; Huang, X.-T.; Tang, X.-C.; Zhang, H.-Y. Decreased Accumulation of Subcellular Amyloid-β with Improved Mitochondrial Function Mediates the Neuroprotective Effect of Huperzine A. J. Alzheimer’s Dis. 2012, 31, 131–142. [Google Scholar] [CrossRef]
- Zhang, H.-Y. New insights into Huperzine A for the treatment of Alzheimer’s disease. Acta Pharmacol. Sin. 2012, 33, 1170–1175. [Google Scholar] [CrossRef]


| Article | Animal Data | Administration of Hup A | Methods |
|---|---|---|---|
| Cheng, D. H. 1998 [23] | Sprague-Dawley rats (male, 280–350 g); i.c.v. AF64A 3 nmol/side | dosage: 0.3, 0.5, 0.8 mg/kg/day; ad: i.g.; duration: 3 weeks | behavioral test (radial maze test); biochemical experiments (choline acetyltransferase (ChAT) activity, acetylcholinesterase (AChE) activity, cholinesterase (ChE) activity) |
| Feng, J. H. 2017 [24] | Sprague-Dawley (SD) rats (male and female, 3–4 months old, 250–280 g); Aβ40 peptide 10 μg injection into left hippocampal CA1 area | dosage: 40.5 μg/kg/day ad: i.g.; duration: 4 weeks | behavioral test (Morris water maze); hematoxylin-eosin staining (neuronal morphology); western blot (Cdk 5 protein); RT-qPCR (cdk5 mRNA); |
| Huang, D. B. 2013 [25] | Wistar rats (male, 400–550 g, 10 months old); i.c.v. quinoline acid 150 nmol | dosage: 0.3 mg/kg/day; ad: i.p.; duration: 34 days; | behavioral test (morris water maze); biochemical experiments (acetylcholine and choline level, cholinesterase activity); tissue observation (hematoxylin and eosin staining of hippocampus) |
| Huang, X. T. 2014 [26] | APPswe/PS1dE9 transgenic mice (2 months old) | dosage: 0.1 mg/kg/day; ad: i.g.; duration: 6 months | Immunohistochemistry (tau plaque); Aβ quantification; western blot (metal transporter with or without iron-responsive element); brain iron measurement |
| Huang, Z. S. 2009 [27] | senescence-accelerated mouse prone/8 (SAMP8); normal control: senescence-accelerated mouse resistant 1 (SAMR1) | dosage: 0.02 mg/kg/day; ad: i.g.; duration: 50 days; | immunohistochemistry (Aβ expression in the cortex and hippocampus); RT-PCR (mRNA levels of BACE and APP) |
| Liang, Y. Q. 2008 [28] | Sprague-Dawley rats (male, 250–300 g) 10 μg Aβ injection into nucleus basalis magnocellularis (NBM) | dosage: 012, 0.18 mg/kg; ad: i.g.; duration: 21 days | HPLC (ACh level detection, monoamine level detection); immunohistochemistry (detemine Aβ deposition) |
| Nie, H. 2013 [29] | APPswe/PS1dE9 mice with C57BL/6J background (male and female, 30–35 g) | dosage: 0.25 mg/kg twice a day; ad: i.g.; duration: 4 weeks | behavioral test (independent activity test, Morris water maze); |
| Rispoli, V. 2013 [30] | Wistar rats (male, 200–250 g, 3 months old); i.c. excitotoxic AMPA injection into NBM | dosage: 0.5 mg/kg; ad: i.p.; duration: 3 weeks | electroencephalogram (EEG) (theta rhythm); behavioral test (Morris water maze; Object recognition test (ORT)) |
| Teng, Y. 2014 [31] | Kunming mice (male, 8–10 weeks old, 38–42 g) i.c.v. Aβ 25–35 10 μg induced AD model | dosage: 0.4 mg/kg; ad: i.g.; duration: 14 days | behavioral test (Morris water maze); ELISA [Aβ42, (ChAT), IL-6, TNF-α,receptor of activated protein kinase C1 (RACK1) and brain-derived neurotrophic factor (BDNF)]; Brain histopathology (neuronal damage); RT-PCR (β-actin, IL-6, RACK1 and BDNF); |
| Turkseven, C. H. 2017 [32] | Sprague-Dawley rats (female, 13 weeks old, 180–200 g) 100 mg/kg/day d-galactose i.p. | dosage: 0.1 mg/kg/day; ad: i.p.; duration: 3 weeks | behavioral test (Morris water maze, open field test); electro-biophysical tests (extensor digitorum longus muscle contractile properties); immunohistochemistry (Aβ level); RT-PCR, ΔΔCT-PCR (several miRNA level) |
| Wang, C. Y. 2011 [33] | APP/PS1 (APPswe/PSEN1dE9) transgenic mice; wild-type C57BL/6 mice (male, 6 months old) | dosage: 167, 500 μg/kg; ad: nasal; duration: 1 months | BrdU Staining (neurogenesis); Evan’s Blue Leakage Assay; AChE, ChAT, GSH-PX, CAT Activity; Immunohistochemistry and Confocal Laser Scanning Microscopy (distribution of Aβ in brain); RT-PCR (GAPDH, APP mRNA level); Sandwich Elisa |
| Wang, R. 2001 [34] | Sprague-Dawley rats (male, 220–280 g) 800 pmol Aβ 40 i.c.v. at day 5, 8, 12 | dosage: 0.1, 0.2 mg/kg/day; ad: i.p.; duration: 12 days | neuron morphology (HE staining); TUNEL staining (detect apoptosis); immunohistochemistry (detect plaque); behavioral test (Morris water maze); measurement of ChAT activity |
| Wang, T. 1998 [35] | Sprague-Dawley rats (male, 220–270 g) scopolamine 0.15 mg/kg i.p. induced memory impairment | dosage: 0.1, 0.2, 0.3, 0.4, 0.5 mg/kg; ad: p.o.; duration: 30 min before test; dosage: 0.05, 0.1, 0.2, 0.4 mg/kg; ad: i.p.; duration: 30 min before test | radial arm maze task; |
| Wang, Y. 2012 [36] | APPswe/PS1dE9 transgenic mice (7 months old) high iron diet (2.5% carbonyl iron) | dosage: 0.1 mg/kg; ad: i.g.; duration: 5 months | Golgi staining (observe dendritic spine density); Thioflavin S Staining (assess the deposition of fibrous amyloid); Western blotting; Real-Time PCR Analysis; |
| Xiao, X. 2019 [37] | APPswe/PS1dE9 transgenic mice (male, 2 months old, 20–30 g) | dosage: 0.1 mg/kg; ad: i.p. duration: 6 months | AChE activity measurement; cell viability; mitochondria activity (ROS level detection, ATP level detection, ABAD immunohistochemistry, cytochrome c release; western blot); measure the level and disposition of Aβ (thioflavin S immunostaining, ELISA quantity); |
| Xu, Z. W. 2006 [38] | Kunming mice (male, 4 weeks old, 16–25 g) 4 mg/kg scopolamine-induced brain lesion | dosage: 0.1, 0.2, 0.3 mg/kg; ad: i.p.; duration: 30 min before test | behavioral test (step-through passive avoidance test, Y-water maze test) |
| Model | Mechanism | Main Use | Disadvantages |
|---|---|---|---|
| AF64A induced | A cholinergic neuron-specific neurotoxin and an irreversible inhibitor of choline acetyltransferase | Studying of cholinergic damage as a similar characteristic of AD | Fail to reflect the typical pathological changes of AD senile plaques and NFTs |
| AMPA induced | Cholinergic neuron toxicity | Simulating cholinergic damage of AD | Fail to reflect the typical pathological changes of AD |
| quinolinic acid induced | Inducing tau phosphorylation | Simulating tau accumulation of AD | Acute toxicity model cannot simulate the progress of AD. |
| Aβ induced | Aβ peptide-induced neurotoxicity | Studying of Aβ accumulation and toxicity of AD | A large amount of Aβ accumulates locally at the injection site Instead of spreading to the brain |
| D-galactose induced | D-galactose induced glucose metabolism disorders and increasing free radical level | Studying mechanism of age-related diseases | The mechanism is unclear and cannot reflect pathological features well |
| scopolamine induced | Non-selective cholinergic receptor blocker | Simulating cholinergic damage of AD | Lack of the characteristics necessary to study the pathophysiology of AD |
| APPswe/PS1dE9 transgenic mice | Aβ plaques accumulation | Studying the role of APP and PSEN1 in the development of AD | Lack of neurofibrillary tangles and cost highly |
| Senescence-accelerated mouse prone/8 (SAMP8) | Naturally occurring rapid aging | Studying mechanism of age-related memory defect | The short life is not suitable for long cycle experiment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.-P.; Chen, J.-Y.; Lu, J.-H. Disease-Modifying Activity of Huperzine A on Alzheimer’s Disease: Evidence from Preclinical Studies on Rodent Models. Int. J. Mol. Sci. 2022, 23, 15238. https://doi.org/10.3390/ijms232315238
Yan Y-P, Chen J-Y, Lu J-H. Disease-Modifying Activity of Huperzine A on Alzheimer’s Disease: Evidence from Preclinical Studies on Rodent Models. International Journal of Molecular Sciences. 2022; 23(23):15238. https://doi.org/10.3390/ijms232315238
Chicago/Turabian StyleYan, Ye-Piao, Jia-Yue Chen, and Jia-Hong Lu. 2022. "Disease-Modifying Activity of Huperzine A on Alzheimer’s Disease: Evidence from Preclinical Studies on Rodent Models" International Journal of Molecular Sciences 23, no. 23: 15238. https://doi.org/10.3390/ijms232315238
APA StyleYan, Y.-P., Chen, J.-Y., & Lu, J.-H. (2022). Disease-Modifying Activity of Huperzine A on Alzheimer’s Disease: Evidence from Preclinical Studies on Rodent Models. International Journal of Molecular Sciences, 23(23), 15238. https://doi.org/10.3390/ijms232315238







