Mechanism of [CO2] Enrichment Alleviated Drought Stress in the Roots of Cucumber Seedlings Revealed via Proteomic and Biochemical Analysis
Abstract
1. Introduction
2. Results
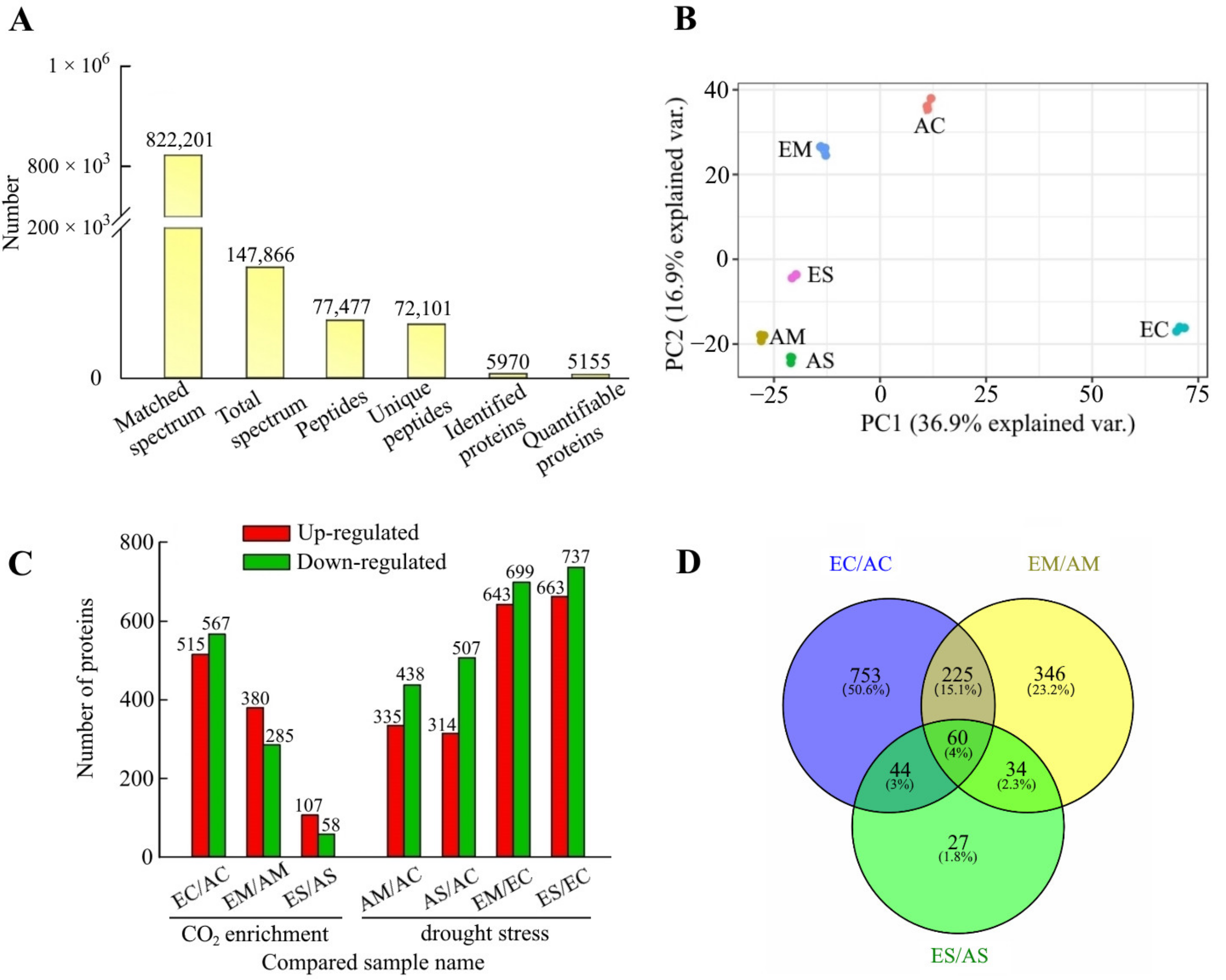
2.1. Overview of Quantitative Proteomic Responses to [CO2] Enrichment and Drought Stress
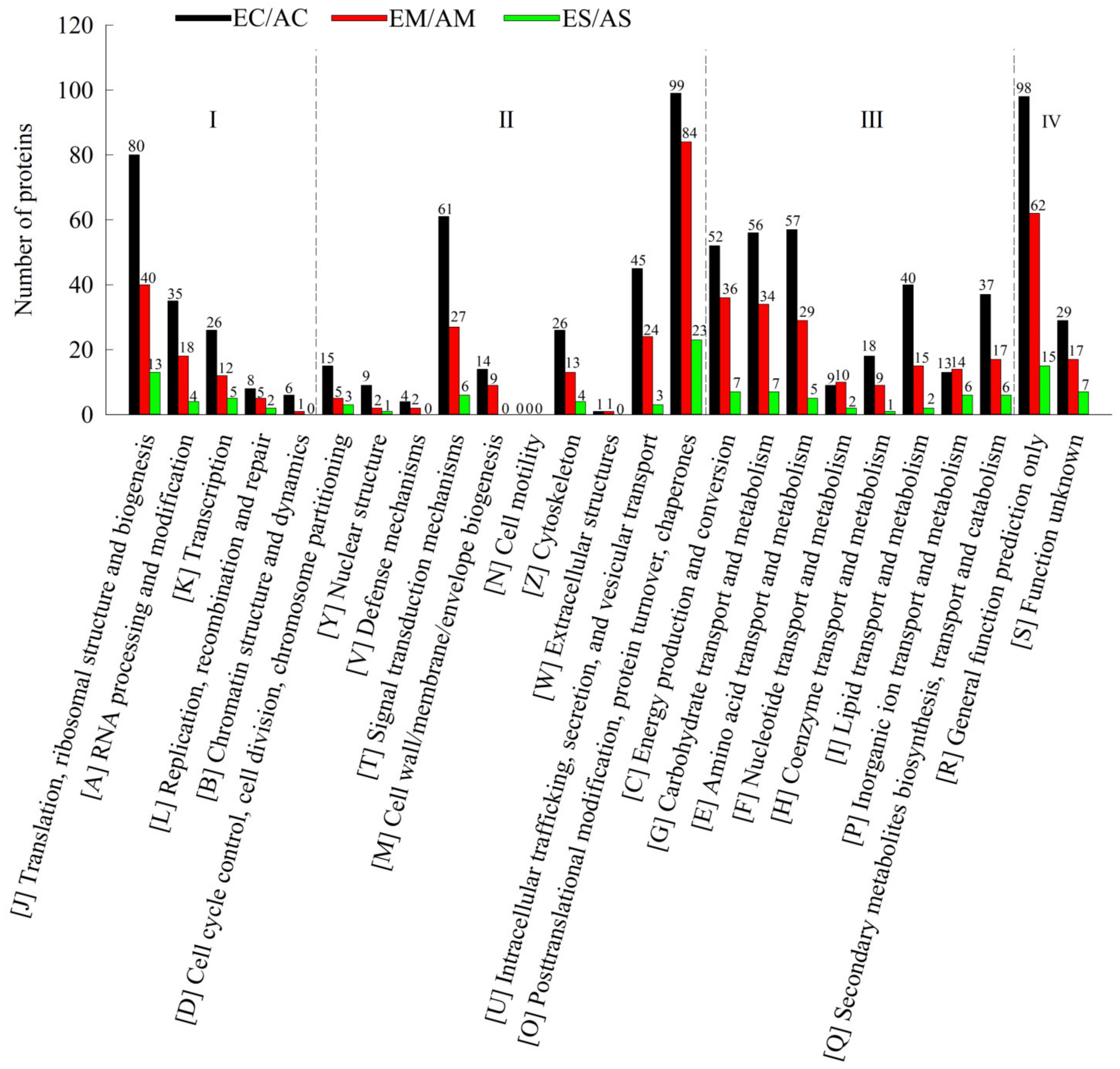
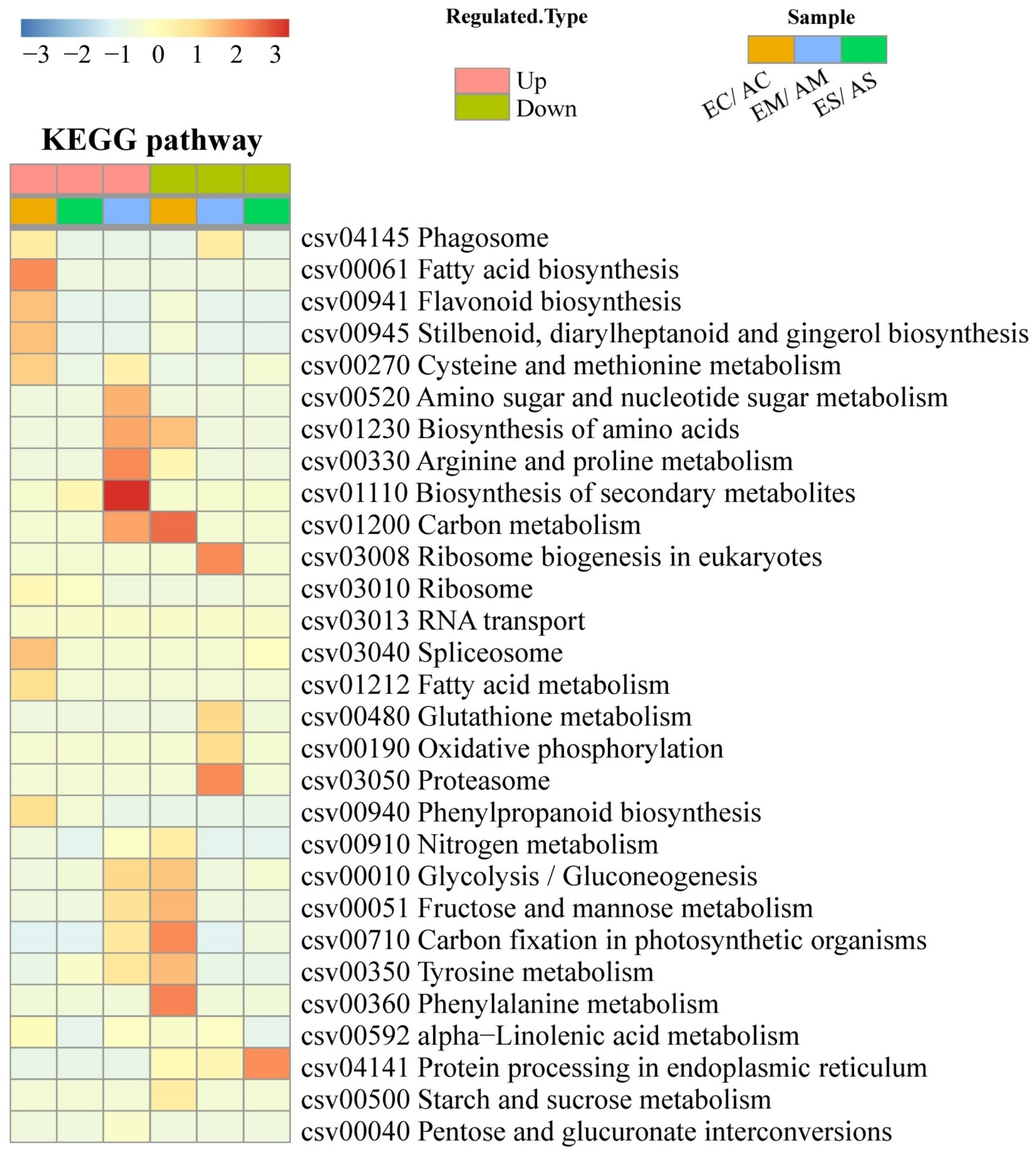
2.2. Hierarchical Clustering and Functional Classification Analysis of DAP Response to [CO2] Enrichment and Drought Stress
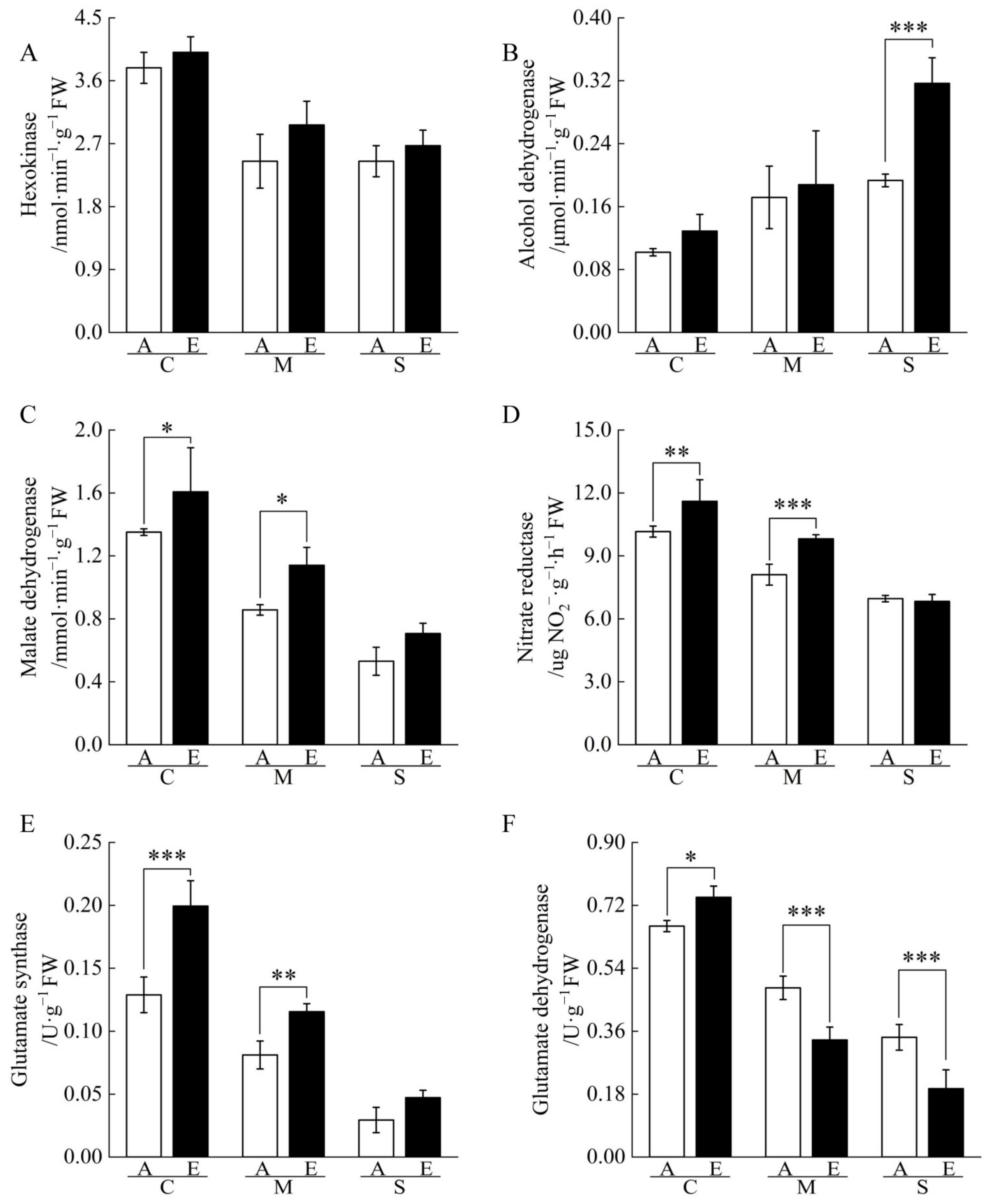
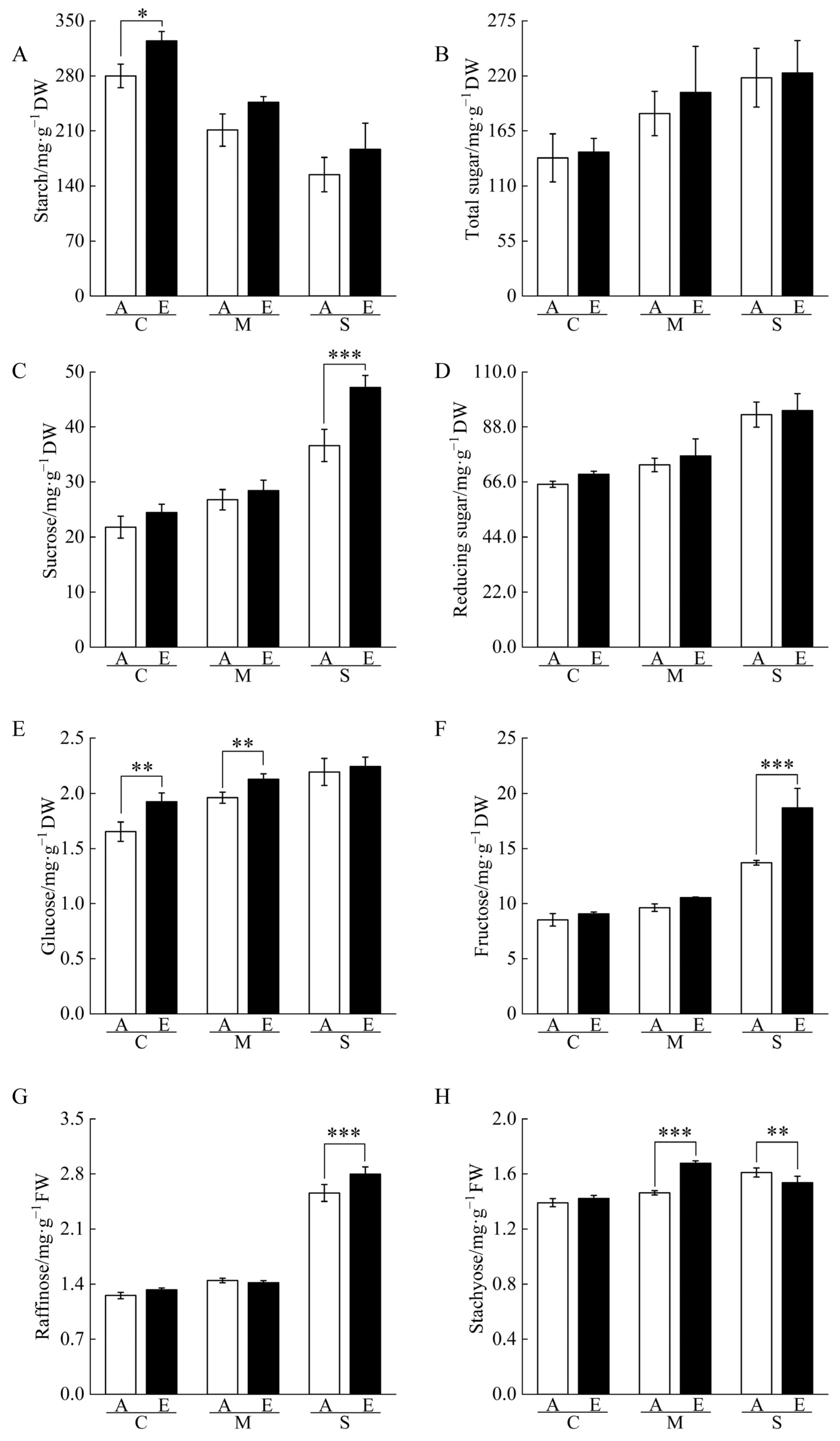
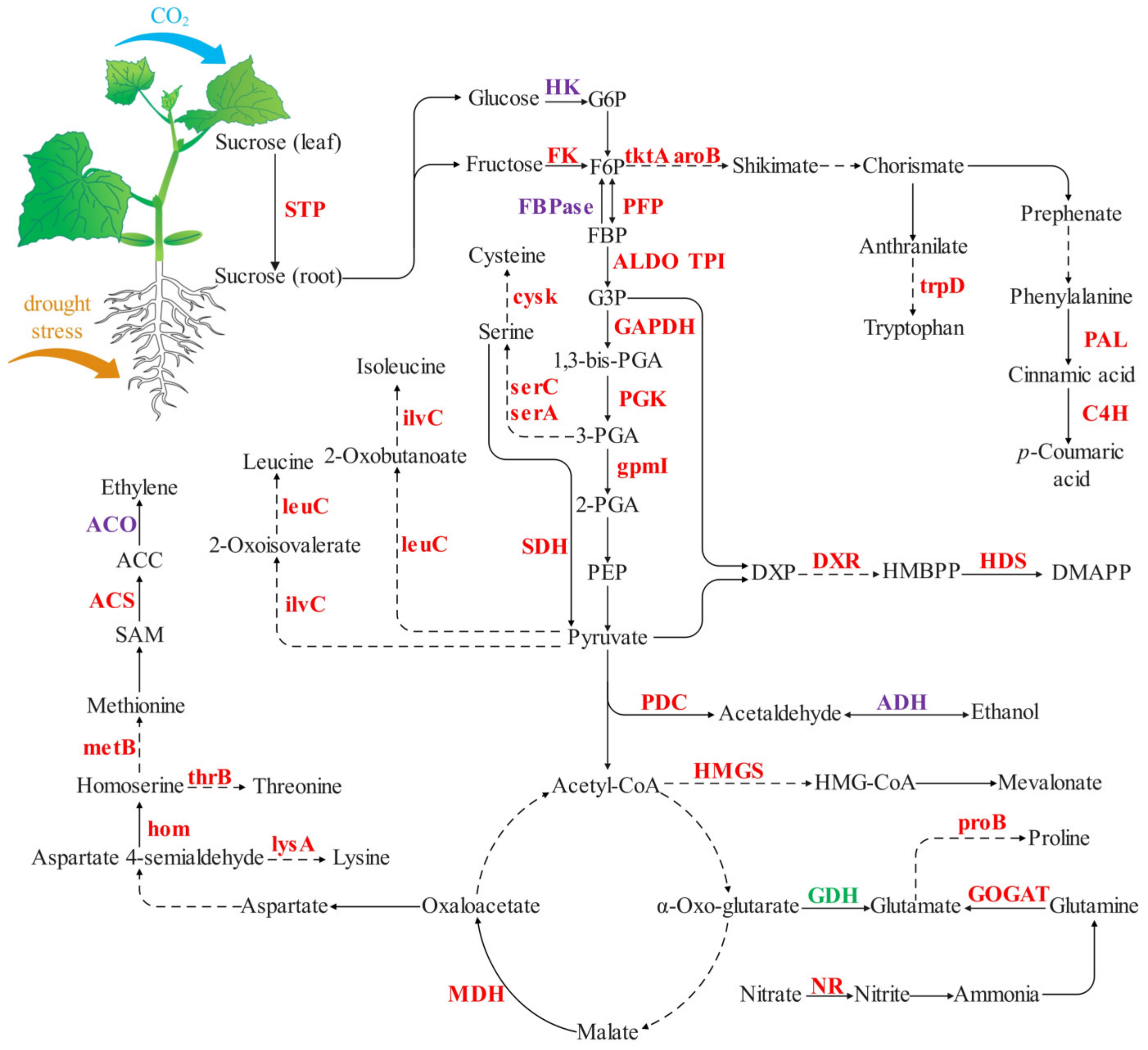
2.3. Non-Structural Carbohydrate Contents of Cucumber Seedling Roots under Drought Stress Changed by [CO2] Enrichment
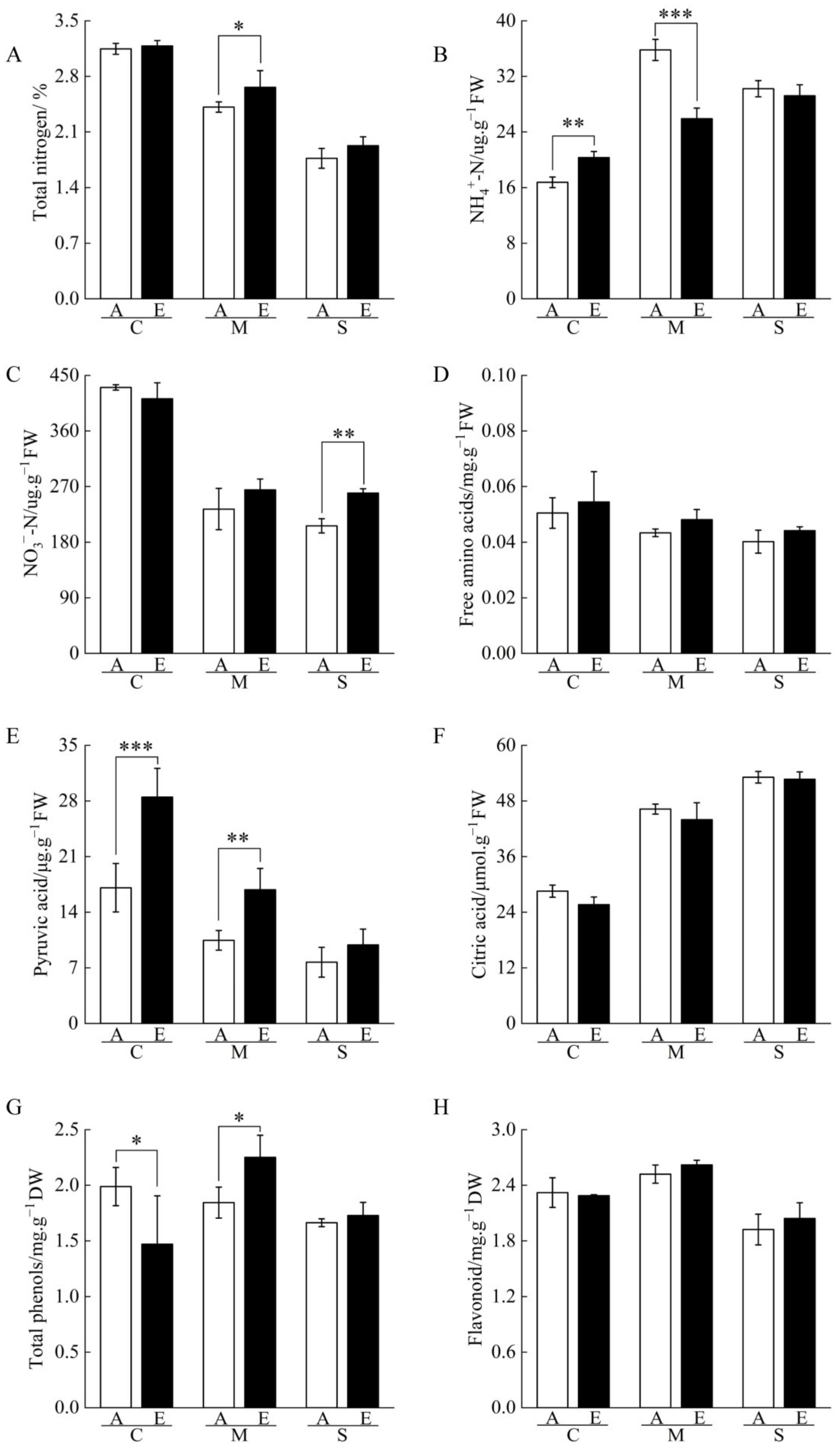
2.4. Nitrogen and Organic Acid Contents of Cucumber Seedling Roots under Drought Stress Regulated by [CO2] Enrichment
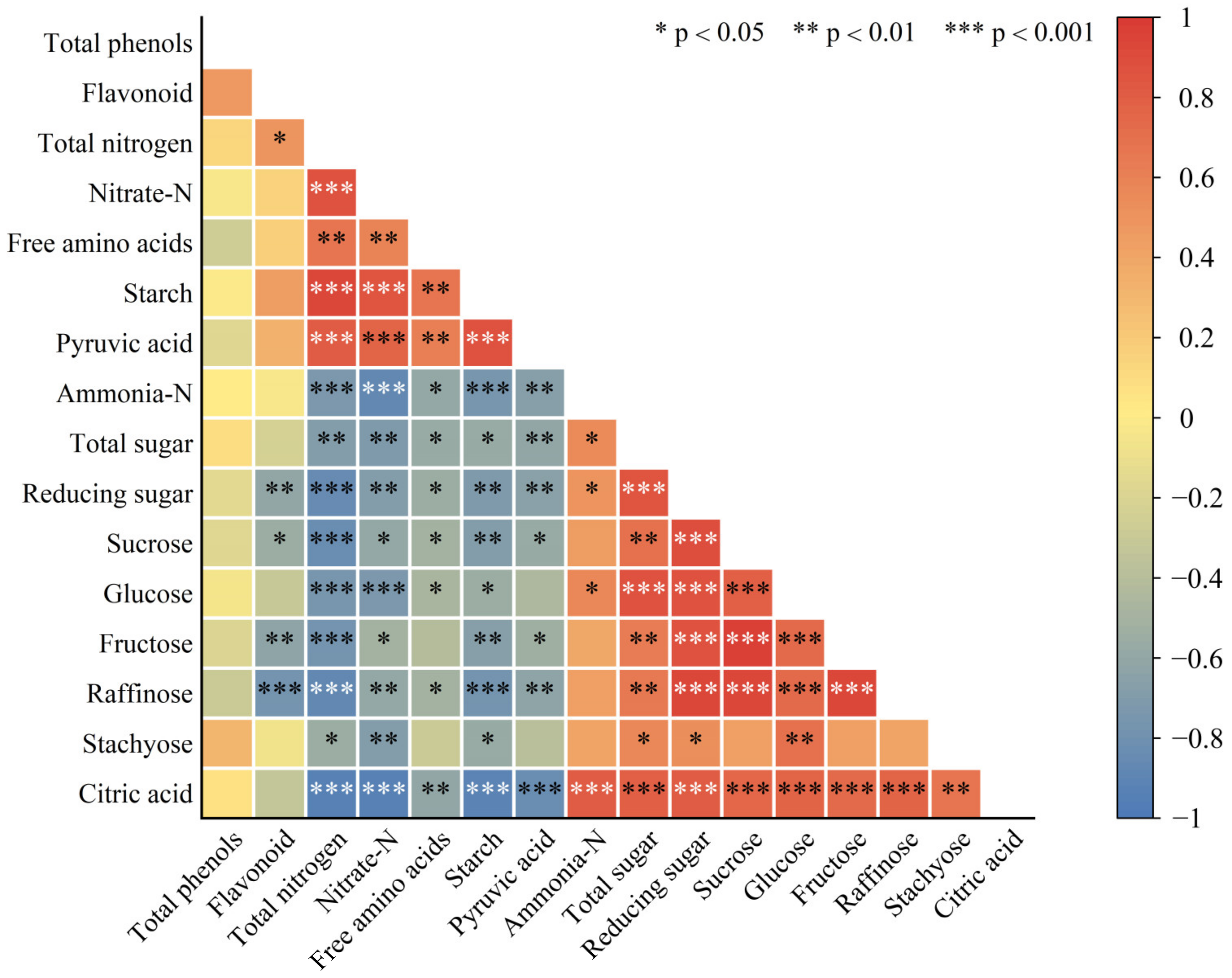
2.5. Correlation Analysis
3. Discussion
3.1. [CO2] Enrichment Improves Carbohydrates Content and Energy Metabolism
3.2. [CO2] Enrichment Improves Amino Acid Metabolism and N Remobilization
3.3. [CO2] Enrichment Reduces Drought-Induced Damage in Roots
4. Materials and Methods
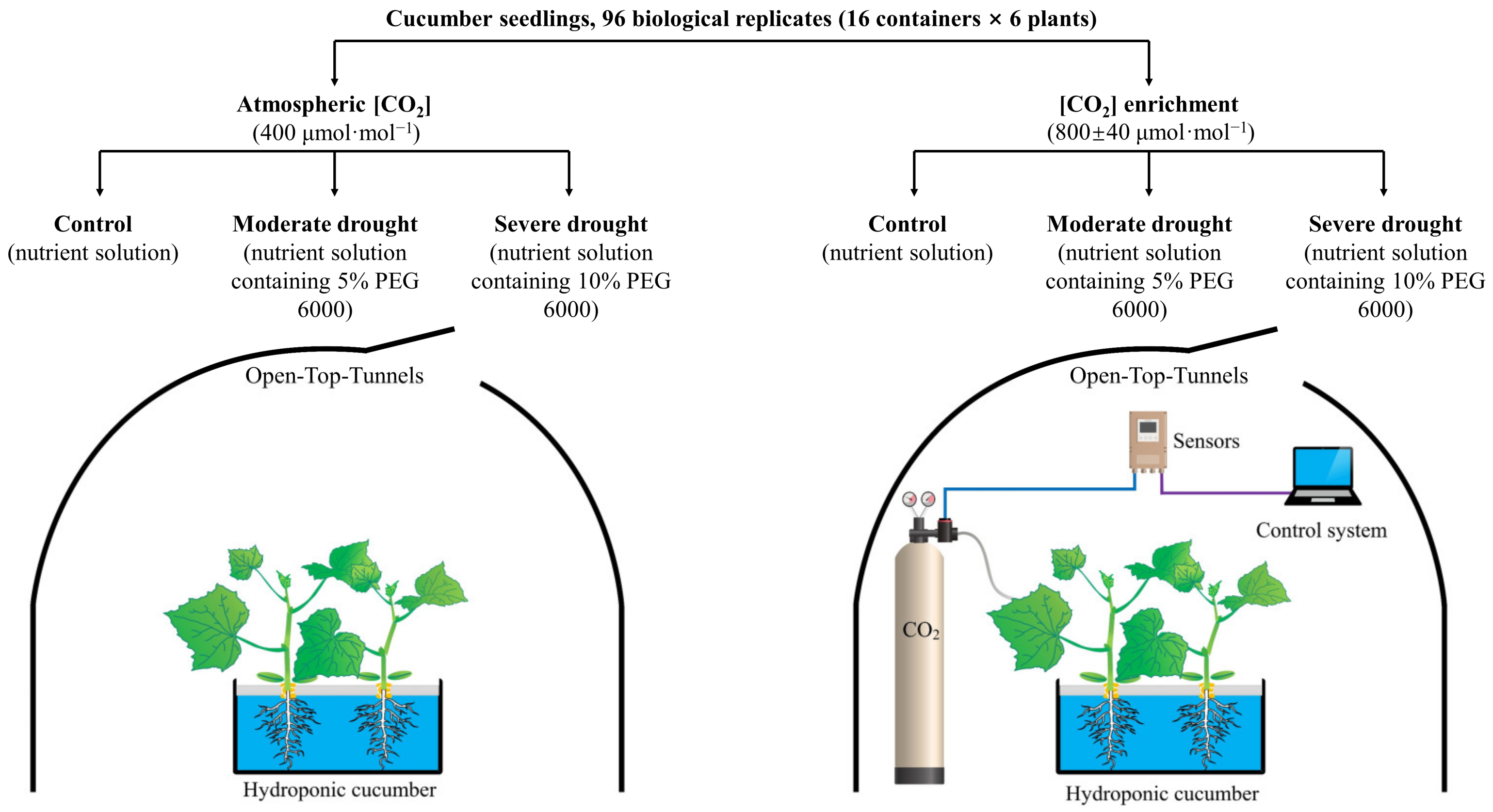
4.1. Plant Material, Growth Conditions, and Experimental Design
4.2. Measurements of Biochemical Indices
4.3. Protein Extraction, Trypsin Digestion, and TMT Labeling
4.4. LC-MS/MS Analysis, Database Search, and Functional Classification
4.5. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zargar, S.M.; Nagar, P.; Deshmukh, R.; Nazir, M.; Wani, A.A.; Masoodi, K.Z. Aquaporins as potential drought tolerance inducing proteins: Towards instigating stress tolerance. J. Proteom. 2017, 169, 233–238. [Google Scholar] [CrossRef]
- Feng, Q.; Ma, H.; Jiang, X.M.; Wang, X.; Cao, S. What has caused desertification in China? Sci. Rep. 2015, 51, 15998. [Google Scholar] [CrossRef] [PubMed]
- Van der Kooi, C.J.; Reich, M.; Low, M.; De Kok, L.J.; Tausz, M. Growth and yield stimulation under elevated CO2 and drought: A meta-analysis on crops. Environ. Exp. Bot. 2016, 122, 150–157. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air [CO2] enrichment FACE? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New. Phytol. 2005, 165, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Arndal, M.F.; Tolver, A.; Larsen, K.S.; Beier, C.; Schmidt, I.K. Fine root growth and vertical distribution in response to elevated CO2, warming and drought in a mixed heathland–grassland. Ecosystems 2018, 21, 15–30. [Google Scholar] [CrossRef]
- Pacholski, A.; Manderscheid, R.; Weigel, H.J. Effects of free air [CO2] enrichment on root growth of barley, sugar beet and wheat grown in a rotation under different nitrogen supply. Eur. J. Agron. 2015, 63, 36–46. [Google Scholar] [CrossRef]
- Li, Y.M.; He, X.R.; Li, Q.M.; Liu, B.B.; Li, S.H.; Ai, X.Z. Effect of [CO2] enrichment on antioxidant system in cucumber seedling root system under drought stress in Chinese with English abstract. Plant Physiol. Commun. 2018, 55, 1011–1019. [Google Scholar] [CrossRef]
- Li, Y.M.; Li, S.H.; He, X.R.; Jiang, W.L.; Zhang, D.L.; Liu, B.B. [CO2] enrichment enhanced drought resistance by regulating growth, hydraulic conductivity and phytohormone contents in the root of cucumber seedlings. Plant Physiol. Biochem. 2020, 152, 62–71. [Google Scholar] [CrossRef]
- Mohammadi, P.P.; Moieni, A.; Hiraga, S.; Komatsu, S. Organ-specific proteomic analysis of drought-stressed soybean seedlings. J. Proteom. 2011, 75, 1906–1923. [Google Scholar] [CrossRef]
- Mohammadi, P.P.; Moieni, A.; Komatsu, S. Comparative proteome analysis of drought-sensitive and drought-tolerant rapeseed roots and their hybrid F1 line under drought stress. Amino Acids 2012, 43, 2137–2152. [Google Scholar] [CrossRef]
- Ghaffari, M.; Toorchi, M.; Valizadeh, M.; Komatsu, S. Differential response of root proteome to drought stress in drought sensitive and tolerant sunflower inbred lines. Funct. Plant Biol. 2013, 40, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.J.; Li, N.Y.; Sun, J.; Hou, P.C.; Jing, X.S.; Zhu, H.P. Exogenous hydrogen peroxide, nitric oxide and calcium mediate root ion fluxes in two non-secretor mangrove species subjected to NaCl stress. Tree Physiol. 2013, 33, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Kaur, V.; Mahla, R.; Behl, R. Research Article High temperature, drought and their interaction induced protein alterations in sensitive and tolerant wheat varieties. Electron. J. Plant Breed. 2014, 5, 641–650. Available online: https://www.researchgate.net/publication/320014897 (accessed on 12 March 2022).
- Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 2013, 14, 630–642. [Google Scholar] [CrossRef]
- Webb, K.M.; Broccardo, C.J.; Prenni, J.E.; Wintermantel, W.M. Proteomic profiling of sugar beet Beta vulgaris leaves during rhizomania compatible interactions. Proteomes 2014, 2, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Bian, B.T.; Gong, T.Y.; Liao, W.B. Comparative proteomic analysis of key proteins during abscisic acid-hydrogen peroxide-induced adventitious rooting in cucumber (Cucumis sativus L.) under drought stress. J. Plant Physiol. 2018, 229, 185–194. [Google Scholar] [CrossRef]
- Burgess, P.; Huang, B.R. Root protein metabolism in association with improved root growth and drought tolerance by elevated carbon dioxide in creeping bentgrass. Field Crops Res. 2014, 165, 80–91. [Google Scholar] [CrossRef]
- Wang, X.L.; Cai, X.F.; Xu, C.X.; Wang, Q.H.; Dai, S.J. Drought-responsive mechanisms in plant leaves revealed by proteomics. Int. J. Mol. Sci. 2016, 17, 1706. [Google Scholar] [CrossRef]
- He, J.Q.; Hu, W.; Li, Y.X.; Zhu, H.H.; Zou, J.; Wang, Y.H. Prolonged drought affects the interaction of carbon and nitrogen metabolism in root and shoot of cotton. Environ. Exp. Bot. 2022, 197, 104839. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Xu, F.X.; Richter, K.T.; McGrath, J.M.; Markelz, R.J.C.; Ort, D.R. Greater antioxidant and respiratory metabolism in field-grown soybean exposed to elevated O3 under both ambient and elevated CO2. Plant Cell Environ. 2012, 35, 169–184. [Google Scholar] [CrossRef]
- Klein, T.; Hoch, G.; Yakir, D.; Korner, C. Drought stress, growth and nonstructural carbohydrate dynamics of pine trees in a semi-arid forest. Tree Physiol. 2014, 34, 981–992. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Change 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Aluko, O.O.; Li, C.; Wang, Q.; Liu, H. Sucrose utilization for improved crop yields: A review article. Int. J. Mol. Sci. 2021, 22, 4704. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, Y.; Zhao, X.; Han, L.N.; Yang, S.J. Effects of CO2 on transplantation of grape plantlets cultured in vitro by promoting photosynthesis. Sci. Hortic. 2021, 287, 110286. [Google Scholar] [CrossRef]
- Calvo, O.C.; Franzaring, J.; Schmid, I.; Fangmeier, A. Root exudation of carbohydrates and cations from barley in response to drought and elevated CO2. Plant Soil 2019, 438, 127–142. [Google Scholar] [CrossRef]
- Li, X.; Dong, J.; Chu, W.; Chen, Y.; Duan, Z. The relationship between root exudation properties and root morphological traits of cucumber grown under different nitrogen supplies and atmospheric CO2 concentrations. Plant Soil 2018, 425, 415–432. [Google Scholar] [CrossRef]
- Du, J.; Guo, S.R.; Sun, J.; Shu, S. Proteomic and physiological analyses reveal the role of exogenous spermidine on cucumber roots in response to Ca(NO3)2 stress. Plant Mol. Biol. 2018, 97, 1–21. [Google Scholar] [CrossRef]
- Guo, X.M.; Ronhovde, K.; Yuan, L.L.; Yao, B.; Soundararajan, M.P.; Elthon, T. Pyrophosphate-dependent fructose-6-phosphate 1-phosphotransferase induction and attenuation of Hsp gene expression during endosperm modification in quality protein maize. Plant Physiol. 2012, 158, 917–929. [Google Scholar] [CrossRef][Green Version]
- Degenkolbe, T.; Do, P.T.; Kopka, J.; Zuther, E.; Hincha, D.K.; Kohl, K.I. Identification of drought tolerance markers in a diverse population of rice cultivars by expression and metabolite profiling. PLoS ONE 2013, 8, 14. [Google Scholar] [CrossRef]
- Kappachery, S.; Baniekal-Hiremath, G.; Yu, J.W.; Park, S.W. Effect of over-and under-expression of glyceraldehyde 3-phosphate dehydrogenase on tolerance of plants to water-deficit stress. Plant Cell Tissue Organ Cult. 2015, 121, 97–107. [Google Scholar] [CrossRef]
- Zhang, X.H.; Rao, X.L.; Shi, H.T.; Li, R.J.; Lu, Y.T. Overexpression of a cytosolic glyceraldehyde-3-phosphate dehydrogenase gene OsGAPC3 confers salt tolerance in rice. Plant Cell Tissue Organ Cult. 2011, 107, 1. [Google Scholar] [CrossRef]
- Diab, A.; Kantety, R.; Ozturk Gokce, N.; Benscher, D.; Nachit, M.; Sorrells, M. Drought—Inducible genes and differentially expressed sequence tags associated with components of drought tolerance in durum wheat. Sci. Res. Essays 2008, 3, 9–26. Available online: http://www.academicjournals.org/journal/SRE/article-abstract/8BF3F7014195 (accessed on 12 March 2022).
- Li, S.H.; Li, Y.M.; Gao, Y.; He, X.R.; Zhang, D.L.; Liu, B.B. Effects of [CO2] enrichment on non-structural carbohydrate metabolism in leaves of cucumber seedlings under salt stress. Sci. Hortic. 2020, 265, 109275. [Google Scholar] [CrossRef]
- Manaa, A.; Ben Ahmed, H.; Valot, B.; Bouchet, J.P.; Aschi-Smiti, S.; Causse, M. Salt and genotype impact on plant physiology and root proteome variations in tomato. J. Exp. Bot. 2011, 62, 2797–2813. [Google Scholar] [CrossRef] [PubMed]
- Zadraznik, T.; Hollung, K.; Egge-Jacobsen, W.; Meglic, V.; Sustar-Vozlic, J. Differential proteomic analysis of drought stress response in leaves of common bean Phaseolus vulgaris L. J. Proteom. 2013, 78, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Chen, G.; Yoo, M.J.; Zhu, N.; Dufresne, D.; Erickson, J.E. Comparative proteomic analysis of brassica napus in response to drought stress. J. Proteome Res. 2015, 14, 3068–3081. [Google Scholar] [CrossRef]
- Li, S.H.; Li, Y.M.; He, X.R.; Li, Q.M.; Liu, B.B.; Ai, X.Z. Response of water balance and nitrogen assimilation in cucumber seedlings to [CO2] enrichment and salt stress. Plant Physiol. Biochem. 2019, 139, 256–263. [Google Scholar] [CrossRef]
- Zeng, W.J.; Peng, Y.L.; Zhao, X.Q.; Wu, B.Y.; Chen, F.Q.; Ren, B. Comparative proteomics analysis of the seedling root response of drought-sensitive and drought-tolerant maize varieties to drought stress. Int. J. Mol. Sci. 2019, 20, 2793. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, M.F.; Chen, H.; Qu, L.Q.; Chen, F.; Shen, S.H. Abscisic acid pretreatment enhances salt tolerance of rice seedlings: Proteomic evidence. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2010, 1804, 929–940. [Google Scholar] [CrossRef]
- Prinsi, B.; Espen, L. Mineral nitrogen sources differently affect root glutamine synthetase isoforms and amino acid balance among organs in maize. BMC Plant Biol. 2015, 15, 13. [Google Scholar] [CrossRef]
- Raveneau, M.P.; Benamar, A.; Macherel, D. Water content, adenylate kinase, and mitochondria drive adenylate balance in dehydrating and imbibing seeds. J. Exp. Bot. 2017, 68, 3501–3512. [Google Scholar] [CrossRef]
- Bannenberg, G.; Martínez, M.; Hamberg, M.; Castresana, C. Diversity of the enzymatic activity in the lipoxygenase gene family of arabidopsis thaliana. Lipids 2008, 44, 85. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.Q.; Li, Y.M.; He, X.R.; Li, S.H.; Zhong, X.; Liu, B.B. Physiological and iTRAQ based proteomics analyses reveal the mechanism of elevated CO2 concentration alleviating drought stress in cucumber Cucumis sativus L. seedlings. Plant Physiol. Biochem. 2019, 143, 142–153. [Google Scholar] [CrossRef]
- Jedmowski, C.; Ashoub, A.; Beckhaus, T.; Berberich, T.; Karas, M.; Brüggemann, W. Comparative analysis of sorghum bicolor proteome in response to drought stress and following recovery. Int. J. Proteom. 2014, 2014, 395905. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.H.; Palaniyandi, S.A.; Yang, S.H.; Suh, J.W. Expression of potato S-adenosyl-L-methionine synthase SbSAMS gene altered developmental characteristics and stress responses in transgenic Arabidopsis plants. Plant Physiol. Biochem. 2015, 87, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Y.; Zhang, J.F.; Zhang, H.W.; Zhang, Z.J.; Quan, R.D.; Zhou, S.R. Transcriptional activation of OsDERF1 in OsERF3 and OsAP2-39 negatively modulates ethylene synthesis and drought tolerance in rice. PLoS ONE 2011, 6, e25216. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bharalee, R.; Bhorali, P.; Bandyopadhyay, T.; Gohain, B.; Agarwal, N. Identification of drought tolerant progenies in tea by gene expression analysis. Funct. Integr. Genom. 2012, 12, 543–563. [Google Scholar] [CrossRef]
- Lyzenga, W.J.; Stone, S.L. Abiotic stress tolerance mediated by protein ubiquitination. J. Exp. Bot. 2012, 63, 599–616. [Google Scholar] [CrossRef]
- Wan, X.; Mo, A.; Liu, S.; Yang, L.; Li, L. Constitutive expression of a peanut ubiquitin-conjugating enzyme gene in Arabidopsis confers improved water-stress tolerance through regulation of stress-responsive gene expression. J. Biosci. Bioeng. 2010, 111, 478–484. [Google Scholar] [CrossRef]
- Piasecka, A.; Sawikowska, A.; Kuczynska, A.; Ogrodowicz, P.; Mikolajczak, K.; Krystkowiak, K. Drought-related secondary metabolites of barley Hordeum vulgare L. leaves and their metabolomic quantitative trait loci. Plant J. 2017, 89, 898–913. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.M.; Zhang, W.D.; Li, S.H.; Gao, Y.; Ai, X.Z. Metabolomics analysis reveals that elevated atmospheric CO2 alleviates drought stress in cucumber seedling leaves. Anal. Biochem. 2018, 559, 71–85. [Google Scholar] [CrossRef]
- Kiba, T.; Takebayashi, Y.; Kojima, M.; Sakakibara, H. Sugar-induced de novo cytokinin biosynthesis contributes to Arabidopsis growth under elevated CO2. Sci. Rep. 2019, 9, 7765. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Tyagi, K.; Maoz, I.; Kochanek, B.; Sela, N.; Lerno, L.; Ebeler, S.E. Cytokinin but not gibberellin application had major impact on the phenylpropanoid pathway in grape. Hortic. Res. 2021, 8, 51. [Google Scholar] [CrossRef]
- Ma, Q.L.; Kang, J.M.; Long, R.C.; Zhang, T.J.; Xiong, J.B.; Zhang, K. Comparative proteomic analysis of alfalfa revealed new salt and drought stress-related factors involved in seed germination. Mol. Biol. Rep. 2017, 44, 261–272. [Google Scholar] [CrossRef]
- Costa, M.C.D.; Righetti, K.; Nijveen, H.; Yazdanpanah, F.; Ligterink, W.; Buitink, J. A gene co-expression network predicts functional genes controlling the re-establishment of desiccation tolerance in germinated Arabidopsis thaliana seeds. Planta 2015, 242, 435–449. [Google Scholar] [CrossRef]
- Cho, S.K.; Kim, J.E.; Park, J.; Eom, T.J.; Kim, W.T. Constitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Lett. 2006, 580, 3136–3144. [Google Scholar] [CrossRef]
- Jin, H.J.; Xu, M.J.; Chen, H.; Zhang, S.R.; Han, X.Y.; Tang, Z.H. Comparative proteomic analysis of differentially expressed proteins in Amaranthus hybridus L. roots under cadmium stress. Water Air Soil Pollut. 2016, 227, 12. [Google Scholar] [CrossRef]
- Takac, T.; Pechan, T.; Richter, H.; Muller, J.; Eck, C.; Bohm, N. Proteomics on brefeldin a-treated arabidopsis roots reveals profilin 2 as a new protein involved in the cross-talk between vesicular trafficking and the actin cytoskeleton. J. Proteome Res. 2011, 10, 488–501. [Google Scholar] [CrossRef]
- Mondal, H.A.; Louis, J.; Archer, L.; Patel, M.; Nalam, V.J.; Sarowar, S. Arabidopsis actin-depolymerizing factor3 is required for controlling aphid feeding from the phloem. Plant Physiol. 2018, 176, 879–890. [Google Scholar] [CrossRef]
- Zhong, D.H.; Du, H.M.; Wang, Z.L.; Huang, B.R. Genotypic variation in fatty acid composition and unsaturation levels in bermudagrass associated with leaf dehydration tolerance. J. Am. Soc. Hortic. Sci. 2011, 136, 35–40. [Google Scholar] [CrossRef]
- Gasulla, F.; vom Dorp, K.; Dombrink, I.; Zahringer, U.; Gisch, N.; Dormann, P. The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: A comparative approach. Plant J. 2013, 75, 726–741. [Google Scholar] [CrossRef]
- Rosa, M.; Hilal, M.; González, J.A.; Prado, F.E. Low-temperature effect on enzyme activities involved in sucrose–starch partitioning in salt-stressed and salt-acclimated cotyledons of quinoa Chenopodium quinoa Willd. seedlings. Plant Physiol. Biochem. 2009, 47, 300–307. [Google Scholar] [CrossRef]
- Wang, D.H.; Shi, Q.H.; Wang, X.F.; Wei, M.; Hu, J.Y.; Liu, J. Influence of cow manure vermicompost on the growth, metabolite contents, and antioxidant activities of Chinese cabbage Brassica campestris ssp. chinensis. Biol. Fertil. Soils 2010, 46, 689–696. [Google Scholar] [CrossRef]
- Lü, J.G.; Sui, X.L.; Ma, S.; Li, X.; Liu, H.; Zhang, Z.X. Suppression of cucumber stachyose synthase gene CsSTS inhibits phloem loading and reduces low temperature stress tolerance. Plant Mol. Biol. 2017, 95, 1–15. [Google Scholar] [CrossRef]
- Aurisano, N.; Bertani, A.; Reggiani, R. Involvement of calcium and calmodulin in protein and amino acid metabolism in rice roots under anoxia. Plant Cell Physiol. 1995, 36, 1525–1529. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Solorzano, L. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol. Oceanogr. 1969, 14, 799–801. [Google Scholar] [CrossRef]
- Datta, R.; Sharma, R. Temporal and spatial regulation of nitrate reductase and nitrite reductase in greening maize leaves. Plant Sci. 1999, 144, 77–83. [Google Scholar] [CrossRef]
- Ma, J.; Chen, T.; Wu, S.; Yang, C.; Bai, M.; Shu, K. iProX: An integrated proteome resource. Nucleic Acids Res. 2019, 47, D1211–D1217. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, W.; Zhang, D.; Zheng, Y.; Xu, Y.; Liu, B.; Li, Q. Mechanism of [CO2] Enrichment Alleviated Drought Stress in the Roots of Cucumber Seedlings Revealed via Proteomic and Biochemical Analysis. Int. J. Mol. Sci. 2022, 23, 14911. https://doi.org/10.3390/ijms232314911
Li Y, Zhang W, Zhang D, Zheng Y, Xu Y, Liu B, Li Q. Mechanism of [CO2] Enrichment Alleviated Drought Stress in the Roots of Cucumber Seedlings Revealed via Proteomic and Biochemical Analysis. International Journal of Molecular Sciences. 2022; 23(23):14911. https://doi.org/10.3390/ijms232314911
Chicago/Turabian StyleLi, Yiman, Wendong Zhang, Dalong Zhang, Yinjian Zheng, Yaliang Xu, Binbin Liu, and Qingming Li. 2022. "Mechanism of [CO2] Enrichment Alleviated Drought Stress in the Roots of Cucumber Seedlings Revealed via Proteomic and Biochemical Analysis" International Journal of Molecular Sciences 23, no. 23: 14911. https://doi.org/10.3390/ijms232314911
APA StyleLi, Y., Zhang, W., Zhang, D., Zheng, Y., Xu, Y., Liu, B., & Li, Q. (2022). Mechanism of [CO2] Enrichment Alleviated Drought Stress in the Roots of Cucumber Seedlings Revealed via Proteomic and Biochemical Analysis. International Journal of Molecular Sciences, 23(23), 14911. https://doi.org/10.3390/ijms232314911







