Effect of Stress Signals and Ib-rolB/C Overexpression on Secondary Metabolite Biosynthesis in Cell Cultures of Ipomoea batatas
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biomass Accumulation of I. batatas Calli under Stress Factors and Chemical Elicitor Treatment
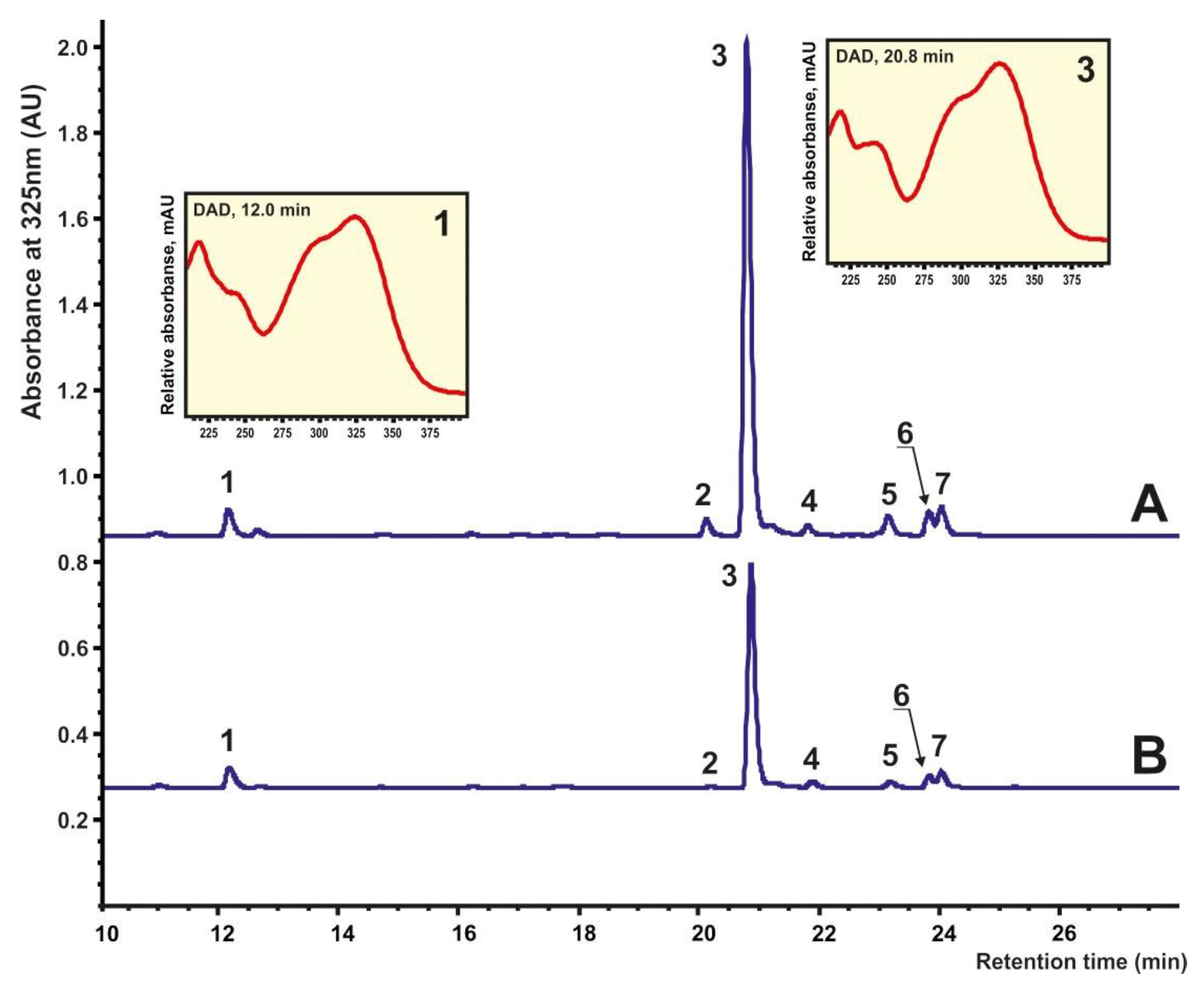
2.2. Accumulation of Phytochemicals in I. batatas Calli under Various Stimuli
2.3. Expression Pattern of Biosynthetic Genes in I. batatas Calli
2.4. Analysis of IbT-DNA2 Genes Expression in I. batatas Calli
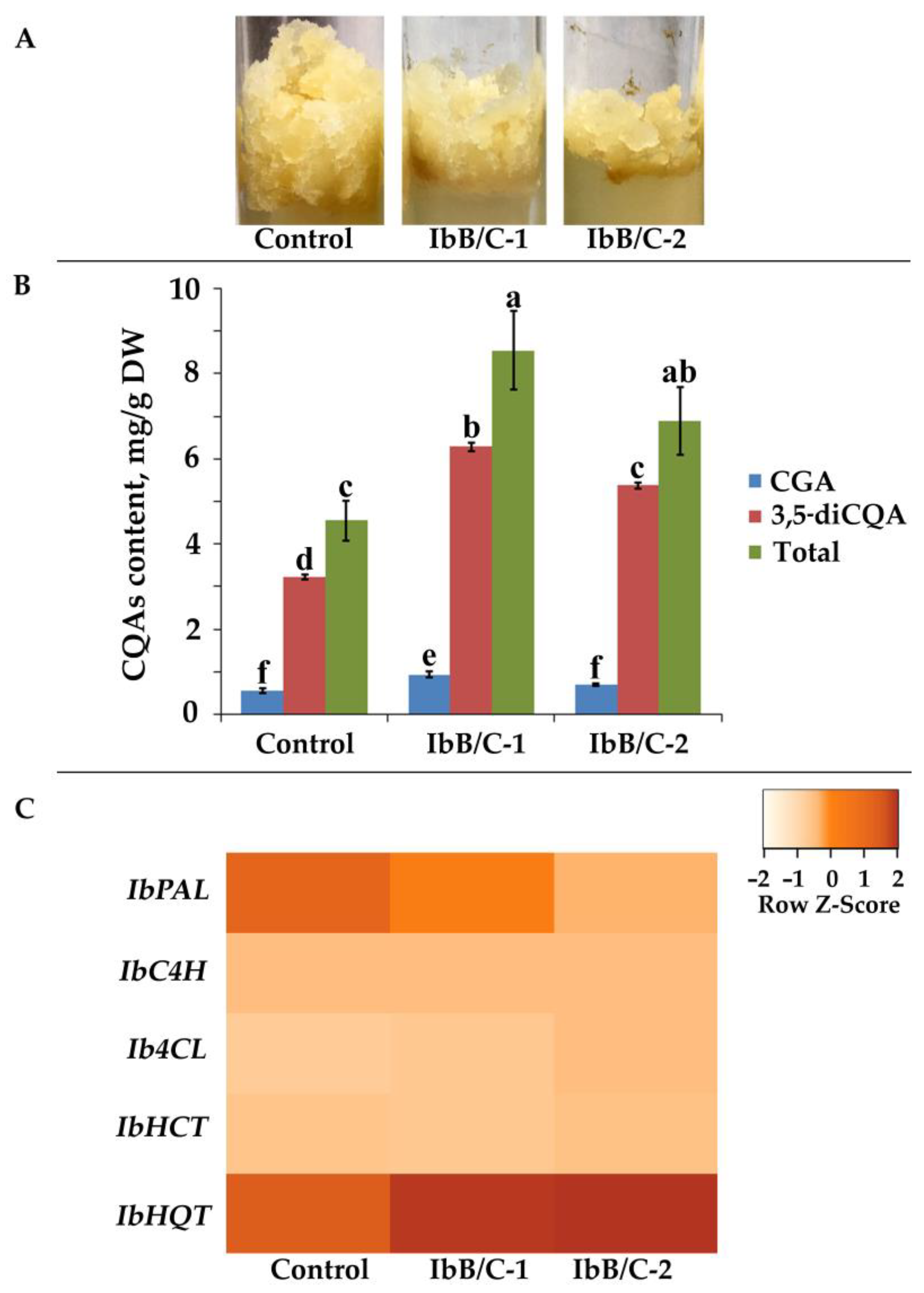
2.5. Effect of Ib-rolB/C Gene Expression on Secondary Metabolism of Transgenic I. batatas Calli
3. Materials and Methods
3.1. Plant Cell Cultures and Treatments
3.2. High-Performance Liquid Chromatography (HPLC) Analysis
3.2.1. Chemicals
3.2.2. Sample Preparation for Analytical Chromatography
3.2.3. Analytical Chromatography and Mass-Spectrometry
3.3. Obtaining Ib-rolB/C–Expressing Cell Cultures
3.4. Synthesis of cDNA and Real-Time PCR Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayeleso, T.B.; Ramachela, K.; Mukwevho, E. A review of therapeutic potentials of sweet potato: Pharmacological activities and influence of the cultivar. Trop. J. Pharm. Res. 2016, 15, 2751–2761. [Google Scholar] [CrossRef] [Green Version]
- Loebenstein, G. Sweet Potato, A Research neglected important food crop, regarding virus research and propagation systems: A review. Austin J. Plant Biol. 2016, 2, 1012. [Google Scholar]
- Drapal, M.; Rossel, G.; Heider, B.; Fraser, P.D. Metabolic diversity in sweet potato (Ipomoea batatas, Lam.) leaves and storage roots. Hortic. Res. 2019, 6, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavva, S.; Nedunchezhiyan, M. Global status of sweet potato cultivation. Fruit. Veg. Cereal Sci. Biotech. 2012, 1, 143–147. [Google Scholar]
- Panda, V.; Sonkamble, M. Phytochemical constituents and pharmacological activities of Ipomoea batatas L. (Lam)—A review. Int. J. Res. Phytochem. Pharmacol. 2012, 2, 25–34. [Google Scholar]
- Naomi, R.; Bahari, H.; Yazid, M.D.; Othman, F.; Zakaria, Z.A.; Hussain, M.K. Potential effects of sweet potato (Ipomoea batatas) on hyperglycemia and dyslipidemia—A systematic review in diabetic retinopathy context. Int. J. Mol. Sci. 2021, 22, 10816. [Google Scholar] [CrossRef]
- Escobar-Puentes, A.A.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Villegas-Ochoa, M.A.; González-Aguilar, G.A.; Olivas-Aguirre, F.J.; Wall-Medrano, A. Sweet potato (Ipomoea batatas L.) phenotypes: From agroindustry to health effects. Foods 2022, 11, 1058. [Google Scholar] [CrossRef]
- Islam, M.S.; Yoshimoto, M.; Yahara, S.; Okuno, S.; Ishiguro, K.; Yamakawa, O. Identification and characterization of foliar polyphenolic composition in sweet potato (Ipomoea batatas L.) genotypes. J. Agric. Food Chem. 2002, 50, 3718–3722. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kurata, R.; Kai, Y. Seasonal variation in the yield and polyphenol content of sweet potato (Ipomoea batatas L.) foliage. Hort. J. 2019, 88, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhu, J.; Lin, Y.; Zhu, H.; Tang, L.; Wang, X.; Wang, X. Comparative transcriptome and weighted correlation network analyses reveal candidate genes involved in chlorogenic acid biosynthesis in sweet potato. Sci. Rep. 2022, 12, 2770. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In Plant Signaling Molecules: Role and Regulation under Stressful Environments; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–168. ISBN 9780128164518. [Google Scholar]
- Yan, K.; Cui, M.; Zhao, S.; Chen, X.; Tang, X. Salinity stress is beneficial to the accumulation of chlorogenic acids in honeysuckle (Lonicera japonica Thunb.). Front. Plant Sci. 2016, 7, 1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant. Biol. 2018, 18, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Macías, P.; Ordidge, M.; Vysini, E.; Waroonphan, S.; Battey, N.H.; Gordon, M.H.; Hadley, P.; John, P.; Lovegrove, J.A.; Wagstaffe, A. Changes in the flavonoid and phenolic acid contents and antioxidant activity of red leaf lettuce (Lollo Rosso) due to cultivation under plastic films varying in ultraviolet transparency. J. Agric. Food Chem. 2007, 55, 10168–10172. [Google Scholar] [CrossRef] [PubMed]
- Reimer, J.J.; Thiele, B.; Biermann, R.T.; Junker-Frohn, L.V.; Wiese-Klinkenberg, A.; Usadel, B.; Wormit, A. Tomato leaves under stress—A comparison of stress response to mild abiotic stress between a cultivated and a wild tomato species. Plant Mol. Biol. 2021, 107, 177–206. [Google Scholar] [CrossRef]
- Kikowska, M.; Budzianowski, J.; Krawczyk, A.; Thiem, B. Accumulation of rosmarinic, chlorogenic and caffeic acids in in vitro cultures of Eryngium planum L. Acta Physiol. Plant. 2012, 34, 2425–2433. [Google Scholar] [CrossRef] [Green Version]
- Kamarauskaite, J.; Baniene, R.; Raudone, L.; Vilkickyte, G.; Vainoriene, R.; Motiekaityte, V.; Trumbeckaite, S. Antioxidant and mitochondria-targeted activity of caffeoylquinic-acid-rich fractions of wormwood (Artemisia absinthium L.) and silver wormwood (Artemisia ludoviciana nutt.). Antioxidants 2021, 10, 1405. [Google Scholar] [CrossRef]
- Mei, Y.; Sun, H.; Du, G.; Wang, X.; Lyu, D. Exogenous chlorogenic acid alleviates oxidative stress in apple leaves by enhancing antioxidant capacity. Sci. Hortic. 2020, 274, 109676. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Hasanuzzaman, M. Plant phenolic compounds for abiotic stress tolerance. In Managing Plant Production Under Changing Environment; Springer: Singapore, 2022; pp. 193–237. [Google Scholar]
- Naik, P.M.; Al-Khayri, J.M. Impact of abiotic elicitors on in vitro production of plant secondary metabolites: A review. J. Adv. Res. Biotechnol. 2016, 1, 7. [Google Scholar] [CrossRef]
- D’Alessandro, R.; Docimo, T.; Graziani, G.; D’Amelia, V.; De Palma, M.; Cappetta, E.; Tucci, M. Abiotic stresses elicitation potentiates the productiveness of cardoon calli as bio-factories for specialized metabolites production. Antioxidants 2022, 11, 1041. [Google Scholar] [CrossRef]
- Baenas, N.; Garcia-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [Green Version]
- Ragaey, M.M.; Sadak, M.S.; Dawood, M.F.A.; Mousa, N.H.S.; Hanafy, R.S.; Latef, A.A.H.A. Role of signaling molecules sodium nitroprusside and arginine in alleviating salt-Induced oxidative stress in wheat. Plants 2022, 11, 1786. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.B.; Chen, J.G.; Yin, Z.P.; Shangguan, X.-C.; Peng, D.-Y.; Lu, T.; Lin, P. Methyl jasmonate and salicylic acid elicitation increase content and yield of chlorogenic acid and its derivatives in Gardenia jasminoides cell suspension cultures. Plant Cell Tiss. Organ. Cult. 2018, 134, 79–93. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Thiruvengadam, M.; Chung, I.-M. Identification of elicitors enhances the polyphenolic compounds and pharmacological potential in hairy root cultures of Aster scaber. S. Afr. J. Bot. 2019, 125, 92–101. [Google Scholar] [CrossRef]
- Alcalde, M.A.; Perez-Matas, E.; Escrich, A.; Cusido, R.M.; Palazon, J.; Bonfill, M. Biotic elicitors in adventitious and hairy root cultures: A review from 2010 to 2022. Molecules 2022, 27, 5253. [Google Scholar] [CrossRef] [PubMed]
- Payyavula, R.S.; Shakya, R.; Sengoda, V.G.; Munyaneza, J.E.; Swamy, P.; Navarre, D.A. Synthesis and regulation of chlorogenic acid in potato: Rerouting phenylpropanoid flux in HQT-silenced lines. Plant Biotechnol. J. 2015, 13, 551–564. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.N.; Kerimi, A.; Williamson, G. Bioavailability and metabolism of chlorogenic acids (acyl-quinic acids) in humans. Compr. Rev. Food. Sci. Food Saf. 2020, 19, 1299–1352. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current advances in naturally occurring caffeoylquinic acids: Structure, bioactivity, and synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [Green Version]
- Hammerschmidt, R. Chlorogenic acid: A versatile defense compound. Physiol. Mol. Plant Pathol. 2014, 88, 3–6. [Google Scholar] [CrossRef]
- Kyndt, T.; Quispe, D.; Zhai, H.; Jarret, R.; Ghislain, M.; Liu, Q.; Gheysen, G.; Kreuze, J.F. The genome of cultivated sweet potato contains Agrobacterium T-DNAs with expressed genes: An example of a naturally transgenic food crop. Proc. Natl. Acad. Sci. USA 2015, 112, 5844–5849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quispe-Huamanquispe, D.G.; Gheysen, G.; Yang, J.; Jarret, R.; Rossel, G.; Kreuze, J.K. The horizontal gene transfer of Agrobacterium T-DNAs into the series Batatas (Genus Ipomoea) genome is not confined to hexaploid sweet potato. Sci. Rep. 2019, 9, 12584. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Shkryl, Y.N.; Veremeichik, G.N.; Gorpenchenko, T.Y.; Inyushkina, Y.V. Application of Agrobacterium rol genes in plant biotechnology: A natural phenomenon of secondary metabolism regulation, genetic transformation. In Genetic Transformation; Alvarez, M.A., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 261–270. [Google Scholar] [CrossRef] [Green Version]
- Matveeva, T.V.; Lutova, L.A. Horizontal gene transfer from Agrobacterium to plants. Front. Plant Sci. 2014, 5, 326. [Google Scholar] [CrossRef] [Green Version]
- Otten, L. The Agrobacterium phenotypic plasticity (plast) genes. Curr. Top. Microbiol. Immunol. 2018, 418, 375–419. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Vereshchagina, Y.V.; Bulgakov, D.V.; Veremeichik, G.N.; Shkryl, Y.N. The rolB plant oncogene affects multiple signaling protein modules related to hormone signaling and plant defense. Sci. Rep. 2018, 8, 2285. [Google Scholar] [CrossRef] [Green Version]
- Kruglova, N.N.; Seldimirova, O.; Zinatullina, A. In vitro callus as a model system for the study of plant stress resistance to abiotic factors (on the example of cereals). Biol. Bull. Rev. 2018, 8, 518–526. [Google Scholar] [CrossRef]
- Nie, H.; Wang, Y.; Wei, C.; Grover, C.E.; Su, Y.; Wendel, J.F.; Hua, J. Embriogenic calli induction and salt stress response revealed by RNA-Seq in diploid wild species Gossypiu sturtianum and Gossypium raimondii. Front. Plant Sci. 2021, 12, 715041. [Google Scholar] [CrossRef]
- Martinez-Silvestre, K.E.; Santiz-Gómez, J.A.; Luján-Hidalgo, M.C.; Ruiz-Lau, N.; Sánchez-Roque, Y.; Gutiérrez-Miceli, F.A. Effect of UV-B radiation on flavonoids and phenols accumulation in Tempisque (Sideroxylon capiri pittier) callus. Plants 2022, 11, 473. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Park, Y.G.; Jeong, B.R. Chemical elicitor-induced modulation of antioxidant metabolism and enhancement of secondary metabolite accumulation in cell suspension cultures of Scrophularia kakudensis. Int. J. Mol. Sci. 2016, 17, 399. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.L.; Costantino, P.; Bettini, P.P. The never ending story of rol genes: A century after. Plant Cell Tiss. Organ. Cult. 2017, 131, 201–212. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef] [PubMed]
- Guru, A.; Dwivedia, P.; Kaurb, P.; Pandey, D.K. Exploring the role of elicitors in enhancing medicinal values of plants under in vitro condition. S. Afr. J. Bot. 2022, 149, 1029–1043. [Google Scholar] [CrossRef]
- Terahara, N.; Konczak-Islam, I.; Nakatani, M.; Yamakawa, O.; Goda, Y.; Honda, T. Anthocyanins in callus induced from purple storage root of Ipomoea batatas. Phytochemistry 2000, 54, 919–922. [Google Scholar] [CrossRef]
- Padmanabhan, K.; Cantliffe, D.; Koch, K. Auxin-regulated gene expression and embryogenic competence in callus cultures of sweet potato, Ipomoea batatas (L.) Lam. Plant Cell Rep. 2001, 20, 187–192. [Google Scholar] [CrossRef]
- Konczak, I.; Okuno, S.; Yoshimoto, M.; Yamakawa, O. Caffeoylquinic acids generated in vitro in a high-anthocyanin-accumulating sweet potato cell line. J. Biomed. Biotechnol. 2004, 5, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.Y.; Yap, W.S.; Yong, W.T.L. Comparison of anthocyanin and phenolic contents between tuber and callus of Ipomoea batatas (L). Petranika J. Trop. Agric. Sci. 2012, 35, 9–14. [Google Scholar]
- Xin, Q.; Liu, B.; Sun, J.; Fan, X.; Li, X.; Jiang, L.; Hao, G.; Pei, H.; Zhou, X. Heat shock treatment promoted callus formation on postharvest sweet potato by adjusting active oxygen and phenylpropanoid metabolism. Agriculture 2022, 12, 1351. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Bucinski, A.; Luczkiewicz, M. Light and temperature conditions affect bioflavonoid accumulation in callus cultures of Cyclopia subternata Vogel (honeybush). Plant Cell Tiss. Organ. Cult. 2014, 118, 589–593. [Google Scholar] [CrossRef] [Green Version]
- Summart, J.; Panichajakul, S.; Prathepha, P.; Thanonkeo, P. Callus induction and influence of culture condition and culture medium on growth of Thai aromatic rice, Khao Dawk Mali 105, cell culture. World Appl. Sci. J. 2008, 5, 246–251. [Google Scholar]
- Amir, M.; Aqil, M.; Ismail, M.V.; Akhtar, M.; Khan, A.H.; Mujeeb, M. Effect of carbon source and incubation temperature on total content of secondary metabolites of callus culture of Solanum nigrum. World J. Pharm. Res. 2017, 6, 905–922. [Google Scholar]
- Shkryl, Y.N.; Veremeichik, G.N.; Avramenko, T.V.; Gorpenchenko, T.Y.; Tchernoded, G.K.; Bulgakov, V.P. Transcriptional regulation of enzymes involved in ROS metabolism and abiotic stress resistance in rolC-transformed cell cultures. Plant Growth Regul. 2022, 97, 485–497. [Google Scholar] [CrossRef]
- El-Dawayati, M.M.; El-Sharabasy, S.; Gantait, S. Light intensity-induced morphogenetic response and enhanced β-sitosterol accumulation in date palm (Phoenix dactylifera L. cv. Hayani) callus culture. Sugar Tech. 2020, 22, 1122–1129. [Google Scholar] [CrossRef]
- Gollan, P.J.; Aro, E.-M. Photosynthetic signalling during high light stress and recovery: Targets and dynamics. Phil. Trans. R. Soc. B 2020, 375, 20190406. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H. Light-induced fluctuations in the biomass accumulation, secondary metabolites production and antioxidant activity in the cell suspension cultures of Artemisia absinthium L. J. Photochem. Photobiol. B Biol. 2014, 140, 223–227. [Google Scholar] [CrossRef]
- Aanisia, Z.; Mehjabeen, A.; John, S.A.; Shukla, P.K.; Pragati, M. Salicylic acid induced changes in biomass and elicits primary metabolites under in vitro cultured callus of snow mountain garlic. Int. J. Plant Res. 2017, 30, 329–335. [Google Scholar] [CrossRef]
- Khan, T.; Khan, T.; Hano, C.; Abbasi, B.H. Effects of chitosan and salicylic acid on the production of pharmacologically attractive secondary metabolites in callus cultures of Fagonia indica. Ind. Crops Prod. 2019, 129, 525–535. [Google Scholar] [CrossRef]
- Mahendran, G.; Kumar, D.; Verma, S.K.; Chandran, A.; Warsi, Z.I.; Husain, Z.; Afroz, S.; Rout, P.K.; Rahman, L.U. Sodium nitroprusside enhances biomass and gymnemic acids production in cell suspension of Gymnema sylvestre (Retz.) R. Br. ex. Sm. Plant Cell Tiss. Organ Cult. 2021, 146, 161–170. [Google Scholar] [CrossRef]
- Giri, L.; Dhyani, P.; Rawat, S.; Bhatt, I.D.; Nandi, S.K.; Rawal, R.S.; Pande, V. In vitro production of phenolic compounds and antioxidant activity in callus suspension cultures of Habenaria edgeworthii: A rare Himalayan medicinal orchid. Ind. Crops Prod. 2012, 39, 1–6. [Google Scholar] [CrossRef]
- Koo, H.B.; Hwang, H.S.; Han, J.Y.; Cheong, E.J.; Kwon, Y.-S.; Choi, Y.E. Enhanced production of pinosylvin stilbene with aging of Pinus strobus callus and nematicidal activity of callus extracts against pinewood nematodes. Sci. Rep. 2022, 12, 770. [Google Scholar] [CrossRef] [PubMed]
- Szymczyk, P.; Szymańska, G.; Kuźma, Ł.; Jeleń, A.; Balcerczak, E. Methyl jasmonate activates the 2C methyl-D-erithrytol 2,4-cyclodiphosphate synthase gene and stimulates tanshinone accumulation in Salvia miltiorrhiza solid callus cultures. Molecules 2022, 27, 1772. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Clifford, M.N.; Marks, S.; Knight, S.; Kuhnert, N. Characterization by LC-MSn of four new classes of p-coumaric acid-containing diacyl chlorogenic acids in green coffee beans. J. Agric. Food Chem. 2006, 54, 4095–4101. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Karakas, F.P.; Cingoz, G.S.; Turker, A.U. The effects of oxidative stress on phenolic composition and antioxidant metabolism in callus culture of common daisy. Afr. J. Tradit. Comple. Altern. Med. 2016, 13, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Reine, D.; Pan, R.; Liu, Y.; Rao, L.; Zhang, W.; Yang, X. Chlorogenic acid metabolism: The evolution and roles in plant response to abiotic stress. Phyton-Int. J. Exp. Bot. 2022, 91, 239–255. [Google Scholar] [CrossRef]
- Karakas, F.P.; Bozat, B.G. Fluctuation in secondary metabolite production and antioxidant defense enzymes in in vitro callus cultures of goat’s rue (Galega officinalis) under different abiotic stress treatments. Plant Cell Tiss. Organ. Cult. 2020, 142, 401–414. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, Q.; Guo, Y.Q.; Zhu, W.H. Elicitor-induced indole alkaloid biosynthesis in Catharanthus roseus cell cultures is related to Ca2+ influx and the oxidative burst. Plant Sci. 2001, 161, 423–431. [Google Scholar] [CrossRef]
- Nadeem, M.; Ahmad, W.; Zahir, A.; Hano, C.; Abbasi, B.H. Salicylic acid-enhanced biosynthesis of pharmacologically iportant lignans and neo lignans in cell suspension culture of Linum ussitatsimum L. Eng. Life Sci. 2018, 19, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Maciel, G.; Lopes, A.A.; Cantrell, C.L.; de Castro França, S.; Bertoni, B.W.; Lourenço, M.V. Jasmonates promote enhanced prduction of bioactive caffeoylquinic acid derivative in Eclipta prostrata (L.) L. hairy roots. Plant Cell Tiss. Organ. Cult. 2022, 149, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Moglia, A.; Lanteri, S.; Comino, C.; Acquadro, A.; de Vos, R.; Beekwilder, J. Stress-induced biosynthesis of dicaffeoylquinic acids in globe artichoke. J. Agric. Food Chem. 2008, 56, 8641–8649. [Google Scholar] [CrossRef]
- Zheng, L.P.; Guo, Y.T.; Wang, J.W.; Tan, R.X. Nitric oxide potentiates oligosaccharide-induced artemisinin production in Artemisia annua hairy roots. J. Integr. Plant Biol. 2008, 50, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Zheng, L.P.; Wu, J.Y.; Tan, R.X. Involvement of nitric oxide in oxidative burst, phenylalanine ammonia-lyase activation and taxol production induced by low-energy ultrasound in Taxus yunnanensis cell suspension cultures. Nitric Oxide 2006, 15, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Claude, S.J.; Park, S.; Park, S.J. Transcriptome-wide identification and quantification of caffeoylquinic acid biosynthesis pathway and prediction of its putative BAHDs gene complex in A. spathulifolius. Int. J. Mol. Sci. 2021, 22, 6333. [Google Scholar] [CrossRef]
- Biala, W.; Jasinski, M. The phenylpropanoid case—It is transport that matters. Front. Plant Sci. 2018, 9, 1610. [Google Scholar] [CrossRef] [Green Version]
- Park, W.T.; Yeo, S.K.; Sathasivam, R.; Park, J.S.; Kim, J.K.; Park, S.U. Influence of light-emitting diodes on phenylpropanoid biosynthetic gene expression and phenylpropanoid accumulation in Agastache rugosa. Appl. Biol. Chem. 2020, 63, 25. [Google Scholar] [CrossRef]
- Han, G.; Bai, G.; Wu, Y.; Zhou, Y.; Yao, W.; Li, L. Comparative transcriptome analysis to identify candidate genes related to chlorogenic acid and flavonoids biosynthesis in iridaceae. Forests 2022, 13, 1632. [Google Scholar] [CrossRef]
- Weitzel, C.; Petersen, M. Enzymes of phenylpropanoid metabolism in the important medicinal plant Melissa officinalis L. Planta 2010, 232, 731–742. [Google Scholar] [CrossRef]
- Ravazzolo, L.; Ruperti, B.; Frigo, M.; Bertaiola, O.; Pressi, G.; Malagoli, M.; Quaggiotti, S. C3H expression is crucial for methyl jasmonate induction of chicoric acid production by Echinacea purpurea (L.) moench cell suspension cultures. Int. J. Mol. Sci. 2022, 23, 11179. [Google Scholar] [CrossRef]
- Gutierrez-Valdes, N.; Häkkinen, S.T.; Lemasson, C.; Guillet, M.; Oksman-Caldentey, K.M.; Ritala, A.; Cardon, F. Hairy root vultures-a versatile tool with multiple applications. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matveeva, T.V.; Sokornova, S.V.; Lutova, L.A. Influence of Agrobacterium oncogenes on secondary metabolism of plants. Phytochem. Rev. 2015, 14, 541–554. [Google Scholar] [CrossRef]
- Chandra, S. Natural plant genetic engineer Agrobacterium rhizogenes: Role of T-DNA in plant secondary metabolism. Biotechnol. Lett. 2012, 34, 407–415. [Google Scholar] [CrossRef]
- Dilshad, E.; Cusido, R.M.; Ramirez-Estrada, K.; Bonfill, M.; Mirza, B. Genetic transformation of Artemisia carvifolia buch with rol genes enhances artemisinin accumulation. PLoS ONE 2015, 10, e0140266. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, T.; Ozeki, Y.; Syono, K. Evidence for the expression of the rol genes of Nicotiana glauca in genetic tumors of N. glauca × N. langsdorffii. Mol. Gen. Genet. 1990, 220, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Kawaoka, A.; Sekine, M.; Ichikawa, T.; Fujita, T.; Shinmyo, A.; Syono, K. Sequence of the cellular T-DNA in the untransformed genome of Nicotiana glauca that is homologous to ORFs 13 and 14 of the Ri plasmid and analysis of its expression in genetic tumors of N. glauca × N. langsdorffii. Mol. Gen. Genet. 1994, 243, 706–710. [Google Scholar] [CrossRef]
- Aoki, S.; Syno, K. Horizontal gene transfer and mutation: Ngrol genes in the genome of Nicotiana glauca. Proc. Natl. Acad. Sci. USA 1999, 96, 13229–13234. [Google Scholar] [CrossRef] [Green Version]
- Veremeichik, G.N.; Shkryl, Y.N.; Pinkus, S.A.; Bulgakov, V.P. Expression profiles of calcium-dependent protein kinase genes (CDPK1-14) in Agrobacterium rhizogenes pRiA4-transformed calli of Rubia cordifolia under temperature- and salt-induced stresses. J. Plant Physiol. 2014, 171, 467–474. [Google Scholar] [CrossRef]
- Veremeichik, G.N.; Bulgakov, V.P.; Shkryl, Y.N.; Silantieva, S.A.; Makhazen, D.S.; Tchernoded, G.K.; Mischenko, N.P.; Fedoreyev, S.A.; Vasileva, E.A. Activation of anthraquinone biosynthesis in long-cultured callus culture of Rubia cordifolia transformed with the rolA plant oncogene. J. Biotechnol. 2019, 306, 38–46. [Google Scholar] [CrossRef]
- Zeng, F.; Qian, J.; Luo, W.; Zhan, Y.; Xin, Y.; Yang, C. Stability of transgenes in long-term micropropagation of plants of transgenic birch (Betula platyphylla). Biotechnol. Lett. 2010, 32, 151–156. [Google Scholar] [CrossRef]
- Kaeppler, S.M.; Kaeppler, H.F.; Rhee, Y. Epigenetic aspects of somaclonal variation in plants. Plant. Mol. Biol. 2000, 43, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.L.; Kaeppler, S.M.; Olhoft, P. Genetic instability of plant tissue cultures: Breakdown of normal controls. Proc. Natl. Acad. Sci. USA 1994, 91, 5222–5226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shkryl, Y.; Yugay, Y.; Vasyutkina, E.; Chukhlomina, E.; Rusapetova, T.; Bulgakov, V. The RolB/RolC homolog from sweet potato promotes early flowering and triggers premature leaf senescence in transgenic Arabidopsis thaliana plants. Plant Physiol. Biochem. 2022, 193, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Comino, C.; Hehn, A.; Moglia, A.; Menin, B.; Bourgaud, F.; Lanteri, S.; Portis, E. The isolation and mapping of a novel hydroxycinnamoyltransferase in the globe artichoke chlorogenic acid pathway. BMC Plant Biol. 2009, 9, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonnante, G.; D’Amore, R.; Blanco, E.; Pierri, C.L.; de Palma, M.; Luo, J.; Tucci, M.; Martin, C. Novel hydroxycinnamoyl-coenzyme A quinate transferase genes from artichoke are involved in the synthesis of chlorogenic acid. Plant Physiol. 2010, 153, 1224–1238. [Google Scholar] [CrossRef]
- Navarre, D.A.; Payyavula, R.S.; Shakya, R.; Knowles, N.R.; Pillai, S.S. Changes in potato phenylpropanoid metabolism during tuber development. Plant Physiol. Biochem. 2013, 65, 89–101. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plantarum 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Tzfira, T.; Tian, G.-W.; Lacroix, B.; Vyas, S.; Li, J.; Leitner-Dagan, Y.; Krichevsky, A.; Taylor, T.; Vainstein, A.; Citovsky, V. pSAT vectors: A modular series of plasmids for autofluorescent protein tagging and expression of multiple genes in plants. Plant Mol. Biol. 2005, 57, 503–516. [Google Scholar] [CrossRef]
- Shkryl, Y.N.; Veremeichik, G.N.; Bulgakov, V.P.; Avramenko, T.V.; Günter, E.A.; Ovodov, Y.S.; Muzarok, T.I.; Zhuravlev, Y.N. The production of class III plant peroxidases in transgenic callus cultures transformed with the rolB gene of Agrobacterium rhizogenes. J. Biotechnol. 2013, 168, 64–70. [Google Scholar] [CrossRef]
- Hood, E.E.; Gelvin, S.B.; Melchers, L.S.; Hoekema, A. New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res. 1993, 2, 208–218. [Google Scholar] [CrossRef]
- Shkryl, Y.N.; Veremeichik, G.N.; Makhazen, D.S.; Silantieva, S.A.; Mishchenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A.; Bulgakov, V.P. Increase of anthraquinone content in Rubia cordifolia cells transformed by native and constitutively active forms of the AtCPK1 gene. Plant Cell Rep. 2016, 35, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Oki, T.; Kobayashi, T.; Kai, Y.; Okuno, S. Single-laboratory validation for the determination of caffeic acid and seven caffeoylquinic acids in sweet potato leaves. Biosci. Biotechnol. Biochem. 2014, 78, 2073–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Tu, Z.; Wang, H.; Fu, Z.; Wen, Q.; Chang, X.; Huang, X. Comparison of different methods for extracting polyphenols from Ipomoea batatas leaves, and identification of antioxidant constituents by HPLC-QTOF-MS2. Int. Food Res. J. 2015, 70, 101–109. [Google Scholar] [CrossRef]
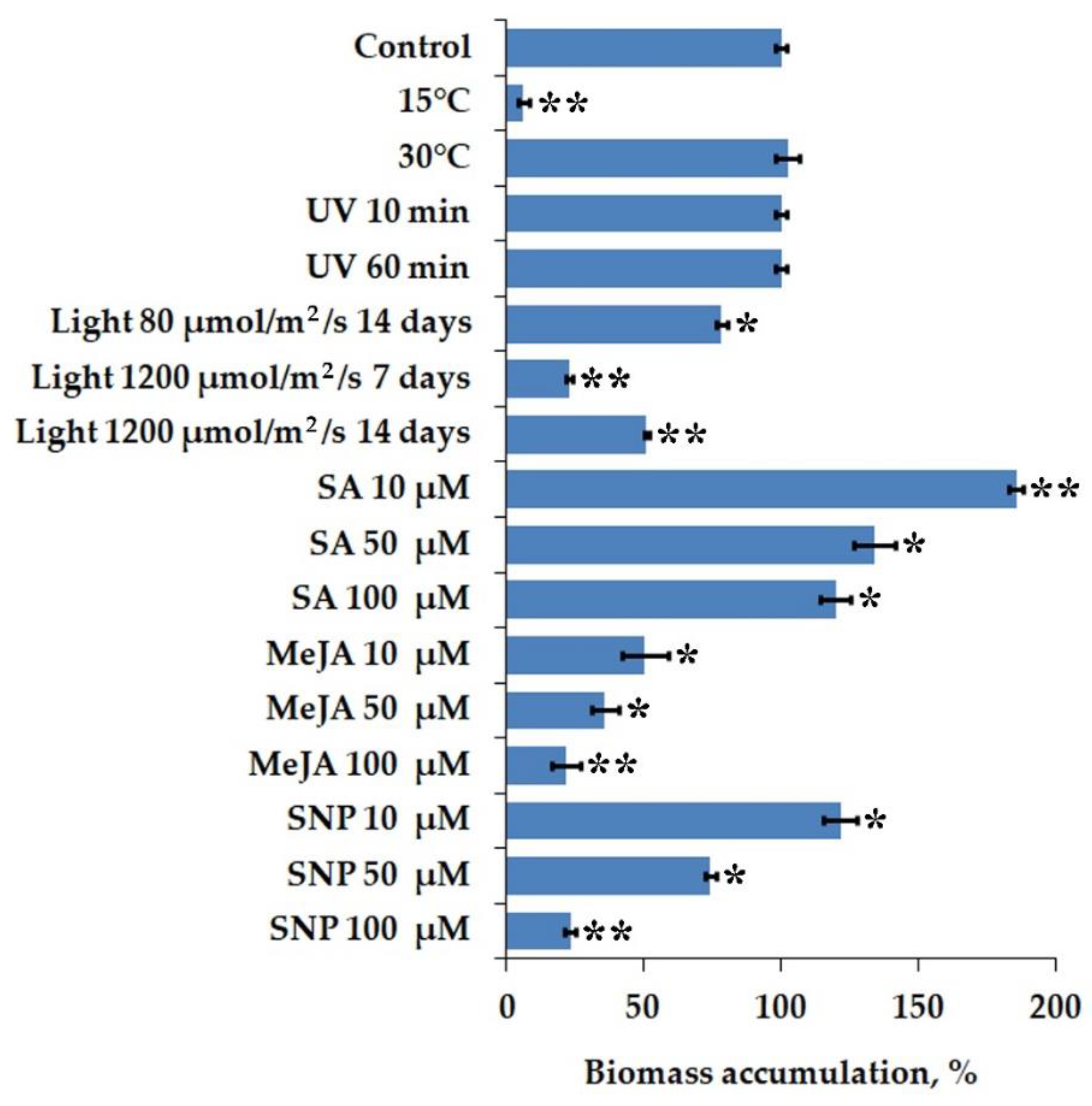

| Callus Line | CQAs Content, mg/g DW | Dry Weight (g/L) | CQAs Production, mg/L | ||||
|---|---|---|---|---|---|---|---|
| CGA | 3,5-diCQA | Total CQAs | CGA | 3,5-diCQA | Total CQAs | ||
| Control | 0.56 ± 0.05 | 3.23 ± 0.08 | 4.55 ± 0.47 | 14.67 ± 0.29 | 8.23 ± 0.16 | 47.40 ± 0.95 | 66.78 ± 1.34 |
| 15 °C | 0.14 ± 0.02 | 0.58 ± 0.08 | 0.98 ± 0.08 | 0.88 ± 0.28 | 0.13 ± 0.04 | 0.51 ± 0.16 | 0.86 ± 0.27 |
| 30 °C | 1.10 ± 0.14 | 5.29 ± 0.74 | 7.34 ± 0.78 | 15.01 ± 0.62 | 16.58 ± 0.68 | 79.39 ± 3.27 | 110.14 ± 4.53 |
| Normal light * | 0.29 ± 0.04 | 1.99 ± 0.28 | 2.68 ± 0.29 | 11.47 ± 0.28 | 3.35 ± 0.09 | 22.83 ± 0.58 | 30.74 ± 0.79 |
| High light (7 d) ** | 0.22 ± 0.03 | 1.40 ± 0.20 | 1.97 ± 0.20 | 3.34 ± 0.14 | 0.75 ± 0.03 | 4.68 ± 0.21 | 6.59 ± 0.29 |
| High light (14 d) *** | 0.25 ± 0.03 | 1.33 ± 0.19 | 1.90 ± 0.19 | 7.46 ± 0.15 | 1.87 ± 0.04 | 9.90 ± 0.19 | 14.16 ± 0.28 |
| UV 10 min | 0.81 ± 0.10 | 5.50 ± 0.77 | 7.33 ± 0.81 | 14.67 ± 0.29 | 11.84 ± 0.24 | 80.76 ± 1.62 | 107.51 ± 2.15 |
| UV 60 min | 0.52 ± 0.07 | 4.15 ± 0.58 | 5.41 ± 0.61 | 14.67 ± 0.29 | 7.64 ± 0.15 | 60.93 ± 1.22 | 79.42 ± 1.59 |
| SA 10 μM | 1.00 ± 0.13 | 8.59 ± 1.20 | 11.59 ± 1.25 | 27.25 ± 0.39 | 27.24 ± 0.39 | 234.08 ± 3.39 | 315.83 ± 4.58 |
| SA 50 μM | 0.85 ± 0.11 | 6.88 ± 0.96 | 9.35 ± 1.00 | 19.65 ± 1.10 | 16.74 ± 0.94 | 135.08 ± 7.54 | 183.76 ± 10.26 |
| SA 100 μM | 0.48 ± 0.06 | 3.60 ± 0.50 | 4.77 ± 0.53 | 17.57 ± 0.82 | 8.39 ± 0.39 | 63.34 ± 2.97 | 83.91 ± 3.94 |
| MeJA 10 μM | 0.85 ± 0.11 | 7.52 ± 1.05 | 9.85 ± 1.11 | 7.36 ± 1.23 | 6.24 ± 1.05 | 55.34 ± 9.28 | 72.51 ± 12.15 |
| MeJA 50 μM | 1.22 ± 0.16 | 14.47 ± 2.03 | 18.32 ± 2.14 | 5.24 ± 0.75 | 6.41 ± 0.92 | 75.82 ± 10.85 | 96.01 ± 13.74 |
| MeJA 100 μM | 0.91 ± 0.12 | 9.87 ± 1.38 | 13.66 ± 1.43 | 3.14 ± 0.78 | 2.86 ± 0.71 | 31.02 ± 7.72 | 42.95 ± 10.69 |
| SNP 10 μM | 0.76 ± 0.10 | 5.66 ± 0.79 | 7.57 ± 0.83 | 17.84 ± 0.90 | 13.47 ± 0.68 | 101.03 ± 5.08 | 135.11 ± 6.79 |
| SNP 50 μM | 0.38 ± 0.05 | 1.98 ± 0.28 | 2.69 ± 0.29 | 10.87 ± 0.30 | 4.10 ± 0.11 | 21.54 ± 0.59 | 29.28 ± 0.80 |
| SNP 100 μM | 0.38 ± 0.05 | 1.98 ± 0.28 | 2.65 ± 0.29 | 3.39 ± 0.31 | 1.29 ± 0.12 | 6.71 ± 0.62 | 8.96 ± 0.82 |
| ↓ > 2 | ↓ < 2 | 1 | ↑ < 2 | ↑ > 2 | |||
| Ib-rolB/C | ORF13 | ORF14, ORF17n, ORF18/17n | |
|---|---|---|---|
| Control | UD | UD | UD |
| 15 °C | UD | 1.07 ± 0.08 | UD |
| 30 °C | UD | UD | UD |
| Normal light * | 5.17 ± 0.12 | UD | UD |
| High light ** | UD | UD | UD |
| UV 10 min | 2.49 ± 0.09 | UD | UD |
| UV 60 min | 3.21 ± 0.11 | UD | UD |
| Chemical elicitors (MeJA, SA, and SNP) | UD | UD | UD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasyutkina, E.A.; Yugay, Y.A.; Grigorchuk, V.P.; Grishchenko, O.V.; Sorokina, M.R.; Yaroshenko, Y.L.; Kudinova, O.D.; Stepochkina, V.D.; Bulgakov, V.P.; Shkryl, Y.N. Effect of Stress Signals and Ib-rolB/C Overexpression on Secondary Metabolite Biosynthesis in Cell Cultures of Ipomoea batatas. Int. J. Mol. Sci. 2022, 23, 15100. https://doi.org/10.3390/ijms232315100
Vasyutkina EA, Yugay YA, Grigorchuk VP, Grishchenko OV, Sorokina MR, Yaroshenko YL, Kudinova OD, Stepochkina VD, Bulgakov VP, Shkryl YN. Effect of Stress Signals and Ib-rolB/C Overexpression on Secondary Metabolite Biosynthesis in Cell Cultures of Ipomoea batatas. International Journal of Molecular Sciences. 2022; 23(23):15100. https://doi.org/10.3390/ijms232315100
Chicago/Turabian StyleVasyutkina, Elena A., Yulia A. Yugay, Valeria P. Grigorchuk, Olga V. Grishchenko, Maria R. Sorokina, Yulia L. Yaroshenko, Olesya D. Kudinova, Varvara D. Stepochkina, Victor P. Bulgakov, and Yury N. Shkryl. 2022. "Effect of Stress Signals and Ib-rolB/C Overexpression on Secondary Metabolite Biosynthesis in Cell Cultures of Ipomoea batatas" International Journal of Molecular Sciences 23, no. 23: 15100. https://doi.org/10.3390/ijms232315100
APA StyleVasyutkina, E. A., Yugay, Y. A., Grigorchuk, V. P., Grishchenko, O. V., Sorokina, M. R., Yaroshenko, Y. L., Kudinova, O. D., Stepochkina, V. D., Bulgakov, V. P., & Shkryl, Y. N. (2022). Effect of Stress Signals and Ib-rolB/C Overexpression on Secondary Metabolite Biosynthesis in Cell Cultures of Ipomoea batatas. International Journal of Molecular Sciences, 23(23), 15100. https://doi.org/10.3390/ijms232315100






