Shedding Light on the Role of Na,K-ATPase as a Phosphatase during Matrix-Vesicle-Mediated Mineralization †
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of MV
2.2. ATP Hydrolysis by MV
2.3. Mineral Formation Monitored by Turbidity
| Sample | Protein (mg·mL−1) | pNPP (U·mg−1) | Diameter (nm) | PI |
|---|---|---|---|---|
| MV (native) | 3.7 ± 0.3 | 4.4 ± 0.9 | 217 ± 23 | 0.4 |
| cMV (partial cleaved TNAP) | 1.9 ± 0.3 | 1.5 ± 0.3 | 286 ± 61 | 0.5 |
| cTNAP (cleaved from MV) | 0.4 ± 0.04 | 5.1 ± 1.8 | -- | -- |
2.4. Na,K-ATPase Proteoliposomes as MV Biomimetic Models
2.5. Spectroscopic Analysis of Minerals Formed by Na, K-ATPase-Liposomes
3. Materials and Methods
3.1. Materials
3.2. Matrix Vesicles Isolated from Chicken Embryo Femurs
3.3. TNAP Enzymatically Cleaved from MV by PI-PLC
3.4. Mineralization Assay for MV and for cMV
3.5. Preparation of Na,K-ATPase
3.6. Liposomes and Na,K-ATPase Proteoliposomes
3.7. Mineralization Assay for Na,K-ATPase Proteoliposomes
3.8. Atomic Force Microscopy
3.9. Determination of ATP Hydrolysis by Colorimetric Assay and by 31P NMR
3.10. Determination of p-Nitrophenolphosphate Hydrolysis by Colorimetric Assay
3.11. FTIR Chemical Analysis of Mineral Formed
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | adenosine 5′-Diphosphate |
| AFM | Atomic Force Microcopy |
| AMP | Adenosine 5′-Monophosphate |
| ATP | Adenosine 5′-Triphosphate |
| ATR-FTIR | Fourier transformed infrared spectroscopy using an attenuated total reflectance accessory |
| BSA | Bovine Serum Albumin |
| C12E8 | dodecyloctaglycol |
| CD39 | nucleoside triphosphate diphosphohydrolase 1 |
| CPPD | Calcium pyrophosphate dehydrate |
| DLS | Dynamic Light Scattering |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| DPPE | 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine |
| GPI | Glycosylphosphatidylinositol |
| HA | apatite |
| HEPES | N-(2-hydroxyethyl) piperazine-N′-ethanesulfonic acid |
| LUVs | large unillamelar vesicles |
| MVs | Matrix vesicles |
| cMV | Matrix vesicles with partial cleaved NAP (Treated with PI-PL-C) |
| NKA | Na,K,-ATPase |
| NPP1 | Nucleotide Pyrophosphatases/Phosphodiesterases |
| PC | phosphocholine |
| PC-1 | glycoprotein-1 |
| PHOSPHO1 | Orphan phosphatase 1 |
| Pi | phosphate |
| PI | polydispersity index |
| PI-PLC | phosphatidylinositol phospholipase C |
| PiT-1 | phosphate transporter 1 |
| PMP | potential of mineral propagation |
| PPi | Pyrophosphate |
| PS-CPLX | phosphtidylserine complex containing calcium and phosphate |
| SCL | Synthetic Cartilage Lymph |
| SDS | sodium dodecyl sulfate |
| SM | sphingomyelin |
| SMPD3 | sphingomyelin phosphodiesterase 3 |
| TCA | trichloroacetic acid |
| TNAP | tissue-nonspecific alkaline phosphatase |
| Tris | Tris[hydroxymethyl]aminomethane |
References
- Schlesinger, P.H.; Braddock, D.T.; Larrouture, Q.C.; Ray, E.C.; Riazanski, V.; Nelson, D.J.; Tourkova, I.L.; Blair, H.C.; Louis, S.; New, Y.; et al. Phylogeny and Chemistry of Biological Mineral Transport. Bone 2020, 141, 115621. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C. Molecular Biology of Matrix Vesicles. Clin. Orthop. Relat. Res. 1995, 314, 266–280. [Google Scholar] [CrossRef]
- Anderson, H.C.; Garimella, R.; Tague, S.E. The Role of Matrix Vesicles in Growth Plate Development and Biomineralization. Front. Biosci. 2005, 10, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E. Role of Matrix Vesicles in Biomineralization. Biochim. Biophys. Acta—Gen. Subj. 2009, 1790, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E. Biomineralization and Matrix Vesicles in Biology and Pathology. Semin. Immunopathol. 2011, 33, 409–417. [Google Scholar] [CrossRef]
- Wuthier, R.E.; Lipscomb, G.F. Matrix Vesicles: Structure, Composition, Formation and Function in Calcification. Front. Biosci. 2011, 16, 2812–2902. [Google Scholar] [CrossRef]
- Cui, L.; Houston, D.A.; Farquharson, C.; MacRae, V.E. Characterisation of Matrix Vesicles in Skeletal and Soft Tissue Mineralisation. Bone 2016, 87, 147–158. [Google Scholar] [CrossRef]
- Ciancaglini, P.; Simão, A.M.S.; Camolezi, F.L.; Millán, J.L.; Pizauro, J.M. Contribution of Matrix Vesicles and Alkaline Phosphatase to Ectopic Bone Formation. Braz. J. Med. Biol. Res. 2006, 39, 603–610. [Google Scholar] [CrossRef]
- Bolean, M.; Borin, I.A.; Simão, A.M.S.; Bottini, M.; Bagatolli, L.A.; Hoylaerts, M.F.; Millán, J.L.; Ciancaglini, P. Topographic Analysis by Atomic Force Microscopy of Proteoliposomes Matrix Vesicle Mimetics Harboring TNAP and AnxA5. Biochim. Biophys. Acta—Biomembr. 2017, 1859, 1911–1920. [Google Scholar] [CrossRef]
- Bottini, M.; Mebarek, S.; Anderson, K.L.; Strzelecka-Kiliszek, A.; Bozycki, L.; Simão, A.M.S.; Bolean, M.; Ciancaglini, P.; Pikula, J.B.; Pikula, S.; et al. Matrix Vesicles from Chondrocytes and Osteoblasts: Their Biogenesis, Properties, Functions and Biomimetic Models. Biochim. Biophys. Acta—Gen. Subj. 2018, 1862, 532–546. [Google Scholar] [CrossRef]
- Cruz, M.A.E.; Ferreira, C.R.; Tovani, C.B.; de Oliveira, F.A.; Bolean, M.; Caseli, L.; Mebarek, S.; Luis Millán, J.; Buchet, R.; Bottini, M.; et al. Phosphatidylserine Controls Calcium Phosphate Nucleation and Growth on Lipid Monolayers: A Physicochemical Understanding of Matrix Vesicle-Driven Biomineralization. J. Struct. Biol. 2020, 212, 107607. [Google Scholar] [CrossRef]
- Anderson, H.C. Electron Microscopic Studies of Induced Cartilage Development and Calcification. J. Cell Biol. 1967, 35, 81–101. [Google Scholar] [CrossRef]
- Bonucci, E. Fine Structure of Early Cartilage Calcification. J. Ultrasruct. Res. 1967, 20, 33–50. [Google Scholar] [CrossRef]
- Anderson, H.C. Vesicles Associated with Calcification in the Matrix of Epiphyseal Cartilage. J. Cell Biol. 1969, 41, 59–72. [Google Scholar] [CrossRef]
- Bonucci, E. Fine Structure and Histochemistry of “Calcifying Globules” in Epiphyseal Cartilage. Z. Zellforsch. Mikrosk. Anat. 1970, 103, 192–217. [Google Scholar] [CrossRef]
- Rabinovitch, A.L.; Anderson, H.C. Biogenesis of Matrix Vesicles in Cartilage Growth Plates. Fed. Proc. 1976, 35, 112–116. [Google Scholar]
- Borg, T.K.; Runyan, R.; Wuthier, R.E. A Freeze-fracture Study of Avian Epiphyseal Cartilage Differentiation. Anat. Rec. 1981, 199, 449–457. [Google Scholar] [CrossRef]
- Akisaka, T.; Shigenaga, Y. Ultrastructure of Growing Epiphyseal Cartilage Processed by Rapid Freezing and Freeze-Substitution. Microscopy 1983, 32, 305–320. [Google Scholar] [CrossRef]
- Akisaka, T.; Kawaguchi, H.; Subita, G.P.; Shigenaga, Y.; Gay, C.V. Ultrastructure of Matrix Vesicles in Chick Growth Plate as Revealed by Quick Freezing and Freeze Substitution. Calcif. Tissue Int. 1988, 42, 383–393. [Google Scholar] [CrossRef]
- Wu, L.N.Y.; Genge, B.R.; Lloyd, G.C.; Wuthier, R.E. Collagen-Binding Proteins in Collagenase-Released Matrix Vesicles from Cartilage. Interaction between Matrix Vesicles Proteins and Different Types of Collagen. J. Biol. Chem. 1991, 266, 1195–1203. [Google Scholar] [CrossRef]
- Kirsch, T.; Pfäffle, M. Selective Binding of Anchorin CII (Annexin V) to Type II and X Collagen and to Chondrocalcin (C-Propeptide of Type II Collagen) Implications for Anchoring Function between Matrix Vesicles and Matrix Proteins. FEBS Lett. 1992, 310, 143–147. [Google Scholar] [CrossRef]
- Hale, J.E.; Wuthier, R.E. The Mechanism of Matrix Vesicle Formation. Studies on the Composition of Chondrocyte Microvilli and on the Effects of Microfilament-Perturbing Agents on Cellular Vesiculation. J. Biol. Chem. 1987, 262, 1916–1925. [Google Scholar] [CrossRef]
- Warner, G.P.; Lee Hubbard, H.; Lloyd, G.C.; Wuthier, R.E. 32Pi- And45Ca-Metabolism by Matrix Vesicle-Enriched Microsomes Prepared from Chicken Epiphyseal Cartilage by Isosmotic Percoll Density-Gradient Fractionation. Calcif. Tissue Int. 1983, 35, 327–338. [Google Scholar] [CrossRef]
- Solomon, D.H.; Browning, J.A.; Wilkins, R.J. Inorganic Phosphate Transport in Matrix Vesicles from Bovine Articular Cartilage. Acta Physiol. 2007, 190, 119–125. [Google Scholar] [CrossRef]
- Valhmu, W.B.; Wu, L.N.Y.; Wuthier, R.E. Effects of Ca Pi Ratio, Ca2+ × Pi Ion Product, and PH of Incubation Fluid on Accumulation of 45Ca2+ by Matrix Vesicles in Vitro. Bone Miner. 1990, 8, 195–209. [Google Scholar] [CrossRef]
- Montessuit, C.; Caverzasio, J.; Bonjour, J.P. Characterization of a P(i) Transport System in Cartilage Matrix Vesicles: Potential Role in the Calcification Process. J. Biol. Chem. 1991, 266, 17791–17797. [Google Scholar] [CrossRef]
- Wu, L.N.Y.; Sauer, G.R.; Genge, B.R.; Valhmu, W.B.; Wuthier, R.E. Effects of Analogues of Inorganic Phosphate and Sodium Ion on Mineralization of Matrix Vesicles Isolated from Growth Plate Cartilage of Normal Rapidly Growing Chickens. J. Inorg. Biochem. 2003, 94, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.C.; Bottini, M.; Cory, E.; Bhattacharya, K.; Kuss, P.; Narisawa, S.; Sah, R.L.D.; Beck, L.; Fadeel, B.; Farquharson, C.; et al. Skeletal Mineralization Deficits and Impaired Biogenesis and Function of Chondrocyte-Derived Matrix Vesicles in Phospho1−/− and Phospho1/Pit1 Double Knockout Mice HHS Public Access. J Bone Min. Res. 2016, 31, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Plaut, J.S.; Strzelecka-Kiliszek, A.; Bozycki, L.; Pikula, S.; Buchet, R.; Mebarek, S.; Chadli, M.; Bolean, M.; Simao, A.M.S.; Ciancaglini, P.; et al. Quantitative Atomic Force Microscopy Provides New Insight into Matrix Vesicle Mineralization. Arch. Biochem. Biophys. 2019, 667, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.N.Y.; Genge, B.R.; Sauer, G.R.; Wuthier, R.E. Characterization and Reconstitution of the Nucleational Complex Responsible for Mineral Formation by Growth Plate Cartilage Matrix Vesicles. Connect. Tissue Res. 1996, 35, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.N.Y.; Genge, B.R.; Dunkelberger, D.G.; Legeros, R.Z.; Concannon, B.; Wuthier, R.E. Physicochemical Characterization of the Nucleational Core of Matrix Vesicles. J. Biol. Chem. 1997, 272, 4404–4411. [Google Scholar] [CrossRef]
- Wu, L.N.Y.; Genge, B.R.; Wuthier, R.E. Analysis and Molecular Modeling of the Formation, Structure, and Activity of the Phosphatidylserine-Calcium-Phosphate Complex Associated with Biomineralization. J. Biol. Chem. 2008, 283, 3827–3838. [Google Scholar] [CrossRef]
- Wu, L.N.Y.; Yoshimori, T.; Genge, B.R.; Sauer, G.R.; Kirsch, T.; Ishikawa, Y.; Wuthier, R.E. Characterization of the Nucleational Core Complex Responsible for Mineral Induction by Growth Plate Cartilage Matrix Vesicles. J. Biol. Chem. 1993, 268, 25084–25094. [Google Scholar] [CrossRef]
- Peress, N.S.; Anderson, H.C.; Sajdera, S.W. The Lipids of Matrix Vesicles from Bovine Fetal Epiphyseal Cartilage. Calcif. Tissue Res. 1974, 14, 275–281. [Google Scholar] [CrossRef]
- Wuthier, R.E. Lipids of Matrix Vesicles. Fed. Proc. 1976, 35, 117–121. [Google Scholar]
- Wuthier, R.E. Lipid Composition of Isolated Epiphyseal Cartilage Cells, Membranes and Matrix Vesicles. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1975, 409, 128–143. [Google Scholar] [CrossRef]
- Wu, L.N.Y.; Genge, B.R.; Kang, M.W.; Arsenault, A.L.; Wuthier, R.E. Changes in Phospholipid Extractability and Composition Accompany Mineralization of Chicken Growth Plate Cartilage Matrix Vesicles. J. Biol. Chem. 2002, 277, 5126–5133. [Google Scholar] [CrossRef]
- Genge, B.R.; Wu, L.N.Y.; Wuthier, R.E. Separation and Quantification of Chicken and Bovine Growth Plate Cartilage Matrix Vesicle Lipids by High-Performance Liquid Chromatography Using Evaporative Light Scattering Detection. Anal. Biochem. 2003, 322, 104–115. [Google Scholar] [CrossRef]
- Terkeltaub, R.A. Invited Review Inorganic Pyrophosphate Generation and Disposition in Pathophysiology. Am. J. Physiol. Cell Physiol. 2001, 281, C1–C11. [Google Scholar] [CrossRef]
- Huang, R.; Rosenbach, M.; Vaughn, R.; Prowedini, D.; Rebbe, N.; Hickman, S.; Goding, J.; Terkeltaub, R.; Diego, S. Expression of the Murine Plasma Cell Nucleotide Pyrophosphohydrolase PC-1 Is Shared by Human Liver, Bone, and Cartilage Cells. Regulation of PC-1 Expression in Osteosarcoma Cells by Transforming Growth Factor-β. J. Clin. Investig. 1994, 94, 560–567. [Google Scholar] [CrossRef]
- Andrilli, L.H.S.; Sebinelli, H.G.; Favarin, B.Z.; Cruz, M.A.E.; Ramos, A.P.; Bolean, M.; Millán, J.L.; Bottini, M.; Ciancaglini, P. NPP1 and TNAP Hydrolyze ATP Synergistically during Biomineralization. Purinergic Signal. 2022. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Ochs, R.L.; Rosen, F.; Quach, J.; Mccabe, G.; Solan, J.; Seegmiller, J.E.; Terkeltaub, R.; Lotz, M. Chondrocyte-Derived Apoptotic Bodies and Calcification of Articular Cartilage. Proc. Natl. Acad. Sci. USA 1998, 95, 3094–3099. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Hashimoto, S.; Lotz, M.; Pritzker, K.; Goding, J.; Terkeltaub, R. Up-regulated Expression of the Phosphodiesterase Nucleotide Pyrophosphatase Family Member PC-1 Is a Marker and Pathogenic Factor for Knee Meniscal Cartilage Matrix Calcification. Arthritis Rheum. 2001, 44, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Terkeltaub, R.; Rosenbach, M.; Fong, F.; Goding, J. Causal Link between Nucleotide Pyrophosphohydrolase Overactivity and Increased Intracellular Inorganic Pyrophosphate Generation Demonstrated by Transfection of Cultured Fibroblasts and Osteoblasts with Plasma Cell Membrane Glycoprotein–1. Arthritis Rheum. 1994, 37, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Chuck, A.J.; Arie, E.A.; Green, D.J.; Doherty, M. Diseases Associated with Calcium Pyrophosphate Deposition Disease. Semin. Arthritis Rheum. 1992, 22, 188–202. [Google Scholar] [CrossRef]
- Ciancaglini, P.; Yadav, M.C.; Simão, A.M.S.; Narisawa, S.; Pizauro, J.M.; Farquharson, C.; Hoylaerts, M.F.; Millán, J.L. Kinetic Analysis of Substrate Utilization by Native and TNAP-, NPP1-, or PHOSPHO1-Deficient Matrix Vesicles. J. Bone Miner. Res. 2010, 25, 716–723. [Google Scholar] [CrossRef]
- Simão, A.M.S.; Yadav, M.C.; Ciancaglini, P.; Millán, J.L. Proteoliposomes as Matrix Vesicles’ Biomimetics to Study the Initiation of Skeletal Mineralization. Braz. J. Med. Biol. Res. 2010, 43, 234–241. [Google Scholar] [CrossRef]
- Simão, A.M.S.; Bolean, M.; Hoylaerts, M.F.; Millán, J.L.; Ciancaglini, P. Effects of PH on the Production of Phosphate and Pyrophosphate by Matrix Vesicles’ Biomimetics. Calcif. Tissue Int. 2013, 93, 222–232. [Google Scholar] [CrossRef]
- Bolean, M.; Simão, A.M.S.; Barioni, M.B.; Favarin, B.Z.; Sebinelli, H.G.; Veschi, E.A.; Janku, T.A.B.; Bottini, M.; Hoylaerts, M.F.; Itri, R.; et al. Biophysical Aspects of Biomineralization. Biophys. Rev. 2017, 9, 747–760. [Google Scholar] [CrossRef]
- Latini, S.; Pedata, F. Adenosine in the Central Nervous System: Release Mechanisms and Extracellular Concentrations. J. Neurochem. 2001, 79, 463–484. [Google Scholar] [CrossRef]
- Stewart, A.J.; Roberts, S.J.; Seawright, E.; Davey, M.G.; Fleming, R.H.; Farquharson, C. The Presence of PHOSPHO1 in Matrix Vesicles and Its Developmental Expression Prior to Skeletal Mineralization. Bone 2006, 39, 1000–1007. [Google Scholar] [CrossRef][Green Version]
- Yadav, M.C.; Simão, A.M.S.; Narisawa, S.; Huesa, C.; McKee, M.D.; Farquharson, C.; Millán, J.L. Loss of Skeletal Mineralization by the Simultaneous Ablation of PHOSPHO1 and Alkaline Phosphatase Function: A Unified Model of the Mechanisms of Initiation of Skeletal Calcification. J. Bone Miner. Res. 2011, 26, 286–297. [Google Scholar] [CrossRef]
- Hsu, H.H.T.; Clarke Anderson, H. A Role for ATPase in the Mechanisms of ATP-Dependent Ca and Phosphate Deposition by Isolated Rachitic Matrix Vesicles. Int. J. Biochem. Cell Biol. 1995, 27, 1349–1356. [Google Scholar] [CrossRef]
- Anderson, H.C.; Sipe, J.B.; Hessle, L.; Dhamyamraju, R.; Atti, E.; Camacho, N.P.; Millán, J.L. Impaired Calcification Around Matrix Vesicles of Growth Plate and Bone in Alkaline Phosphatase-Deficient Mice. Am. J. Pathol. 2004, 164, 841–847. [Google Scholar] [CrossRef]
- Morth, J.P.; Pedersen, B.P.; Buch-Pedersen, M.J.; Andersen, J.P.; Vilsen, B.; Palmgren, M.G.; Nissen, P. A Structural Overview of the Plasma Membrane Na+,K +-ATPase and H+-ATPase Ion Pumps. Nat. Rev. Mol. Cell Biol. 2011, 12, 60–70. [Google Scholar] [CrossRef]
- Lingrel, J. The Physiological Significance of the Cardiotonic Steroid/Ouabain-Binding Site of the Na,K-ATPase. Annu. Rev. Physiol. 2010, 17, 395–4112. [Google Scholar] [CrossRef]
- Kaplan, J.H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002, 71, 511–535. [Google Scholar] [CrossRef]
- Sheen, C.R.; Kuss, P.; Narisawa, S.; Yadav, M.C.; Nigro, J.; Wang, W.; Chhea, T.N.; Sergienko, E.A.; Kapoor, K.; Jackson, M.R.; et al. Pathophysiological Role of Vascular Smooth Muscle Alkaline Phosphatase in Medial Artery Calcification. J. Bone Miner. Res. 2015, 30, 824–836. [Google Scholar] [CrossRef]
- Pinkerton, A.B.; Sergienko, E.A.; Bravo, Y.; Dahl, R.; Ma, C.-T.; Sun, Q.; Jackson, M.R.; Cosford, N.D.P.; Millán, J.L. Discovery of 5-((5-Chloro-2- Methoxyphenyl)Sulfonamido)Nicotinamide (SBI-425), a Potent and Orally Bioavailable Tissue-Nonspecific Alkaline Phosphatase (TNAP) Inhibitor. Bioorg. Med. Chem. Lett. 2018, 28, 31–34. [Google Scholar] [CrossRef]
- Ciancaglini, P.; Simão, A.M.S.; Bolean, M.; Millán, J.L.; Rigos, C.F.; Yoneda, J.S.; Colhone, M.C.; Stabeli, R.G. Proteoliposomes in Nanobiotechnology. Biophys. Rev. 2012, 4, 67–81. [Google Scholar] [CrossRef]
- Cornelius, F.; Habeck, M.; Kanai, R.; Toyoshima, C.; Karlish, S.J.D. General and Specific Lipid-Protein Interactions in Na,K-ATPase. Biochim. Biophys. Acta—Biomembr. 2015, 1848, 1729–1743. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.L.; Lamas, R.P.; Ciancaglini, P. Solubilization of Na,K-ATPase from Rabbit Kidney Outer Medulla Using Only C12E8. Braz. J. Med. Biol. Res. 2002, 35, 277–288. [Google Scholar] [CrossRef] [PubMed]
- De Lima Santos, H.; Lopes, M.L.; Maggio, B.; Ciancaglini, P. Na,K-ATPase Reconstituted in Liposomes: Effects of Lipid Composition on Hydrolytic Activity and Enzyme Orientation. Colloids Surf. B Biointerfaces 2005, 41, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, J.S.; Scanavachi, G.; Sebinelli, H.G.; Borges, J.C.; Barbosa, L.R.S.; Ciancaglini, P.; Itri, R. Multimeric Species in Equilibrium in Detergent-Solubilized Na,K-ATPase. Int. J. Biol. Macromol. 2016, 89, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, J.S.; Sebinelli, H.G.; Itri, R.; Ciancaglini, P. Overview on Solubilization and Lipid Reconstitution of Na,K-ATPase: Enzyme Kinetic and Biophysical Characterization. Biophys. Rev. 2020, 12, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Sebinelli, H.G.; Borin, I.A.; Ciancaglini, P.; Bolean, M. Topographical and Mechanical Properties of Liposome Surfaces Harboring Na,K-ATPase by Means of Atomic Force Microscopy. Soft Matter 2019, 15, 2737–2745. [Google Scholar] [CrossRef]
- Majeska, R.J.; Wuthier, R.E. Studies on Matrix Vesicles Isolated from Chick Epiphyseal Cartilage Association of Pyrophosphatase and ATPase Activities with Alkaline Phosphatase. BBA—Enzymol. 1975, 391, 51–60. [Google Scholar] [CrossRef]
- Van Belle, H. Alkaline Phosphatase. I. Kinetics and Inhibition by Levamisole of Purified Isoenzymes from Humans. Clin. Chem. 1976, 22, 972–976. [Google Scholar] [CrossRef]
- Heinonen, J.K.; Lahti, R.J. A New and Convenient Colorimetric Determination of Inorganic Orthophosphate and Its Application to the Assay of Inorganic Pyrophosphatase. Anal. Biochem. 1981, 113, 313–317. [Google Scholar] [CrossRef]
- Lévesque, S.A.; Lavoie, É.G.; Lecka, J.; Bigonnesse, F.; Sévigny, J. Specificity of the Ecto-ATPase Inhibitor ARL 67156 on Human and Mouse Ectonucleotidases. Br. J. Pharmacol. 2007, 152, 141–150. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, W.; Gu, J.; Zhang, X.; Dang, J.; Wang, J.; Zheng, Y.; Huang, F.; Yuan, J.; Xue, Y.; et al. Human Gingival Tissue-Derived MSC Suppress Osteoclastogenesis and Bone Erosion via CD39-Adenosine Signal Pathway in Autoimmune Arthritis. EBioMedicine 2019, 43, 620–631. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, Z.; Chen, Y.; Deng, Y.; Zeng, D.; Liu, Y.; Huang, F.; Wang, J.; Liu, Y.; Bellanti, J.A.; et al. CD39 Produced from Human GMSCs Regulates the Balance of Osteoclasts and Osteoblasts through the Wnt/β-Catenin Pathway in Osteoporosis. Mol. Ther. 2020, 28, 1518–1532. [Google Scholar] [CrossRef]
- Camolezi, F.L.; Daghastanli, K.R.P.; Magalhães, P.P.; Pizauro, J.M.; Ciancaglini, P. Construction of an Alkaline Phosphatase-Liposome System: A Tool for Biomineralization Study. Int. J. Biochem. Cell Biol. 2002, 34, 1091–1101. [Google Scholar] [CrossRef]
- Zhang, L.; Balcerzak, M.; Radisson, J.; Thouverey, C.; Pikula, S.; Rard Azzar, G.; Buchet, R. Phosphodiesterase Activity of Alkaline Phosphatase in ATP-Initiated Ca(2+) and Phosphate Deposition in Isolated Chicken Matrix Vesicles. J. Biol. Chem. 2005, 280, 37289–37296. [Google Scholar] [CrossRef]
- Buchet, R.; Pikula, S.; Magne, D.; Mebarek, S. Isolation and Characteristics of Matrix Vesicles. In Phosphatase Modulators; Millán, J.L., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1053, pp. 115–124. ISBN 978-1-62703-561-3. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kozlenkov, A.; Le Du, M.H.; Cuniasse, P.; Ny, T.; Hoylaerts, M.F.; Millán, J.L. Residues Determining the Binding Specificity of Uncompetitive Inhibitors to Tissue-Nonspecific Alkaline Phosphatase. J. Bone Miner. Res. 2004, 19, 1862–1872. [Google Scholar] [CrossRef]
- Meyer, J.L. Can Biological Calcification Occur in the Presence of Pyrophosphate? Arch. Biochem. Biophys. 1984, 231, 1–8. [Google Scholar] [CrossRef]
- Johnson, K.; Moffa, A.; Chen, Y.; Pritzker, K.; Goding, J.; Terkeltaub, R. Matrix Vesicle Plasma Cell Membrane Glycoprotein-1 Regulates Mineralization by Murine Osteoblastic MC3T3 Cells. J. Bone Miner. Res. 1999, 14, 883–892. [Google Scholar] [CrossRef]
- Hessle, L.; Johnson, K.A.; Anderson, H.C.; Narisawa, S.; Sali, A.; Goding, J.W.; Terkeltaub, R.; Millán, J.L. Tissue-Nonspecific Alkaline Phosphatase and Plasma Cell Membrane Glycoprotein-1 Are Central Antagonistic Regulators of Bone Mineralization. Proc. Natl. Acad. Sci. USA 2002, 99, 9445–9449. [Google Scholar] [CrossRef]
- Shinoda, T.; Ogawa, H.; Cornelius, F.; Toyoshima, C. Crystal Structure of the Sodium-Potassium Pump at 2.4 Resolution. Nature 2009, 459, 446–450. [Google Scholar] [CrossRef]
- Simão, A.M.S.; Bolean, M.; Favarin, B.Z.; Veschi, E.A.; Tovani, C.B.; Ramos, A.P.; Bottini, M.; Buchet, R.; Millán, J.L.; Ciancaglini, P. Lipid Microenvironment Affects the Ability of Proteoliposomes Harboring TNAP to Induce Mineralization without Nucleators. J. Bone Miner. Metab. 2019, 37, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Tejera-Garcia, R.; Ranjan, S.; Zamotin, V.; Sood, R.; Kinnunen, P.K.J. Making Unilamellar Liposomes Using Focused Ultrasound. Langmuir 2011, 27, 10088–10097. [Google Scholar] [CrossRef] [PubMed]
- De Lima Santos, H.; Ciancaglini, P. Kinetic Characterization of Na,K-ATPase from Rabbit Outer Renal Medulla: Properties of the (Aβ)2 Dimer. Comp. Biochem. Physiol.—B Biochem. Mol. Biol. 2003, 135, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Rigos, C.F.; Santos, H.D.L.; Thedei, G.; Ward, R.J.; Ciancaglini, P. Influence of Enzyme Conformational Changes on Catalytic Activity Investigated by Circular Dichroism Spectroscopy. Biochem. Mol. Biol. Educ. 2003, 31, 329–332. [Google Scholar] [CrossRef]
- Santos, H.D.L.; Rigos, C.F.; Tedesco, A.C.; Ciancaglini, P. Rose Bengal Located within Liposome Do Not Affect the Activity of Inside-out Oriented Na,K-ATPase. Biochim. Biophys. Acta—Biomembr. 2005, 1715, 96–103. [Google Scholar] [CrossRef]
- Rigos, C.F.; Santos, H.D.L.; Ward, R.J.; Ciancaglini, P. Lipid Bilayer Stabilization of the Na,K-ATPase Reconstituted in DPPC/DPPE Liposomes. Cell Biochem. Biophys. 2006, 44, 438–445. [Google Scholar] [CrossRef]
- Harmey, D.; Hessle, L.; Narisawa, S.; Johnson, K.A.; Terkeltaub, R.; Millán, J.L. Concerted Regulation of Inorganic Pyrophosphate and Osteopontin by Akp2, Enpp1, and Ank: An Integrated Model of the Pathogenesis of Mineralization Disorders. Am. J. Pathol. 2004, 164, 1199–1209. [Google Scholar] [CrossRef]
- Li, L.; Buchet, R.; Wu, Y. Dimethyl Sulfoxide-Induced Hydroxyapatite Formation: A Biological Model of Matrix Vesicle Nucleation to Screen Inhibitors of Mineralization. Anal. Biochem. 2008, 381, 123–128. [Google Scholar] [CrossRef]
- Genge, B.R.; Wu, L.N.Y.; Wuthier, R.E. In Vitro Modeling of Matrix Vesicle Nucleation: Synergistic Stimulation of Mineral Formation by Annexin A5 and Phosphatidylserine. J. Biol. Chem. 2007, 282, 26035–26045. [Google Scholar] [CrossRef]
- Veschi, E.A.; Bolean, M.; da Silva Andrilli, L.H.; Sebinelli, H.G.; Strzelecka-Kiliszek, A.; Bandorowicz-Pikula, J.; Pikula, S.; Granjon, T.; Mebarek, S.; Magne, D.; et al. Mineralization Profile of Annexin A6-Harbouring Proteoliposomes: Shedding Light on the Role of Annexin A6 on Matrix Vesicle-Mediated Mineralization. Int. J. Mol. Sci. 2022, 23, 8945. [Google Scholar] [CrossRef]
- Favarin, B.F.; Andrade, M.A.R.; Bolean, M.; Simão, A.M.S.; Ramos, A.P.; Hoylaerts, M.F.; Millán, J.L.; Ciancaglini, P. Effect of the Presence of Cholesterol in the Interfacial Microenvironment on the Modulation of the Alkaline Phosphatase Activity during in Vitro Mineralization. Colloids Surf. B Biointerfaces 2017, 155, 466–476. [Google Scholar] [CrossRef]
- Bolean, M.; Simão, M.S.; Kiffer-Moreira, T.; Hoylaerts, M.F.; Luis Millán, J.; Itri, R.; Ciancaglini, P. Proteoliposomes with the Ability to Transport Ca2+ into the Vesicles and Hydrolyze Phosphosubstrates on Their Surface Graphical Abstract HHS Public Access. Arch. Biochem. Biophys. 2015, 584, 79–89. [Google Scholar] [CrossRef]
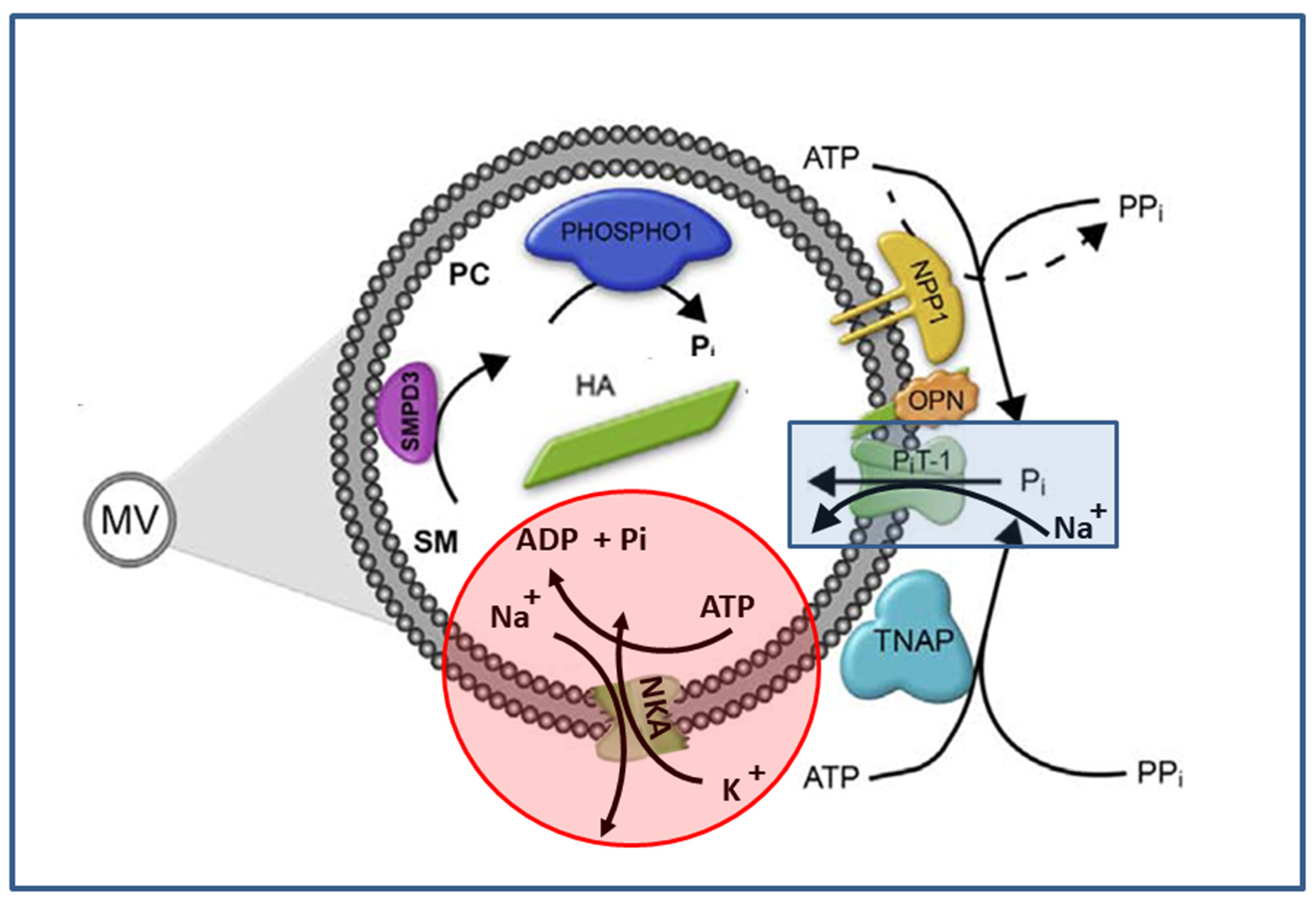

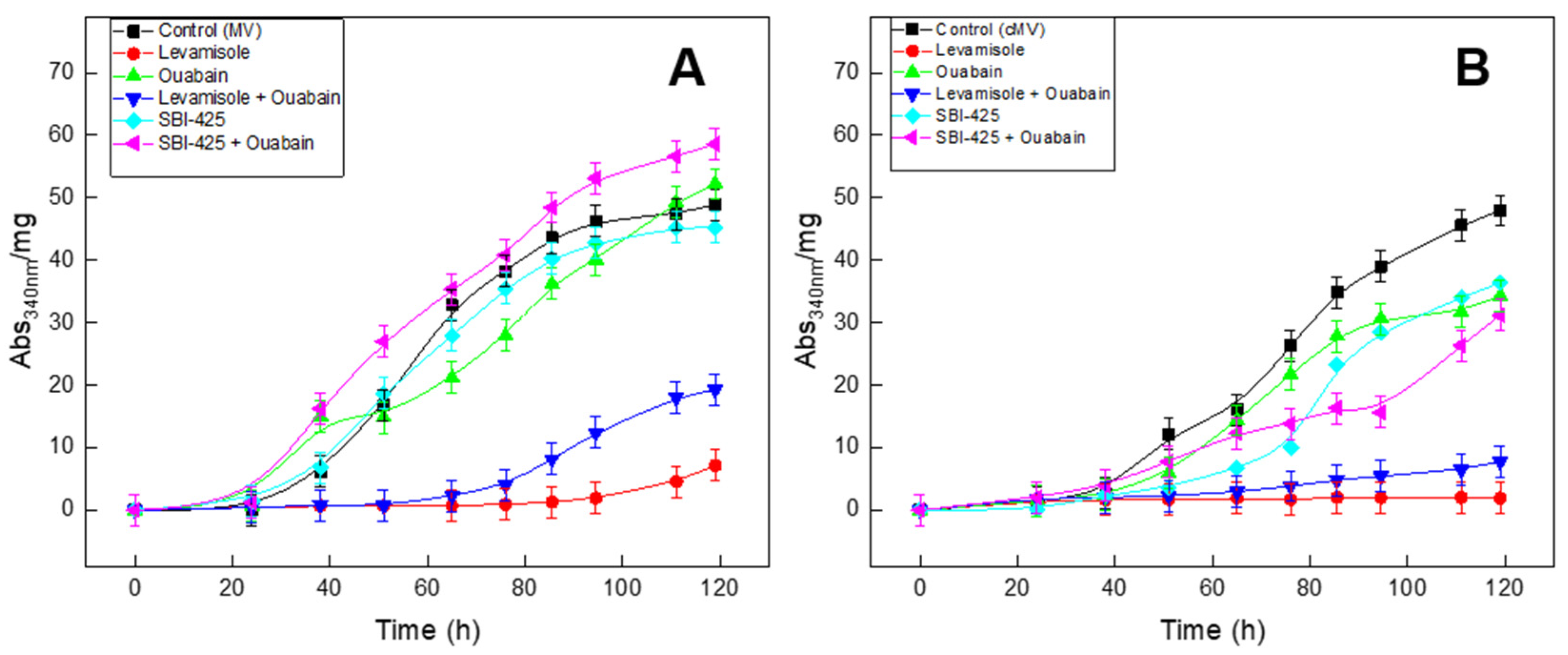
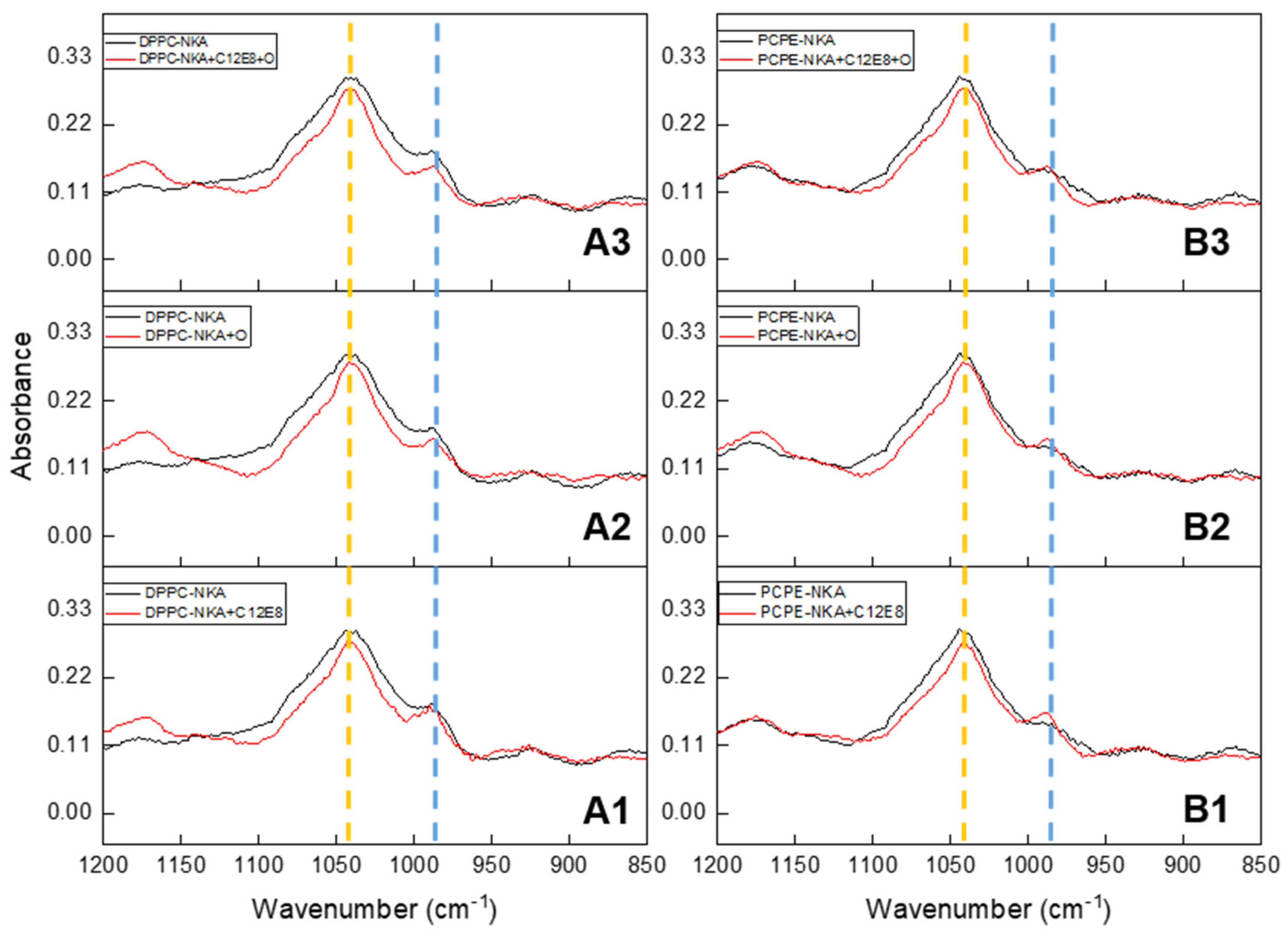
| ATPase Activity Method | Ouabain (3 mM) | Levamisole (5 mM) | ARL (0.1 mM) | SBI-425 (5 μM) | SBI-425 (10 μM) |
|---|---|---|---|---|---|
| Colorimetric | 93.2 ± 3.6 | 27.8 | 92.0 ± 5.6 | 78.5 | 64.6 |
| 31P NMR | 97.6 ± 2.9 | 31.4 ± 3.9 | 86.3 ± 4.2 | ND | ND |
| Inhibitor (Concentration) | ti (h) | tf (h) | tmax rate (h) | Umax (Abs340nm·mg−1) | PMP (h−1) | |
|---|---|---|---|---|---|---|
| MV | Without | 20.0 ± 0.4 | 120.0 ± 0.6 | 57.9 ± 0.8 | 50.1 ± 0.8 | 0.86 ± 0.04 |
| Levamisole (5 mM) | ND | ND | ND | ND | ND | |
| Ouabain (3 mM) | 24.3 ± 0.7 | 160.5 ± 7.6 | 74.4 ± 0.7 | 54.6 ± 3.3 | 0.73 ± 0.03 | |
| Levamisole (5mM) + Ouabain (3 mM) | 60.0 ± 0.3 | 119.0 ± 1.6 | 91.7 ± 0.5 | 21.4 ± 0.8 | 0.23 ± 0.02 | |
| SBI-425 (10 μM) | 15.0 ± 0.4 | 120.0 ± 2.4 | 58.9 ± 0.3 | 48.8 ± 1.1 | 0.83 ± 0.05 | |
| SBI-425 (10 μM) + Ouabain (3 mM) | 14.6 ± 0.2 | 140.7 ± 1.5 | 62.3 ± 5.6 | 71.6 ± 2.3 | 1.15 ± 0.07 | |
| cMV | Without | 34.1 ± 1.3 | 140.9 ± 5.2 | 79.7 ± 3.4 | 58.6 ± 3.7 | 0.74 ± 0.04 |
| Levamisole (5 mM) | ND | ND | ND | ND | ND | |
| Ouabain (3 mM) | 35.9 ± 1.6 | 125.6 ± 4.1 | 35.2 ± 1.0 | 69.8 ± 1.2 | 1.98 ± 0.09 | |
| Levamisole (5 mM) + Ouabain (3 mM) | ND | ND | ND | ND | ND | |
| SBI-425 (10 μM) | 48.9 ± 1.8 | 128.2 ± 3.9 | 37.4 ± 2.3 | 83.3 ± 2.1 | 2.22 ± 0.11 | |
| SBI-425 (10 μM) + Ouabain (3 mM) | 37.6 ± 3.8 | 94.4 ± 2.4 | 15.6 ± 2.2 | 55.9 ± 4.8 | 3.58 ± 0.14 |
| Sample | NKA (mg·mL−1) | ATPase (U·mg−1) | Diameter (nm) | IP | Abs340nm·mg−1 |
|---|---|---|---|---|---|
| NKA solubilized | 0.19 | 0.336 | 19.1 ± 1 * | -- | 51.9 ± 5 |
| DPPC | --- | --- | 365 ± 35 | 0.7 | --- |
| DPPC:DPPE | --- | --- | 504 ± 50 | 1.3 | --- |
| NKA-DPPC | 0.61 | 0.141 | 634 ± 60 | 1.4 | 38.4 ± 4 |
| NKA-DPPC:DPPE | 0.53 | 0.290 | 899 ± 90 | 1.4 | 51.4 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebinelli, H.G.; Andrilli, L.H.S.; Favarin, B.Z.; Cruz, M.A.E.; Bolean, M.; Fiore, M.; Chieffo, C.; Magne, D.; Magrini, A.; Ramos, A.P.; et al. Shedding Light on the Role of Na,K-ATPase as a Phosphatase during Matrix-Vesicle-Mediated Mineralization. Int. J. Mol. Sci. 2022, 23, 15072. https://doi.org/10.3390/ijms232315072
Sebinelli HG, Andrilli LHS, Favarin BZ, Cruz MAE, Bolean M, Fiore M, Chieffo C, Magne D, Magrini A, Ramos AP, et al. Shedding Light on the Role of Na,K-ATPase as a Phosphatase during Matrix-Vesicle-Mediated Mineralization. International Journal of Molecular Sciences. 2022; 23(23):15072. https://doi.org/10.3390/ijms232315072
Chicago/Turabian StyleSebinelli, Heitor Gobbi, Luiz Henrique Silva Andrilli, Bruno Zoccaratto Favarin, Marcos Aantonio Eufrasio Cruz, Maytê Bolean, Michele Fiore, Carolina Chieffo, David Magne, Andrea Magrini, Ana Paula Ramos, and et al. 2022. "Shedding Light on the Role of Na,K-ATPase as a Phosphatase during Matrix-Vesicle-Mediated Mineralization" International Journal of Molecular Sciences 23, no. 23: 15072. https://doi.org/10.3390/ijms232315072
APA StyleSebinelli, H. G., Andrilli, L. H. S., Favarin, B. Z., Cruz, M. A. E., Bolean, M., Fiore, M., Chieffo, C., Magne, D., Magrini, A., Ramos, A. P., Millán, J. L., Mebarek, S., Buchet, R., Bottini, M., & Ciancaglini, P. (2022). Shedding Light on the Role of Na,K-ATPase as a Phosphatase during Matrix-Vesicle-Mediated Mineralization. International Journal of Molecular Sciences, 23(23), 15072. https://doi.org/10.3390/ijms232315072









