Red Cell Microparticles Suppress Hematoma Growth Following Intracerebral Hemorrhage in Chronic Nicotine-Exposed Rats
Abstract
1. Introduction
2. Results
2.1. Physiological Parameters
2.2. Stages of the Estrous Cycle
2.3. RMP Treatment Limited Hematoma Growth Following sICH in Nicotine-Exposed Rats of Both Sexes
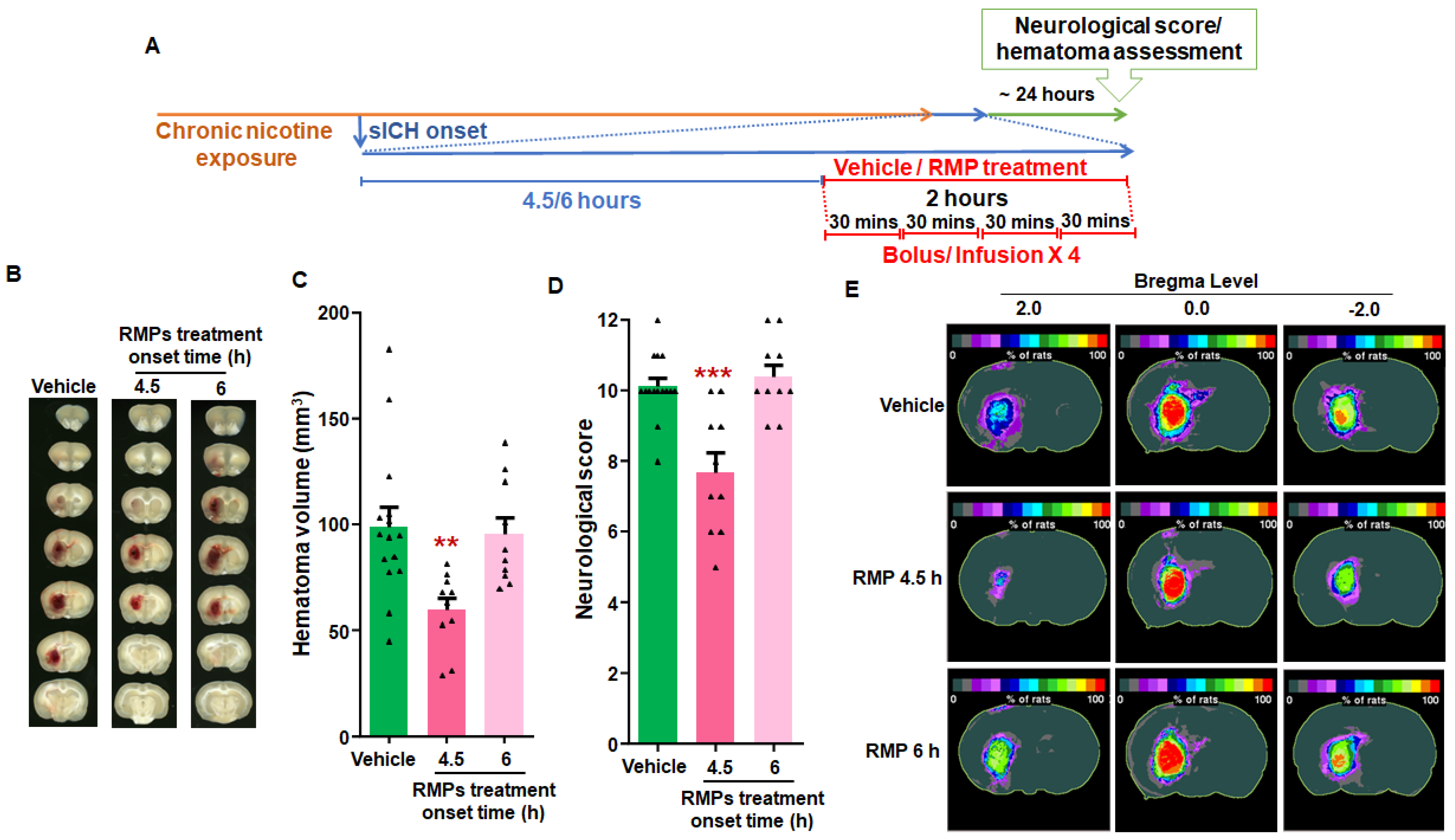
2.4. RMP Therapy Limited Hematoma Expansion When Administered as Late as 4.5 h Post-sICH in Nicotine-Exposed Rats of Both Sexes
2.5. The Effect of Treatment on Hematoma Growth in Male and Female Rats
3. Discussion
4. Methods
4.1. Animals
4.2. Nicotine Administration
4.3. Production of RMPs
4.4. Monitoring of the Stage of the Estrous Cycle
4.5. sICH Induction
4.6. Determination of Neurological Score
4.7. Animal Perfusion and Brain Isolation
4.8. Assessment of Hematoma Volume
4.9. Experimental Protocol
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.M.; Broderick, J.; Hennerici, M.; Brun, N.C.; Diringer, M.N.; Mayer, S.A.; Begtrup, K.; Steiner, T.; Recombinant Activated Factor V I I Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006, 66, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Rehni, A.K.; Dave, K.R. Tobacco Use: A Major Risk Factor of Intracerebral Hemorrhage. J. Stroke 2021, 23, 37–50. [Google Scholar] [CrossRef]
- Hauer, A.J.; Ruigrok, Y.M.; Algra, A.; van Dijk, E.J.; Koudstaal, P.J.; Luijckx, G.J.; Nederkoorn, P.J.; van Oostenbrugge, R.J.; Visser, M.C.; Wermer, M.J.; et al. Age-Specific Vascular Risk Factor Profiles According to Stroke Subtype. J. Am. Heart Assoc. 2017, 6, e005090. [Google Scholar] [CrossRef]
- Faigle, R.; Marsh, E.B.; Llinas, R.H.; Urrutia, V.C.; Gottesman, R.F. Race-Specific Predictors of Mortality in Intracerebral Hemorrhage: Differential Impacts of Intraventricular Hemorrhage and Age Among Blacks and Whites. J. Am. Heart Assoc. 2016, 5, e003540. [Google Scholar] [CrossRef]
- Rehni, A.K.; Cho, S.; Zhang, Z.; Zhao, W.; Raval, A.P.; Perez-Pinzon, M.A.; Dave, K.R. Chronic Nicotine Exposure Increases Hematoma Expansion following Collagenase-Induced Intracerebral Hemorrhage in Rats. Biomolecules 2022, 12, 621. [Google Scholar] [CrossRef]
- Lord, A.S.; Gilmore, E.; Choi, H.A.; Mayer, S.A.; Vista-Ich Collaboration. Time course and predictors of neurological deterioration after intracerebral hemorrhage. Stroke 2015, 46, 647–652. [Google Scholar] [CrossRef]
- Staykov, D.; Huttner, H.B.; Kohrmann, M.; Bardutzky, J.; Schellinger, P.D. Novel approaches to the treatment of intracerebral haemorrhage. Int. J. Stroke 2010, 5, 457–465. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Aviv, R.I.; Demchuk, A.M.; Hill, M.D.; Thorpe, K.E.; Khoury, J.C.; Sucharew, H.J.; Al-Ajlan, F.; Butcher, K.; Dowlatshahi, D.; et al. Effect of Recombinant Activated Coagulation Factor VII on Hemorrhage Expansion Among Patients With Spot Sign-Positive Acute Intracerebral Hemorrhage: The SPOTLIGHT and STOP-IT Randomized Clinical Trials. JAMA Neurol. 2019, 76, 1493–1501. [Google Scholar] [CrossRef]
- Baharoglu, M.I.; Cordonnier, C.; Al-Shahi Salman, R.; de Gans, K.; Koopman, M.M.; Brand, A.; Majoie, C.B.; Beenen, L.F.; Marquering, H.A.; Vermeulen, M.; et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): A randomised, open-label, phase 3 trial. Lancet 2016, 387, 2605–2613. [Google Scholar] [CrossRef]
- Dowlatshahi, D.; Butcher, K.S.; Asdaghi, N.; Nahirniak, S.; Bernbaum, M.L.; Giulivi, A.; Wasserman, J.K.; Poon, M.C.; Coutts, S.B.; Canadian, P.C.C.R.I. Poor prognosis in warfarin-associated intracranial hemorrhage despite anticoagulation reversal. Stroke 2012, 43, 1812–1817. [Google Scholar] [CrossRef]
- Sonni, S.; Lioutas, V.A.; Selim, M.H. New avenues for treatment of intracranial hemorrhage. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 277. [Google Scholar] [CrossRef][Green Version]
- Jy, W.; Johansen, M.E.; Bidot, C., Jr.; Horstman, L.L.; Ahn, Y.S. Red cell-derived microparticles (RMP) as haemostatic agent. Thromb. Haemost. 2013, 110, 751–760. [Google Scholar] [CrossRef]
- Berckmans, R.J.; Nieuwland, R.; Boing, A.N.; Romijn, F.P.; Hack, C.E.; Sturk, A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb. Haemost. 2001, 85, 639–646. [Google Scholar]
- Chang, C.P.; Zhao, J.; Wiedmer, T.; Sims, P.J. Contribution of platelet microparticle formation and granule secretion to the transmembrane migration of phosphatidylserine. J. Biol. Chem. 1993, 268, 7171–7178. [Google Scholar] [CrossRef]
- Rehni, A.K.; Cho, S.; Quero, H.N.; Shukla, V.; Zhang, Z.; Dong, C.; Zhao, W.; Perez-Pinzon, M.A.; Koch, S.; Jy, W.; et al. Red Blood Cell Microparticles Limit Hematoma Growth in Intracerebral Hemorrhage. Stroke 2022, 53, 3182–3191. [Google Scholar] [CrossRef]
- Woodward, M.; Lam, T.H.; Barzi, F.; Patel, A.; Gu, D.; Rodgers, A.; Suh, I.; Asia Pacific Cohort Studies, C. Smoking, quitting, and the risk of cardiovascular disease among women and men in the Asia-Pacific region. Int. J. Epidemiol. 2005, 34, 1036–1045. [Google Scholar] [CrossRef]
- Shah, R.S.; Cole, J.W. Smoking and stroke: The more you smoke the more you stroke. Expert Rev. Cardiovasc. Ther. 2010, 8, 917–932. [Google Scholar] [CrossRef]
- MacLellan, C.L.; Silasi, G.; Poon, C.C.; Edmundson, C.L.; Buist, R.; Peeling, J.; Colbourne, F. Intracerebral hemorrhage models in rat: Comparing collagenase to blood infusion. J. Cereb. Blood Flow Metab. 2008, 28, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kang, K.; Kang, J.; Koo, J.; Kim, D.H.; Kim, B.J.; Kim, W.J.; Kim, E.G.; Kim, J.G.; Kim, J.M.; et al. Executive Summary of Stroke Statistics in Korea 2018: A Report from the Epidemiology Research Council of the Korean Stroke Society. J. Stroke 2019, 21, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.S.; Heeley, E.; Huang, Y.; Wang, J.; Stapf, C.; Delcourt, C.; Lindley, R.; Robinson, T.; Lavados, P.; Neal, B.; et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N. Engl. J. Med. 2013, 368, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Institute of Health Metrics. Global Burden of Disease [database]. Washington DC. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 17 July 2021).
- National Center for Health Statistics. Electronic Cigarette Use Among U.S. Adults. Available online: https://www.cdc.gov/nchs/products/databriefs/db365.htm (accessed on 13 January 2022).
- Benowitz, N.L.; Fraiman, J.B. Cardiovascular effects of electronic cigarettes. Nat. Rev. Cardiol. 2017, 14, 447–456. [Google Scholar] [CrossRef]
- Kaisar, M.A.; Villalba, H.; Prasad, S.; Liles, T.; Sifat, A.E.; Sajja, R.K.; Abbruscato, T.J.; Cucullo, L. Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: Is Metformin a viable countermeasure? Redox Biol. 2017, 13, 353–362. [Google Scholar] [CrossRef]
- Lippi, G.; Favaloro, E.J. An Update on Biological and Clinical Associations between E-Cigarettes and Myocardial Infarction. Semin. Thromb. Hemost. 2020, 46, 512–514. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Broderick, J.P.; Brott, T.G.; Duldner, J.E.; Tomsick, T.; Huster, G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 1993, 24, 987–993. [Google Scholar] [CrossRef]
- Elkhatib, T.H.M.; Shehta, N.; Bessar, A.A. Hematoma Expansion Predictors: Laboratory and Radiological Risk Factors in Patients with Acute Intracerebral Hemorrhage: A Prospective Observational Study. J. Stroke Cerebrovasc. Dis. 2019, 28, 2177–2186. [Google Scholar] [CrossRef]
- Jy, W.; Rehni, A.K.; Bidot, C., Jr.; Navarro-Quero, H.; Haase, C.R.; Koch, S.; Ahn, Y.S.; Dave, K.R. Pharmacokinetics of Human Red Blood Cell Microparticles Prepared Using High-Pressure Extrusion Method. Front. Pharmacol. 2018, 9, 599. [Google Scholar] [CrossRef]
- Fisher, M.; Feuerstein, G.; Howells, D.W.; Hurn, P.D.; Kent, T.A.; Savitz, S.I.; Lo, E.H.; Group, S. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009, 40, 2244–2250. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Fitzgerald, G.A.; Wilson, M.; Zhang, Q. Nicotine effects on eicosanoid formation and hemostatic function: Comparison of transdermal nicotine and cigarette smoking. J. Am. Coll. Cardiol. 1993, 22, 1159–1167. [Google Scholar] [CrossRef]
- Hawkins, R.I. Smoking, platelets and thrombosis. Nature 1972, 236, 450–452. [Google Scholar] [CrossRef]
- Levine, P.H. An acute effect of cigarette smoking on platelet function. A possible link between smoking and arterial thrombosis. Circulation 1973, 48, 619–623. [Google Scholar] [CrossRef]
- Renaud, S.; Blache, D.; Dumont, E.; Thevenon, C.; Wissendanger, T. Platelet function after cigarette smoking in relation to nicotine and carbon monoxide. Clin. Pharmacol. Ther. 1984, 36, 389–395. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, M.H.; Choi, K.C.; Lee, K.; Kim, K.S.; Shim, S.M. Oxidative Stress Induced by Cigarette Smoke Extracts in Human Brain Cells (T98G) and Human Brain Microvascular Endothelial Cells (HBMEC) in Mono- and Co-Culture. J. Toxicol. Environ. Health A 2015, 78, 1019–1027. [Google Scholar] [CrossRef]
- Singh, J.M.; Singh, M.D. Alkaloids of tobacco and blood coagulation: Effect of nicotine on thrombin and fibrinogen. Clin. Toxicol. 1975, 8, 43–52. [Google Scholar] [CrossRef]
- Rehni, A.K.; Shukla, V.; Navarro Quero, H.; Bidot, C., Jr.; Haase, C.R.; Crane, E.A.A.; Patel, S.G.; Koch, S.; Ahn, Y.S.; Jy, W.; et al. Preclinical Evaluation of Safety and Biodistribution of Red Cell Microparticles: A Novel Hemostatic Agent. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 474–483. [Google Scholar] [CrossRef]
- Kazui, S.; Naritomi, H.; Yamamoto, H.; Sawada, T.; Yamaguchi, T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke 1996, 27, 1783–1787. [Google Scholar] [CrossRef]
- Mayer, S.A.; Brun, N.C.; Begtrup, K.; Broderick, J.; Davis, S.; Diringer, M.N.; Skolnick, B.E.; Steiner, T.; Investigators, F.T. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N. Engl. J. Med. 2008, 358, 2127–2137. [Google Scholar] [CrossRef]
- Naganuma, M.; Toyoda, K.; Nonogi, H.; Yokota, C.; Koga, M.; Yokoyama, H.; Okayama, A.; Naritomi, H.; Minematsu, K. Early hospital arrival improves outcome at discharge in ischemic but not hemorrhagic stroke: A prospective multicenter study. Cerebrovasc. Dis. 2009, 28, 33–38. [Google Scholar] [CrossRef]
- Shigematsu, K.; Watanabe, Y.; Nakano, H.; Kyoto Stroke Registry, C. Lower hazard ratio for death in women with cerebral hemorrhage. Acta Neurol. Scand. 2015, 132, 59–64. [Google Scholar] [CrossRef]
- Marini, S.; Morotti, A.; Ayres, A.M.; Crawford, K.; Kourkoulis, C.E.; Lena, U.K.; Gurol, E.M.; Viswanathan, A.; Goldstein, J.N.; Greenberg, S.M.; et al. Sex differences in intracerebral hemorrhage expansion and mortality. J. Neurol. Sci. 2017, 379, 112–116. [Google Scholar] [CrossRef]
- James, M.L.; Cox, M.; Xian, Y.; Smith, E.E.; Bhatt, D.L.; Schulte, P.J.; Hernandez, A.; Fonarow, G.C.; Schwamm, L.H. Sex and Age Interactions and Differences in Outcomes After Intracerebral Hemorrhage. J. Womens Health 2017, 26, 380–388. [Google Scholar] [CrossRef]
- Ganti, L.; Jain, A.; Yerragondu, N.; Jain, M.; Bellolio, M.F.; Gilmore, R.M.; Rabinstein, A. Female gender remains an independent risk factor for poor outcome after acute nontraumatic intracerebral hemorrhage. Neurol. Res. Int. 2013, 2013, 219097. [Google Scholar] [CrossRef]
- Nakamura, T.; Xi, G.; Hua, Y.; Schallert, T.; Hoff, J.T.; Keep, R.F. Intracerebral hemorrhage in mice: Model characterization and application for genetically modified mice. J. Cereb. Blood Flow Metab. 2004, 24, 487–494. [Google Scholar] [CrossRef]
- Schwartz, J.B. The influence of sex on pharmacokinetics. Clin. Pharm. 2003, 42, 107–121. [Google Scholar] [CrossRef]
- Whitley, H.; Lindsey, W. Sex-based differences in drug activity. Am. Fam. Physician 2009, 80, 1254–1258. [Google Scholar]
- Farkouh, A.; Riedl, T.; Gottardi, R.; Czejka, M.; Kautzky-Willer, A. Sex-Related Differences in Pharmacokinetics and Pharmacodynamics of Frequently Prescribed Drugs: A Review of the Literature. Adv. Ther. 2020, 37, 644–655. [Google Scholar] [CrossRef]
- Liu, K.A.; Mager, N.A. Women’s involvement in clinical trials: Historical perspective and future implications. Pharm. Pract. 2016, 14, 708. [Google Scholar] [CrossRef]
- Murrin, L.C.; Ferrer, J.R.; Zeng, W.Y.; Haley, N.J. Nicotine administration to rats: Methodological considerations. Life Sci. 1987, 40, 1699–1708. [Google Scholar] [CrossRef]
- Raval, A.P.; Hirsch, N.; Dave, K.R.; Yavagal, D.R.; Bramlett, H.; Saul, I. Nicotine and estrogen synergistically exacerbate cerebral ischemic injury. Neuroscience 2011, 181, 216–225. [Google Scholar] [CrossRef]
- Wang, L.; Kittaka, M.; Sun, N.; Schreiber, S.S.; Zlokovic, B.V. Chronic nicotine treatment enhances focal ischemic brain injury and depletes free pool of brain microvascular tissue plasminogen activator in rats. J. Cereb. Blood Flow Metab. 1997, 17, 136–146. [Google Scholar] [CrossRef]
- Raval, A.P.; Saul, I.; Dave, K.R.; DeFazio, R.A.; Perez-Pinzon, M.A.; Bramlett, H. Pretreatment with a single estradiol-17beta bolus activates cyclic-AMP response element binding protein and protects CA1 neurons against global cerebral ischemia. Neuroscience 2009, 160, 307–318. [Google Scholar] [CrossRef]
- Zhang, Z.; Cho, S.; Rehni, A.K.; Quero, H.N.; Dave, K.R.; Zhao, W. Automated Assessment of Hematoma Volume of Rodents Subjected to Experimental Intracerebral Hemorrhagic Stroke by Bayes Segmentation Approach. Transl. Stroke Res. 2020, 11, 789–798. [Google Scholar] [CrossRef]
- Belayev, L.; Alonso, O.F.; Busto, R.; Zhao, W.; Ginsberg, M.D. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 1996, 27, 1616–1623. [Google Scholar] [CrossRef]
- Zhao, W.; Ginsberg, M.D.; Prado, R.; Belayev, L. Depiction of infarct frequency distribution by computer-assisted image mapping in rat brains with middle cerebral artery occlusion. Comparison of photothrombotic and intraluminal suture models. Stroke 1996, 27, 1112–1117. [Google Scholar] [CrossRef]




| Groups | Sampling Time | Body Weight (g) | Body Temperature (°C) | Head Temperature (°C) | pH | pCO2 (mmHg) | pO2 (mmHg) | MABP (mmHg) | Blood Glucose (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|
| Vehicle-treated (n = 10) | Before | 297 ± 3 | 36.8 ± 0.1 | 36.6 ± 0.0 | 7.30 ± 0.01 | 37 ± 1 | 113 ± 7 | 98 ± 3 | 135 ± 8 |
| During | 36.9 ± 0.1 | 36.6 ± 0.0 | 7.31 ± 0.01 | 36 ± 1 | 110 ± 6 | 94 ± 2 | |||
| 0.5 h after | 36.9 ± 0.1 | 36.8 ± 0.1 | 7.31 ± 0.01 | 36 ± 0 | 108 ± 5 | 97 ± 2 | |||
| 1 h after | 37.2 ± 0.1 | 36.9 ± 0.1 | 7.29 ± 0.01 | 38 ± 1 | 122 ± 6 | 101 ± 3 | |||
| 3 h after | 37.2 ± 0.1 | 36.8 ± 0.1 | 7.30 ± 0.01 | 36 ± 1 | 110 ± 5 | 98 ± 2 | |||
| 3.5 h after | 37.1 ± 0.1 | 36.8 ± 0.1 | 7.29 ± 0.01 | 37 ± 1 | 108 ± 7 | 96 ± 2 | |||
| 24 h after | 37.3 ± 0.1 | 36.7 ± 0.0 | 7.30 ± 0.01 | 40 ± 0 | 124 ± 6 | 101 ± 5 | |||
| RMP- treated (n = 10) | Before | 299 ± 7 | 36.7 ± 0.1 | 36.9 ± 0.1 ** | 7.31 ± 0.01 | 38 ± 1 | 95 ± 3 * | 97 ± 2 | 134 ± 8 |
| During | 36.8 ± 0.1 | 36.7 ± 0.1 | 7.31 ± 0.01 | 38 ± 1 | 95 ± 4 | 98 ± 2 | |||
| 0.5 h after | 36.9 ± 0.1 | 36.8 ± 0.1 | 7.32 ± 0.01 | 36 ± 0 | 105 ± 5 | 98 ± 2 | |||
| 2 h after | 36.9 ± 0.1 | 36.9 ± 0.0 | 7.29 ± 0.01 | 37 ± 1 | 121 ± 6 | 98 ± 3 | |||
| 3 h after | 36.8 ± 0.1 ** | 36.8 ± 0.1 | 7.30 ± 0.01 | 37 ± 0 | 124 ± 6 | 98 ± 2 | |||
| 3.5 h after | 37.0 ± 0.1 | 36.8 ± 0.1 | 7.30 ± 0.00 | 36 ± 1 | 123 ± 9 | 97 ± 2 | |||
| 24 h after | 37.2 ± 0.1 | 36.7 ± 0.1 | 7.31 ± 0.01 | 39 ± 1 | 124 ± 3 | 100 ± 3 |
| Groups | Sampling Time | Body Weight (g) | Body Temperature (°C) | Head Temperature (°C) | pH | pCO2 (mmHg) | pO2 (mmHg) | MABP (mmHg) | Blood Glucose (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|
| Vehicle-treated (n = 10) | Before | 272 ± 3 | 36.9 ± 0.1 | 36.7 ± 0.1 | 7.28 ± 0.01 | 35 ± 1 | 114 ± 5 | 96 ± 2 | 127 ± 4 |
| During | 36.9 ± 0.1 | 36.7 ± 0.0 | 7.28 ± 0.01 | 35 ± 1 | 109 ± 5 | 97 ± 2 | |||
| 0.5 h after | 37.0 ± 0.1 | 36.9 ± 0.1 | 7.28 ± 0.01 | 36 ± 1 | 109 ± 5 | 101 ± 1 | |||
| 2 h after | 37.0 ± 0.1 | 36.8 ± 0.1 | 7.25 ± 0.01 | 37 ± 1 | 119 ± 7 | 102 ± 3 | |||
| 3 h after | 37.0 ± 0.1 | 36.9 ± 0.1 | 7.25 ± 0.01 | 36 ± 1 | 120 ± 6 | 98 ± 3 | |||
| 3.5 h after | 37.0 ± 0.1 | 36.9 ± 0.1 | 7.26 ± 0.01 | 36 ± 0 | 118 ± 6 | 93 ± 2 | |||
| 24 h after | 37.1 ± 0.1 | 36.7 ± 0.0 | 7.27 ± 0.01 | 37 ± 1 | 123 ± 5 | 92 ± 2 | |||
| RMP- treated (n = 10) | Before | 285 ± 5 | 36.9 ± 0.1 | 36.6 ± 0.1 | 7.27 ± 0.01 | 37 ± 1 | 103 ± 10 | 96 ± 3 | 138 ± 6 |
| During | 36.9 ± 0.1 | 36.9 ± 0.1 | 7.26 ± 0.01 * | 38 ± 1 * | 104 ± 8 | 98 ± 2 | |||
| 0.5 h after | 37.0 ± 0.1 | 36.8 ± 0.1 | 7.28 ± 0.00 | 37 ± 0 | 110 ± 9 | 100 ± 2 | |||
| 2 h after | 36.9 ± 0.1 | 36.8 ± 0.1 | 7.27 ± 0.01 | 37 ± 1 | 112 ± 6 | 98 ± 2 | |||
| 3 h after | 37.0 ± 0.1 | 37.0 ± 0.1 | 7.27 ± 0.01 | 36 ± 1 | 106 ± 5 | 101 ± 3 | |||
| 3.5 h after | 37.0 ± 0.1 | 37.0 ± 0.1 | 7.26 ± 0.01 | 37 ± 1 | 108 ± 8 | 98 ± 2 | |||
| 24 h after | 37.1 ± 0.1 | 36.8 ± 0.1 | 7.27 ± 0.01 | 37 ± 1 | 116 ± 8 | 88 ± 2 |
| Groups | Sampling Time | Body Weight (g) | Body Temperature (°C) | Head Temperature (°C) | pH | pCO2 (mmHg) | pO2 (mmHg) | MABP (mmHg) | Blood Glucose (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|
| Vehicle-treated (n = 15) | Before | 315 ± 4 | 36.8 ± 0.1 | 36.7 ± 0.0 | 7.46 ± 0.01 | 37 ± 1 | 105 ± 4 | 96 ± 1 | 122 ± 4 |
| During | 36.8 ± 0.1 | 36.6 ± 0.1 | 7.47 ± 0.01 | 36 ± 1 | 103 ± 4 | 93 ± 2 | |||
| 0.5 h after | 37.0 ± 0.1 | 36.8 ± 0.1 | 7.48 ± 0.00 | 34 ± 0 | 103 ± 3 | 91 ± 2 | |||
| 4.5 h after (n = 10) | 37.0 ± 0.1 | 36.7 ± 0.1 | 7.43 ± 0.01 | 39 ± 1 | 120 ± 7 | 92 ± 2 | |||
| 5.5 h after (n = 10) | 36.9 ± 0.1 | 36.7 ± 0.1 | 7.42 ± 0.01 | 38 ± 1 | 114 ± 8 | 91 ± 1 | |||
| 6 h after (n = 5) | 37.1 ± 0.2 | 36.9 ± 0.2 | 7.44 ± 0.01 | 39 ± 1 | 104 ± 3 | 98 ± 3 | |||
| 6.5 h after (n = 10) | 37.0 ± 0.1 | 36.8 ± 0.1 | 7.43 ± 0.01 | 37 ± 1 | 110 ± 5 | 89 ± 1 | |||
| 7 h after (n = 5) | 37.3 ± 0.1 | 36.9 ± 0.2 | 7.45 ± 0.00 | 37 ± 1 | 94 ± 2 | 98 ± 2 | |||
| 8 h after (n = 5) | 37.3 ± 0.1 | 37.0 ± 0.2 | 7.45 ± 0.00 | 37 ± 1 | 98 ± 2 | 100 ± 4 | |||
| 24 h after | 37.3 ± 0.1 | 36.7 ± 0.0 | 7.46 ± 0.01 | 36 ± 1 | 112 ± 5 | 88 ± 1 | |||
| 4.5 h window (n = 10) | Before | 315 ± 5 | 36.8 ± 0.1 | 36.6 ± 0.0 | 7.46 ± 0.01 | 37 ± 1 | 100 ± 7 | 98 ± 2 | 120 ± 5 |
| During | 36.9 ± 0.1 | 36.7 ± 0.1 | 7.47 ± 0.01 | 36 ± 1 | 99 ± 7 | 95 ± 2 | |||
| 0.5 h after | 37.0 ± 0.1 | 36.8 ± 0.1 | 7.47 ± 0.01 | 36 ± 1 * | 103 ± 6 | 93 ± 2 | |||
| 4.5 h after | 37.0 ± 0.1 | 37.0 ± 0.1 | 7.45 ± 0.01 | 37 ± 1 | 112 ± 6 | 96 ± 3 | |||
| 5.5 h after | 37.0 ± 0.1 | 37.1 ± 0.1 ** | 7.43 ± 0.01 | 37 ± 1 | 103 ± 6 | 96 ± 2 | |||
| 6 h after | 37.0 ± 0.1 | 37.0 ± 0.1 * | 7.44 ± 0.01 | 37 ± 1 | 103 ± 6 | 91 ± 2 | |||
| 24 h after | 37.3 ± 0.2 | 36.7 ± 0.1 | 7.46 ± 0.01 | 37 ± 1 | 108 ± 7 | 91 ± 1 | |||
| 6 h window (n = 10) | Before | 310 ± 3 | 36.9 ± 0.1 | 36.7 ± 0.1 | 7.47 ± 0.01 | 36 ± 1 | 103 ± 6 | 94 ± 2 | 107 ± 7 |
| During | 36.8 ± 0.1 | 36.6 ± 0.1 | 7.47 ± 0.01 | 36 ± 1 | 104 ± 6 | 94 ± 3 | |||
| 0.5 h after | 37.0 ± 0.1 | 36.7 ± 0.1 | 7.47 ± 0.01 | 35 ± 0 | 103 ± 5 | 94 ± 2 | |||
| 6 h after | 37.0 ± 0.1 | 36.8 ± 0.1 | 7.42 ± 0.01 | 38 ± 1 | 129 ± 8 | 94 ± 3 | |||
| 7 h after | 37.2 ± 0.1 | 37.0 ± 0.1 | 7.42 ± 0.01 * | 38 ± 0 | 113 ± 5 | 94 ± 2 | |||
| 8 h after | 37.1 ± 0.1 | 36.8 ± 0.1 | 7.42 ± 0.01 ** | 38 ± 0 | 112 ± 6 | 89 ± 2 | |||
| 24 h after | 37.5 ± 0.1 | 36.8 ± 0.0 | 7.45 ± 0.01 | 36 ± 1 | 123 ± 7 | 89 ± 2 |
| Groups | Sampling Time | Body Weight (g) | Body Temperature (°C) | Head Temperature (°C) | pH | pCO2 (mmHg) | pO2 (mmHg) | MABP (mmHg) | Blood Glucose (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|
| Vehicle-treated (n = 15) | Before | 248 ± 3 | 36.9 ± 0.1 | 36.7 ± 0.1 | 7.37 ± 0.03 | 38 ± 1 | 103 ± 4 | 98 ± 1 | 113 ± 3 |
| During | 36.9 ± 0.1 | 36.7 ± 0.1 | 7.37 ± 0.02 | 37 ± 0 | 104 ± 4 | 97 ± 2 | |||
| 0.5 h after | 37.0 ± 0.1 | 36.8 ± 0.1 | 7.38 ± 0.03 | 35 ± 1 | 104 ± 3 | 99 ± 1 | |||
| 4.5 h after (n = 10) | 37.1 ± 0.1 | 36.7 ± 0.1 | 7.32 ± 0.02 | 38 ± 1 | 117 ± 8 | 99 ± 1 | |||
| 5.5 h after (n = 10) | 37.2 ± 0.1 | 36.8 ± 0.1 | 7.33 ± 0.03 | 36 ± 1 | 110 ± 6 | 93 ± 2 | |||
| 6 h after (n = 5) | 36.9 ± 0.2 | 36.7 ± 0.1 | 7.45 ± 0.01 | 36 ± 1 | 96 ± 6 | 97 ± 4 | |||
| 6.5 h after (n = 10) | 37.2 ± 0.1 | 36.8 ± 0.1 | 7.32 ± 0.00 | 36 ± 1 | 110 ± 3 | 91 ± 1 | |||
| 7 h after (n = 5) | 36.9 ± 0.1 | 36.7 ± 0.1 | 7.45 ± 0.01 | 37 ± 1 | 96 ± 5 | 99 ± 3 | |||
| 8 h after (n = 5) | 37.1 ± 0.2 | 37.0 ± 0.1 | 7.44 ± 0.01 | 36 ± 1 | 91 ± 4 | 97 ± 1 | |||
| 24 h after | 37.4 ± 0.1 | 36.7 ± 0.1 | 7.38 ± 0.02 | 37 ± 1 | 115 ± 5 | 94 ± 2 | |||
| 4.5 h window (n = 10) | Before | 258 ± 6 | 36.9 ± 0.1 | 36.8 ± 0.1 | 7.35 ± 0.01 | 37 ± 0 | 107 ± 9 | 98 ± 2 | 119 ± 5 |
| During | 37.0 ± 0.1 | 36.7 ± 0.1 | 7.35 ± 0.02 | 36 ± 1 | 111 ± 9 | 98 ± 2 | |||
| 0.5 h after | 37.2 ± 0.1 | 36.8 ± 0.1 | 7.36 ± 0.03 | 35 ± 1 | 108 ± 8 | 96 ± 2 | |||
| 4.5 h after | 37.0 ± 0.1 | 37.0 ± 0.1 * | 7.35 ± 0.03 | 36 ± 1 | 126 ± 8 | 97 ± 3 | |||
| 5.5 h after | 37.1 ± 0.1 | 37.1 ± 0.1 | 7.34 ± 0.03 | 35 ± 1 | 116 ± 6 | 93 ± 2 | |||
| 6.5 h after | 37.0 ± 0.1 | 36.8 ± 0.1 | 7.35 ± 0.03 | 35 ± 1 | 112 ± 5 | 95 ± 2 | |||
| 24 h after | 37.3 ± 0.2 | 36.7 ± 0.0 | 7.37 ± 0.04 | 36 ± 1 | 110 ± 6 | 92 ± 1 | |||
| 6 h window (n = 10) | Before | 253 ± 5 | 36.8 ± 0.1 | 36.7 ± 0.1 | 7.45 ±0.01 * | 37 ± 1 | 103 ± 5 | 96 ± 1 | 106 ± 3 |
| During | 36.8 ± 0.1 | 36.7 ± 0.0 | 7.45 ± 0.01 | 38 ± 1 | 103 ± 4 | 95 ± 2 | |||
| 0.5 h after | 37.0 ± 0.1 | 36.7 ± 0.1 | 7.48 ±0.01 ** | 35 ± 1 | 99 ± 3 | 94 ± 2 | |||
| 6 h after | 37.0 ± 0.1 | 36.8 ± 0.1 | 7.46 ± 0.01 | 37 ± 1 | 114 ± 7 | 96 ± 2 | |||
| 7 h after | 37.0 ± 0.1 | 36.9 ± 0.1 | 7.46 ± 0.01 | 36 ± 1 | 111 ± 5 | 97 ± 2 | |||
| 8 h after | 37.2 ± 0.1 | 37.0 ± 0.1 | 7.44 ± 0.01 | 37 ± 1 | 106 ± 4 | 93 ± 2 | |||
| 24 h after | 37.8 ± 0.1 ** | 36.8 ± 0.1 | 7.47 ± 0.01 * | 37 ± 1 | 104 ± 4 | 95 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehni, A.K.; Cho, S.; Zhang, Z.; Khushal, P.; Raval, A.P.; Koch, S.; Perez-Pinzon, M.A.; Zhao, W.; Jy, W.; Dave, K.R. Red Cell Microparticles Suppress Hematoma Growth Following Intracerebral Hemorrhage in Chronic Nicotine-Exposed Rats. Int. J. Mol. Sci. 2022, 23, 15167. https://doi.org/10.3390/ijms232315167
Rehni AK, Cho S, Zhang Z, Khushal P, Raval AP, Koch S, Perez-Pinzon MA, Zhao W, Jy W, Dave KR. Red Cell Microparticles Suppress Hematoma Growth Following Intracerebral Hemorrhage in Chronic Nicotine-Exposed Rats. International Journal of Molecular Sciences. 2022; 23(23):15167. https://doi.org/10.3390/ijms232315167
Chicago/Turabian StyleRehni, Ashish K., Sunjoo Cho, Zhexuan Zhang, Priyanka Khushal, Ami P. Raval, Sebastian Koch, Miguel A. Perez-Pinzon, Weizhao Zhao, Wenche Jy, and Kunjan R. Dave. 2022. "Red Cell Microparticles Suppress Hematoma Growth Following Intracerebral Hemorrhage in Chronic Nicotine-Exposed Rats" International Journal of Molecular Sciences 23, no. 23: 15167. https://doi.org/10.3390/ijms232315167
APA StyleRehni, A. K., Cho, S., Zhang, Z., Khushal, P., Raval, A. P., Koch, S., Perez-Pinzon, M. A., Zhao, W., Jy, W., & Dave, K. R. (2022). Red Cell Microparticles Suppress Hematoma Growth Following Intracerebral Hemorrhage in Chronic Nicotine-Exposed Rats. International Journal of Molecular Sciences, 23(23), 15167. https://doi.org/10.3390/ijms232315167