Transdermal Delivery of 2-PAM as a Tool to Increase the Effectiveness of Traditional Treatment of Organophosphate Poisoning
Abstract
:1. Introduction
2. Results
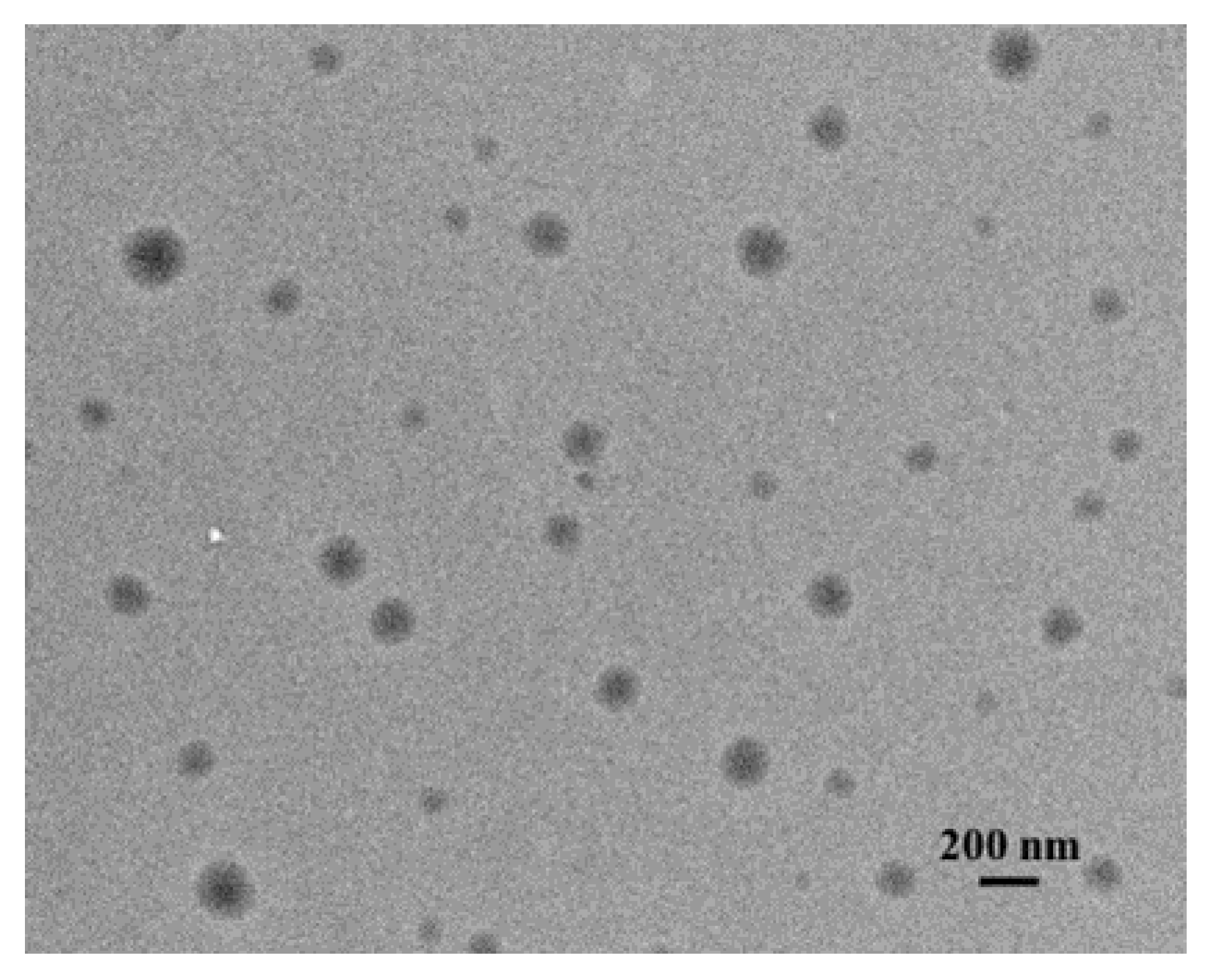
2.1. Transfersome Preparation and Characterization
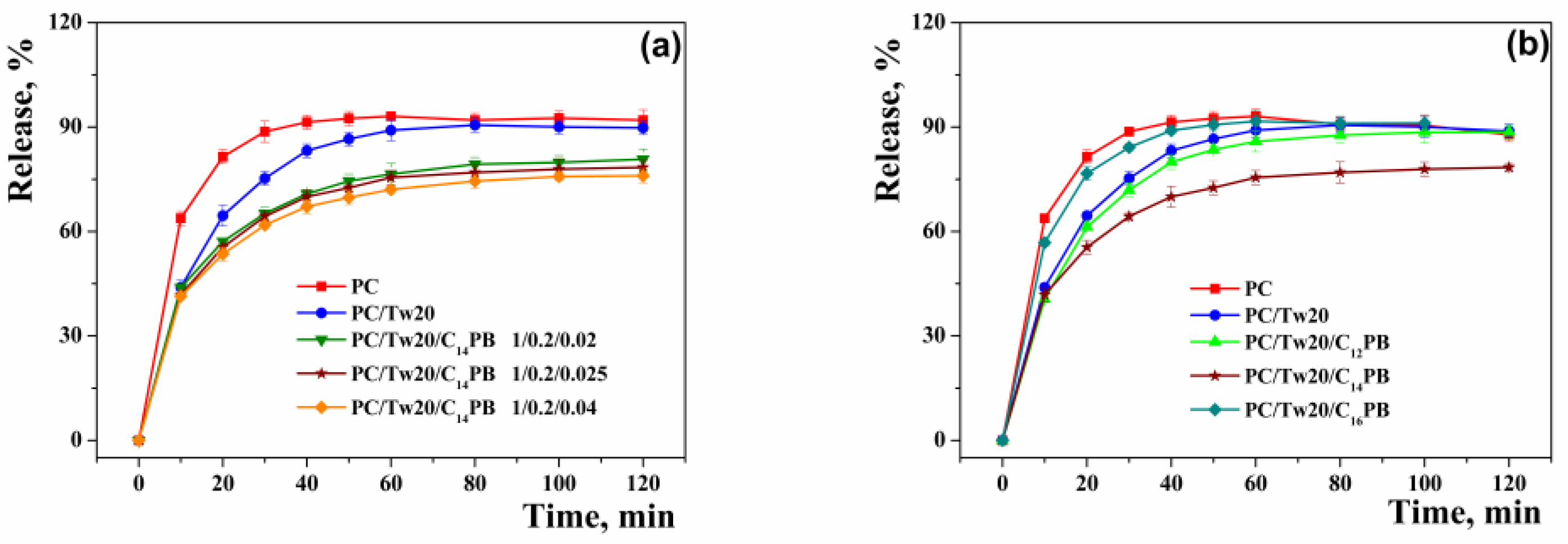
2.2. In Vitro/Ex Vivo Release Study
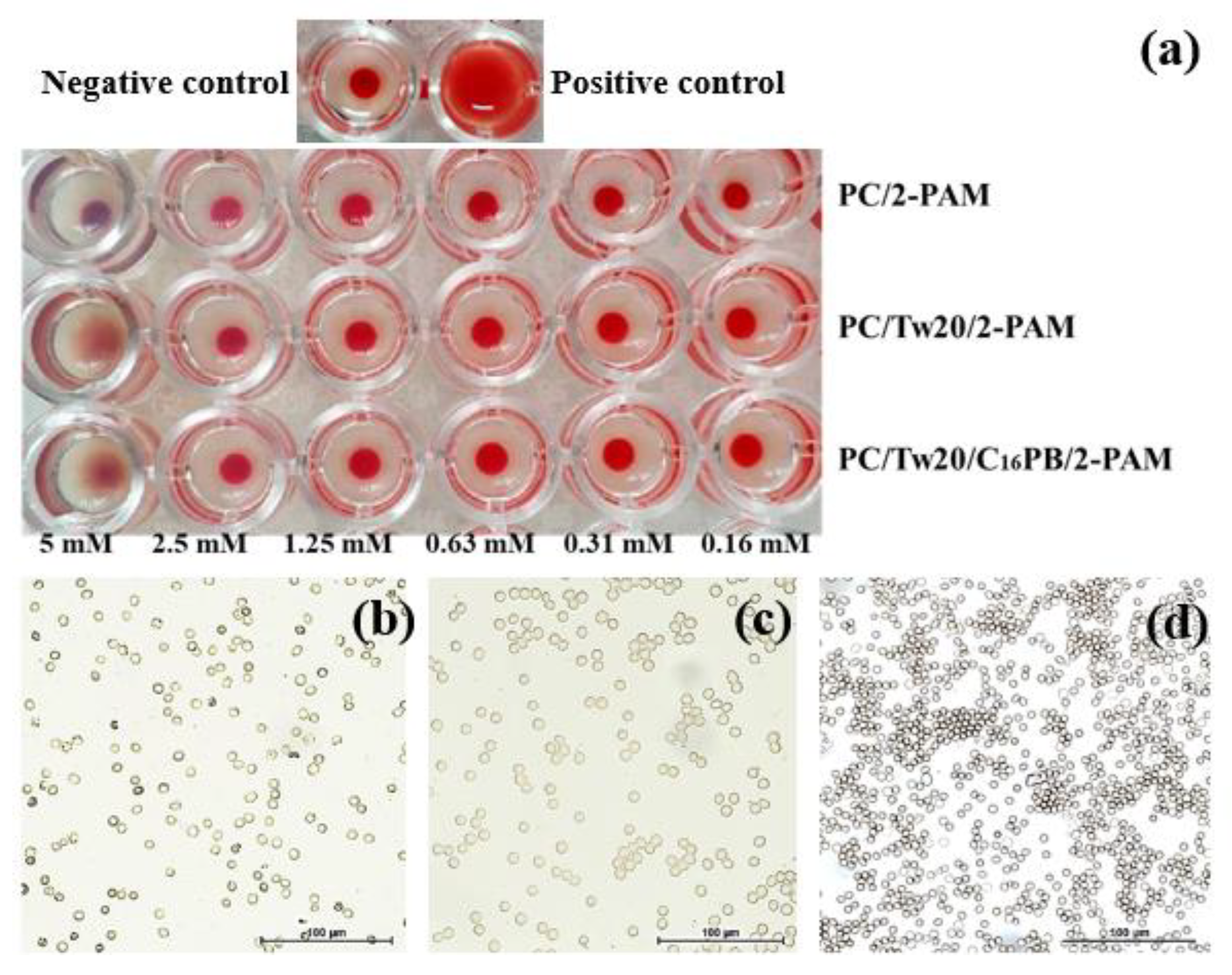
2.3. In Vitro Toxicity Assessment
2.4. Evaluation of Effectiveness In Vivo
3. Discussion
4. Materials and Methods
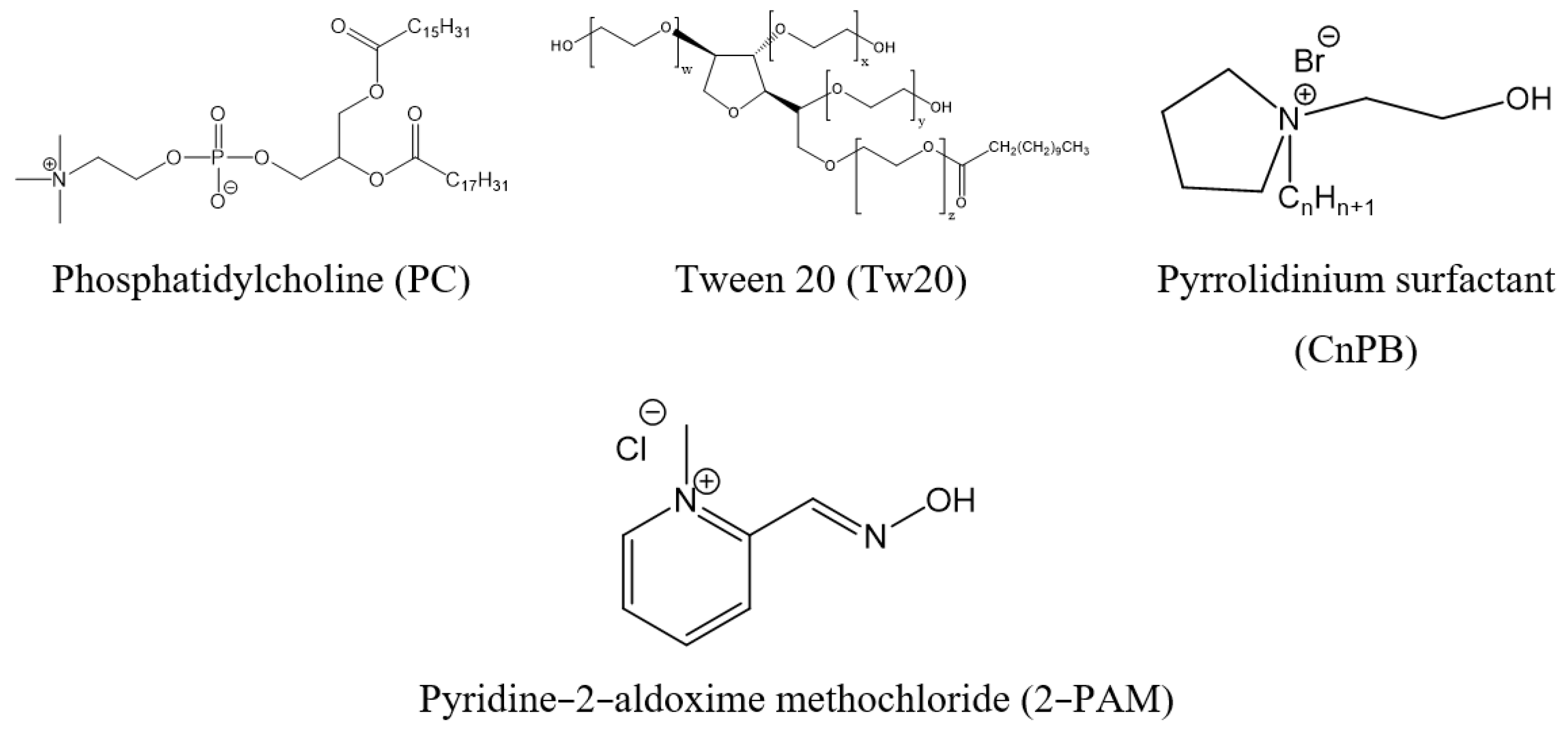
4.1. Materials
4.2. Animals
4.3. Vesicle Preparation
4.4. Particle Zeta Potential and Size Distribution Analysis
4.5. Transmission Electron Microscopy (TEM)
4.6. Potentiometry
4.7. Drug Loading and Quantification of Encapsulation Efficiency (EE%)
4.8. In Vitro Substrate Release Rate Analysis
4.9. In Vitro Toxicity Assessment
4.10. Ex Vivo Substrate Release Studies
4.11. AChE Reactivation in Rat Blood
4.12. 2-PAM Pharmacokinetics in Rat Plasma
4.13. Rat Survival
4.14. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobrlova, T.; Korabecny, J.; Soukup, O. Current Approaches to Enhancing Oxime Reactivator Delivery into the Brain. Toxicology 2019, 423, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, Z.; Maček, N.; Sit, R.K.; Radić, Z.; Fokin, V.V.; Barry Sharpless, K.; Taylor, P. Centrally Acting Oximes in Reactivation of Tabun-Phosphoramidated AChE. Chem.-Biol. Interact. 2013, 203, 77–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercey, G.; Verdelet, T.; Renou, J.; Kliachyna, M.; Baati, R.; Nachon, F.; Jean, L.; Renard, P.-Y. Reactivators of Acetylcholinesterase Inhibited by Organophosphorus Nerve Agents. Acc. Chem. Res. 2012, 45, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Hulse, E.J.; Davies, J.O.J.; Simpson, A.J.; Sciuto, A.M.; Eddleston, M. Respiratory Complications of Organophosphorus Nerve Agent and Insecticide Poisoning: Implications for Respiratory and Critical Care. Am. J. Respir. Crit. Care Med. 2014, 190, 1342–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization & United Nations Environment Programme. Public Health Impact of Pesticides Used in Agriculture. 1990. Available online: http://www.who.int/iris/handle/10665/39772 (accessed on 13 March 2019).
- King, A.M.; Aaron, C.K. Organophosphate and Carbamate Poisoning. Emerg. Med. Clin. N. Am. 2015, 33, 133–151. [Google Scholar] [CrossRef]
- Aroniadou-Anderjaska, V.; Apland, J.P.; Figueiredo, T.H.; De Araujo Furtado, M.; Braga, M.F. Acetylcholinesterase Inhibitors (Nerve Agents) as Weapons of Mass Destruction: History, Mechanisms of Action, and Medical Countermeasures. Neuropharmacology 2020, 181, 108298. [Google Scholar] [CrossRef] [PubMed]
- Petroianu, G.A.; Nurulain, S.M.; Shafiullah, M.; Hasan, M.Y.; Kuča, K.; Lorke, D.E. Usefulness of Administration of Non-Organophosphate Cholinesterase Inhibitors before Acute Exposure to Organophosphates: Assessment Using Paraoxon: Non-Organophosphate Cholinesterase Inhibitors as Pre-Treatment. J. Appl. Toxicol. 2013, 33, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D.E.; Petroianu, G.A. Reversible Cholinesterase Inhibitors as Pretreatment for Exposure to Organophosphates. A Review: Cholinesterase Inhibitors as Pretreatment for Organophosphate Exposure. J. Appl. Toxicol. 2019, 39, 101–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorke, D.E.; Nurulain, S.M.; Hasan, M.Y.; Kuča, K.; Petroianu, G.A. Combined Pre- and Posttreatment of Paraoxon Exposure. Molecules 2020, 25, 1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajgar, J. Organophosphates/Nerve Agent Poisoning: Mechanism of Action, Diagnosis, Prophylaxis, And Treatment. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2004; Volume 38, pp. 151–216. ISBN 978-0-12-010338-6. [Google Scholar]
- Bajgar, J.; Kuca, K.; Fusek, J.; Jun, D.; Bartosova, L. Cholinesterase Reactivators as Prophylactics Against Nerve Agents. CBC 2010, 6, 2–8. [Google Scholar] [CrossRef]
- Bajgar, J.; Fusek, J.; Kassa, J.; Kuca, K.; Jun, D. Chemical Aspects of Pharmacological Prophylaxis Against Nerve Agent Poisoning. CMC 2009, 16, 2977–2986. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Chattopadhyay, P.; Ghosh, A.; Pathak, M.P.; Gogoi, J.; Veer, V. Protection by a Transdermal Patch Containing Eserine and Pralidoxime Chloride for Prophylaxis against (±)-Anatoxin A Poisoning in Rats. Eur. J. Pharm. Sci. 2014, 56, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wei, T.; Goldberg, H.; Wang, W.; Cullion, K.; Kohane, D.S. Getting Drugs Across Biological Barriers. Adv. Mater. 2017, 29, 1606596. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Wischke, C.; Neffe, A.T.; Ma, N.; Alexiev, U.; Lendlein, A. Nanocarriers for drug delivery into and through the skin—Do existing technologies match clinical challenges? J. Control. Release 2016, 242, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative pharmaceutical developments based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaynanova, G.; Vasileva, L.; Kashapov, R.; Kuznetsova, D.; Kushnazarova, R.; Tyryshkina, A.; Vasilieva, E.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembling Drug Formulations with Tunable Permeability and Biodegradability. Molecules 2021, 26, 6786. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyay, P.; Ghosh, A.; Pathak, M.P.; Singh, S.; Veer, V. Acute Dermal Irritation, Sensitization, and Acute Toxicity Studies of a Transdermal Patch for Prophylaxis Against (±) Anatoxin-A Poisoning. Int. J. Toxicol. 2013, 32, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Singh, S.; Policegoudra, R.; Chattopadhyay, P.; Ghosh, A.; Veer, V. Evaluation of the Mutagenic Potential of a Combinational Prophylactic Transdermal Patch by Ames Test. Immuno-Anal. Biol. Spe. 2013, 28, 322–326. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyay, P.; Ghosh, A.; Bhattacharya, S.S.; Kundu, A.; Veer, V. Accelerated Stability Testing of a Transdermal Patch Composed of Eserine and Pralidoxime Chloride for Prophylaxis against (±)-Anatoxin A Poisoning. J. Food Drug Anal. 2014, 22, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Chattopadhyay, P.; Ghosh, A.; Bhatnagar, A.; Veer, V. Pharmacokinetic and Biodistribution Study of Eserine and Pralidoxime Chloride in Rabbits Following a Single Application of a Transdermal Patch. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 219–230. [Google Scholar] [CrossRef]
- Bajgar, J.; Fusek, J.; Sevelova, L.; Kassa, J. Original Transdermal Prophylactic Antidote Against Nerve Agents—TRANSANT. In Proceedings of the CB Medical Treatment Symposium, Spiez, Switzerland, 25–30 April 2004; p. 14. [Google Scholar]
- Vasilieva, E.A.; Lukashenko, S.S.; Voloshina, A.D.; Strobykina, A.S.; Vasileva, L.A.; Zakharova, L.Y. The Synthesis and Properties of Homologous Series of Surfactants Containing the Pyrrolidinium Head Group with Hydroxyethyl Moiety. Russ. Chem. Bull. (Int. Ed) 2018, 67, 1280–1286. [Google Scholar] [CrossRef]
- Balata, G.F.; Faisal, M.M.; Elghamry, H.A.; Sabry, S.A. Preparation and Characterization of Ivabradine HCl Transfersomes for Enhanced Transdermal Delivery. J. Drug Deliv. Sci. Technol. 2020, 60, 101921. [Google Scholar] [CrossRef]
- Estupiñán, Ó.; Rendueles, C.; Suárez, P.; Rey, V.; Murillo, D.; Morís, F.; Gutiérrez, G.; Blanco-López, M.D.C.; Matos, M.; Rodríguez, R. Nano-Encapsulation of Mithramycin in Transfersomes and Polymeric Micelles for the Treatment of Sarcomas. JCM 2021, 10, 1358. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Manca, M.L.; Peris, J.E.; Usach, I.; Diez-Sales, O.; Matos, M.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Tocopherol-Loaded Transfersomes: In Vitro Antioxidant Activity and Efficacy in Skin Regeneration. Int. J. Pharm. 2018, 551, 34–41. [Google Scholar] [CrossRef] [PubMed]
- De Marco Almeida, F.; Silva, C.N.; de Araujo Lopes, S.C.; Santos, D.M.; Torres, F.S.; Cardoso, F.L.; Martinelli, P.M.; da Silva, E.R.; de Lima, M.E.; Miranda, L.A.F.; et al. Physicochemical Characterization and Skin Permeation of Cationic Transfersomes Containing the Synthetic Peptide PnPP-19. CDD 2018, 15, 1064–1071. [Google Scholar] [CrossRef]
- Gadag, S.; Narayan, R.; Sabhahit, J.N.; Hari, G.; Nayak, Y.; Pai, K.S.R.; Garg, S.; Nayak, U.Y. Transpapillary Iontophoretic Delivery of Resveratrol Loaded Transfersomes for Localized Delivery to Breast Cancer. Biomater. Adv. 2022, 140, 213085. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, L.A.; Gaynanova, G.A.; Nizameev, I.R.; Petrova, A.A.; Kadirov, M.K.; Gorshkova, T.A.; Zakharova, L.Y. Enhanced Potato Tuber Penetration of Carboxin via Ultradeformable Liposomes. Food Biosci. 2022, 50, 102003. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Boonpisutiinant, K.; Chutoprapat, R. Preparation, Characterization and Permeation Study of Topical Gel Loaded with Transfersomes Containing Asiatic Acid. Molecules 2022, 27, 4865. [Google Scholar] [CrossRef]
- Fan, Y.; Lu, Y.; Cheng, B.; Wei, Y.; Wei, Y.; Piao, J.; Li, F.; Zheng, H. Correlation between in Vivo Microdialysis Pharmacokinetics and Ex Vivo Permeation for Sinomenine Hydrochloride Transfersomes with Enhanced Skin Absorption. Int. J. Pharm. 2022, 621, 121789. [Google Scholar] [CrossRef]
- Moqejwa, T.; Marimuthu, T.; Kondiah, P.P.D.; Choonara, Y.E. Development of Stable Nano-Sized Transfersomes as a Rectal Colloid for Enhanced Delivery of Cannabidiol. Pharmaceutics 2022, 14, 703. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Lee, E.H.; Choi, S.H.; Kim, C.K. In Vitro and in Vivo Transfection Efficiency of a Novel Ultradeformable Cationic Liposome. Biomaterials 2004, 25, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, D.B.; ElMeshad, A.N.; Fadel, M.; Tawfik, A.; Ramez, S.A. Photodynamic Therapy Fortified with Topical Oleyl Alcohol-Based Transethosomal 8-Methoxypsoralen for Ameliorating Vitiligo: Optimization and Clinical Study. Int. J. Pharm. 2022, 614, 121459. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, D.; Ahyayauch, H.; Goñi, F.M. The Mechanism of Detergent Solubilization of Lipid Bilayers. Biophys. J. 2013, 105, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, M.H.; Abu Lila, A.S.; Shawky, S.M.; Almansour, K.; Alshammari, F.; Khafagy, E.-S.; Makram, T.S. Experimental Design and Optimization of Nano-Transfersomal Gel to Enhance the Hypoglycemic Activity of Silymarin. Polymers 2022, 14, 508. [Google Scholar] [CrossRef] [PubMed]
- Elsana, H.; Olusanya, T.O.B.; Carr-Wilkinson, J.; Darby, S.; Faheem, A.; Elkordy, A.A. Evaluation of Novel Cationic Gene Based Liposomes with Cyclodextrin Prepared by Thin Film Hydration and Microfluidic Systems. Sci. Rep. 2019, 9, 15120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuznetsova, D.A.; Gaynanova, G.A.; Vasilieva, E.A.; Pavlov, R.V.; Zueva, I.V.; Babaev, V.M.; Kuznetsov, D.M.; Voloshina, A.D.; Petrov, K.A.; Zakharova, L.Y.; et al. Oxime Therapy for Brain AChE Reactivation and Neuroprotection after Organophosphate Poisoning. Pharmaceutics 2022, 14, 1950. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Xu, Z.; Sun, M.; Fu, S.; Zhao, F.; Wang, D.; He, Z.; Zhai, Y.; Sun, J. Rebamipide Liposome as an Effective Ocular Delivery System for the Management of Dry Eye Disease. J. Drug Deliv. Sci. Technol. 2022, 75, 103654. [Google Scholar] [CrossRef]
- Gomes, A.S.; Reis, F.M.P.; Ceravolo, I.P.; Dias-Souza, M.V. Effectiveness of Free and Liposome-Entrapped Antitumoral Drugs against Hepatocellular Carcinoma: A Comparative In vitro Study. Biointerface Res. Appl. Chem. 2023, 13, 122. [Google Scholar] [CrossRef]
- Hadidi, N.; Saffari, M.; Faizi, M. Optimized Transferosomal Bovine Lactoferrin (BLF) as a Promising Novel Non-Invasive Topical Treatment for Genital Warts Caused by Human Papiluma Virus (HPV). Iran J. Pharm. Res. 2018, 17, 12–23. [Google Scholar]
- Pavlović, N.; Mijalković, J.; Đorđević, V.; Pecarski, D.; Bugarski, B.; Knežević-Jugović, Z. Ultrasonication for Production of Nanoliposomes with Encapsulated Soy Protein Concentrate Hydrolysate: Process Optimization, Vesicle Characteristics and In Vitro Digestion. Food Chem. X 2022, 15, 100370. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Transferrin-Functionalized Liposomes Loaded with Vitamin VB12 for Alzheimer’s Disease Therapy. Int. J. Pharm. 2022, 626, 122167. [Google Scholar] [CrossRef] [PubMed]
- Dadparvar, M.; Wagner, S.; Wien, S.; Kufleitner, J.; Worek, F.; von Briesen, H.; Kreuter, J. HI 6 Human Serum Albumin Nanoparticles—Development and Transport over an in Vitro Blood–Brain Barrier Model. Toxicol. Lett. 2011, 206, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Dadparvar, M.; Wagner, S.; Wien, S.; Worek, F.; von Briesen, H.; Kreuter, J. Freeze-Drying of HI-6-Loaded Recombinant Human Serum Albumin Nanoparticles for Improved Storage Stability. Eur. J. Pharm. Biopharm. 2014, 88, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Kufleitner, J.; Zensi, A.; Dadparvar, M.; Wien, S.; Bungert, J.; Vogel, T.; Worek, F.; Kreuter, J.; von Briesen, H. Nanoparticulate Transport of Oximes over an In Vitro Blood-Brain Barrier Model. PLoS ONE 2010, 5, e14213. [Google Scholar] [CrossRef] [Green Version]
- Kufleitner, J.; Wagner, S.; Worek, F.; von Briesen, H.; Kreuter, J. Adsorption of Obidoxime onto Human Serum Albumin Nanoparticles: Drug Loading, Particle Size and Drug Release. J. Microencapsul. 2010, 27, 506–513. [Google Scholar] [CrossRef]
- Yang, J.; Fan, L.; Wang, F.; Luo, Y.; Sui, X.; Li, W.; Zhang, X.; Wang, Y. Rapid-Releasing of HI-6 via Brain-Targeted Mesoporous Silica Nanoparticles for Nerve Agent Detoxification. Nanoscale 2016, 8, 9537–9547. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Zueva, I.V.; Petrov, K.A.; Lukashenko, S.S.; Nizameev, I.R.; Kulik, N.V.; Voloshina, A.D.; Almasy, L.; Kadirov, M.K.; Masson, M.; et al. Mixed Cationic Liposomes for Brain Delivery of Drugs by the Intranasal Route: The Acetylcholinesterase Reactivator 2-PAM as Encapsulated Drug Model. Colloids Surf. B 2018, 171, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, R.V.; Gaynanova, G.A.; Kuznetsova, D.A.; Vasileva, L.A.; Zueva, I.V.; Sapunova, A.S.; Buzyurova, D.N.; Babaev, V.M.; Voloshina, A.D.; Lukashenko, S.S.; et al. Biomedical Potentialities of Cationic Geminis as Modulating Agents of Liposome in Drug Delivery across Biological Barriers and Cellular Uptake. Int. J. Pharm. 2020, 587, 119640. [Google Scholar] [CrossRef]
- Orbesteanu, A.-M.; Cojocaru, V.; Ailiesei, I.; Mircioiu, C.; Cinteza, L.O. Studies on the Formulation of Nanostructured Carriers for Increasing the Bioavailability of Pralidoxime Chloride. Stud. Univ. Vasile Goldis Arad Ser. Stiintele Vietii 2014, 24, 357–361. [Google Scholar]
- Pashirova, T.N.; Braïki, A.; Zueva, I.V.; Petrov, K.A.; Babaev, V.M.; Burilova, E.A.; Samarkina, D.A.; Rizvanov, I.K.; Souto, E.B.; Jean, L.; et al. Combination Delivery of Two Oxime-Loaded Lipid Nanoparticles: Time-Dependent Additive Action for Prolonged Rat Brain Protection. J. Control. Release 2018, 290, 102–111. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Zueva, I.V.; Petrov, K.A.; Babaev, V.M.; Lukashenko, S.S.; Rizvanov, I.K.; Souto, E.B.; Nikolsky, E.E.; Zakharova, L.Y.; Masson, P.; et al. Nanoparticle-Delivered 2-PAM for Rat Brain Protection against Paraoxon Central Toxicity. ACS Appl. Mater. Interfaces 2017, 9, 16922–16932. [Google Scholar] [CrossRef] [PubMed]
- Buzyurova, D.N.; Pashirova, T.N.; Zueva, I.V.; Burilova, E.A.; Shaihutdinova, Z.M.; Rizvanov, I.K.; Babaev, V.M.; Petrov, K.A.; Souto, E.B. Surface Modification of Pralidoxime Chloride-Loaded Solid Lipid Nanoparticles for Enhanced Brain Reactivation of Organophosphorus-Inhibited AChE: Pharmacokinetics in Rat. Toxicology 2020, 444, 152578. [Google Scholar] [CrossRef] [PubMed]
- Chigumira, W.; Maposa, P.; Gadaga, L.L.; Dube, A.; Tagwireyi, D.; Maponga, C.C. Preparation and Evaluation of Pralidoxime-Loaded PLGA Nanoparticles as Potential Carriers of the Drug across the Blood Brain Barrier. J. Nanomater. 2015, 2015, 8. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, X.; Wang, X.; Li, Q.; Yin, D. C(RGDyK)-Mediated Pluronic-PBCA Nanoparticles through the Blood-Brain Barrier to Enhance the Treatment of Central Organophosphorus Intoxication. J. Nanopart. Res. 2020, 22, 330. [Google Scholar] [CrossRef]
- Abd, E.; Yousuf, S.; Pastore, M.; Telaprolu, K.; Mohammed, Y.; Namjoshi, S.; Grice, J.; Roberts, M. Skin Models for the Testing of Transdermal Drugs. CPAA 2016, 8, 163–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuznetsova, D.A.; Gaynanova, G.A.; Vasileva, L.A.; Sibgatullina, G.V.; Samigullin, D.V.; Sapunova, A.S.; Voloshina, A.D.; Galkina, I.V.; Petrov, K.A.; Zakharova, L.Y. Mitochondria-Targeted Cationic Liposomes Modified with Alkyltriphenylphosphonium Bromides Loaded with Hydrophilic Drugs: Preparation, Cytotoxicity and Colocalization Assay. J. Mater. Chem. B 2019, 7, 7351–7362. [Google Scholar] [CrossRef] [PubMed]
- Cyboran-Mikołajczyk, S.; Bonarska-Kujawa, D.; Kleszczyńska, H.; Łuczyński, J. Effects of Interaction of Gemini Ester Quat Surfactants with Biological Membranes. Tenside Surfact. Det. 2016, 53, 20–28. [Google Scholar] [CrossRef]
- De Oliveira, T.C.; Tavares, M.E.V.; Soares-Sobrinho, J.L.; Chaves, L.L. The Role of Nanocarriers for Transdermal Application Targeted to Lymphatic Drug Delivery: Opportunities and Challenges. J. Drug Deliv. Sci. Technol. 2022, 68, 103110. [Google Scholar] [CrossRef]
- Malaiya, M.K.; Jain, A.; Pooja, H.; Jain, A.; Jain, D. Controlled Delivery of Rivastigmine Using Transdermal Patch for Effective Management of Alzheimer’s Disease. J. Drug Deliv. Sci. Technol. 2018, 45, 408–414. [Google Scholar] [CrossRef]
- Banerjee, N.; Sengupta, S.; Roy, A.; Ghosh, P.; Das, K.; Das, S. Functional Alteration of a Dimeric Insecticidal Lectin to a Monomeric Antifungal Protein Correlated to Its Oligomeric Status. PLoS ONE 2011, 6, e18593. [Google Scholar] [CrossRef] [Green Version]
- Kushnazarova, R.A.; Mirgorodskaya, A.B.; Kuznetsov, D.M.; Tyryshkina, A.A.; Voloshina, A.D.; Gumerova, S.K.; Lenina, O.A.; Nikitin, E.N.; Zakharova, L.Y. Modulation of Aggregation Behavior, Antimicrobial Properties and Catalytic Activity of Piperidinium Surfactants by Modifying Their Head Group with a Polar Fragment. J. Mol. Liq. 2021, 336, 116318. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Vasileva, L.A.; Gaynanova, G.A.; Vasilieva, E.A.; Lenina, O.A.; Nizameev, I.R.; Kadirov, M.K.; Petrov, K.A.; Zakharova, L.Y.; Sinyashin, O.G. Cationic Liposomes Mediated Transdermal Delivery of Meloxicam and Ketoprofen: Optimization of the Composition, in Vitro and in Vivo Assessment of Efficiency. Int. J. Pharm. 2021, 605, 120803. [Google Scholar] [CrossRef] [PubMed]
- Worek, F.; Mast, U.; Kiderlen, D.; Diepold, C.; Eyer, P. Improved Determination of Acetylcholinesterase Activity in Human Whole Blood. Clin. Chim. Acta 1999, 288, 73–90. [Google Scholar] [CrossRef] [PubMed]

| System | Molar Ratio | Dh, nm | PdI | ζ, mV | Dh, nm | PdI | ζ, mV |
|---|---|---|---|---|---|---|---|
| 1 Day | 2.5 Months | ||||||
| PC | - | 113 ± 1 | 0.086 ± 0.009 | −14 ± 1 | 118 ± 1 | 0.119 ± 0.043 | −11 ± 1 |
| PC/Tw20 | 1/0.2 | 107 ± 1 | 0.057 ± 0.002 | −11 ± 1 | 153 ± 13 | 0.232 ± 0.003 | −1 ± 1 |
| PC/Tw20/C14PB | 1/0.2/0.02 | 111 ± 1 | 0.072 ± 0.019 | 21 ± 1 | 115 ± 1 | 0.071 ± 0.005 | 15 ± 1 |
| PC/Tw20/C14PB | 1/0.2/0.025 | 117 ± 2 | 0.084 ± 0.004 | 29 ± 1 | 121 ± 1 | 0.075 ± 0.006 | 20 ± 1 |
| PC/Tw20/C14PB | 1/0.2/0.04 | 120 ± 2 | 0.096 ± 0.003 | 38 ± 1 | 126 ± 1 | 0.083 ± 0.014 | 23 ± 1 |
| PC/Tw20/C12PB | 1/0.2/0.025 | 110 ± 2 | 0.089 ± 0.015 | 23 ± 1 | 112 ± 1 | 0.076 ± 0.019 | 17 ± 1 |
| PC/Tw20/C16PB | 1/0.2/0.025 | 116 ± 2 | 0.101 ± 0.004 | 24 ± 1 | 121 ± 1 | 0.093 ± 0.004 | 22 ± 1 |
| System | Molar Ratio | EE, % | Dh, nm | PdI | ζ, mV | Dh, nm | PdI | ζ, mV |
|---|---|---|---|---|---|---|---|---|
| 1 Day | 1 Month | |||||||
| PC | - | 60.3 ± 2.8 | 118 ± 1 | 0.082 ± 0.012 | −2 ± 0.5 | 116 ± 2 | 0.115 ± 0.004 | −4 ± 1 |
| PC/Tw20 | 1/0.2 | 55.3 ± 0.9 | 110 ± 1 | 0.061 ± 0.010 | −2 ± 0.2 | 139 ± 2 | 0.242 ± 0.006 | −3 ± 1 |
| PC/Tw20/C14PB | 1/0.2/0.02 | 63.5 ± 3.3 | 105 ± 1 | 0.041 ± 0.009 | 12 ± 1 | 118 ± 1 | 0.111 ± 0.009 | 3 ± 1 |
| PC/Tw20/C14PB | 1/0.2/0.025 | 64.7 ± 5.3 | 107 ± 2 | 0.061 ± 0.014 | 17 ± 1 | 103 ± 1 | 0.047 ± 0.017 | 5 ± 1 |
| PC/Tw20/C14PB | 1/0.2/0.04 | 66.2 ± 2.1 | 114 ± 2 | 0.078 ± 0.009 | 24 ± 1 | 121 ± 2 | 0.099 ± 0.012 | 13 ± 1 |
| PC/Tw20/C12PB | 1/0.2/0.025 | 56.9 ± 0.1 | 110 ± 1 | 0.076 ± 0.010 | 10 ± 1 | 110 ± 1 | 0.143 ± 0.020 | 6 ± 1 |
| PC/Tw20/C16PB | 1/0.2/0.025 | 56.1 ± 1.5 | 117 ± 1 | 0.074 ± 0.016 | 9 ± 1 | 104 ± 1 | 0.085 ± 0.001 | 8 ± 1 |
| System | Molar Ratio | IC50, mM * | |
|---|---|---|---|
| M-HeLa | WI-38 | ||
| PC | - | 3.60 ± 0.28 | >5 |
| PC/Tw20 | 1/0.2 | 1.68 ± 0.13 | 1.64 ± 0.12 |
| PC/Tw20/C12PB | 1/0.2/0.025 | 0.48 ± 0.04 | 1.25 ± 0.10 |
| PC/Tw20/C14PB | 1/0.2/0.025 | 0.51 ± 0.04 | 1.44 ± 0.11 |
| PC/Tw20/C16PB | 1/0.2/0.025 | 0.75 ± 0.06 | 1.00 ± 0.08 |
| Blood Sampling Time after Applying, h | Group of Treatment | |||
|---|---|---|---|---|
| POX, 1 h, n = 5 | POX, 1 h, + free 2- PAM, n = 5 | POX, 1 h, + PC/Tw20/2-PAM, n = 5 | POX, 1 h, + PC/Tw20/C14PB/2-PAM 1/0.2/0.025, n = 5 | |
| AChE reactivation in rat erythrocytes, % | ||||
| 2 | 1.66 ± 3.79 p = 0.940 | 1.35 ± 3.40 p = 0.706 | 1.36 ± 5.57 p = 1.000 | 8.40 ± 7.52 p = 0.449 |
| 4 | 1.80 ± 3.74 p = 0.571 | 1.46 ± 5.95 p = 1.000 | 1.41 ± 5.20 p = 0.821 | 8.73 ± 7.18 p = 0.304 |
| 6 | 0.62 ± 3.16 p = 0.735 | 4.46 ± 9.30 p = 0.483 | 23.65 ± 7.23 p = 0.005 * | 11.98 ± 4.06 p = 0.05 |
| 24 | 2.85 ± 7.39 p = 0.678 | 7.25 ± 6.13 p = 0.404 | 24.2 ± 9.73 p = 0.021 * | 20.10 ± 11.80 p = 0.113 |
| Group of Treatment | n/N * | % of Surviving Rats |
|---|---|---|
| POX | 0/20 | 0% |
| POX + 2-PAM intravenously 10 min after POX | 11/20 | 55% |
| PC/Tw20/2-PAM transdermally for 24 h + POX | 8/20 | 40% |
| PC/Tw20/2-PAM transdermally for 24 h + POX + 2-PAM intravenously 10 min after POX | 18/20 | 90% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileva, L.; Gaynanova, G.; Zueva, I.; Lyubina, A.; Amerhanova, S.; Buzyurova, D.; Babaev, V.; Voloshina, A.; Petrov, K.; Zakharova, L. Transdermal Delivery of 2-PAM as a Tool to Increase the Effectiveness of Traditional Treatment of Organophosphate Poisoning. Int. J. Mol. Sci. 2022, 23, 14992. https://doi.org/10.3390/ijms232314992
Vasileva L, Gaynanova G, Zueva I, Lyubina A, Amerhanova S, Buzyurova D, Babaev V, Voloshina A, Petrov K, Zakharova L. Transdermal Delivery of 2-PAM as a Tool to Increase the Effectiveness of Traditional Treatment of Organophosphate Poisoning. International Journal of Molecular Sciences. 2022; 23(23):14992. https://doi.org/10.3390/ijms232314992
Chicago/Turabian StyleVasileva, Leysan, Gulnara Gaynanova, Irina Zueva, Anna Lyubina, Syumbelya Amerhanova, Daina Buzyurova, Vasily Babaev, Alexandra Voloshina, Konstantin Petrov, and Lucia Zakharova. 2022. "Transdermal Delivery of 2-PAM as a Tool to Increase the Effectiveness of Traditional Treatment of Organophosphate Poisoning" International Journal of Molecular Sciences 23, no. 23: 14992. https://doi.org/10.3390/ijms232314992
APA StyleVasileva, L., Gaynanova, G., Zueva, I., Lyubina, A., Amerhanova, S., Buzyurova, D., Babaev, V., Voloshina, A., Petrov, K., & Zakharova, L. (2022). Transdermal Delivery of 2-PAM as a Tool to Increase the Effectiveness of Traditional Treatment of Organophosphate Poisoning. International Journal of Molecular Sciences, 23(23), 14992. https://doi.org/10.3390/ijms232314992


_Kim.png)




