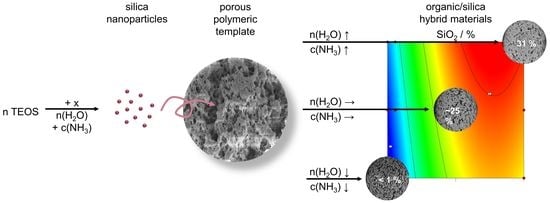
Monodisperse Porous Silica/Polymer Nanocomposite Microspheres with Tunable Silica Loading, Morphology and Porosity
Abstract
1. Introduction
2. Results
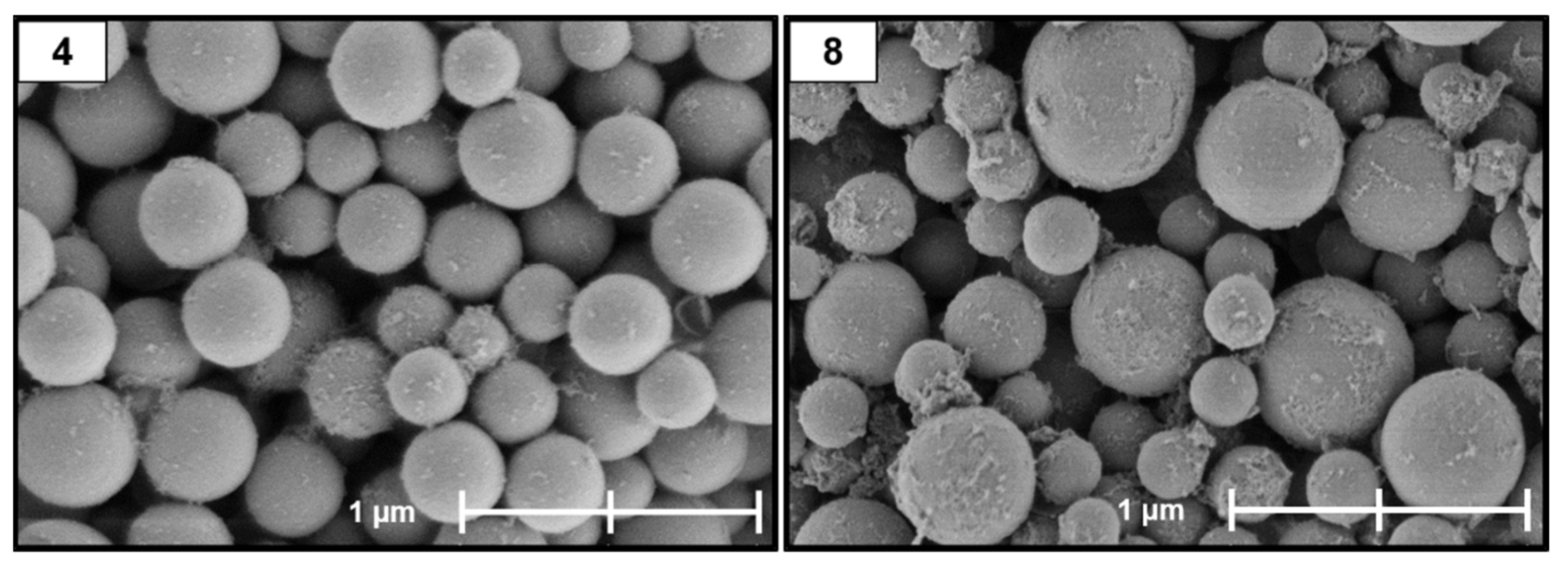
2.1. Size and Dispersity
2.2. SiO2 Content
2.3. Pore Size
2.4. Specific Surface Area
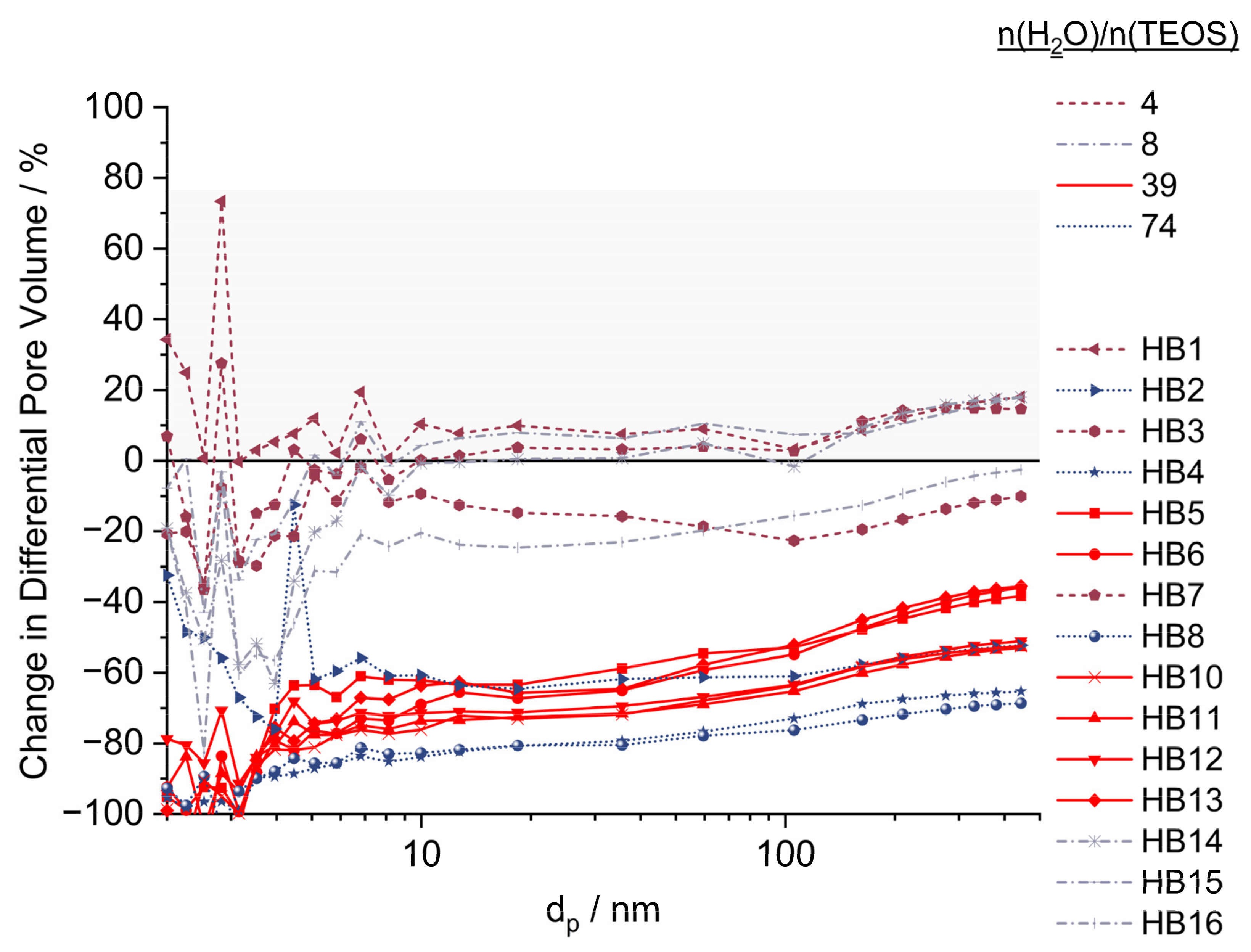
2.5. Pore Volume
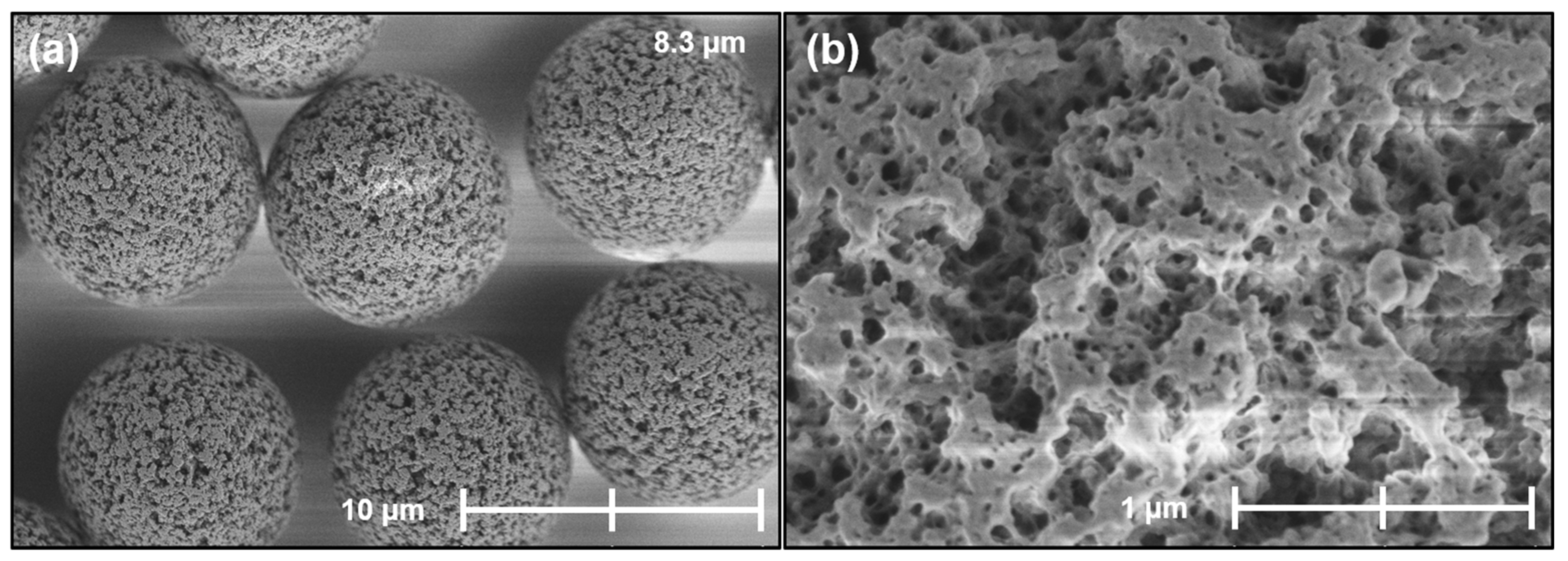
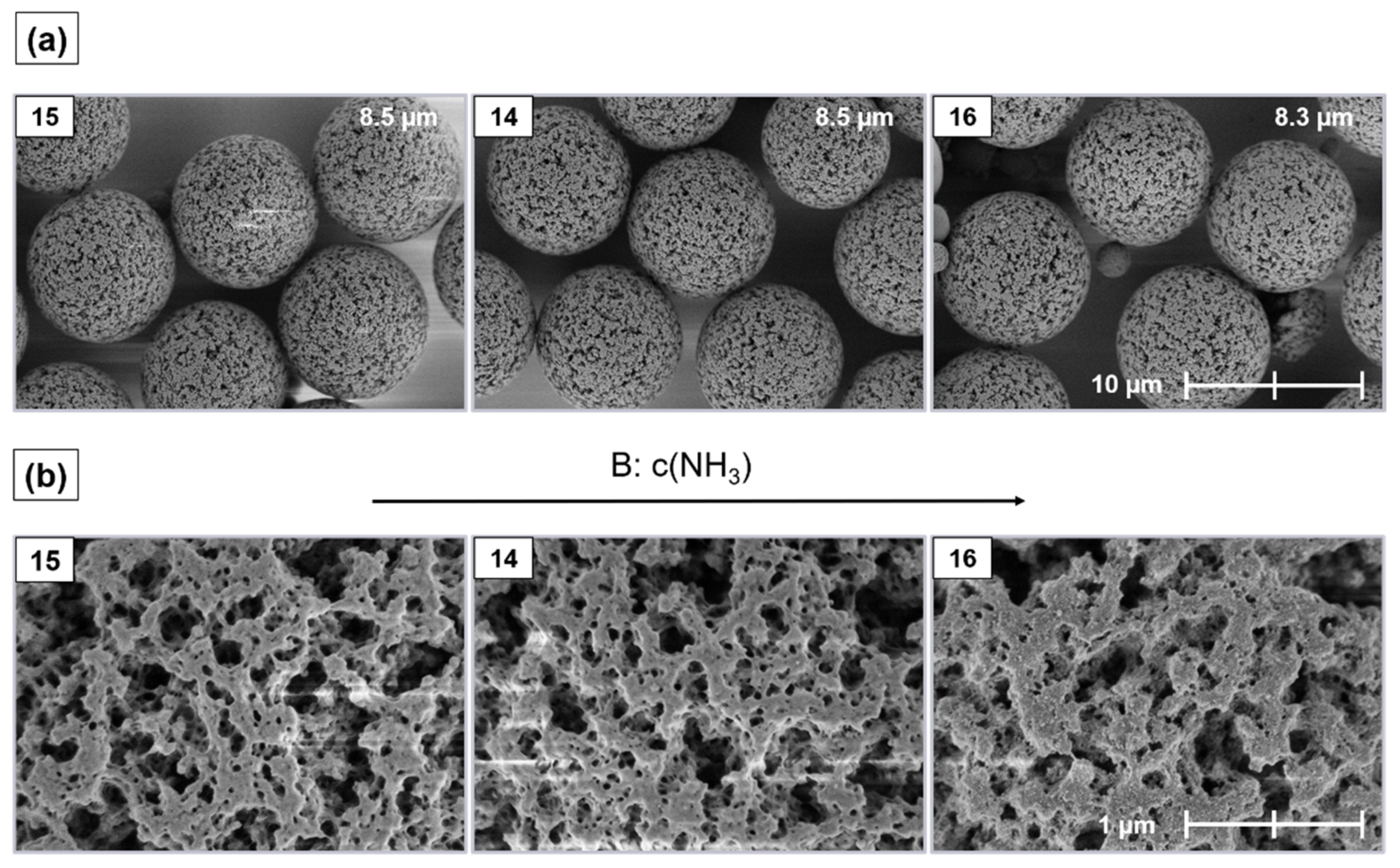
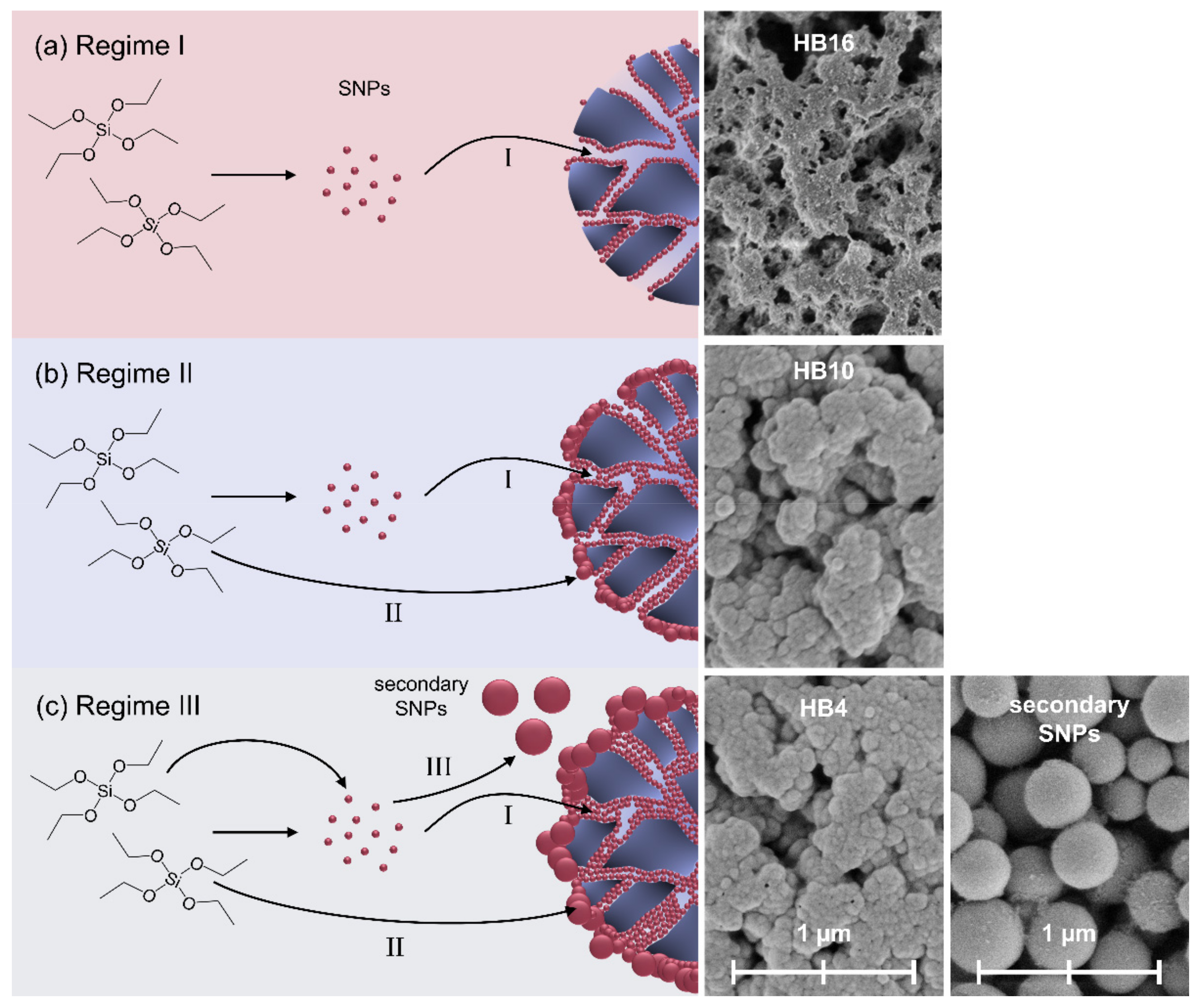
2.6. Morphology and Silica Nanoparticle Size
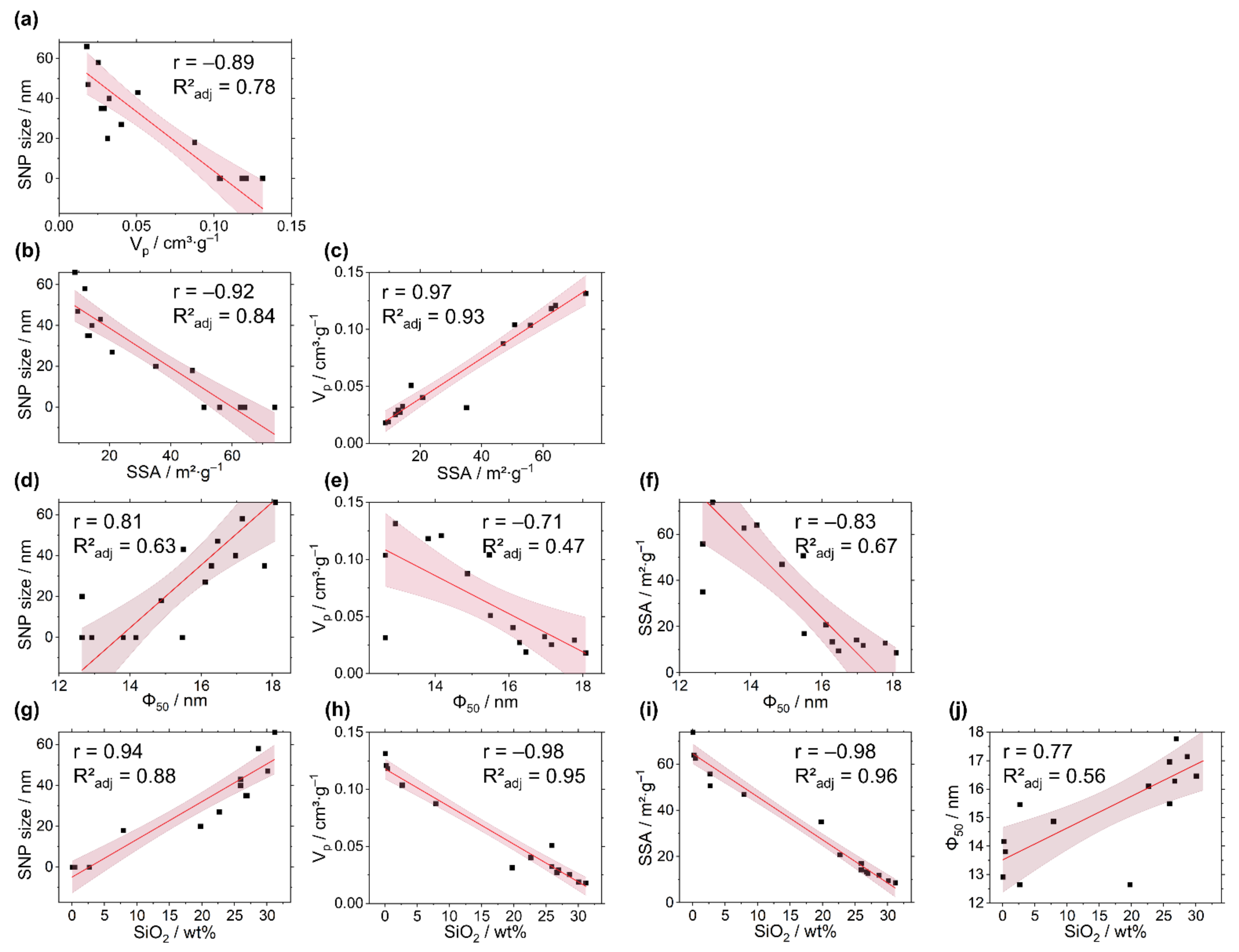
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of HBs
4.3. Nitrogen Adsorption Measurements
4.4. Scanning Electron Microscopy Images (SEM)
4.5. Thermogravimetric (TGA) Determination of SiO2 Content
4.6. Experimental Design
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Preparation of Amino-Functionalized Porous Polymer Template
Appendix B. Supplementary SEM Images
Appendix C. ANOVA Tables for the RSMs
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.0561 | 1 | 0.0561 | 7.45 | 0.0172 | significant |
| A-n(H2O)/n(TEOS) | 0.0561 | 1 | 0.0561 | 7.45 | 0.0172 | |
| Residual | 0.0980 | 13 | 0.0075 | |||
| Lack of Fit | 0.0943 | 9 | 0.0105 | 11.26 | 0.0163 | significant |
| Pure Error | 0.0037 | 4 | 0.0009 | |||
| Cor Total | 0.1541 | 14 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 2187.31 | 3 | 729.10 | 162.91 | <0.0001 | significant |
| A-n(H2O)/n(TEOS) | 1381.35 | 1 | 1381.35 | 308.65 | <0.0001 | |
| B-c(NH3) | 86.00 | 1 | 86.00 | 19.21 | 0.0011 | |
| A² | 432.20 | 1 | 432.20 | 96.57 | <0.0001 | |
| Residual | 49.23 | 11 | 4.48 | |||
| Lack of Fit | 39.41 | 7 | 5.63 | 2.29 | 0.2204 | not significant |
| Pure Error | 9.82 | 4 | 2.45 | |||
| Cor Total | 2236.54 | 14 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 38.43 | 4 | 9.61 | 12.20 | 0.0007 | significant |
| A-n(H2O)/n(TEOS) | 7.93 | 1 | 7.93 | 10.07 | 0.0099 | |
| B-c(NH3) | 10.03 | 1 | 10.03 | 12.74 | 0.0051 | |
| AB | 8.81 | 1 | 8.81 | 11.19 | 0.0074 | |
| A² | 10.08 | 1 | 10.08 | 12.79 | 0.0050 | |
| Residual | 7.88 | 10 | 0.7879 | |||
| Lack of Fit | 4.84 | 6 | 0.8074 | 1.06 | 0.4990 | not significant |
| Pure Error | 3.03 | 4 | 0.7586 | |||
| Cor Total | 46.31 | 14 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 7539.12 | 4 | 1884.78 | 153.80 | <0.0001 | significant |
| A-n(H2O)/n(TEOS) | 3882.36 | 1 | 3882.36 | 316.80 | <0.0001 | |
| B-c(NH3) | 681.83 | 1 | 681.83 | 55.64 | <0.0001 | |
| A² | 989.61 | 1 | 989.61 | 80.75 | <0.0001 | |
| B² | 106.41 | 1 | 106.41 | 8.68 | 0.0146 | |
| Residual | 122.55 | 10 | 12.25 | |||
| Lack of Fit | 79.47 | 6 | 13.25 | 1.23 | 0.4397 | not significant |
| Pure Error | 43.08 | 4 | 10.77 | |||
| Cor Total | 7661.67 | 14 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.0249 | 3 | 0.0083 | 179.66 | <0.0001 | significant |
| A-n(H2O)/n(TEOS) | 0.0174 | 1 | 0.0174 | 375.80 | <0.0001 | |
| B-c(NH3) | 0.0010 | 1 | 0.0010 | 21.98 | 0.0007 | |
| A² | 0.0036 | 1 | 0.0036 | 76.88 | <0.0001 | |
| Residual | 0.0005 | 11 | 0.0000 | |||
| Lack of Fit | 0.0001 | 7 | 0.0000 | 0.2028 | 0.9670 | not significant |
| Pure Error | 0.0004 | 4 | 0.0001 | |||
| Cor Total | 0.0254 | 14 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 6865.92 | 4 | 1716.48 | 52.49 | <0.0001 | significant |
| A-n(H2O)/n(TEOS) | 3797.04 | 1 | 3797.04 | 116.11 | <0.0001 | |
| B-c(NH3) | 1346.92 | 1 | 1346.92 | 41.19 | <0.0001 | |
| AB | 517.00 | 1 | 517.00 | 15.81 | 0.0026 | |
| A² | 699.79 | 1 | 699.79 | 21.40 | 0.0009 | |
| Residual | 327.01 | 10 | 32.70 | |||
| Lack of Fit | 179.01 | 6 | 29.84 | 0.8064 | 0.6132 | not significant |
| Pure Error | 148.00 | 4 | 37.00 | |||
| Cor Total | 7192.93 | 14 |
References
- Lee, K.; Sathyagal, A.N.; McCormick, A.V. A closer look at an aggregation model of the Stöber process. Colloids Surf. A Physicochem. Eng. Asp. 1998, 144, 115–125. [Google Scholar] [CrossRef]
- Meer, S.; Kausar, A.; Iqbal, T. Attributes of Polymer and Silica Nanoparticle Composites: A Review. Polym. Plast. Technol. Eng. 2016, 55, 826–861. [Google Scholar] [CrossRef]
- Krasucka, P.; Stefaniak, W.; Kierys, A.; Goworek, J. Polymer–silica composites and silicas produced by high-temperature degradation of organic component. Thermochim. Acta 2015, 615, 43–50. [Google Scholar] [CrossRef]
- Rahman, I.A.; Padavettan, V. Synthesis of Silica Nanoparticles by Sol-Gel: Size-Dependent Properties, Surface Modification, and Applications in Silica-Polymer Nanocomposites—A Review. J. Nanomater. 2012, 2012, 8. [Google Scholar] [CrossRef]
- Kwon, S.; Adachi, T.; Araki, W.; Yamaji, A. Thermo-viscoelastic properties of silica particulate-reinforced epoxy composites: Considered in terms of the particle packing model. Acta Mater. 2006, 54, 3369–3374. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Cho, U.R. Mechanical Properties of Styrene-Butadiene Rubber Reinforced with Silica by in situ Tetraethoxysilane Hydrolysis over Acid Catalyst. Elastomers Compos. 2018, 53, 57–66. [Google Scholar] [CrossRef]
- Kelly, T.L.; Che, S.P.Y.; Yamada, Y.; Yano, K.; Wolf, M.O. Influence of surface morphology on the colloidal and electronic behavior of conjugated polymer-silica microspheres. Langmuir 2008, 24, 9809–9815. [Google Scholar] [CrossRef]
- McInnes, S.J.P.; Voelcker, N.H. Silicon-polymer hybrid materials for drug delivery. Future Med. Chem. 2009, 1, 1051–1074. [Google Scholar] [CrossRef]
- Kiasat, A.R.; Nazari, S.; Davarpanah, J. Facile synthesis of an organic–inorganic nanocomposite, PEG–silica, by sol–gel method; its characterization and application as an efficient catalyst in regioselective nucleophilic ring opening of epoxides: Preparation of β-azido alcohols and β-cyanohydrins. C. R. Chim. 2014, 17, 124–130. [Google Scholar] [CrossRef]
- Tumnantong, D.; Rempel, G.L.; Prasassarakich, P. Preparation of poly(methyl methacrylate)-Silica nanoparticles via differential microemulsion polymerization and physical properties of NR/PMMA-SiO 2 hybrid membranes. Polym. Eng. Sci. 2018, 58, 759–766. [Google Scholar] [CrossRef]
- Guo, J.; Mattos, B.D.; Tardy, B.L.; Moody, V.M.; Xiao, G.; Ejima, H.; Cui, J.; Liang, K.; Richardson, J.J. Porous Inorganic and Hybrid Systems for Drug Delivery: Future Promise in Combatting Drug Resistance and Translation to Botanical Applications. Curr. Med. Chem. 2019, 26, 6107–6131. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Wan, G.; Yang, F.; Wang, J.; Bai, Q. Preparation of monodisperse large-porous silica microspheres with polymer microspheres as the templates for protein separation. Mater. Lett. 2016, 180, 19–22. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, L.; Ren, L.; Teng, C.; Wang, Y.; Jiang, B.; He, J. Fabrication of Monodisperse Porous Silica Microspheres with a Tunable Particle Size and Pore Size for Protein Separation. ACS Appl. Bio Mater. 2018, 1, 604–612. [Google Scholar] [CrossRef]
- He, J.; Yang, C.; Xiong, X.; Jiang, B. Preparation and characterization of monodisperse porous silica microspheres with controllable morphology and structure. J. Polym. Sci. A Polym. Chem. 2012, 50, 2889–2897. [Google Scholar] [CrossRef]
- Grama, S.; Horák, D. Preparation of monodisperse porous silica particles using poly(glycidyl methacrylate) microspheres as a template. Physiol. Res. 2015, 64, S11–S17. [Google Scholar] [CrossRef]
- Yuan, Z.-Y.; Su, B.-L. Insights into hierarchically meso–macroporous structured materials. J. Mater. Chem. UR 2006, 16, 663–677. [Google Scholar] [CrossRef]
- Steinbach, J.C.; Fait, F.; Wagner, S.; Wagner, A.; Brecht, M.; Mayer, H.A.; Kandelbauer, A. Rational Design of Pore Parameters in Monodisperse Porous Poly(glycidyl methacrylate-co-ethylene glycol dimethacrylate) Particles Based on Response Surface Methodology. Polymers 2022, 14, 382. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, S.; Park, J.I.; Gourevich, I.; Chan, C.; Deetz, M.; Kumacheva, E. Toward Controlling the Surface Morphology of Macroporous Copolymer Particles. Macromolecules 2009, 42, 1990–1994. [Google Scholar] [CrossRef]
- Gokmen, M.T.; Du Prez, F.E. Porous polymer particles—A comprehensive guide to synthesis, characterization, functionalization and applications. Prog. Polym. Sci. 2012, 37, 365–405. [Google Scholar] [CrossRef]
- Costa, L.C.; Monteiro, R.C.; Castro, H.M.A.; Ribeiro, T.S.; Oliveira, M.A.; Torquato, E.C.C.; Arcanjo, M.E.; Marques, M.R.C. Glycidyl Methacrylate-ethylene Glycol Dimethacrylate Copolymers with Varied Pore Structures Prepared with Different Reaction Parameters. Mater. Res. 2020, 23, e20190550. [Google Scholar] [CrossRef]
- Roy, R. Nanocomposites: Retrospecr and Prospect. MRS Online Proc. Libr. 1992, 286, 241–250. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L.M.; Pagliaro, M. The sol-gel route to advanced silica-based materials and recent applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, K.D.; Kim, H.T. Preparation of silica nanoparticles: Determination of the optimal synthesis conditions for small and uniform particles. Colloids Surf. A Physicochem. Eng. Asp. 2002, 197, 7–17. [Google Scholar] [CrossRef]
- Plumeré, N.; Ruff, A.; Speiser, B.; Feldmann, V.; Mayer, H.A. Stöber silica particles as basis for redox modifications: Particle shape, size, polydispersity, and porosity. J. Colloid Interface Sci. 2012, 368, 208–219. [Google Scholar] [CrossRef] [PubMed]
- LaMer, V.K.; Dinegar, R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Matsoukas, T.; Gulari, E. Dynamics of growth of silica particles from ammonia-catalyzed hydrolysis of tetra-ethyl-orthosilicate. J. Colloid Interface Sci. 1988, 124, 252–261. [Google Scholar] [CrossRef]
- Bogush, G.; Zukoski, C. Studies of the kinetics of the precipitation of uniform silica particles through the hydrolysis and condensation of silicon alkoxides. J. Colloid Interface Sci. 1991, 142, 1–18. [Google Scholar] [CrossRef]
- Particles in the Stöber Method: In Situ Seeded Growth Model. Langmuir 2017, 33, 5879–5890. [CrossRef]
- Sadasivan, S.; Dubey, A.K.; Li, Y.; Rasmussen, D.H. Alcoholic Solvent Effect on Silica Synthesis—NMR and DLS Investigation. J. Solgel. Sci. Technol. 1998, 12, 5–14. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 2nd ed.; Wiley: Hoboken, NJ, USA, 2016; ISBN 9781118916025. [Google Scholar]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2005; ISBN 0471718130. [Google Scholar]
- Ryan, T.P. Modern Experimental Design; Wiley-Interscience: Hoboken, NJ, USA, 2007; ISBN 978-0-471-21077-1. [Google Scholar]
- Seidl, R.; Weiss, S.; Zikulnig-Rusch, E.M.; Kandelbauer, A. Response surface optimization for improving the processing behavior of melamine formaldehyde impregnation resins. J. Appl. Polym. Sci. 2021, 138, 50181. [Google Scholar] [CrossRef]
- Ulitzsch, S.; Bäuerle, T.; Chassé, T.; Lorenz, G.; Kandelbauer, A. Optimizing the Process Efficiency of Reactive Extrusion in the Synthesis of Vinyltrimethoxysilane-Grafted Ethylene-Octene-Copolymer (EOC-g-VTMS) by Response Surface Methodology. Polymers 2020, 12, 2798. [Google Scholar] [CrossRef]
- Wahrendorff, P.; Stefanakis, M.; Steinbach, J.C.; Allnoch, D.; Zuber, R.; Kapfhammer, R.; Brecht, M.; Kandelbauer, A.; Rebner, K. Simultaneous Determination of Droplet Size, pH Value and Concentration to Evaluate the Aging Behavior of Metalworking Fluids. Sensors 2021, 21, 8299. [Google Scholar] [CrossRef]
- Thébault, M.; Kutuzova, L.; Jury, S.; Eicher, I.; Zikulnig-Rusch, E.-M.; Kandelbauer, A. Effect of Phenolation, Lignin-Type and Degree of Substitution on the Properties of Lignin-Modified Phenol-Formaldehyde Impregnation Resins: Molecular Weight Distribution, Wetting Behavior, Rheological Properties and Thermal Curing Profiles. J. Renew. Mater.: JRM 2020, 8, 603–630. [Google Scholar] [CrossRef]
- Arantes, T.M.; Pinto, A.H.; Leite, E.R.; Longo, E.; Camargo, E.R. Synthesis and optimization of colloidal silica nanoparticles and their functionalization with methacrylic acid. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 209–217. [Google Scholar] [CrossRef]
- Chiang, Y.-D.; Lian, H.-Y.; Leo, S.-Y.; Wang, S.-G.; Yamauchi, Y.; Wu, K.C.-W. Controlling Particle Size and Structural Properties of Mesoporous Silica Nanoparticles Using the Taguchi Method. J. Phys. Chem. C 2011, 115, 13158–13165. [Google Scholar] [CrossRef]
- Davies, G.-L.; Barry, A.; Gun’ko, Y.K. Preparation and size optimisation of silica nanoparticles using statistical analyses. Chem. Phys. Lett. 2009, 468, 239–244. [Google Scholar] [CrossRef]
- González-Álvarez, R.J.; Naranjo-Rodríguez, I.; Hernández-Artiga, M.P.; Palacios-Santander, J.M.; Cubillana-Aguilera, L.; Bellido-Milla, D. Experimental design applied to optimisation of silica nanoparticles size obtained by sonosynthesis. J. Sol-Gel Sci. Technol. 2016, 80, 378–388. [Google Scholar] [CrossRef]
- Wang, H.-C.; Wu, C.-Y.; Chung, C.-C.; Lai, M.-H.; Chung, T.-W. Analysis of Parameters and Interaction between Parameters in Preparation of Uniform Silicon Dioxide Nanoparticles Using Response Surface Methodology. Ind. Eng. Chem. Res. 2006, 45, 8043–8048. [Google Scholar] [CrossRef]
- Grama, S.; Plichta, Z.; Trchová, M.; Kovářová, J.; Beneš, M.; Horák, D. Monodisperse macroporous poly(glycidyl methacrylate) microspheres coated with silica: Design, preparation and characterization. React. Funct. Polym. 2014, 77, 11–17. [Google Scholar] [CrossRef]
- Muzammil, E.M.; Khan, A.; Stuparu, M.C. Post-polymerization modification reactions of poly(glycidyl methacrylate)s. RSC Adv. 2017, 7, 55874–55884. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, Q.; Tang, C.; Liu, J.; Yi, Y.; Bai, Q. Synthesis and application of 5 μm monodisperse porous silica microspheres with controllable pore size using polymeric microspheres as templates for the separation of small solutes and proteins by high-performance liquid chromatography. J. Chromatogr. A 2022, 1675, 463165. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Bourebrab, M.A.; Oben, D.T.; Durand, G.G.; Taylor, P.G.; Bruce, J.I.; Bassindale, A.R.; Taylor, A. Influence of the initial chemical conditions on the rational design of silica particles. J. Solgel. Sci. Technol. 2018, 88, 430–441. [Google Scholar] [CrossRef]
- Xu, P.; Wang, H.; Tong, R.; Du, Q.; Zhong, W. Preparation and morphology of SiO2/PMMA nanohybrids by microemulsion polymerization. Colloid Polym. Sci. 2006, 284, 755–762. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.L.; Maurin, G. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Elsevier/AP: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. (Eds.) Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht, The Netherlands, 2004; ISBN 978-1-4020-2303-3. [Google Scholar]
- Bäuerle, T.; Ulitzsch, S.; Lorenz, A.; Rebner, K.; Chassé, T.; Kandelbauer, A.; Lorenz, G. Effects of process parameters on silane grafting of liquid ethylene-propylene copolymer by reactive extrusion as quantified by response surface methodology. Polymer 2020, 202, 122601. [Google Scholar] [CrossRef]

 = low setting,
= low setting,  = medium setting,
= medium setting,  = high setting) and visualizing the strengths of the synergistic interactions (d,n); contour plots displaying a heatmap for each response variable dependent on both process factors (b,e,i,l,o); response surface plots showing the values for the responses predicted by the response surface model across the design space for each combination of process factor settings (c,f,j,m,p). Note that, in the 3D-plots (j,m), the axes of n(H2O)/n(TEOS) are given in opposite direction to (c,f,p) for better visibility. Dashed lines indicate 95% confidence intervals for one factor and interaction plots. Red areas correspond to high and blue areas correspond to low response values in the response surface models. The responses are as follows: (a–c) SiO2 content, blue line indicates maximum SiO2 deposition; (d–f) median pore size (ϕ50), the black arrow indicates a shift in maximal ϕ50 across the design space; (g–j) specific surface area (SSA) with the minimal SSA within the experimental space indicated by a flag; (k–m) pore volume (Vp); (n–p) SNP size.
= high setting) and visualizing the strengths of the synergistic interactions (d,n); contour plots displaying a heatmap for each response variable dependent on both process factors (b,e,i,l,o); response surface plots showing the values for the responses predicted by the response surface model across the design space for each combination of process factor settings (c,f,j,m,p). Note that, in the 3D-plots (j,m), the axes of n(H2O)/n(TEOS) are given in opposite direction to (c,f,p) for better visibility. Dashed lines indicate 95% confidence intervals for one factor and interaction plots. Red areas correspond to high and blue areas correspond to low response values in the response surface models. The responses are as follows: (a–c) SiO2 content, blue line indicates maximum SiO2 deposition; (d–f) median pore size (ϕ50), the black arrow indicates a shift in maximal ϕ50 across the design space; (g–j) specific surface area (SSA) with the minimal SSA within the experimental space indicated by a flag; (k–m) pore volume (Vp); (n–p) SNP size.
 = low setting,
= low setting,  = medium setting,
= medium setting,  = high setting) and visualizing the strengths of the synergistic interactions (d,n); contour plots displaying a heatmap for each response variable dependent on both process factors (b,e,i,l,o); response surface plots showing the values for the responses predicted by the response surface model across the design space for each combination of process factor settings (c,f,j,m,p). Note that, in the 3D-plots (j,m), the axes of n(H2O)/n(TEOS) are given in opposite direction to (c,f,p) for better visibility. Dashed lines indicate 95% confidence intervals for one factor and interaction plots. Red areas correspond to high and blue areas correspond to low response values in the response surface models. The responses are as follows: (a–c) SiO2 content, blue line indicates maximum SiO2 deposition; (d–f) median pore size (ϕ50), the black arrow indicates a shift in maximal ϕ50 across the design space; (g–j) specific surface area (SSA) with the minimal SSA within the experimental space indicated by a flag; (k–m) pore volume (Vp); (n–p) SNP size.
= high setting) and visualizing the strengths of the synergistic interactions (d,n); contour plots displaying a heatmap for each response variable dependent on both process factors (b,e,i,l,o); response surface plots showing the values for the responses predicted by the response surface model across the design space for each combination of process factor settings (c,f,j,m,p). Note that, in the 3D-plots (j,m), the axes of n(H2O)/n(TEOS) are given in opposite direction to (c,f,p) for better visibility. Dashed lines indicate 95% confidence intervals for one factor and interaction plots. Red areas correspond to high and blue areas correspond to low response values in the response surface models. The responses are as follows: (a–c) SiO2 content, blue line indicates maximum SiO2 deposition; (d–f) median pore size (ϕ50), the black arrow indicates a shift in maximal ϕ50 across the design space; (g–j) specific surface area (SSA) with the minimal SSA within the experimental space indicated by a flag; (k–m) pore volume (Vp); (n–p) SNP size.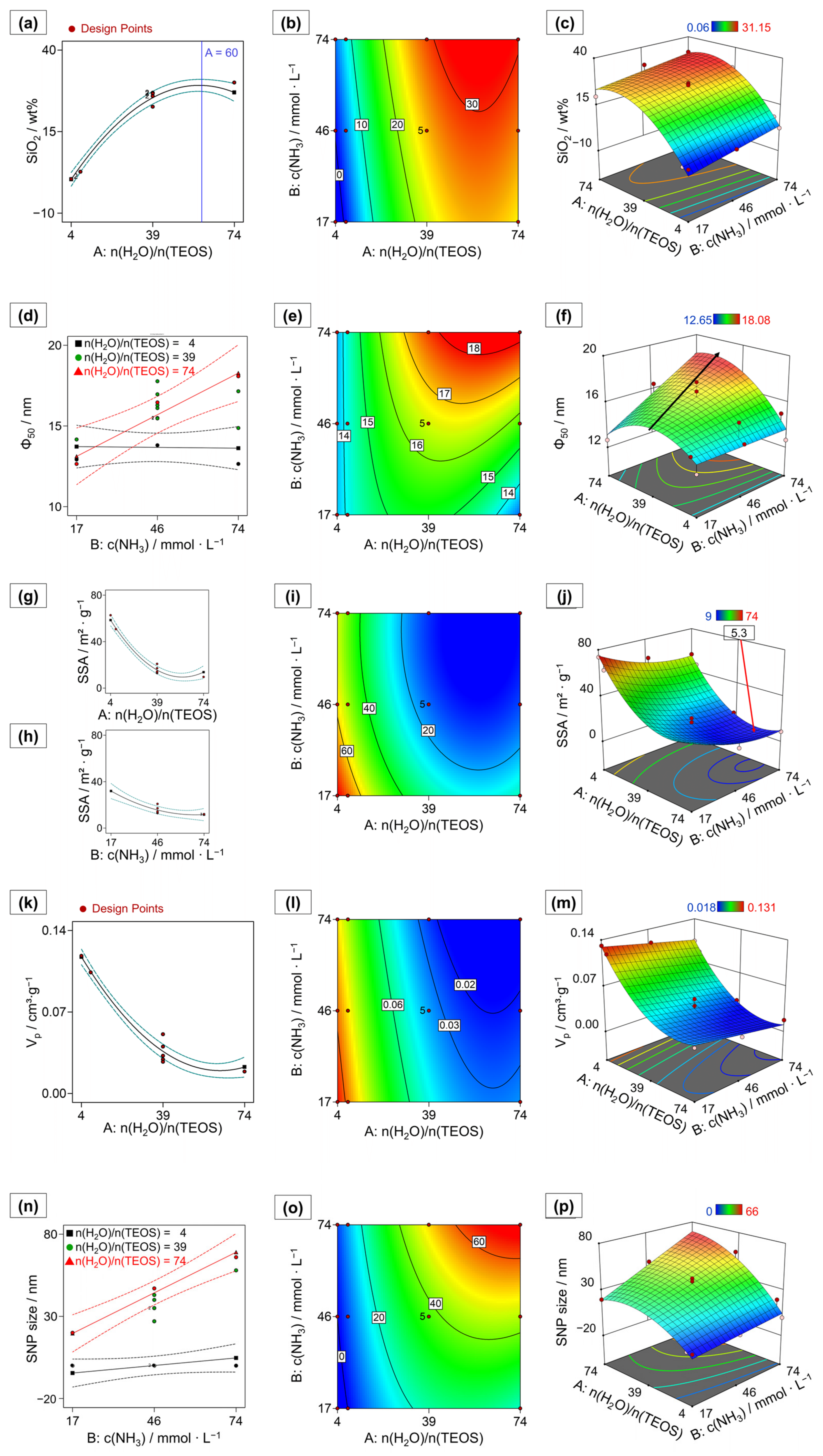
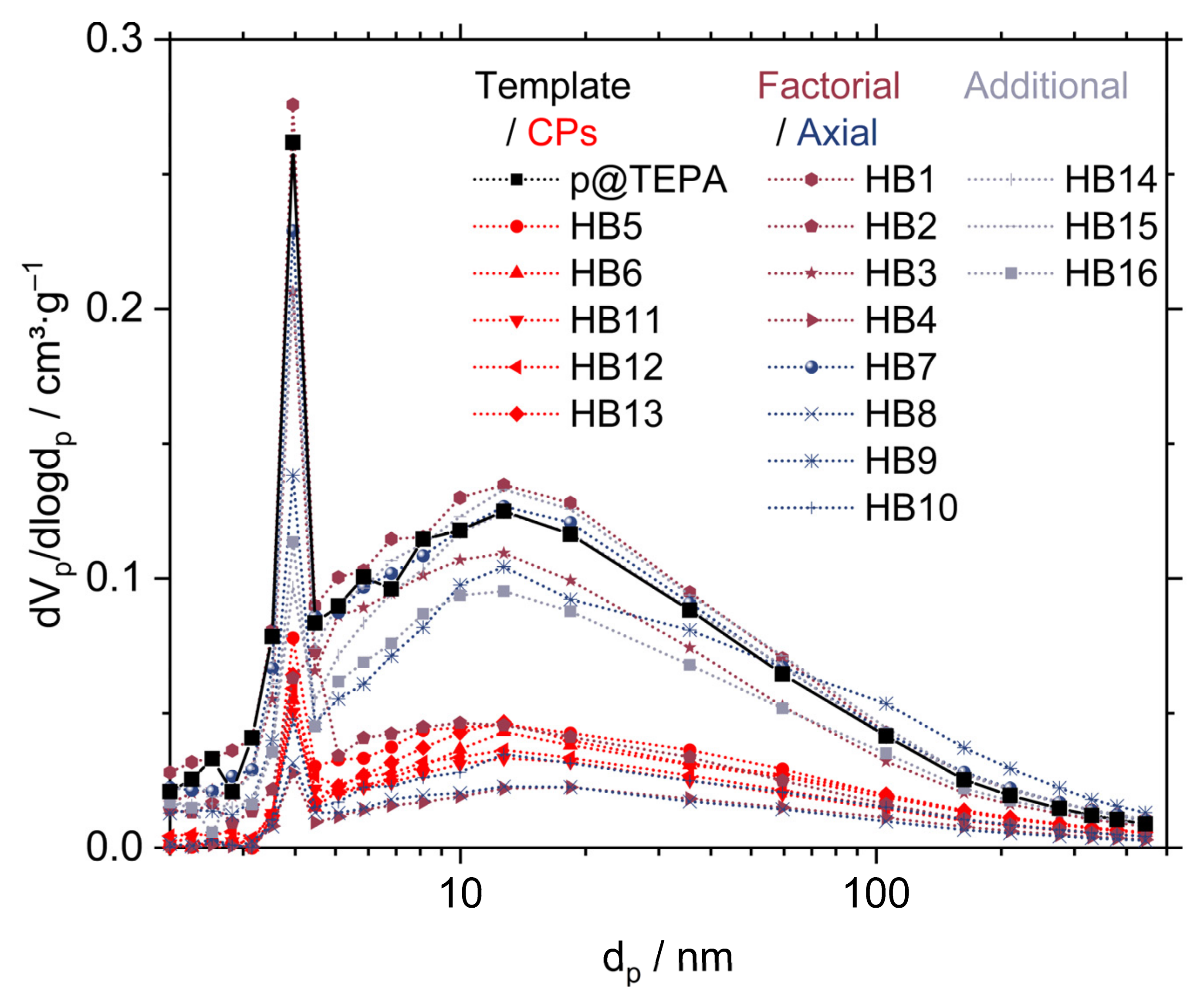

| Factor-Level Settings | Response Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A n(H2O)/ n(TEOS) | B n(NH3) | Particle Size | d90/d10 | SSA | Pore Diameter Φ50 | Pore Volume Vp | SiO2 Content | SNP Size | |
| /mmol∙L−1 | /µm | /m²∙g−1 | /nm | /cm3∙g−1 | /wt% | /nm | |||
| p@TEPA | - | - | 8.3 | 1.04 | 63.79 | 13.0 | 0.12 | 0 | - |
| HB1 | 4 | 17.1 | 8.3 | 1.11 | 73.87 | 12.9 | 0.13 | 0.1 | 0 † |
| HB2 | 74 | 17.1 | 8.6 | 1.14 | 35.11 | 12.6 | 0.03 | 19.7 | 20 |
| HB3 | 4 | 74.1 | 8.6 | 1.06 | 55.86 | 12.7 | 0.10 | 2.7 | 0 |
| HB4 | 74 | 74.1 | 8.6 | 1.07 | 8.75 | 18.1 | 0.02 | 31.2 | 66 |
| HB5 | 39 | 45.6 | 8.5 | 1.09 | 20.87 | 16.1 | 0.04 | 22.6 | 27 |
| HB6 | 39 | 45.6 | 8.5 | 1.08 | 12.92 | 17.8 | 0.03 | 26.9 | 35 |
| HB7 | 4 | 45.6 | 8.5 | 1.06 | 62.72 | 13.8 | 0.12 | 0.4 | 0 † |
| HB8 | 74 | 45.6 | 8.6 | 1.06 | 9.63 | 16.5 | 0.02 | 30.1 | 47 |
| HB9 * | 39 | 17.1 | 8.6 | 1.05 | 47.38 | 17.5 | * 0.09 | * 13.0 | 24 |
| HB10 | 39 | 74.1 | 8.7 | 1.07 | 12.02 | 17.1 | 0.03 | 28.6 | 58 |
| HB11 | 39 | 45.6 | 8.6 | 1.06 | 13.51 | 16.3 | 0.03 | 26.7 | 35 |
| HB12 | 39 | 45.6 | 8.5 | 1.07 | 17.10 | 15.5 | 0.05 | 25.9 | 43 |
| HB13 | 39 | 45.6 | 8.5 | 1.08 | 14.32 | 17.0 | 0.03 | 25.9 | 40 |
| HB14 | 8 | 45.6 | 8.5 | 1.07 | 50.76 | 15.5 | 0.10 | 2.7 | 0 † |
| HB15 | 8 | 17.1 | 8.5 | 1.06 | 64.03 | 14.2 | 0.12 | 0.2 | 0 † |
| HB16 | 8 | 74.1 | 8.3 | 1.15 | 47.03 | 14.9 | 0.09 | 7.9 | 18 |
| p-Values | ||||||
|---|---|---|---|---|---|---|
| Response | Particle Size | SiO2 | Φ50 | SSA | Vp (p/p0 = 0.95) | SNP Size |
| /µm | /wt% | /nm | /m²∙g−1 | /cm³∙g−1 | /nm | |
| Model | 0.0172 | <0.0001 | 0.0007 | <0.0001 | <0.0001 | <0.0001 |
| A–n(H2O)/n(TEOS) | 0.0172 | <0.0001 | 0.0099 | <0.0001 | 0.0007 | <0.0001 |
| B–c(NH3) | n.s. | 0.0011 | 0.0051 | <0.0001 | <0.0001 | <0.0001 |
| AB | n.s. | n.s. | 0.0074 | n.s. | n.s. | 0.0026 |
| A² | n.s. | <0.0001 | 0.0050 | <0.0001 | <0.0001 | 0.0009 |
| B² | n.s. | n.s. | n.s. | 0.0146 | n.s. | n.s. |
| Lack of fit | 0.0163 | 0.2204 (n.s.) | 0.4990 (n.s.) | 0.4397 (n.s.) | 0.9670 (n.s.) | 0.6132 (n.s.) |
| R² | 0.3624 | 0.9780 | 0.8299 | 0.9840 | 0.9800 | 0.9545 |
| R²adj | 0.3152 | 0.9720 | 0.7618 | 0.9776 | 0.9745 | 0.9364 |
| R²pred | 0.1684 | 0.9459 | 0.5529 | 0.9535 | 0.9635 | 0.8874 |
| SiO2 | Φ50 | SSA | Vp | SNP Size | |
|---|---|---|---|---|---|
| /wt% | /nm | /m²∙g−1 | /cm³∙g−1 | /nm | |
| A–n(H2O)/n(TEOS) | +1.00 | +1.00 | −1.00 | −1.00 | +1.00 |
| B–c(NH3), | +0.27 | +1.25 | −0.45 | −0.26 | +0.66 |
| AB | +1.30 | +0.45 | |||
| A² | −0.88 | −1.78 | +0.92 | +0.71 | −0.68 |
| B² | +0.28 |
| Factor | Name | Low Setting (−) | Center Point (0) | High Setting (+) |
|---|---|---|---|---|
| A | n(H2O)/n(TEOS) | 4 | 39 | 74 |
| B | c(NH3)/mmol | 2.39 | 6.38 | 10.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinbach, J.C.; Fait, F.; Mayer, H.A.; Kandelbauer, A. Monodisperse Porous Silica/Polymer Nanocomposite Microspheres with Tunable Silica Loading, Morphology and Porosity. Int. J. Mol. Sci. 2022, 23, 14977. https://doi.org/10.3390/ijms232314977
Steinbach JC, Fait F, Mayer HA, Kandelbauer A. Monodisperse Porous Silica/Polymer Nanocomposite Microspheres with Tunable Silica Loading, Morphology and Porosity. International Journal of Molecular Sciences. 2022; 23(23):14977. https://doi.org/10.3390/ijms232314977
Chicago/Turabian StyleSteinbach, Julia C., Fabio Fait, Hermann A. Mayer, and Andreas Kandelbauer. 2022. "Monodisperse Porous Silica/Polymer Nanocomposite Microspheres with Tunable Silica Loading, Morphology and Porosity" International Journal of Molecular Sciences 23, no. 23: 14977. https://doi.org/10.3390/ijms232314977
APA StyleSteinbach, J. C., Fait, F., Mayer, H. A., & Kandelbauer, A. (2022). Monodisperse Porous Silica/Polymer Nanocomposite Microspheres with Tunable Silica Loading, Morphology and Porosity. International Journal of Molecular Sciences, 23(23), 14977. https://doi.org/10.3390/ijms232314977