Osteoclast-Driven Osteogenesis, Bone Remodeling and Biomaterial Resorption: A New Profile of BMP2-CPC-Induced Alveolar Bone Regeneration
Abstract
1. Introduction
2. Results
2.1. Clinical Study
2.1.1. Radiographic Evaluation
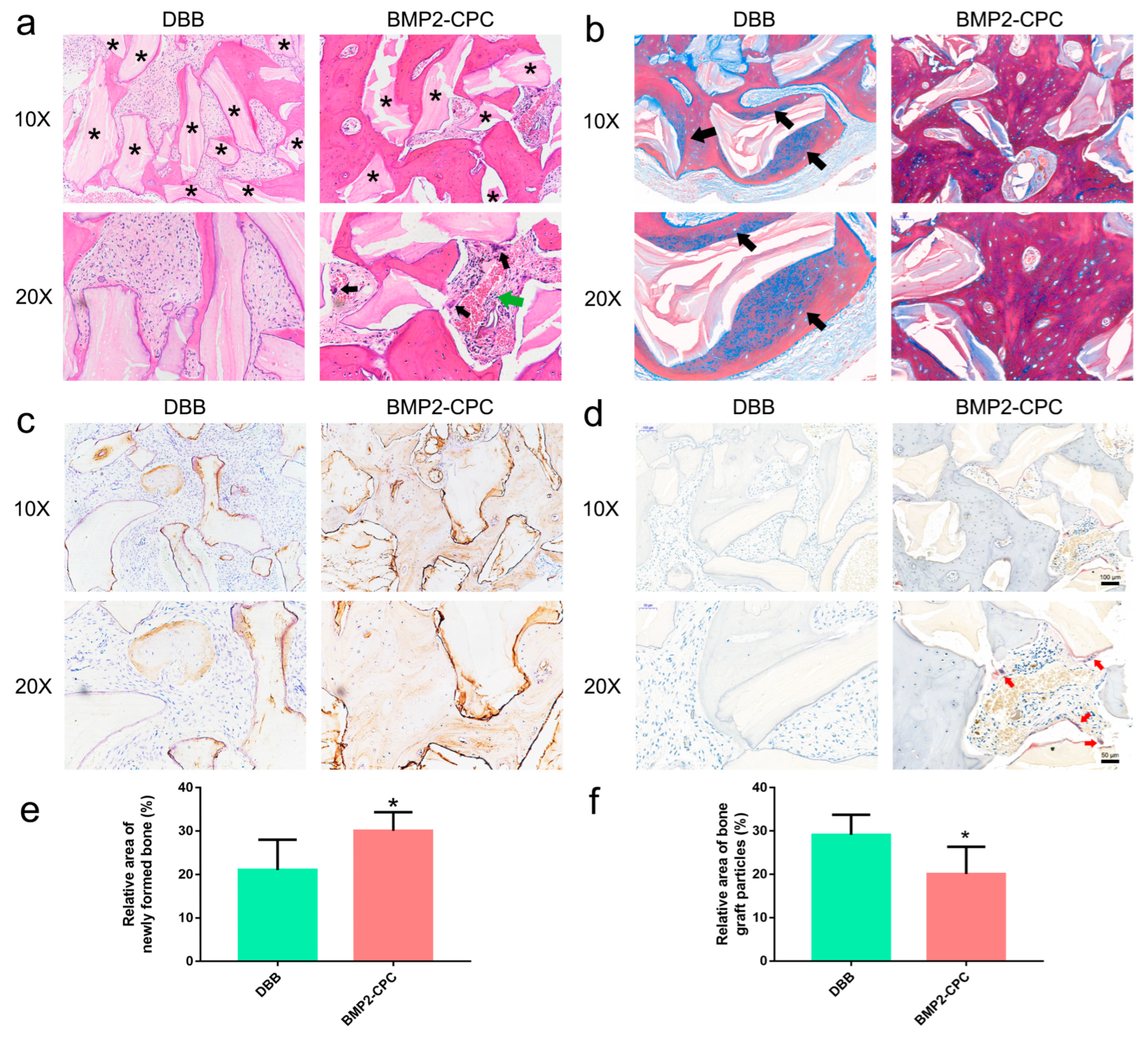
2.1.2. Histological Examination
2.2. In Vitro Study
2.2.1. Biomaterial Microstructure
2.2.2. Cell Viability and Differentiation of Bone Marrow Macrophages (BMMs)
2.2.3. Osteoclast Activity
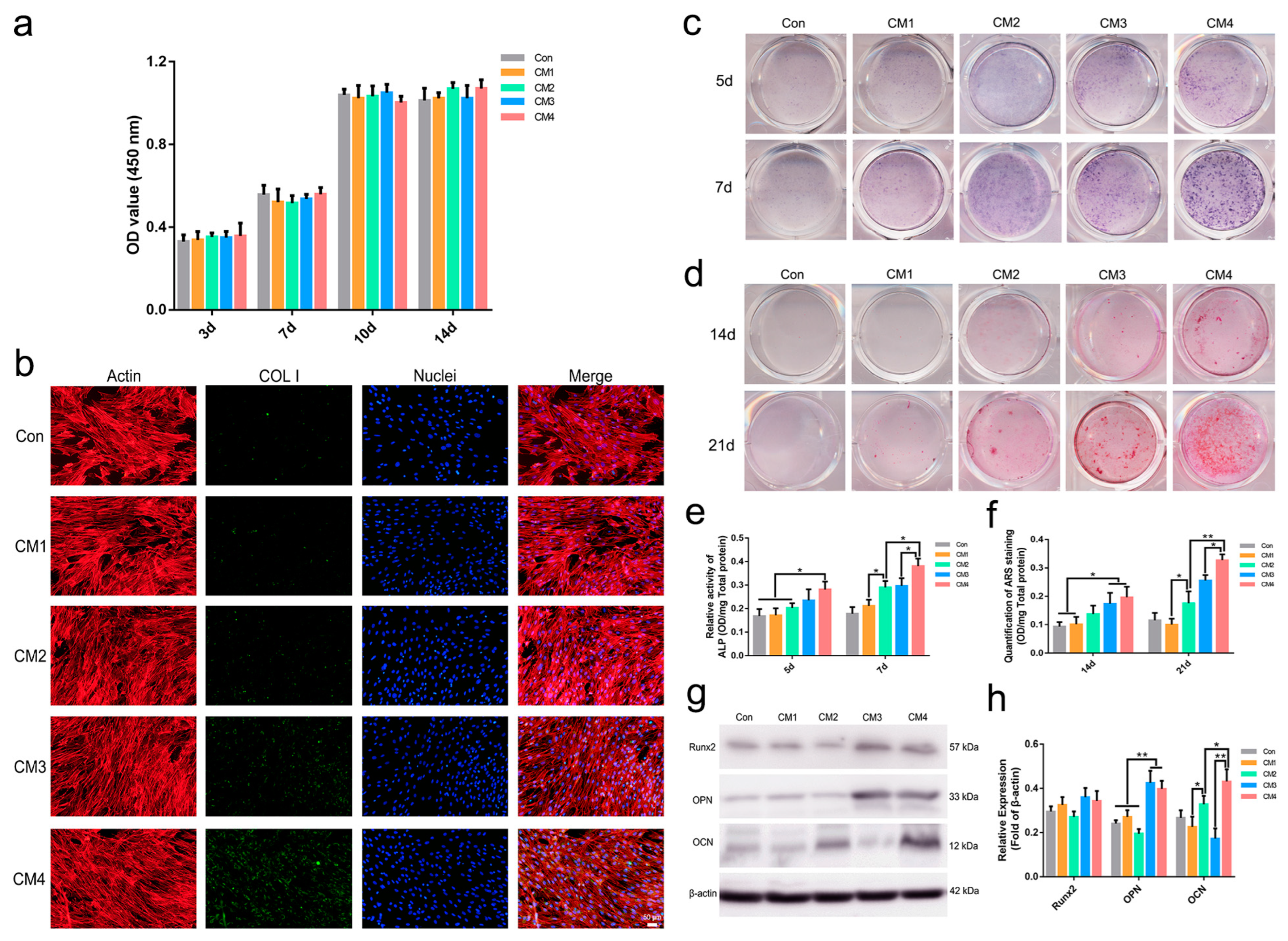
2.2.4. Osteogenic Differentiation of MSCs
2.3. In Vivo Study
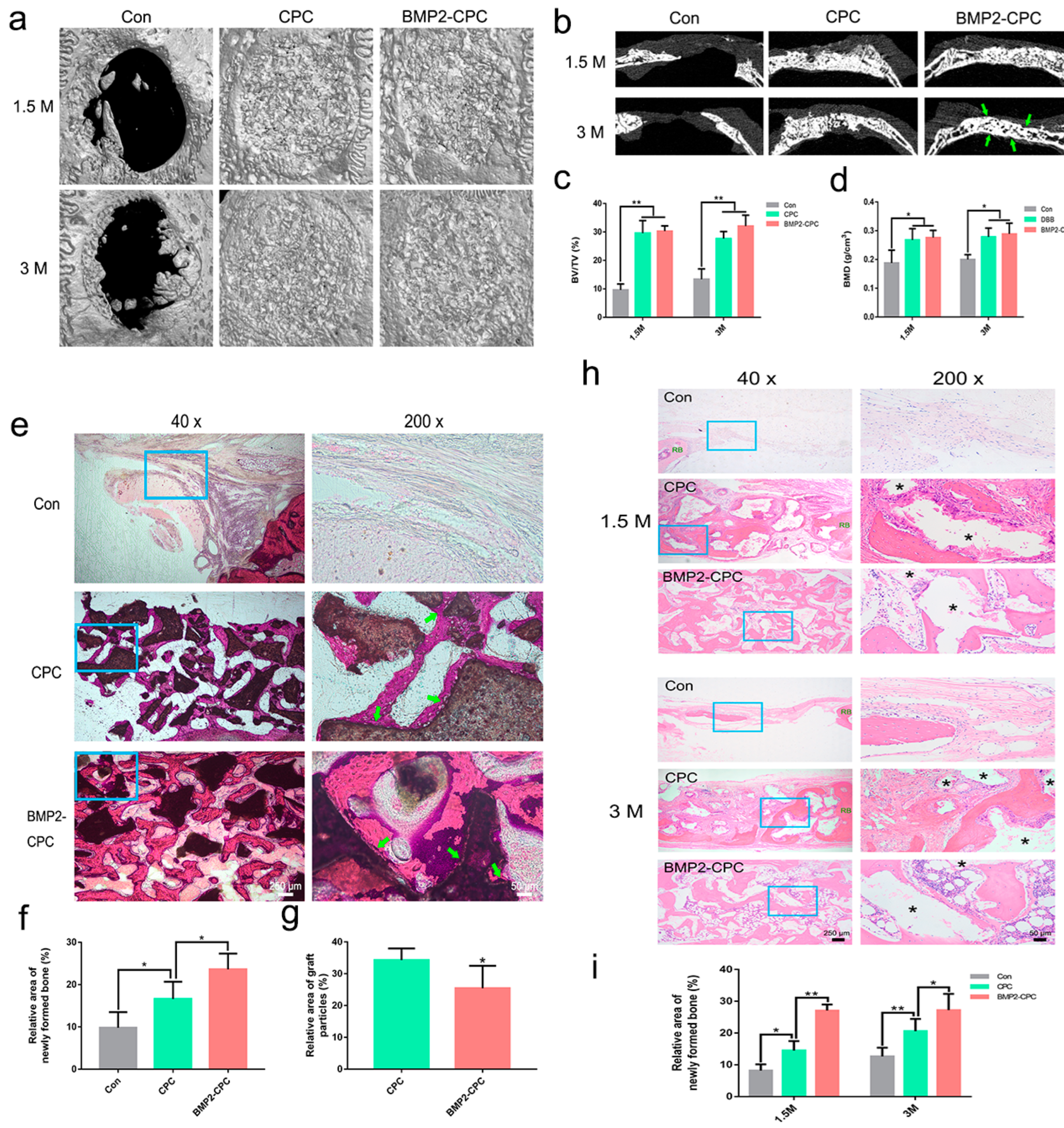
2.3.1. Micro-CT Analysis
2.3.2. Histological Examination (Undecalcified)
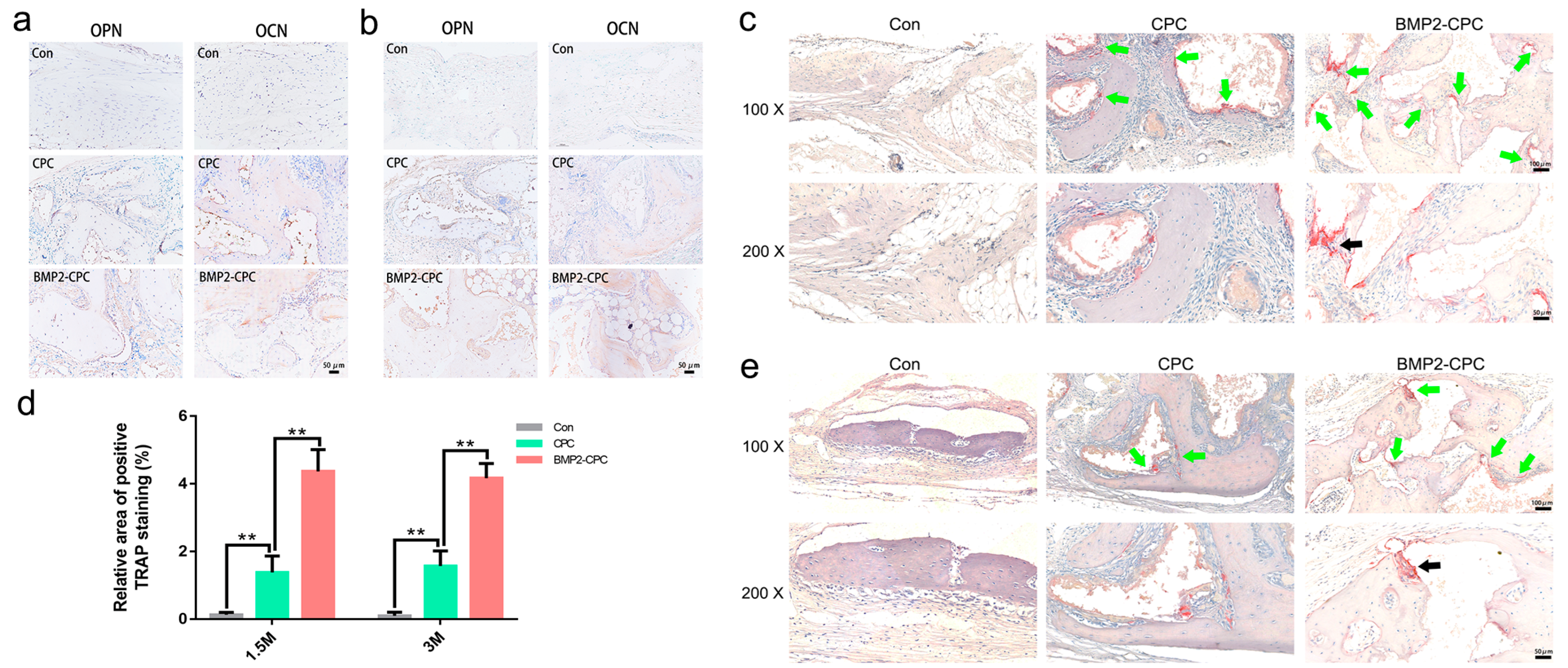
2.3.3. Histological and Immunohistochemical Examination (Decalcified)
2.3.4. Osteoclast Differentiation
3. Discussion
4. Materials and Methods
4.1. Clinical Study
4.1.1. Study Design
4.1.2. Surgical Procedures
4.1.3. Radiographic Evaluation
4.1.4. Histological Examination
4.2. In Vitro Study
4.2.1. Preparation and Characterization of Biomaterials
4.2.2. Osteoclast Culture
Isolation and Culture of BMMs
Cell Viability of BMMs
Tartrate-Resistant Acid Phosphatase (TRAP) Activity
Osteoclast Identification
Calcium Ion Quantification
Osteogenic Cytokine Expression and Secretion
Conditioned Medium (CM) Preparation
4.2.3. MSC Culture
MSC Isolation and Culture
Cell Viability of MSCs
Alkaline Phosphatase (ALP) Activity and Alizarin Red S (ARS) Staining
Osteogenic Differentiation of MSCs
4.3. In Vivo Study
4.3.1. Study Design
4.3.2. Surgical Procedures
4.3.3. Microcomputed Tomography (micro-CT) Examination
4.3.4. Histological Examination (Undecalcified)
4.3.5. Histological and Immunohistochemical Examinations (Decalcified)
4.3.6. TRAP Staining
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Kylmaoja, E.; Nakamura, M.; Tuukkanen, J. Osteoclasts and Remodeling Based Bone Formation. Curr. Stem Cell Res. Ther. 2016, 11, 626–633. [Google Scholar] [CrossRef]
- Lee, I.H.; Chung, C.Y.; Lee, K.M.; Kwon, S.-S.; Moon, S.Y.; Jung, K.J.; Chung, M.K.; Park, M.S. Incidence and risk factors of allograft bone failure after calcaneal lengthening. Clin. Orthop. Relat. Res. 2015, 473, 1765–1774. [Google Scholar] [CrossRef]
- Aponte-Tinao, L.A.; Ayerza, M.A.; Muscolo, D.L.; Farfalli, G.L. What Are the Risk Factors and Management Options for Infection After Reconstruction With Massive Bone Allografts? Clin. Orthop. Relat. Res. 2016, 474, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Byun, H.; Perikamana, S.K.M.; Lee, S.; Shin, H. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv. Healthc. Mater. 2019, 8, e1801106. [Google Scholar] [CrossRef]
- Gillman, C.E.; Jayasuriya, A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C 2021, 130, 112466. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef]
- Zeeshan, S.; Mohamed-Nur, A.; Ahmed, H.; Syed, M.; Haroon, R.; Michael, G. Mechanisms of in Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials. Materials 2015, 8, 7913–7925. [Google Scholar]
- Aghali, A. Craniofacial Bone Tissue Engineering: Current Approaches and Potential Therapy. Cells 2021, 10, 2993. [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Bordukalo-Nikšić, T.; Kufner, V.; Vukičević, S. The Role of BMPs in the Regulation of Osteoclasts Resorption and Bone Remodeling: From Experimental Models to Clinical Applications. Front. Immunol. 2022, 13, 869422. [Google Scholar] [CrossRef]
- Grafe, I.; Alexander, S.; Peterson, J.R.; Snider, T.N.; Levi, B.; Lee, B.; Mishina, Y. TGF-β Family Signaling in Mesenchymal Differentiation. Cold Spring Harb. Perspect. Biol. 2019, 10, a022202. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, J.; Bai, F.; Cao, X.; Fan, C.; Yuan, Y.; Wang, J.; Zhang, J.; Liu, C. Fabrication and clinical application of easy-to-operate pre-cured CPC/rhBMP-2 micro-scaffolds for bone regeneration. Am. J. Transl. Res. 2016, 8, 1379–1396. [Google Scholar]
- Shen, H.; Zhi, Y.; Zhu, F.; Si, J.; Shi, J.; Shen, S.G. Experimental and clinical evaluation of BMP2-CPC graft versus deproteinized bovine bone graft for guided bone regeneration: A pilot study. Dent. Mater. J. 2021, 40, 191–201. [Google Scholar] [CrossRef]
- Shen, H.; Shi, J.; Zhi, Y.; Yang, X.; Yuan, Y.; Si, J.; Shen, S.G.F. Improved BMP2-CPC-stimulated osteogenesis in vitro and in vivo via modulation of macrophage polarization. Mater. Sci. Eng. C 2021, 118, 111471. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Cao, X. Targeting osteoclast-osteoblast communication. Nat. Med. 2011, 17, 1344–1346. [Google Scholar] [CrossRef]
- Kondo, N.; Ogose, A.; Tokunaga, K.; Umezu, H.; Arai, K.; Kudo, N.; Hoshino, M.; Inoue, H.; Irie, H.; Kuroda, K.; et al. Osteoinduction with highly purified beta-tricalcium phosphate in dog dorsal muscles and the proliferation of osteoclasts before heterotopic bone formation. Biomaterials 2006, 27, 4419–4427. [Google Scholar] [CrossRef]
- Gamblin, A.-L.; Brennan, M.A.; Renaud, A.; Yagita, H.; Lézot, F.; Heymann, D.; Trichet, V.; Layrolle, P. Bone tissue formation with human mesenchymal stem cells and biphasic calcium phosphate ceramics: The local implication of osteoclasts and macrophages. Biomaterials 2014, 35, 9660–9667. [Google Scholar] [CrossRef]
- Davison, N.L.; Gamblin, A.-L.; Layrolle, P.; Yuan, H.; de Bruijn, J.D.; Barrère-de Groot, G. Liposomal clodronate inhibition of osteoclastogenesis and osteoinduction by submicrostructured beta-tricalcium phosphate. Biomaterials 2014, 35, 5088–5097. [Google Scholar] [CrossRef]
- Okamoto, K.; Nakashima, T.; Shinohara, M.; Negishi-Koga, T.; Komatsu, N.; Terashima, A.; Sawa, S.; Nitta, T.; Takayanagi, H. Osteoimmunology: The Conceptual Framework Unifying the Immune and Skeletal Systems. Physiol. Rev. 2017, 97, 1295–1349. [Google Scholar] [CrossRef] [PubMed]
- Humbert, P.; Brennan, M.A.; Davison, N.; Rosset, P.; Trichet, V.; Blanchard, F.; Layrolle, P. Immune Modulation by Transplanted Calcium Phosphate Biomaterials and Human Mesenchymal Stromal Cells in Bone Regeneration. Front. Immunol. 2019, 10, 663. [Google Scholar] [CrossRef] [PubMed]
- Fosca, M.; Rau, J.V.; Uskoković, V. Factors influencing the drug release from calcium phosphate cements. Bioact. Mater. 2021, 7, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.B.B.; Menezes, L.d.M.; Azeredo, F.; Filho, L.S.L. Volumetric assessment of alveolar clefts: A literature review. J. Oral Pathol. Med. 2017, 46, 569–573. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar]
- Adamopoulos, I.E.; Mellins, E.D. Alternative pathways of osteoclastogenesis in inflammatory arthritis. Nat. Rev. Rheumatol. 2015, 11, 189–194. [Google Scholar] [CrossRef]
- Itoh, K.; Udagawa, N.; Katagiri, T.; Iemura, S.; Ueno, N.; Yasuda, H.; Higashio, K.; Quinn, J.M.W.; Gillespie, M.T.; Martin, T.J.; et al. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology 2001, 142, 3656–3662. [Google Scholar] [CrossRef]
- Kim, R.Y.; Oh, J.H.; Lee, B.S.; Seo, Y.-K.; Hwang, S.J.; Kim, I.S. The effect of dose on rhBMP-2 signaling, delivered via collagen sponge, on osteoclast activation and in vivo bone resorption. Biomaterials 2014, 35, 1869–1881. [Google Scholar] [CrossRef]
- Granholm, S.; Henning, P.; Lindholm, C.; Lerner, U.H. Osteoclast progenitor cells present in significant amounts in mouse calvarial osteoblast isolations and osteoclastogenesis increased by BMP-2. Bone 2013, 52, 83–92. [Google Scholar] [CrossRef]
- Niu, H.; Ma, Y.; Wu, G.; Duan, B.; Wang, Y.; Yuan, Y.; Liu, C. Multicellularity-interweaved bone regeneration of BMP-2-loaded scaffold with orchestrated kinetics of resorption and osteogenesis. Biomaterials 2019, 216, 119216. [Google Scholar] [CrossRef]
- Davison, N.L.; Luo, X.; Schoenmaker, T.; Everts, V.; Yuan, H.; Groot, F.B.-d.; de Bruijn, J.D. Submicron-scale surface architecture of tricalcium phosphate directs osteogenesis in vitro and in vivo. Eur. Cells Mater. 2014, 27, 281–297; discussion 296–297. [Google Scholar] [CrossRef] [PubMed]
- González-Vázquez, A.; Planell, J.A.; Engel, E. Extracellular calcium and CaSR drive osteoinduction in mesenchymal stromal cells. Acta Biomater. 2014, 10, 2824–2833. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Kohara, Y.; Naoe, Y.; Watanabe, A.; Ito, M.; Ikeda, K.; Takeshita, S. WAIF1 Is a Cell-Surface CTHRC1 Binding Protein Coupling Bone Resorption and Formation. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2018, 33, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yu, W.; Niibe, K.; Zhang, W.; Egusa, H.; Tang, T.; Jiang, X. The Effects of Platelet-Derived Growth Factor-BB on Bone Marrow Stromal Cell-Mediated Vascularized Bone Regeneration. Stem Cells Int. 2018, 2018, 3272098. [Google Scholar] [CrossRef]
- Takeshita, S.; Fumoto, T.; Matsuoka, K.; Park, K.-a.; Aburatani, H.; Kato, S.; Ito, M.; Ikeda, K. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J. Clin. Investig. 2013, 123, 3914–3924. [Google Scholar] [CrossRef]
- Xie, H.; Cui, Z.; Wang, L.; Xia, Z.; Hu, Y.; Xian, L.; Li, C.; Xie, L.; Crane, J.; Wan, M.; et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat. Med. 2014, 20, 1270–1278. [Google Scholar] [CrossRef]
- Seeherman, H.J.; Azari, K.; Bidic, S.; Rogers, L.; Li, X.J.; Hollinger, J.O.; Wozney, J.M. rhBMP-2 delivered in a calcium phosphate cement accelerates bridging of critical-sized defects in rabbit radii. J. Bone Jt. Surg. 2006, 88, 1553–1565. [Google Scholar] [CrossRef]
- Schröter, L.; Kaiser, F.; Stein, S.; Gbureck, U.; Ignatius, A. Biological and mechanical performance and degradation characteristics of calcium phosphate cements in large animals and humans. Acta Biomater. 2020, 117, 1–20. [Google Scholar] [CrossRef]
- Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 2007, 13, 791–801. [Google Scholar] [CrossRef]
- Ma, Z.; Zheng, J.; Yang, C.; Xie, Q.; Liu, X.; Abdelrehem, A. A new modified bone grafting technique for periodontally accelerated osteogenic orthodontics. Medicine 2018, 97, e12047. [Google Scholar] [CrossRef]
- Ma, Z.-G.; Yang, C.; Xie, Q.-Y.; Ye, Z.-X.; Zhang, S.-Y.; Abdelrehem, A. A Novel Surgical Technique for Augmented Corticotomy-Assisted Orthodontics: Bone Grafting With Periosteum. J. Oral Maxillofac. Surg. 2016, 74, 170–180. [Google Scholar] [CrossRef] [PubMed]
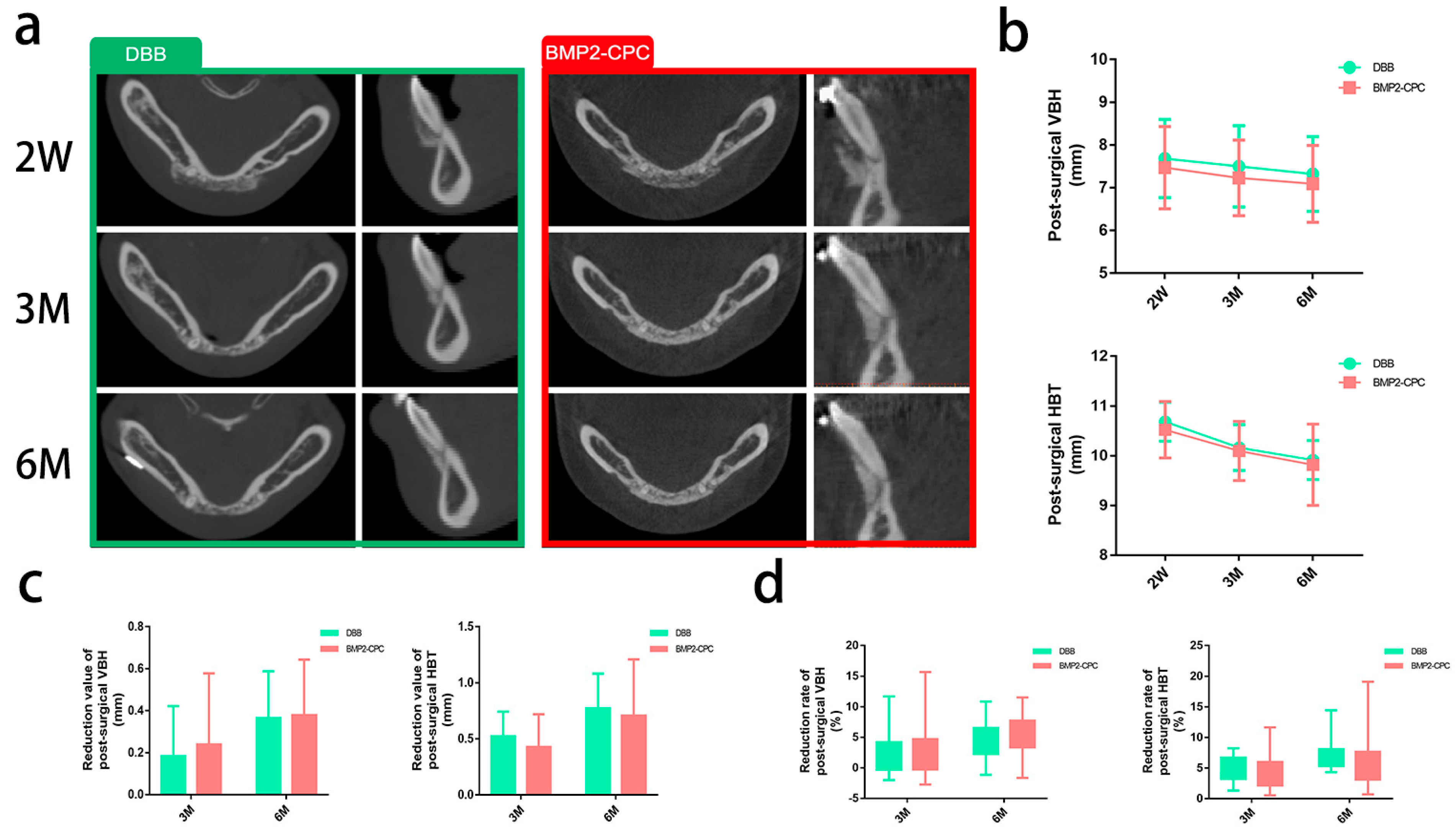


| Parameters | DBB (mm) | BMP2-CPC (mm) | p Value |
|---|---|---|---|
| VBH2W | 7.68 ± 0.89 | 7.47 ± 0.94 | 0.663 |
| VBH3M | 7.50 ± 0.92 | 7.23 ± 0.86 | 0.258 |
| VBH6M | 7.32 ± 0.85 | 7.09 ± 0.88 | 0.358 |
| HBT2W | 10.69 ± 0.38 | 10.52 ± 0.55 | 0.358 |
| HBT 3M | 10.16 ± 0.45 | 10.10 ± 0.58 | 0.893 |
| HBT6M | 9.91 ± 0.38 | 9.82 ± 0.79 | 0.869 |
| Parameters | DBB | BMP2-CPC | p Value |
|---|---|---|---|
| 3-month reduction value of VBH (mm) | 0.18 ± 0.23 | 0.24 ± 0.33 | 0.685 |
| 6-month reduction value of VBH (mm) | 0.36 ± 0.22 | 0.38 ± 0.26 | 0.940 |
| 3-month reduction rate of VBH (%) | 2.45 ± 3.25 | 3.01 ± 4.11 | 0.663 |
| 6-month reduction rate of VBH (%) | 4.71 ± 2.88 | 4.98 ± 3.12 | 0.988 |
| 3-month reduction value of HBT (mm) | 0.52 ± 0.21 | 0.43 ± 0.28 | 0.142 |
| 6-month reduction value of HBT (mm) | 0.77 ± 0.30 | 0.70 ± 0.49 | 0.258 |
| 3-month reduction rate of HBT (%) | 4.90 ± 2.01 | 4.05 ± 2.64 | 0.189 |
| 6-month reduction rate of HBT (%) | 7.21 ± 2.78 | 6.76 ± 4.87 | 0.284 |
| Vertical Bone Height | Horizontal Bone Thickness | |
|---|---|---|
| Reduction value-3M | VBH2W − VBH3M | HBT2W − HBT3M |
| Reduction rate-3M | (VBH2W − VBH3M)/VBH2W × 100% | (HBT2W − HBT3M)/HBT2W × 100% |
| Reduction value-6M | VBH2W − VBH6M | HBT2W − HBT6M |
| Reduction rate-6M | (VBH2W − VBH6M)/VBH2W × 100% | (HBT2W − HBT6M)/HBT2W × 100% |
| Gene | Primer Sequences |
|---|---|
| CTHRC1 | Forward: 5′-AAGCAAAAAGCGCTGATCC-3′ |
| Reverse: 5′-CCTGCTGGTCCTTGTAGACAC-3′ | |
| Wnt10b | Forward: 5′-GGGCTCAGGTTCCTACTTCC-3′ |
| Reverse: 5′-AAGGAGAAGCCTCCCAAGAG-3′ | |
| PDGF-BB | Forward: 5′-CCTCGGCCTGTGACTAGAAG-3′ |
| Reverse: 5′-CCTTGTCATGGGTGTGCTTA-3′ | |
| BMP6 | Forward: 5′-AACCTTTCTTATCAGCATTTACCA-3′ |
| Reverse: 5′-GTGTCCAACAAAAATAGGTCAGAG-3′ | |
| GAPDH | Forward: 5′-TGGTGAAGGTCGGTGTGAAC-3′ |
| Reverse: 5′-CCATGTAGTTGAGGTCAATGAAGG-3′ |
| Group | Material | M-CSF | RANKL | Corresponding Cells | DMEM Containing 10% FBS |
|---|---|---|---|---|---|
| Con | — | — | — | None | + |
| CM1 | CPC | + | — | BMMs | + |
| CM2 | CPC | + | + | Osteoclasts | + |
| CM3 | BMP2-CPC | + | — | BMMs | + |
| CM4 | BMP2-CPC | + | + | Osteoclasts | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Zhuang, Y.; Zhang, C.; Zhang, C.; Yuan, Y.; Yu, H.; Si, J.; Shen, G. Osteoclast-Driven Osteogenesis, Bone Remodeling and Biomaterial Resorption: A New Profile of BMP2-CPC-Induced Alveolar Bone Regeneration. Int. J. Mol. Sci. 2022, 23, 12204. https://doi.org/10.3390/ijms232012204
Shen H, Zhuang Y, Zhang C, Zhang C, Yuan Y, Yu H, Si J, Shen G. Osteoclast-Driven Osteogenesis, Bone Remodeling and Biomaterial Resorption: A New Profile of BMP2-CPC-Induced Alveolar Bone Regeneration. International Journal of Molecular Sciences. 2022; 23(20):12204. https://doi.org/10.3390/ijms232012204
Chicago/Turabian StyleShen, Hongzhou, Yu Zhuang, Chenglong Zhang, Changru Zhang, Yuan Yuan, Hongbo Yu, Jiawen Si, and Guofang Shen. 2022. "Osteoclast-Driven Osteogenesis, Bone Remodeling and Biomaterial Resorption: A New Profile of BMP2-CPC-Induced Alveolar Bone Regeneration" International Journal of Molecular Sciences 23, no. 20: 12204. https://doi.org/10.3390/ijms232012204
APA StyleShen, H., Zhuang, Y., Zhang, C., Zhang, C., Yuan, Y., Yu, H., Si, J., & Shen, G. (2022). Osteoclast-Driven Osteogenesis, Bone Remodeling and Biomaterial Resorption: A New Profile of BMP2-CPC-Induced Alveolar Bone Regeneration. International Journal of Molecular Sciences, 23(20), 12204. https://doi.org/10.3390/ijms232012204








