Surprising Solid-State ESIPT Emission from Apparently Ordinary Salicyliden Glycinates Schiff Bases
Abstract
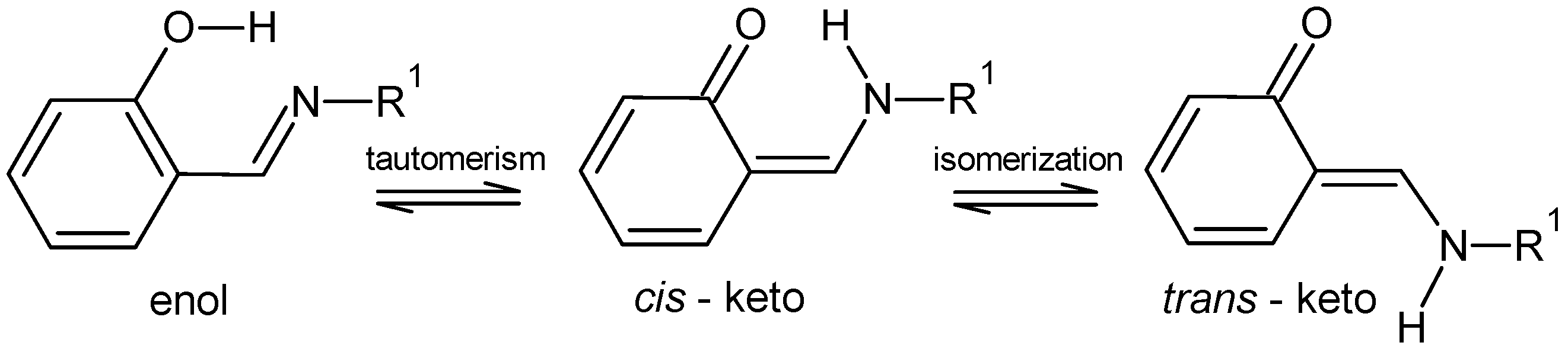
:1. Introduction
2. Results
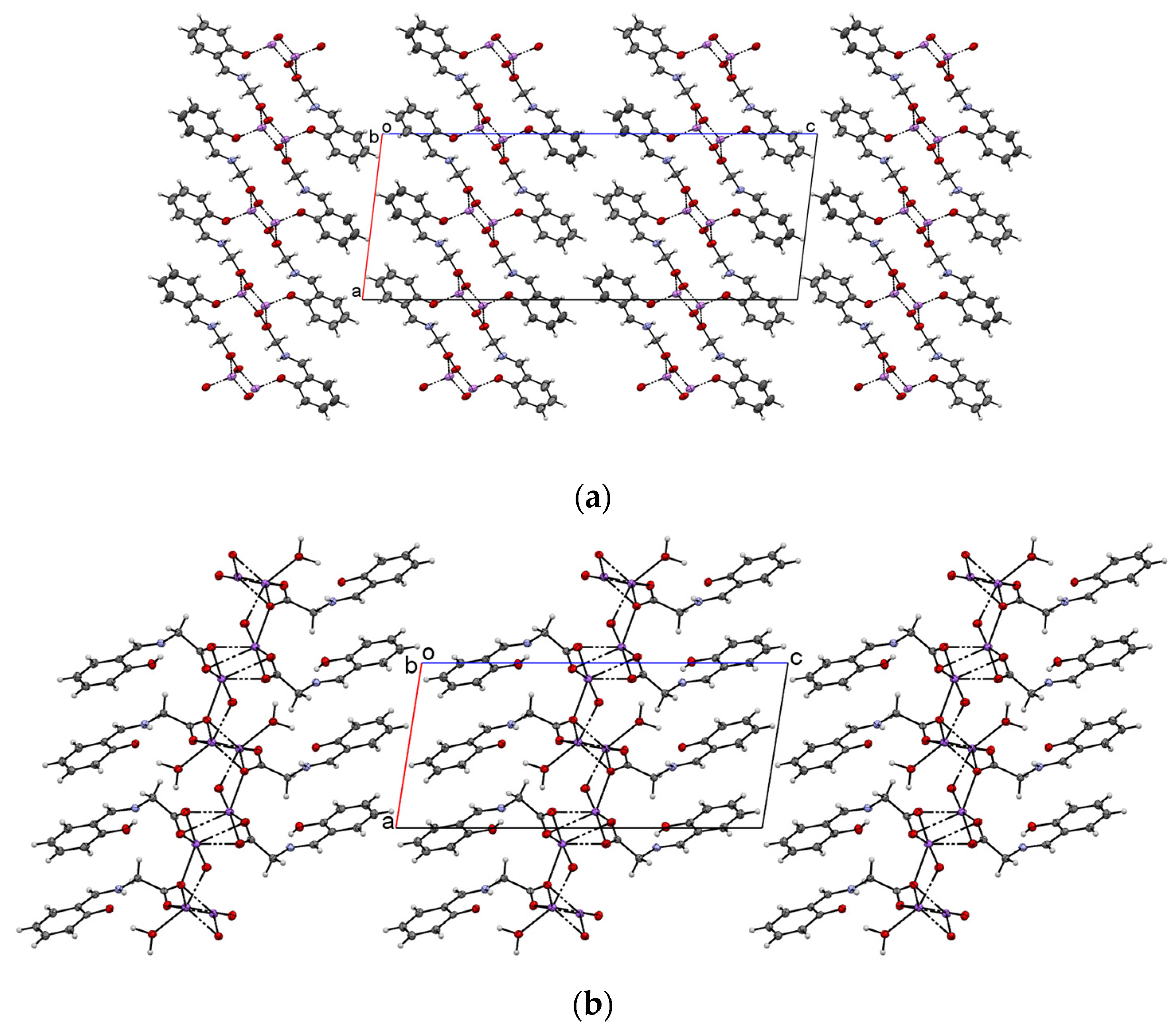
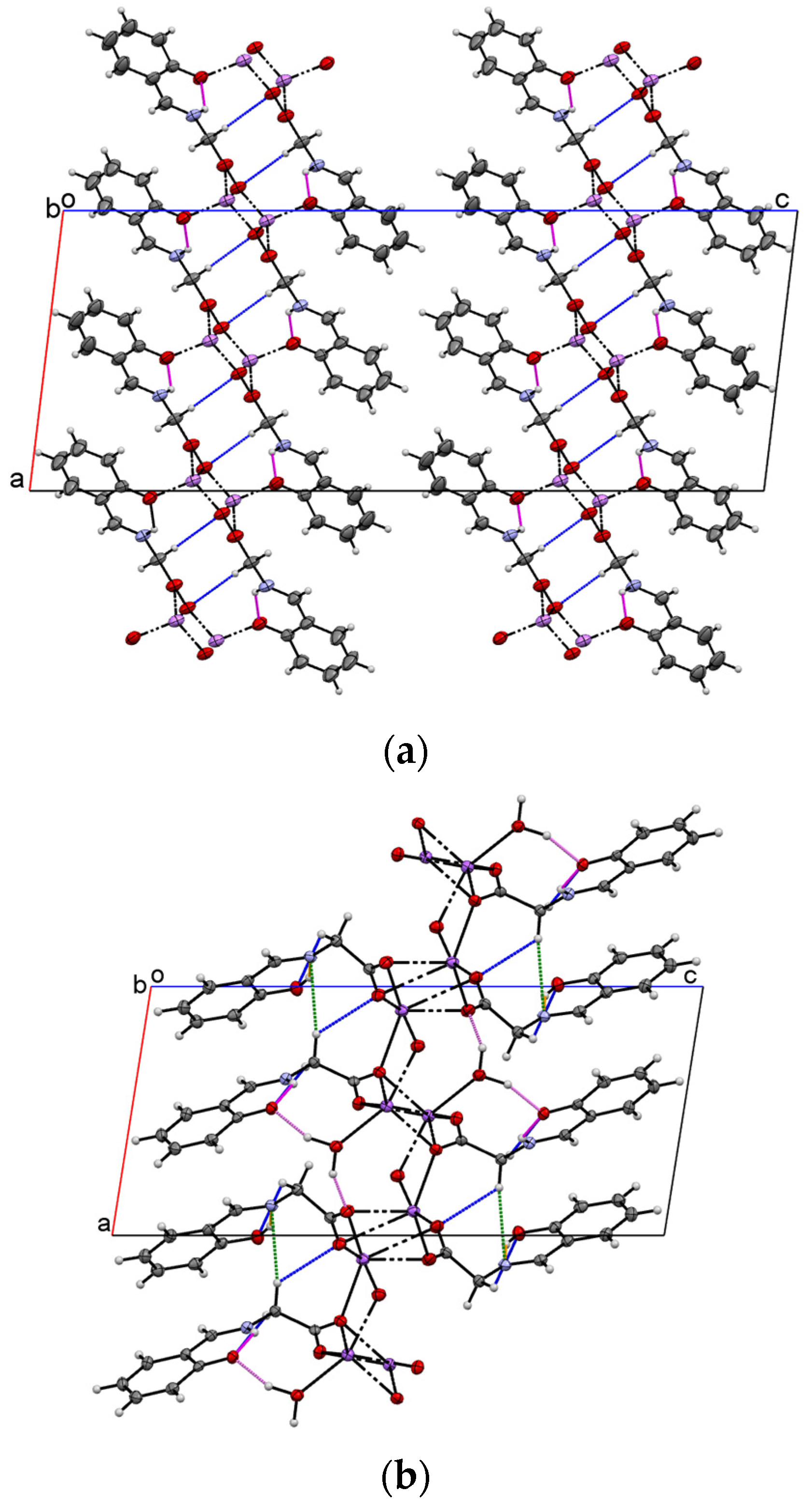
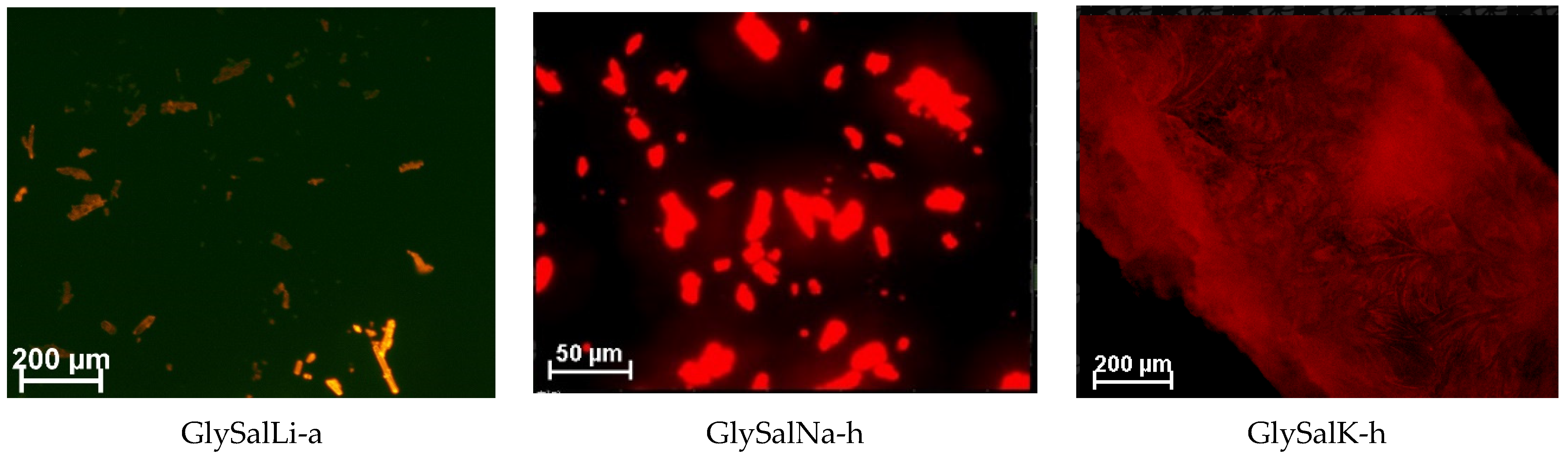
2.1. Crystal Structure
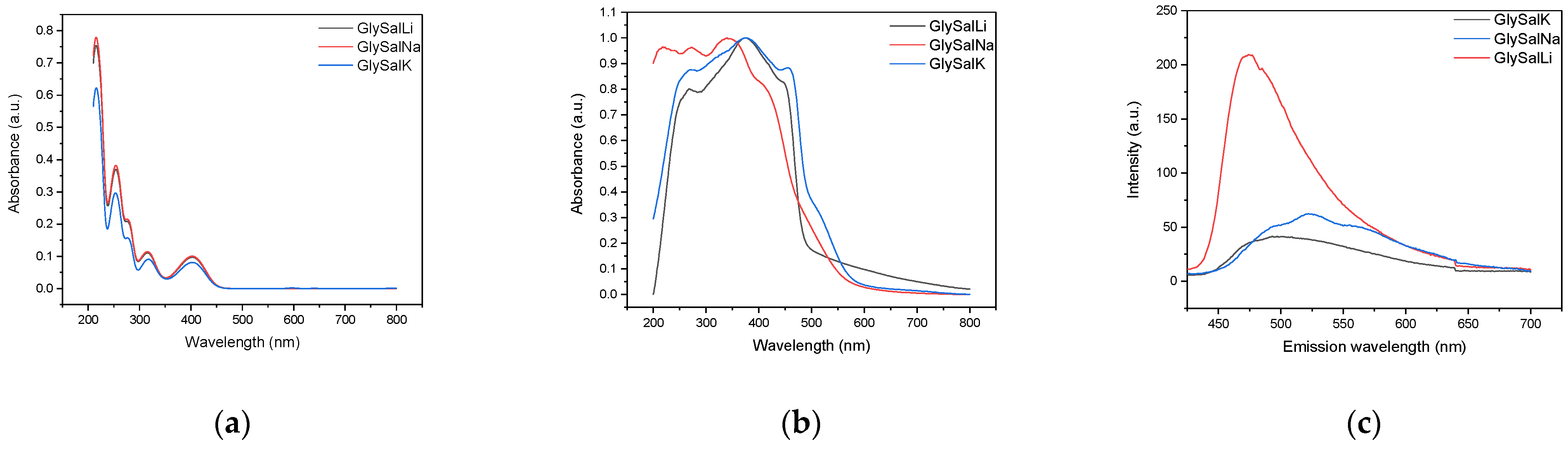
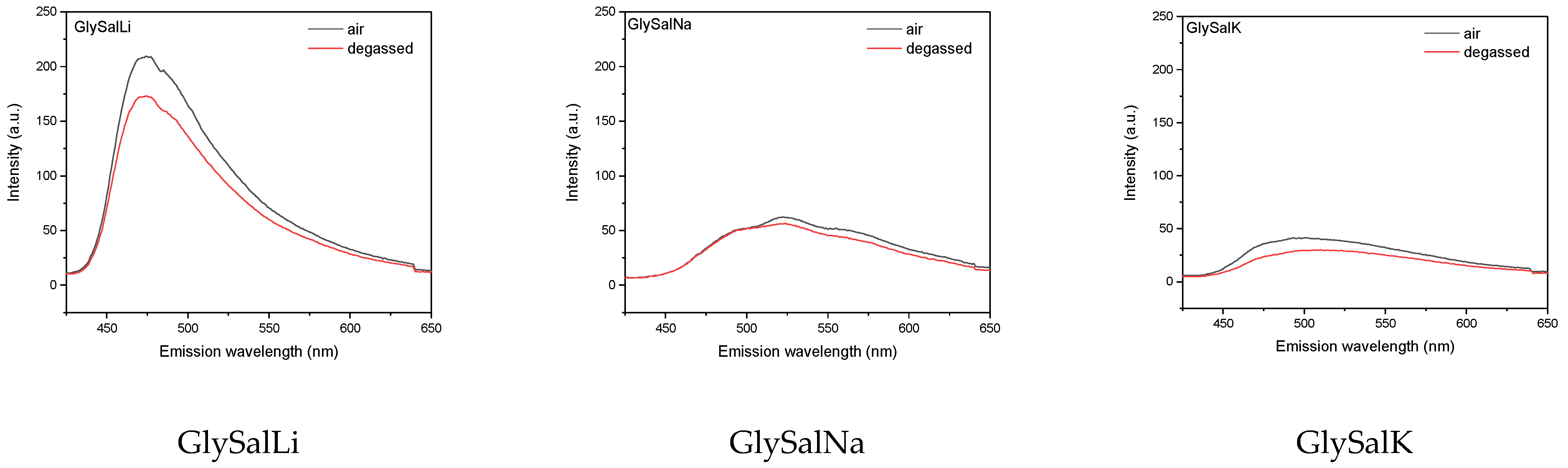
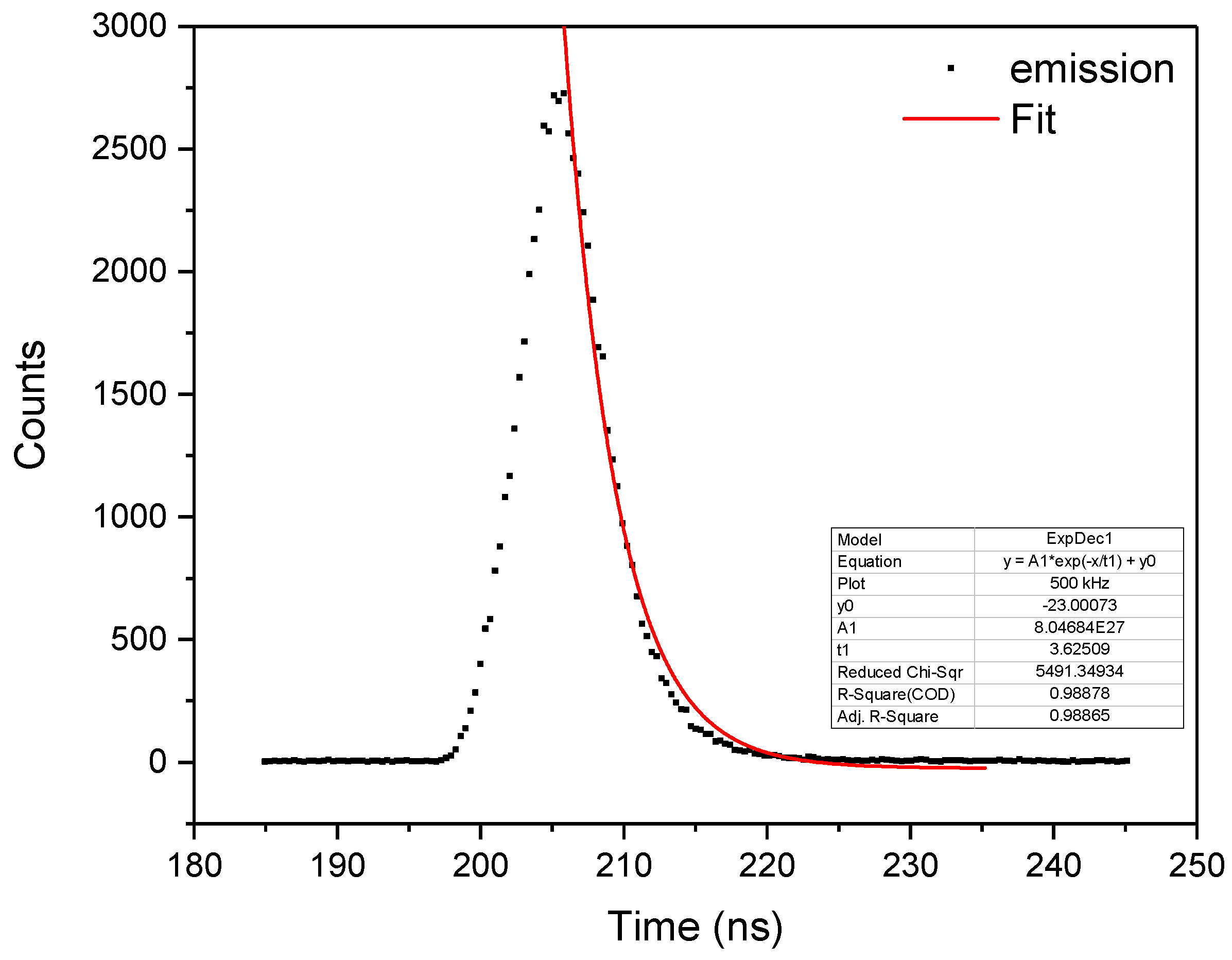
2.2. Absorption and Emission Properties
3. Materials and Methods
3.1. Synthesis of Salicyl-Glycine Schiff Bases Salts GlySalX (GlySalLi or GlySalNa)
3.2. Solid State and Solution Fluorescence Measurements
3.3. UV-Vis Absorption Spectroscopy
3.4. NMR Spectroscopy
3.5. X-Ray Crystallography
3.6. Confocal Microscopy
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padalkar, V.S.; Seki, S. Excited-State Intramolecular Proton-Transfer (ESIPT)-Inspired Solid State Emitters. Chem. Soc. Rev. 2016, 45, 169–202. [Google Scholar] [CrossRef] [PubMed]
- Hadjoudis, E. Photochromic and Thermochromic Anils. Mol. Eng. 1995, 5, 301–337. [Google Scholar] [CrossRef]
- Mitra, S.; Tamai, N. Dynamics of Photochromism in Salicylideneaniline: A Femtosecond Spectroscopic Study. Phys. Chem. Chem. Phys. 2003, 5. [Google Scholar] [CrossRef] [Green Version]
- Ganie, A.A.; Dar, A.A. Water Switched Reversible Thermochromism in Organic Salt of Sulfonated Anil. Cryst. Growth Des. 2021, 21, 3014–3023. [Google Scholar] [CrossRef]
- Cai, M.; Gao, Z.; Zhou, X.; Wang, X.; Chen, S.; Zhao, Y.; Qian, Y.; Shi, N.; Mi, B.; Xie, L.; et al. A Small Change in Molecular Structure, a Big Difference in the AIEE Mechanism. Phys. Chem. Chem. Phys. 2012, 14, 5289. [Google Scholar] [CrossRef] [PubMed]
- Hadjoudis, E.; Mavridis, I.M. Photochromism and Thermochromism of Schiff Bases in the Solid State: Structural Aspects. Chem. Soc. Rev. 2004, 33, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Weller, A. Über Die Fluoreszenz Der Salizylsäure Und Verwandter Verbindungen. Naturwissenschaften 1955, 42, 175–176. [Google Scholar] [CrossRef]
- Shida, T.; Mutai, T.; Araki, K. Sterically Induced Polymorphism: ON-OFF Control of Excited-State Intramolecular Proton Transfer (ESIPT) Luminescence of 1-Methyl-2-(2’-Hydroxyphenyl)Benzimidazole. CrystEngComm 2013, 15, 10179–10182. [Google Scholar] [CrossRef]
- Chou, P.-T.; Chen, Y.; Yu, W.; Chou, Y.; Wei, C.; Cheng, Y.-M. Excited-State Intramolecular Proton Transfer in 10-Hydroxybenzo[h]Quinoline. J. Phys. Chem. A 2001, 105, 1731–1740. [Google Scholar] [CrossRef]
- Oliveira, F.F.D.; Santos, D.C.B.D.; Lapis, A.A.M.; Corrêa, J.R.; Gomes, A.F.; Gozzo, F.C.; Moreira, P.F.; De Oliveira, V.C.; Quina, F.H.; Neto, B.A.D. On the Use of 2,1,3-Benzothiadiazole Derivatives as Selective Live Cell Fluorescence Imaging Probes. Bioorganic Med. Chem. Lett. 2010, 20, 6001–6007. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kaneko, Y.; Arai, T. Photoinduced Intramolecular Hydrogen Atom Transfer of N-Salicylideneaniline in the Triplet State. Chem. Lett. 2000, 7, 756–757. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kato, T.; Huang, H.; Yoshikawa, I.; Mutai, T.; Houjou, H. Photochromism of Salicylideneanilines Bearing Super Bulky Substituents: Single-Crystal UV-Vis Spectroscopic Examination of Bleaching under Variable Temperature and Visible-Light Irradiation. J. Photochem. Photobiol. A Chem. 2019, 385, 112096. [Google Scholar] [CrossRef]
- Bakalorz, K.; Przypis, Ł.; Tomczyk, M.M.; Książek, M.; Grzesik, R.; Kuźnik, N. Unprecedented Water Effect as a Key Element in Salicyl-Glycine Schiff Base Synthesis. Molecules 2020, 25, 1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Sakai, K.; Kawamura, H.; Kobayashi, N.; Ishikawa, T.; Ikeda, C.; Kikuchi, T.; Akutagawa, T. Highly Efficient Solid-State Red Fluorophores Using ESIPT: Crystal Packing and Fluorescence Properties of Alkoxy-Substituted Dibenzothiazolylphenols. CrystEngComm 2014, 16. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. Crysalispro Software System, version 1.171.38.41q; Rigaku Corporation: Wroclaw, Poland, 2015. [Google Scholar]
- Sheldrick, G.M. Crystal refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]

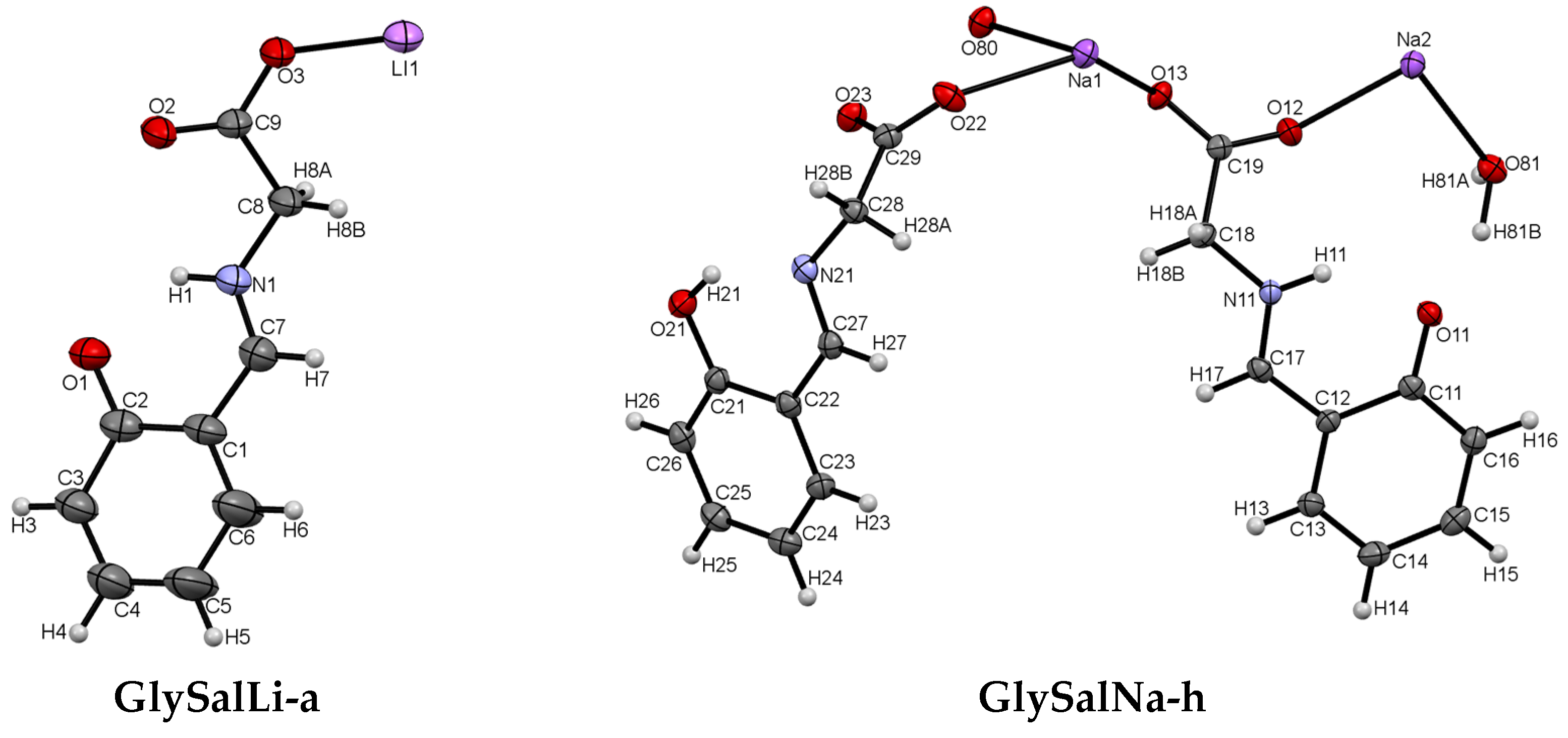
| GlySalLi-a | GlySalNa-h | |
|---|---|---|
| Empirical formula | C9H8LiNO3 | C18H18N2Na2O8 |
| Molecular weight | 185.10 | 435.32 |
| Temperature [K] | 100 | 100 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | I2/a | P21 |
| Unit cell dimensions | ||
| a [Å] | 11.6482(9) | 8.6872(2) |
| b [Å] | 5.0003(4) | 5.8744(1) |
| c [Å] | 30.317(2) | 19.0522(6) |
| β [°] | 96.865(8) | 98.906(2) |
| V [Å3] | 1753.1(2) | 960.55(4) |
| Z | 8 | 2 |
| Density (calculated) [Mg/m3] | 1.403 | 1.509 |
| Absorption coefficient [mm−1] | 0.869 | 1.393 |
| F(000) | 768 | 452 |
| Theta range for data collection | 2.936 to 74.822 | 4.699 to 73.696 |
| Index ranges | −14 ≤ h ≤ 10 | −10 ≤ h ≤ 10 |
| −5 ≤ k ≤ 6 | −7 ≤ k ≤ 6 | |
| −37 ≤ l ≤ 36 | −23 ≤ l ≤ 22 | |
| Reflections collected | 4141 | 6598 |
| Independent reflections | 1685 | 3200 |
| R(int) | 0.0636 | 0.0258 |
| Completeness to theta = 67.684° [%] | 98.4 | 99.9 |
| Data/restraints/parameters | 1685/0/127 | 3200/0/273 |
| Goodness-of-fit on F2 | 1.031 | 1.071 |
| Final R indices [I > 2σ(I)] | R1 = 0.0714 wR2 = 0.1735 | R1 = 0.0361 wR2 = 0.0967 |
| R indices (all data) | R1 = 0.1245 wR2 = 0.2119 | R1 = 0.0381 wR2 = 0.0986 |
| Largest diff. peak and hole [eÅ−3] | 0.289 and −0.328 | 0.480 and −0.310 |
| Structure | Torsion Angle [°] | Conformation |
|---|---|---|
| GlySalLi-a | 135.4(4) | tilted anticlinal |
| GlySalNa-h | 118.3(3); 139.7(3) | anticlinal; tilted anticlinal |
| GlySalK-a * | 172.38(11) | antiperiplanar |
| GlySalK-h * | 124.4(4) | anticlinal |
| Compound | D-H···A | d(D-H) | d(H···A) | d(D···A) | <(DHA) |
|---|---|---|---|---|---|
| GlySalLi-a | N1-H1···O1 | 0.88 | 1.87 | 2.574(4) | 136.0 |
| C8-H8A···O3 (i) | 0.99 | 2.52 | 3.468(5) | 160.6 | |
| GlySalNa-h | N11-H11···O11 | 0.88 | 1.89 | 2.595(4) | 135.9 |
| C18-H18A···O23 | 0.99 | 2.61 | 3.309(3) | 127.3 | |
| C18-H18A···N21 (ii) | 0.99 | 2.67 | 3.651(3) | 168.6 | |
| C18-H18B···O11 (iii) | 0.99 | 2.50 | 3.389(4) | 149.1 | |
| C28-H28A···O21 (ii) | 0.99 | 2.71 | 3.351(4) | 122.8 | |
| O21-H21···N21 | 0.84 | 1.85 | 2.596(4) | 147.2 | |
| O81-H81A···O22 (iv) | 1.04 | 1.68 | 2.659(3) | 154.1 | |
| O81-H81B···O11 | 1.05 | 1.73 | 2.714(3) | 155.0 |
| Compound | Absorption (nm) | Emission (nm) | Stokes Shift (cm−1) | PLQY (%) |
|---|---|---|---|---|
| GlySalLi | 373 | 470 | 5533 | 12.1 |
| GlySalNa | 340 | 520 | 10,181 | 10.1 |
| GlySalK | 378 | 500 | 6455 | 8.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczyk, M.M.; Przypis, Ł.; Shiraki, T.; Kusz, J.; Książek, M.; Janas, D.; Kuźnik, N. Surprising Solid-State ESIPT Emission from Apparently Ordinary Salicyliden Glycinates Schiff Bases. Int. J. Mol. Sci. 2022, 23, 14955. https://doi.org/10.3390/ijms232314955
Tomczyk MM, Przypis Ł, Shiraki T, Kusz J, Książek M, Janas D, Kuźnik N. Surprising Solid-State ESIPT Emission from Apparently Ordinary Salicyliden Glycinates Schiff Bases. International Journal of Molecular Sciences. 2022; 23(23):14955. https://doi.org/10.3390/ijms232314955
Chicago/Turabian StyleTomczyk, Mateusz M., Łukasz Przypis, Tomohiro Shiraki, Joachim Kusz, Maria Książek, Dawid Janas, and Nikodem Kuźnik. 2022. "Surprising Solid-State ESIPT Emission from Apparently Ordinary Salicyliden Glycinates Schiff Bases" International Journal of Molecular Sciences 23, no. 23: 14955. https://doi.org/10.3390/ijms232314955
APA StyleTomczyk, M. M., Przypis, Ł., Shiraki, T., Kusz, J., Książek, M., Janas, D., & Kuźnik, N. (2022). Surprising Solid-State ESIPT Emission from Apparently Ordinary Salicyliden Glycinates Schiff Bases. International Journal of Molecular Sciences, 23(23), 14955. https://doi.org/10.3390/ijms232314955








